Abstract
In schistosomiasis mansoni, helminth eggs secrete soluble egg antigens (SEA) that induce T-cell-mediated granulomatous tissue responses. The cloned 38-kDa peptide (p38) of SEA was shown to induce and elicit Th1-type responsiveness in H-2k mice. Subsequently, the immunodominant T-cell epitope (P4) of p38 was shown to elicit pulmonary granuloma formation and Th1-type cytokine production in sensitized or infected mice. Here, we report that the immune response to p38 or P4 can be polarized to a Th1 or Th2 profile when the peptides are presented intraperitoneally in soluble recombinant interleukin-12 (IL-12) or alum adjuvant, respectively. The Th1 or Th2 profile was verified by cytokine secretion, enzyme-linked spot assay, and antibody isotype characterization. Importantly, the polarized immune response generated two types of pulmonary granulomas around injected P4-coated beads. The type 1 granulomas were smaller and contained mononuclear cells and occasional thin strands of deposited collagen. In contrast, the type 2 lesions were larger and contained mononuclear cells, large numbers of eosinophils, and several thick bands of deposited collagen. By reverse transcription-PCR cytokine, message in the type 1 granuloma-bearing lungs was found for gamma interferon, tumor necrosis factor alpha, and inducible nitric oxide synthase but not for IL-4 or IL-5. Conversely, lungs with type 2 granulomas had message only for IL-4 and IL-5. These results show that in the proper cytokine environment, the response to a strong Th1 inducer peptide can be deviated to a Th2 profile.
Granuloma formation around deposited parasite eggs and subsequent tissue fibrosis are the major pathological manifestation of schistosomiasis mansoni (2, 8). In the murine model, it has been established that this typical inflammatory response is mediated by CD4+ Th helper lymphocytes (27) in response to soluble egg antigens (SEA) secreted by the live miracidia within the eggs (3). During the evolution of granulomas, the pattern of Th cytokine response to SEA was shown to undergo a dynamic switch from an early Th1/Th0 to a predominant Th2 phenotype (15, 32, 36, 41). The predominant SEA peptide-specific Th2-type cytokine response of mice (25) was correlated with the typical histological feature of the mature granulomatous inflammation, i.e., high percentage of eosinophils and large amount of collagen content. Neutralization in infected mice of endogenously produced interleukin-4 (IL-4) (40), infection in IL-4-deficient mice (18, 28, 31), or downregulation of the Th2 response by exogenous recombinant IL-12 (rIL-12) treatment (4, 39) all resulted in diminished granuloma development.
Previously it has been demonstrated that a wide range of larval cross-reactive and egg-specific antigenic fractions of SEA are involved in the Th cell responsiveness during granuloma formation (16, 21, 23, 24). Recently, one of the major protein components of SEA, the cloned 38- to 40-kDa peptide (p38) (6, 29), was shown in S. mansoni-infected H-2k haplotype mice to be primarily responsible for the SEA-specific early Th1-type cytokine response. Immunization of mice intraperitoneally (i.p.) with p38 also induced predominantly a Th1-type cytokine response with mononuclear granulomas around peptide-coated beads (6). These data suggested that p38 is a preferential Th1 inducer that may play an important role in the early phase of granuloma formation. Recently we identified within p38 a 15-mer immunodominant T-cell epitope, P4, which also elicited pulmonary granuloma formation in mice sensitized with p38 and incomplete Freund’s adjuvant (IFA) (10). Another laboratory using T-cell hybridomas localized the immunodominant epitope within the same domain (17). The availability of a short, well-defined immunogenic peptide provided a tool for the analysis of the conditions whereby a Th1- or Th2-type cell responsiveness with corresponding phenotypic changes in granuloma is induced.
Results indicate that depending on the mode of antigen presentation, Th1- or Th2-type granulomas differing in size, cellularity, cytokine profile, and collagen content can be generated.
MATERIALS AND METHODS
Mice.
Female CBA/Jk mice purchased from Jackson Laboratory, Bar Harbor, Maine, were used in all experiments. The mice were maintained under standard laboratory care.
Preparation of antigenic peptides.
rp38 was produced in Escherichia coli which carried the recombinant pGEX vector with the isopropyl-β-d-thiogalactopyranoside-inducible gene for expression of the glutathione S-transferase–p38 fusion protein. The fusion protein was purified by a bulk glutathione S-transferase purification module (Pharmacia Biotechnology, Piscataway, N.J.) as described previously (6). The peptide solution was mixed with n-octyl-β-d-glucopyranoside and run through polymyxin B-bound agarose to remove endotoxin contamination (19). Assay with an Endotect kit (ICN Biochemical Inc., Aurora, Ohio) found no detectable level of endotoxin. After dialysis against phosphate-buffered saline (PBS), the peptide was filter sterilized and the protein content was determined by the Bradford method (Bio-Rad Laboratories, Richmond, Calif.). The P4 peptide was synthesized with a Dupont RaMPS solid-phase peptide synthesizer (kindly provided by R. H. Swanborg, Wayne State University School of Medicine).
Immunization of mice.
Naive 6- to 8-week-old mice were immunized subcutaneously (s.c.) or i.p. in a volume of 0.2 ml with 3 μg of p38 or 1 μg of P4 incorporated into complete Freund’s adjuvant, IFA, 100 μg of alum in 0.1 ml of PBS (Rehydrogel low-viscosity gel; Rehies, Inc., Berkeley Heights, N.J.) or 1 μg of rIL-12 to induce the antigen-specific immune response.
Determination of antigen-specific cytokine production.
Spleens from at least three immunized mice were aseptically removed, and single cell suspensions were prepared after removal of erythrocytes by hypotonic lysis. Cells were resuspended in RPMI 1640 supplemented with 2 mM l-glutamine, 50 U of penicillin per ml, and 50 μg of streptomycin per ml, 10 mM HEPES, 0.2 mM sodium pyruvate, 50 μM 2-mercaptoethanol, and 10% fetal calf serum (FCS). A concentration of 5 × 106 cells per ml was incubated with p38 (5 μg/ml) or P4 peptide (1 μg/ml). Supernatants were collected at 24 h for IL-2 determination and at 48 h for gamma interferon (IFN-γ), IL-4, and IL-5 measurements. IL-2 levels in culture supernatants were determined by using the IL-2-dependent CTLL-20 cell line (a generous gift from Frank Fitch, University of Chicago, Ill.). The specificity of this assay was confirmed by the complete abrogation of proliferative responses with anti-IL-2 monoclonal antibody S4B6 (kindly provided by DNAX Corporation, Palo Alto, Calif.). A standard curve for IL-2 was generated by using murine rIL-2 (generously donated by Cetus Corporation, Emeryville, Calif.). IFN-γ, IL-4, and IL-5 were measured by an enzyme-linked immunosorbent assay (ELISA) using paired antibodies with and without biotinylation (purchased from Pharmingen, San Diego, Calif.) and streptavidin-alkaline phosphatase conjugate (Sigma Chemical Co., St. Louis, Mo.). Color was developed by nitrophenyl diamine diethanolamine as substrate, and the optical densities of wells were measured at 405 nm. Standard curves for IFN-γ, IL-4, and IL-5 were made by using dilutions of recombinant cytokines (rIFN-γ and rIL-4 were generously donated by Genentech Inc., South San Francisco, Calif., and Immunex Corporation, Seattle, Wash., respectively; rIL-5 was purchased from Pharmingen).
Enzyme-linked spot (ELISPOT) assay.
Spleen cell suspensions of immunized mice were prepared as described above. Except for anti-IL-2 antibody (Pharmingen), the antibodies used for determining IFN-γ, IL-4, and IL-5 production were from the same sources as those used for ELISA. Antibodies were coated at 2.5 μg/ml on Immunolon 4 microtiter plates (Costar, Cambridge, Mass.) overnight at 4°C. After three washes with PBS, the plates were blocked with 200 μl of 5% FCS and incubated at room temperature for 2 h. The wells were washed six times with PBS. The spleen cell suspensions were then added at a concentration of 106 per well and at threefold dilutions with or without 1 μg of P4 peptide per ml. Twenty hours later, the plates were washed with distilled water to remove the cells, and 50 μl of 1-μg/ml concentrations of the various biotin-labeled detecting antibodies in PBS with 5% FCS were added. The plates were incubated at room temperature for 1 h and washed, and 50 μl of a 1:40,000 dilution of streptavidin-alkaline phosphatase conjugate (Sigma) in PBS with 5% FCS was added for 1 h. Finally, the plates were washed, and 100 μl of 5-bromo-4-chloro-3-indolylphosphate phosphatase substrate (Sigma) was added. The development of blue spots was stopped in 30 min by washing with running water. The individual cytokine-secreting cells were enumerated by using a 10× eyepiece of a microscope.
Determination of antigen-specific antibody isotype response.
The sera of variously sensitized mice were collected, and levels of p38-specific total immunoglobulin G1 (IgG1) and IgG2a were determined by ELISA. The coating antigen was p38 at 5 μg/ml. The serum samples were added with series of threefold dilution. The detecting antibodies were rat anti-mouse IgG1 or IgG2a (Pharmingen) paired with alkaline phosphatase-conjugated goat anti-rat antibody (Organon Teknika Corp., Westchester, Pa.). Color was developed by nitrophenyl diamine diethanolamine as substrate, and the optical densities of wells were measured at 405 nm. The antibody titer was calculated as the dilution factor of the serum sample that reached the background reading of the normal serum.
Elicitation of pulmonary granulomas.
Groups of mice were sensitized according to the experimental design, and at 14 days after the booster injection they were injected intravenously (i.v.) with 2,500 Sepharose 4B beads covalently bound with P4 peptide (7). The peptide was bound to CNBr-activated Sepharose 4B (Pharmacia) beads, which bind ligands containing primary amino groups. At 4 or 8 days after i.v. injection, mice were sacrificed. A lobe of lung was cut from each mouse; lungs were pooled from the same experimental group and minced in a Waring blender at low speed for 15 s. After a wash, the pellet was frozen immediately in liquid nitrogen and kept at −70°C for RNA extraction. The remainder of the lung was perfused, inflated with 10% buffered formalin, and removed for histological analysis.
Histopathology.
Fixed and paraffin-embedded lung samples were sectioned at 5-μm thickness, stained with hematoxylin and eosin, and examined by light microscopy. Granulomas were measured by using a Microcomp integrated image analysis system (Southern Micro Instruments Inc., Atlanta, Ga.). An average of 30 lesions was measured per sample. The sample sections were also stained with Litt’s modification of the Dominici stain (22) in order to count eosinophils. For collagen, the Mallory trichrome staining was applied.
PCR detection of cytokine mRNA expression.
The pattern of cytokine mRNA expression in the granulomatous lungs was determined by the standard procedure of reverse transcription-PCR. Lung samples were homogenized in 1 ml of Trizol (Sigma) in a tissue grinder (Omni International, Waterbury, Conn.), and total RNA was isolated as recommended by the manufacturer. The RNA was resuspended in diethylpyrocarbonate-treated water and quantitated spectrophotometrically. Reverse transcription of RNA was carried out in a 20-μl final volume containing 1 μg of total RNA, 0.25 mM deoxynucleoside triphosphate 1× reverse transcriptase buffer, 0.5 U of oligo(dT), and 200 U of Moloney murine leukemia virus reverse transcriptase (GIBCO BRL, Gaithersburg, Md.). The reaction mixture was incubated at 42°C for 1 h, heated to 90°C for 5 min to denature the Moloney murine leukemia virus reverse transcriptase. After cooling on ice for 3 min, the final reaction volume was diluted 1:5 by the addition of 80 μl of distilled water and stored at −20°C. The primers for all genes were prepared based on published sequence as shown: for IFN-γ, sense (AACGCTACACACTGCATCTTGG) and antisense (GACTTCAAAGAGTCTGAGG); for TNF-α, sense (GGCAGGTCTACTTTGGAGTCATTGC) and antisense (ACATTCGAGGCTCCAGTGAATTCGG); for iNOS, sense (CATGGCTTGCCCCTGGAAGTTTCTCTTCAAAG) and antisense (GCAGCATCCCCTCTGATGGTGCCATCG); for IL-4, sense (GAATGTACCAGGAGCCATATC), and antisense (CTCAGTACTACGAGTAATCCA); for IL-5, sense (GACAAGCAATGAGACGATGAGG) and antisense (GAACTCTTGCAGGTAATCCAGG).
To calibrate the amount of input cDNA from each sample, the expression of the housekeeping β-actin gene was first determined. The PCR was performed in a 50-μl final volume including the amount of cDNA, based on β-actin calibration, 1× PCR buffer, 0.25 mM deoxynucleoside triphosphate, 20 mM sense and antisense primers, and 1 U of Taq polymerase (GIBCO). Each PCR cycle consisted of 45 s at 94°C, 45 s at 60°C, and 90 s at 72°C. The number of PCR cycles was strictly defined for each primer pair and was selected as follows: β-actin, 23; IFN-γ, 37; TNF-α, 32; iNOS, 35; IL-4, 41; and IL-5, 41. The PCR products were electrophoresed on a 2% agarose gel containing ethidium bromide, and densities of the bands were visualized under UV light.
Statistical analysis.
Differences in granuloma size among the various groups were determined by analysis of variance and Tukey’s test. Data were determined to be significant at P < 0.05. Comparison of granulomas at 4 and 8 days in the p38-alum-sensitized mice was done by the one-tailed Student t test. Significance was determined at P < 0.05.
RESULTS
Induction of Th1- or Th2-type immune responses to p38 and its immunodominant P4 peptide.
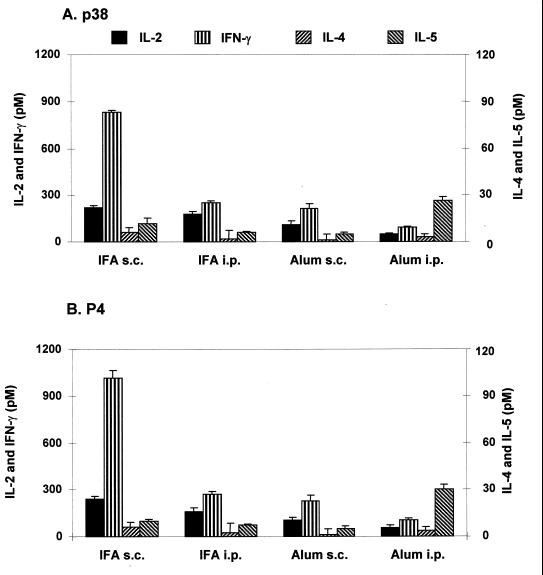
Previously we showed that mice sensitized s.c. with p38 and P4 peptides in IFA, an adjuvant that usually favors the induction of Th2 cells (1), developed a predominant Th1-type response (10). To examine whether such a response can be changed to a Th2 profile, we changed the route of immunization to an i.p. mode and used alum adjuvant, known to promote Th2 cytokine production and humoral responses (5). As Fig. 1A shows, when p38 was given in IFA by the i.p. route, the level of splenic IFN-γ production decreased drastically and no increase in IL-4 or IL-5 production occurred. The peptide presented with alum s.c. also resulted in low IFN-γ production, which was further decreased in mice sensitized by the i.p. route. Splenocytes of the latter group responded also with a significantly (P < 0.05) increased IL-5 production. An identical pattern of response was observed when the immunodominant P4 peptide was used as stimulator antigen (Fig. 1B).
FIG. 1.
Cytokine responses of splenocytes of mice immunized with p38 in IFA or alum. Three mice per group were immunized s.c. or i.p. with 3 μg p38 in IFA or alum. Seven days later, the splenocytes were prepared, pooled, and assayed for cytokine production in response to p38 (5 μg/ml; A) or P4 (1 μg/ml; B) as described in Materials and Methods. Cytokine levels are expressed as net mean ± SD of triplicate cultures after subtraction of the mean for medium only. Data are representative of three separate experiments with similar results.
To confirm that differences in cytokine production were caused by different microenvironments altered by the various adjuvants, a group of five mice was sensitized s.c. with 3 μg r38 mixed with IFA or alum. Seven days later, mice were sacrificed, their spleens were pooled, and splenocytes were stimulated with 5 μg of r38. The mean counts per minute ± standard deviation (SD) of IFA-sensitized mice was 47,591 ± 3,122, with a stimulation index (SI) of 10.7 (value for medium-only cultures, 4,433 ± 1,338). Alum sensitization induced lower proliferative responses, 26,376 ± 3,799, with an SI of 6.9 (value for medium-only cultures, 3,818 ± 1,087). Sensitization s.c. with r38 without adjuvant generated a much weaker response, 2,912 ± 439, with a significant SI of 2.0 (value for medium-only cultures, 1,448 ± 274).
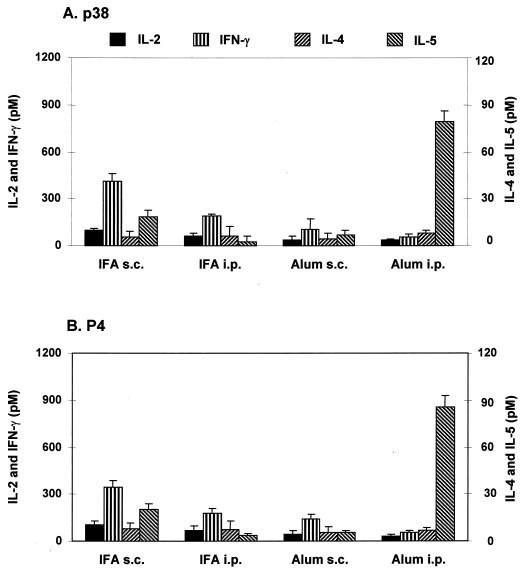
During schistosome infection, the newly arriving eggs provide repeated antigenic stimuli by the secreted peptides. To simulate this condition, we sensitized mice as in the previous experiments and gave them an additional booster injection by the same route. This regimen lowered the previously high level of IFN-γ response in the IFA s.c.-sensitized group and further diminished IFN-γ production in the alum s.c.-immunized animals. Decreased IFN-γ production in the alum i.p.-sensitized group was not antigen dependent; splenocytes of alum-sensitized mice stimulated with 10-fold higher doses of antigen did not show increased IFN-γ production. The conspicuous rise in IL-5 production indicated a strengthened Th2 response in the alum i.p.-sensitized and boosted group. Identical results were observed for p38- or P4-elicited responses (Fig. 2).
FIG. 2.
Cytokine responses of splenocytes of immunized mice after a booster injection with p38 in IFA or alum. Three mice per group were immunized s.c. or i.p. with 3 μg of p38 in IFA or alum and boosted 14 days later by the same route. Fourteen days after the second injection, the splenocytes were prepared and assayed for cytokine production in response to p38 (5 μg/ml; A) or P4 (1 μg/ml; B) as described in Materials and Methods. Cytokine levels are expressed as net mean ± SD of triplicate cultures after subtraction of the mean for medium only. Data are representative of three separate experiments with similar results.
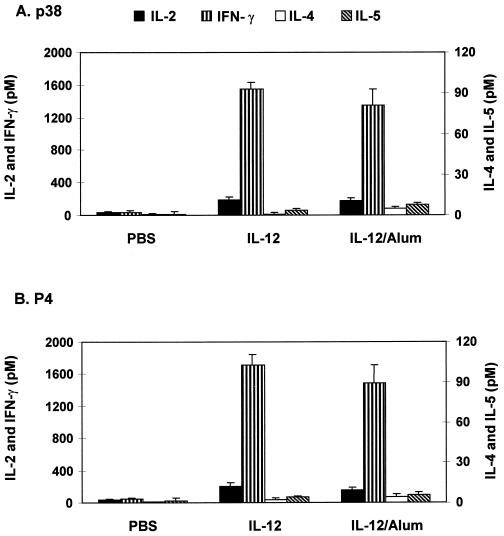
Because sensitization with the peptides in IFA via the s.c. route provided a predominant but not pure Th1 response, we attempted to further polarize the response by using rIL-12, a cytokine known to be important in Th1 cell induction (34). To ascertain the role of IL-12 in Th1 polarization, we deliberately chose the i.p. sensitization route with or without the use of alum, which has been shown to induce a Th2 response (Fig. 1). As Fig. 3 illustrates, soluble rIL-12 mixed with p38 administered i.p. in two consecutive injections followed by a booster dose induced very high levels of IFN-γ with virtually no IL-4 or IL-5 production. Interestingly, when the cytokine-peptide mixture was incorporated into alum, strong IFN-γ production with minimal or no IL-4 or IL-5 secretion was observed.
FIG. 3.
Incorporation of IL-12 into alum promotes a Th1 cytokine response to p38 and P4. Three mice per group were immunized i.p. with 3 μg of rIL-12 at days 1 and 4. The PBS control group received p38 or P4 without adjuvant. Fourteen days later, they received a booster injection with the same regimen. At day 28, splenocytes were prepared and assayed for in vitro secretion of cytokines in response to p38 and P4 (1 μg/ml). Cytokine levels are expressed as net mean ± SD of triplicate cultures after subtraction of the mean for medium only. Data are representative of three separate experiments with similar results.
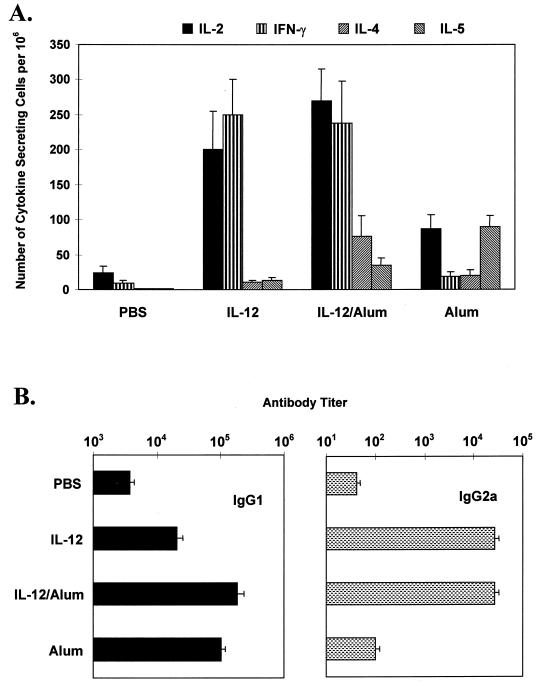
To confirm the validity of the induced polarized Th1 or Th2 response, we used the ELISPOT assay to examine the numbers of P4-specific cytokine-secreting cells in the spleens of p38-sensitized mice. As shown in Fig. 4A, splenocytes of the rIL-12–peptide immunized mice showed a pure Th1 pattern with considerable number of IFN-γ but almost no IL-4 or IL-5 producer cells. Incorporation into alum of the cytokine-peptide mixture generated similarly high numbers of IFN-γ but with higher numbers of IL-5 producer splenocytes.
FIG. 4.
Induction of Th1 or Th2 cytokine responses was confirmed by the frequency of P4-specific cytokine-secreting cells in spleens (A) and the pattern of anti-p38 antibody isotypes in sera (B). Three mice per group were immunized and boosted with p38 as described for Fig. 3. Seven days after the second injection, the number of P4-specific cytokine-secreting cells in spleens (mean ± SD) was determined by ELISPOT assay in the presence of 1 μg of P4 per ml in the cell culture. The titer of p38-specific IgG1 and IgG2a antibody in sera was determined by ELISA. Data are representative of three separate experiments with similar results.
Antibody isotype profile.
The above results were compared with antibody isotype production in the various groups of mice. Figure 4B shows that repeated injections of p38 alone generated minimal IgG2a and low level of IgG1 isotype anti-p38 antibodies. Immunization with alum-peptide caused the level of IgG1 isotype antibodies to increase 20-fold, while that of IgG2a remained low. Adsorption of IL-12–peptide onto alum also induced very high level of IgG1 antibodies, but a strong increase in IgG2a antibody production was also observed. Finally, soluble rIL-12 administered with the peptide generated only a 4-fold increase in IgG1 antibody level compared with p38-alone sensitization but induced a 200-fold increase in IgG2a production.
Th1- and Th2-type granuloma formation.
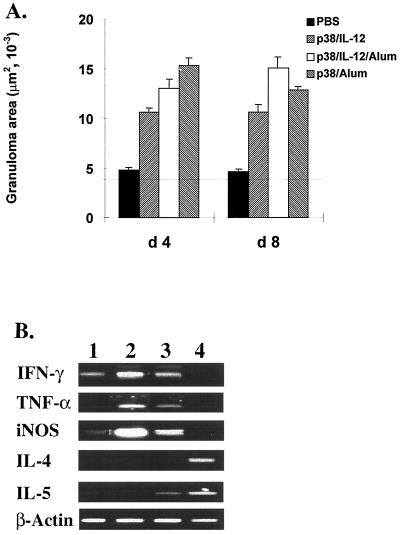
Having established the polarized immune responses to p38, it was of interest to see whether such responses can influence the character of pulmonary granuloma formation around P4 peptide-coated beads. Injection into naive mice of peptide-coupled beads induced minimal, one- or two-layer-thick cellular reactions at 4 days, with no further growth at 8 days (Fig. 5A). The p38–IL-12-sensitized mice developed mid-sized granulomas by day 4, with no further growth on subsequent days. Sensitization with the peptide–IL-12–alum mixture yielded larger granulomas at both day 4 and day 8 after bead challenge. Mice sensitized with p38-alum developed the largest granulomas, which peaked at day 4 and showed a significant decline by day 8 (P < 0.05).
FIG. 5.
The induced Th1 or Th2 responses mediate pulmonary granulomatous reactions to P4-coated beads. Six mice per group were sensitized with p38 as described for Fig. 3. As a control, one group of mice was injected i.p. with PBS without the antigen. Fourteen days after the second injection, the mice received an i.v. injection of 2,500 P4-coated beads. Four or eight days later lungs were removed and prepared for histological staining. (A) Mean granuloma area ± standard error for six mice in each group from two separate experiments. The horizontal line indicates the average of bead size alone. (B) Reverse transcription-PCR assay of cytokine mRNA expression in lung tissues with bead granulomas (day 4) from different groups. Lanes: 1, PBS group; 2, p38–IL-12 group; 3, p38–IL-12–alum group; 4, p38-alum group. β-Actin was the invariant-message control.
Determination by analysis of variance of intragroup differences showed that granulomas formed at 4 day in p38–IL-12-sensitized mice were significantly smaller than those in the p38-alum group (P < 0.05). Compared with the p38–IL-12–alum-sensitized mice, no significant difference was obtained (P > 0.05). By day 8, the only significant difference in size was observed between the p38–IL-12- and p38–IL-12–alum-sensitized animals (P < 0.05).
The cellular composition and collagen deposits of the 4 day-old granulomas were also different. The p38–IL-12-induced lesions were mononuclear with no eosinophil content and had only occasional thin strands of deposited collagen. Granulomas that developed after p38–IL-12–alum-sensitization had low numbers of eosinophils and a few thin strands of collagen. In contrast, the p38-alum-sensitized mice responded with granulomas containing high numbers of eosinophils and several thick bands of deposited collagen (Table 1).
TABLE 1.
Eosinophils and collagen deposits in 4-day-old granulomas around P4-coated beadsa
| Sensitization | No. of eosinophils/granuloma (mean ± SD) | Collagen staining in granulomas |
|---|---|---|
| P38–IL-12 | 0.58 ± 0.62 | +/− |
| P38–IL-12–alum | 6.78 ± 3.24 | + |
| P38-alum | 38.45 ± 15.34 | +++ |
Stained sections were examined at a magnification of ×250. For the eosinophil count, 30 granulomas in each of six mice (two separate experiments) were examined.
To examine whether the cytokine profile conformed to the different patterns of the granulomas, the expression of cytokine mRNA was examined in the pooled lobes of lungs from six mice after 4 days of granuloma growth. As Fig. 5B shows, there was message for IFN-γ and weak message for iNOS in bead-injected naive mice. In lungs of the p38–IL-12-sensitized group, the granulomas contained strong messages for IFN-γ, TNF-α, and iNOS but not for IL-4 or IL-5. Conversely, the p38–IL-12–alum-sensitized group expressed mRNA in the granulomatous lungs for IFN-γ, TNF-α, and iNOS and weakly for IL-5. Finally, the lungs of the p38–alum-sensitized mice had no messages for IFN-γ, TNF-α, or iNOS but expressed the strongest message for IL-4 and IL-5 signals.
DISCUSSION
The switch from a Th1 to a predominant Th2 responsive during the evolution of the schistosome egg-induced granulomas has major implications in the fibrous pathology of the murine disease (8, 33). Recent publications showed that manipulation of the immune response for sustained Th1 responsiveness (4, 37) or infection in IL-4-deficient mice (18, 28, 31) resulted in diminished granuloma formation and ameliorated fibrosis (18). In the present study, we used a cloned major egg antigen the p38 peptide and its immunodominant epitope that preferentially induces the Th1 response to analyze the induction of Th1 or Th2 responsiveness and its effect on the type of the developed granulomatous response.
Results indicate that by the choice of the adjuvants and route of sensitization, we could polarize the immune response to this well-defined peptide into a predominant Th1 or Th2 pathway. The most effective adjuvants were IL-12, a cytokine known to induce Th1 cell differentiation (35), and alum gel, generally used for the induction of antibody production (5). Previous and present observations indicated that soluble p38, alone or incorporated into IFA, induced a strong Th1 response (6). Possibly, the peptide has some intrinsic characteristics whereby its immunodominant P4 epitope can guide the response into the Th1 pathway. The present observations indicate, however, that given the mode of antigen presentation that prefers Th2 cell induction, p38 and its P4 epitope can also induce a Th2 response dominated by IL-5 production. Generally, the s.c. route favored high IFN-γ production by any adjuvant used, indicating that local Langerhans and dendritic cells, which mediate a Th1 response (14), may have participated in antigen presentation. In addition to the route, repeated immunizations also appeared to be important in decreasing the level of the existing Th1 and increasing the Th2 response. Because repeated stimuli with the peptide were given in order to mimic the conditions during infection when newly arriving eggs provide protracted stimulation, it was important that such conditions shift the response away from the Th1 mode. This process is similar to that in infected mice, where we observed a sharp drop in p38-specific IFNγ production by the second week of granuloma development without a concomitant switch to peptide-specific Th2 responsiveness (10). The underlying mechanism for such a shift is not clear. It is not likely that restimulated Th2 memory cells acted in a cross-regulatory manner because no increase in IL-4 or IL-10 secretion was seen in the boosted animals. It is possible that a shift in the type of antigen-presenting cells from Langerhans to B cells favored the stimulation of Th2 cells which provided help for very high levels of specific IgG1 antibody production (Fig. 4).
A noteworthy observation in this study was that the polarized immune response generated two types of pulmonary granulomas. Whereas the IL-12–p38-sensitized mice developed smaller mononuclear granulomas and their lungs contained messages for the Th1-type cytokines IFN-γ and TNF-α as well as for iNOS, the alum-p38-sensitized animals formed larger granulomas with high eosinophil content and IL-4 and IL-5 message, congruent with a Th2 inflammatory response. Moreover, whereas the former lesions contained minimal deposited collagen fibers, the latter contained several thick collagen bands. Collagen production within the two types of granulomas generated by a single peptide has implications for the pathology of murine schistosomiasis. IL-4 has been shown to promote fibroblast activity and collagen production (9), whereas IFN-γ played an opposite role and curtailed the deposition of collagen (13). Thus, a strong, sustained Th1 granulomatous response is likely to be accompanied by less fibrotic healing and fibrosis-induced pathology (portal pressure, etc.) than the Th2 response. This has in part been demonstrated in rIL-12-treated (4, 37) and Stat6-deficient infected mice (18), which had smaller granulomas and less fibrosis. However, it remains to be examined whether prolonged Th1 responsiveness that generates inflammatory cytokines and NO could cause tissue damage as described for several experimental diseases (20, 26, 30, 33, 34).
Our observations are similar to the previous finding that Th1- or Th2-type artificial granulomas could be established with crude antigen (purified protein derivative or SEA)-coated beads, respectively, in the properly sensitized animals (11). Here, a novel observation shows that such granuloma prototypes can be generated also to a single well-defined peptide of SEA when the appropriate sensitizing protocols are used.
Data obtained from the present experiments also have implications for the dynamics of the immune response to components of egg-secreted SEA. We have shown recently that lymphocytes of infected mice during the early phase of granuloma formation respond strongly to p38-P4; therefore, this peptide may well be responsible for the induction of the Th1 phase of the inflammation, which is characterized by IL-12 production by granuloma macrophages and IL-12 receptor β2 expression (unpublished data). With development of the granulomas, the Th2-type cytokine production is gradually enhanced (15) and the p38-directed Th1 response is diminished (10). In the present study, we showed that induction of a strong Th2-type immune environment by alum-p38 sensitization shifted the original Th1 anti-p38 peptide response to a Th2 pattern.
In sum, the present study demonstrated that the immunodominant portion of p38 a major egg antigen and a strong Th1 cell inducer when presented in strong adjuvants could induce either a Th1 or a Th2 immune response. These data emphasize the importance of antigen-presenting cells, costimulatory signals, and cytokine environment in the induction of the egg antigen-specific immune response. By the polarization of the immune response, either Th1- or Th2-type granulomas that differed from one another in size, cellularity, cytokine profile, and collagen content could be generated. This underscores the importance of the induced Th cell activity in the size, duration, and subsequent pathology of the granulomatous inflammation.
Previous studies used IL-12 and eggs for the induction of a strong Th1 response and to protect against the Th2-type granulomatous pathology (37, 39). The model established in this study shows the potential for a single, well-defined strong immunogen to induce sustained Th1 responsiveness and thereby to influence the intensity of granulomatous pathology.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI-12913 from the National Institute of Allergy and Infectious Diseases, Bethesda, Md.
We thank Joel Whitfield for skillful technical support.
REFERENCES
- 1.Allison A C. Adjuvants for a new and improved vaccines. In: Grigoriadis G, McCormack B, Allison A C, editors. Vaccines: new generation immunological adjuvants. New York, N.Y: Plenum Press; 1995. pp. 1–14. [Google Scholar]
- 2.Boros D L. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989;2:250–269. doi: 10.1128/cmr.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boros D L, Warren K S. Delayed hypersensitivity granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boros D L, Whitfield J R. Enhanced Th1 and dampened Th2 responses synergize to inhibit acute granulomatous and fibrotic responses in murine schistosomiasis mansoni. Infect Immun. 1999;67:1187–1193. doi: 10.1128/iai.67.3.1187-1193.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewer J M, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to Freund’s complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26:2062–2066. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 6.Cai Y, Langley J G, Smith D I, Boros D L. A cloned major Schistosoma mansoni egg antigen with homologies to small heat shock proteins elicits Th1 responsiveness. Infect Immun. 1996;64:1750–1755. doi: 10.1128/iai.64.5.1750-1755.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrick L, Jr, Boros D L. The artificial granuloma. I. In vitro lymphokine production by pulmonary artificial hypersensitivity granulomas. Clin Immunol Immunopathol. 1980;17:415–426. doi: 10.1016/0090-1229(80)90113-0. [DOI] [PubMed] [Google Scholar]
- 8.Cheever A W. Schistosomiasis: infection versus disease and hypersensitivity versus immunity. Am J Pathol. 1993;142:699–702. [PMC free article] [PubMed] [Google Scholar]
- 9.Cheever A W, Williams M E, Wynn T A, Finkelman F D, Seder R A, Cox T M, Hieny S, Caspar P, Sher A. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol. 1994;153:753–759. [PubMed] [Google Scholar]
- 10.Chen Y, Boros D L. Identification of the immunodominant T cell epitope of p38, a major egg antigen and characterization of the epitope-specific Th responsiveness during murine schistosomiasis mansoni. J Immunol. 1998;160:5420–5427. [PubMed] [Google Scholar]
- 11.Chensue S W, Warmington K, Ruth J, Lincoln P, Kuo M, Kunkel S L. Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am J Pathol. 1994;145:1105–1113. [PMC free article] [PubMed] [Google Scholar]
- 12.Chikunguwo S M, Quinn J J, Harn D A, Stadecker M J. The cell-mediated response to schistosomal antigens at the clonal level. III. Identification of soluble egg antigens recognized by cloned specific granulomagenic murine CD4+ Th1-type lymphocytes. J Immunol. 1993;150:1413–1421. [PubMed] [Google Scholar]
- 13.Czaja M J, Weiner F R, Takahashi S, Giambrone M A, van der Meide P H, Schellekens H, Biempica L, Zern M A. Gamma-interferon treatment inhibits collagen deposition in murine schistosomiasis. Hepatology. 1989;10:795–800. doi: 10.1002/hep.1840100508. [DOI] [PubMed] [Google Scholar]
- 14.Everson M P, McDuffie D S, Lemak D G, Koopman W J, McGhee J R, Beagley K W. Dendritic cells from different tissues induce production of different T cell cytokine profiles. J Leukoc Biol. 1996;59:494–498. doi: 10.1002/jlb.59.4.494. [DOI] [PubMed] [Google Scholar]
- 15.Gryzch J-M, Pearce E J, Caulada Z A, Caspar P, Hieny S, Lewis F, Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- 16.Harn D A, Danko K, Quinn J J, Stadecker M J. Schistosoma mansoni: the host immune response to egg antigens. I. Partial characterization of cellular and humoral responses to pI fractions of soluble egg antigen. J Immunol. 1989;142:2061–2066. [PubMed] [Google Scholar]
- 17.Hernandez H J, Edson C M, Harn D A, Ianelli C J, Stadecker M J. Schistosoma mansoni: genetic restriction and cytokine profile of the CD4+ T helper cell response to dominant epitope peptide of major egg antigen Sm-p40. Exp Parasitol. 1998;90:122–130. doi: 10.1006/expr.1998.4309. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan M H, Whitfield J R, Boros D L, Grusby M J. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol. 1998;160:1850–1856. [PubMed] [Google Scholar]
- 19.Karplus T E, Ulevitch R J, Wilson C B. A new method for reduction of endotoxin contamination from protein solutions. J Immunol Methods. 1987;105:211–220. doi: 10.1016/0022-1759(87)90268-7. [DOI] [PubMed] [Google Scholar]
- 20.Leonard J P, Waldburger K E, Goldman S J. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin-12. J Exp Med. 1995;181:381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leptak C L, McKerrow T H. Schistosome egg granulomas and hepatic expression of TNF-α are dependent on immune priming during parasite maturation. J Immunol. 1997;158:301–307. [PubMed] [Google Scholar]
- 22.Litt M. Studies in experimental eosinophilia. Am J Pathol. 1963;42:529–534. [PMC free article] [PubMed] [Google Scholar]
- 23.Lukacs N W, Boros D L. Splenic and granuloma T-lymphocyte responses to fractioned soluble egg antigens of Schistosoma mansoni-infected mice. Infect Immun. 1991;59:941–948. doi: 10.1128/iai.59.3.941-948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukacs N W, Boros D L. Identification of larval cross-reactive and egg-specific antigens involved in granuloma formation in murine schistosomiasis mansoni. Infect Immun. 1991;59:3237–3242. doi: 10.1128/iai.59.9.3237-3242.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukacs N W, Boros D L. Utilization of fractionated soluble egg antigens reveals selectively modulated granulomatous and lymphokine responses during murine schistosomiasis mansoni. Infect Immun. 1992;60:3209–3216. doi: 10.1128/iai.60.8.3209-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyons C R. The role of nitric oxide in inflammation. Adv Immunol. 1995;60:323–371. doi: 10.1016/s0065-2776(08)60589-1. [DOI] [PubMed] [Google Scholar]
- 27.Matthew R C, Boros D L. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen induced interleukin-2 production in Schistosoma mansoni infection. Infect Immun. 1986;54:820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metwali A, Elliott D, Blum A M, Li J, Sandor M, Lynch R, Noben-Trauth N, Weinstock J V. The granulomatous response in murine schistosomiasis mansoni does not switch to Th1 in IL-4 deficient C57BL/6 mice. J Immunol. 1996;157:4546–4553. [PubMed] [Google Scholar]
- 29.Nene V, Dunne D W, Johnson K S, Taylor D W, Cordingley J S. Sequence and expression of a major egg antigen from Schistosoma mansoni: homologies to heat shock proteins and alpha-crystallins. Mol Biochem Parasitol. 1986;21:179–188. doi: 10.1016/0166-6851(86)90021-6. [DOI] [PubMed] [Google Scholar]
- 30.Neurath M F, Fuss I, Kelsall B L, Struber E, Strober W. Antibodies to interleukin-12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce E J, Cheever A, Leonard S, Covalsky M, Fernandez-Botran R, Kohler G, Kopf M. Schistosoma mansoni in IL-4-deficient mice. Int Immunol. 1996;8:435–444. doi: 10.1093/intimm/8.4.435. [DOI] [PubMed] [Google Scholar]
- 32.Pearce E J, Caspar P, Gryzch J-M, Lewis F A, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosa Brunet L, Finkelman F D, Cheever A W, Kopf M A, Pearce E J. IL-4 protects against TNF-α-mediated cachexia and death during acute schistosomiasis. J Immunol. 1997;159:777–785. [PubMed] [Google Scholar]
- 34.Trembleau S, Penna G, Bosi E, Mortara A, Gately G K, Adorini L. Interleukin-12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J Exp Med. 1995;181:817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 36.Vella A T, Pearce E J. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly through an early transient Th0-like stage. J Immunol. 1992;148:2283–2290. [PubMed] [Google Scholar]
- 37.Wynn T A, Cheever A W, Jankovic D, Poindexter R W, Caspar P, Lewis F A, Sher A. An IL-12 based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;396:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 38.Wynn T A, Cheever A W. Cytokine regulation of granuloma formation in schistosomiasis. Curr Opin Immunol. 1995;7:505–511. doi: 10.1016/0952-7915(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 39.Wynn T A, Eltoum I, Oswald I P, Cheever A W, Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashita T, Boros D L. IL-4 influences IL-2 production and granulomatous inflammation in murine schistosomiasis mansoni. J Immunol. 1992;149:3659–3664. [PubMed] [Google Scholar]
- 41.Zhu Y, Boros D L. Cloning of Th0- and Th2-type helper lymphocytes from liver granulomas of Schistosoma mansoni-infected mice. Infect Immun. 1994;62:994–999. doi: 10.1128/iai.62.3.994-999.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]