We have recently shown that arginase-1 (Arg1) expression by alternatively activated macrophages promotes tubule cell proliferative repair after ischemia/reperfusion injury (IRI).1 In response, Zhang and colleagues2 have questioned whether the Arg1 expression that we detect is in tubular cells rather than in macrophages. Here, we provide single-cell RNA sequence data and confocal imaging demonstrating that Arg1 mRNA expression is confined to Ccr2-positive macrophages and that Arg1 protein is detected in interstitial cells rather than in tubular cells.
Zhang and colleagues raise the question of whether the Arg1 staining shown in our recent manuscript1 is actually in phagocytic S3 proximal tubule cells rather than in interstitial macrophages. We performed both tubular cell and macrophage staining multiple times for the original manuscript, and never detected either F4/80 or Arg1 staining in tubular cells at baseline or after IRI. Cells expressing either of these markers are only seen in the renal interstitium, most in the medulla. As Figure 1A of this response shows (reproduced from supplemental figure 5B of Shin et al.1), single-cell RNA sequencing demonstrates that Arg1 is expressed almost exclusively in Ccr2+ macrophages, with minimal detection in any of the proximal tubule cell populations. Furthermore, the marked reduction of Arg1 staining in the injured kidneys of Arg1fl/fl mice expressing LysM-Cre (figure 1, E and G, of Shin et al.1) supports macrophages as the source of intrarenal Arg1, because lysozyme M (LysM) is expressed specifically in mononuclear phagocytes and not in tubular cell populations (Figure 1B).
Figure 1.
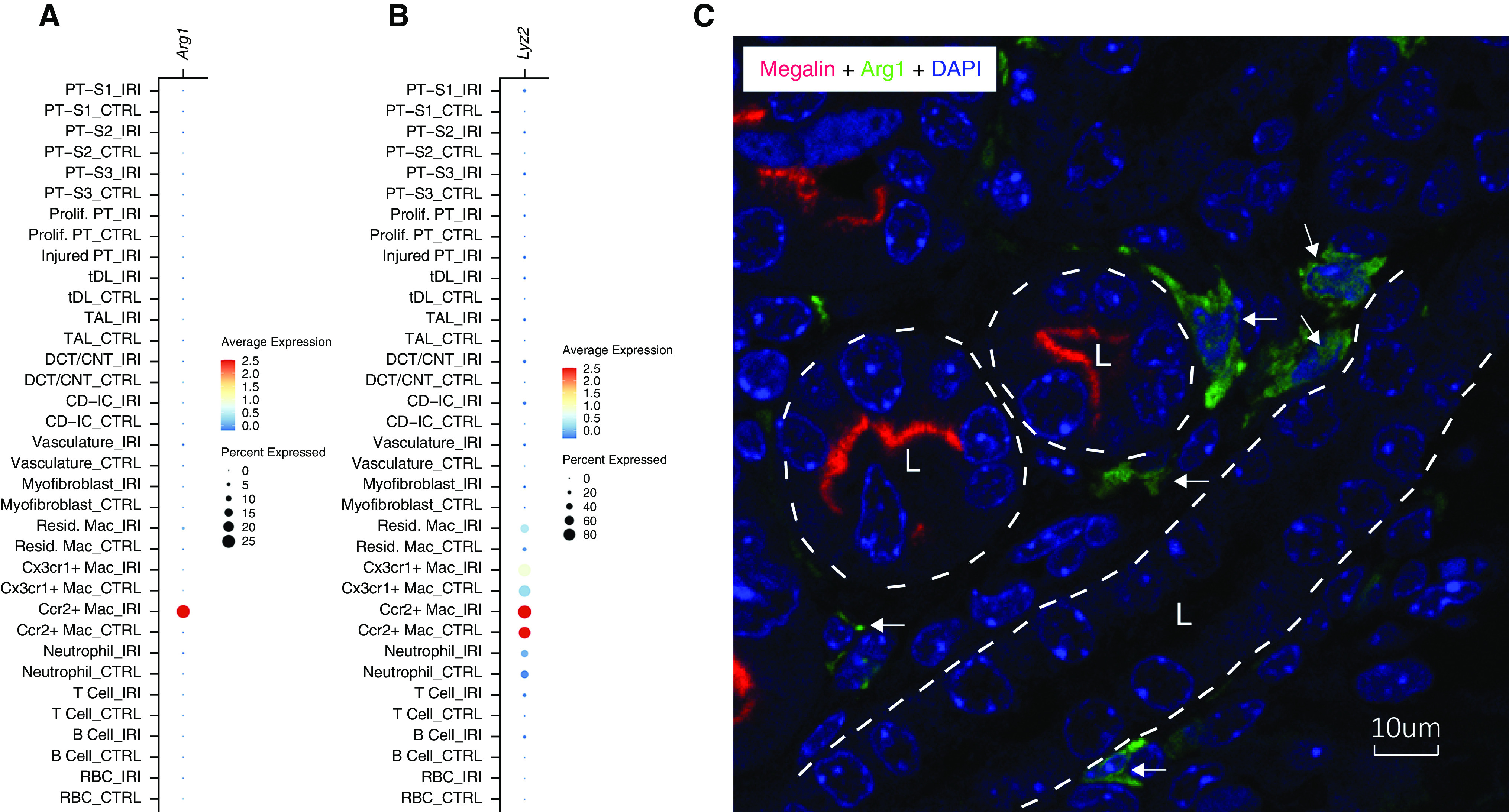
Interstitial macrophages express Arg1. (A) Dot plot of Arg1 mRNA expression in the indicated cell types by single-cell RNA sequencing analysis performed on day 3 after IRI. (B) Dot plot of Lyz2 (gene name for lysozyme 2, also known as LysM) mRNA expression in the indicated cell types by single-cell RNA sequencing analysis performed on day 3 after IRI. (C) Confocal image of renal medulla stained with anti-megalin (red), anti-Arg1 (green), and 4′,6-diamidino-2-phenylindole (DAPI; blue) on day 7 after IRI. Megalin marks the apical membrane of the proximal tubule cells. Tubule outlines are indicated by the superimposed white dashed lines with tubule lumens indicated by “L.” Arrows indicate the interstitial macrophages expressing Arg1.
To further confirm this, we performed immunofluorescence staining of Arg1 and a proximal tubule marker, megalin, in mouse kidney medulla on day 7 after IRI. The high-resolution confocal image shows that Arg1 is expressed in interstitial cells that are immediately adjacent to, but not within, the medullary tubules (Figure 1C).
Disclosures
L.G. Cantley reports having ownership interest in, and research funding from, Arvinas Inc. (spouse’s employer); and having consultancy agreements with Drug Farm, Johnson and Johnson, and Vivace Therapeutics. D. Lindberg reports being employed by, and serving in an advisory or leadership role for, eCLASS AB. A. Marlier reports being employed by Altos Labs. All remaining authors have nothing to disclose.
Funding
This work was funded by R01 DK093771 (L.G. Cantley).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related letter to the editor, “Most Arginase-1 Positive Cells Are Likely Injured S3 Proximal Tubular Cells Carrying Upregulated Phagocytotic Capacity Rather Than M2 Macrophages – Too Many to Be True,” on pages 2123–2124, and original article, “Arginase-1 is Required for Macrophage-Mediated Renal Tubule Regeneration,” in Vol. 33, Iss. 6, on pages 1077–1086.
Author Contributions
L.G. Cantley, N. Doilicho, J. Guo, A. Marlier, and N.S. Shin reviewed and edited the manuscript; L.G. Cantley, J. Guo, and L. Xu wrote the original draft; J. Guo was responsible for data curation and investigation; J. Guo and L. Xu were responsible for formal analysis; and all authors conceptualized the study.
References
- 1.Shin NS, Marlier A, Xu L, Doilicho N, Linberg D, Guo J, et al. : Arginase-1 is required for macrophage-mediated renal tubule regeneration. J Am Soc Nephrol 33: 1077–1086, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang, et al. : Most arginase-1 positive cells are likely injured S3 proximal tubular cells carrying upregulated phagocytotic capacity rather than M2 macrophages. J Am Soc Nephrol 33: 2123–2124, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]


