Significance Statement
Dapagliflozin reduces the risk of kidney failure in patients with CKD but can result in a reversible acute reduction in eGFR on initiation of treatment. This post hoc analysis of the DAPA-CKD trial found that patients who experienced an acute reduction in eGFR>10% after 2 weeks of treatment with dapagliflozin had slower rates of long-term eGFR decline compared with patients who experienced a less pronounced decline or increase in eGFR. Adverse event rates in patients randomized to dapagliflozin were unrelated to the acute change in eGFR. These data suggest that a modest acute reduction in eGFR on dapagliflozin initiation is not associated with higher rates of CKD progression and should not be a reason to discontinue this therapy in the majority of patients.
Keywords: chronic kidney disease, renal function, dapagliflozin, sodium-glucose transporter 2 inhibitors
Visual Abstract
Abstract
Background
Dapagliflozin reduces kidney failure risk in patients with CKD but can result in a reversible acute reduction in eGFR upon treatment initiation. Determinants of this eGFR reduction and its associations with efficacy and safety outcomes are unknown.
Methods
The DAPA-CKD trial randomized 4304 adults with CKD and albuminuria to once-daily dapagliflozin 10 mg or placebo, added to standard care. We prespecified an analysis comparing the effects of dapagliflozin among patients who experienced relative reductions in eGFR (>10% or >0%–10%) or an increase in eGFR from baseline to 2 weeks and assessed long-term efficacy and safety thereafter.
Results
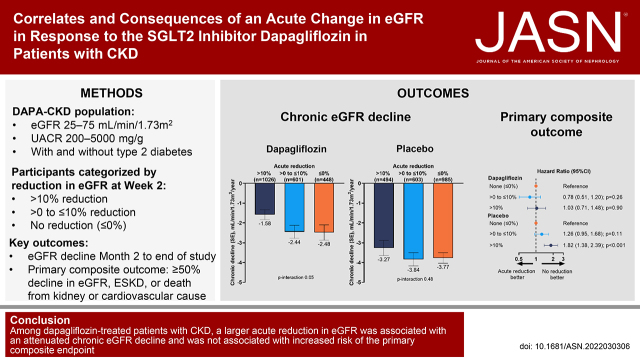
A total of 4157 (96.6%) patients had eGFR data available at baseline and at 2 weeks. In the dapagliflozin and placebo groups, 1026 (49.4%) and 494 (23.7%), respectively, experienced an acute reduction in eGFR >10%. Among patients receiving dapagliflozin, those with an acute reduction in eGFR >10% experienced a long-term eGFR decline of −1.58 ml/min per 1.73 m2 per year compared with −2.44 and −2.48 ml/min per 1.73 m2 per year among those experiencing a less pronounced reduction or increase in eGFR, respectively (P-interaction=0.05). In the placebo group, long-term eGFR decline was −3.27, −3.84, and −3.77 ml/min per 1.73 m2 per year for acute eGFR reduction subgroups of >10%, >0%–10%, or increase in eGFR (P-interaction=0.48). Rates of serious adverse events and adverse events of special interest in patients randomized to dapagliflozin were unrelated to the acute eGFR change.
Conclusions
Among patients with CKD and albuminuria treated with dapagliflozin, an acute reduction in eGFR (from baseline to 2 weeks) is not associated with higher rates of CKD progression.
Clinical Trial registration number: A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease (Dapa-CKD) NCT03036150.
Randomized controlled trials demonstrated the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to slow decline in kidney function and reduce the incidence of ESKD in patients with type 2 diabetes, heart failure, and CKD.1–5 SGLT2 inhibitors are thought to exert nephroprotective effects in part by reducing intraglomerular pressure and glomerular hyperfiltration.6 This protective mechanism is similar to that described with other proven nephroprotective treatments such as angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs).
Clinically, a reduction in intraglomerular pressure is manifested as a decline in eGFR on treatment initiation that is completely reversible after treatment discontinuation, even after several years of treatment.7,8 However, the acute reduction in eGFR observed after initiation of SGLT2 inhibitors has led to concerns about the long-term safety of these agents and may prevent clinicians from initiating or continuing SGLT2 inhibitors despite their well-established safety and efficacy profiles. Emerging data in patients with type 2 diabetes demonstrate that acute reductions in eGFR of up to 30% soon after SGLT2 initiation are not associated with an increased risk of adverse events (AEs), supporting the continued use of these agents despite the acute reduction in eGFR often seen.9,10 Moreover, several studies with ACEis and ARBs have suggested that a larger initial reduction in eGFR is associated with attenuation of CKD progression during prolonged treatment, suggesting that the initial acute reduction in eGFR may serve as a marker of therapeutic benefit.11,12
We performed a prespecified analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial to assess correlates and consequences of an acute reduction in eGFR after initiation of dapagliflozin. We hypothesized that patients treated with dapagliflozin who experienced larger relative and absolute reductions in eGFR would experience slower rates of eGFR decline over the long term than patients with less pronounced or no acute reductions in eGFR.
Methods
DAPA-CKD was a randomized, double-blind, placebo-controlled, multicenter clinical trial with patients recruited at 386 clinical sites over 21 countries from February 2017 to June 2020. Details about the trial design, baseline characteristics, primary results, and trial protocol have been previously published.4,13 All participants provided written informed consent before commencement of any study-specific procedure. Safety of participants in the trial was overseen by an independent data and safety monitoring committee. DAPA-CKD is registered with clinicaltrials.gov, NCT03036150.
Participants
Adult patients with CKD, eGFR 25–75 ml/min per 1.73 m2, and urine albumin-creatinine ratio (UACR) 200–5000 mg/g, with or without type 2 diabetes, were eligible for participation. We required patients to be treated with a stable maximally tolerated dose of an ACEi or ARB for ≥4 weeks unless medically contraindicated. Key exclusion criteria included a documented diagnosis of type 1 diabetes, polycystic kidney disease, lupus nephritis, or anti-neutrophil cytoplasmic antibody-associated vasculitis. Recruitment of patients with eGFR 60–75 ml/min per 1.73 m2 was limited to no more than 10% of trial participants. A complete list of inclusion and exclusion criteria has been previously published.13
Procedures
We randomized participants to dapagliflozin 10 mg once daily or matching placebo. We stratified randomization by diabetes status and UACR (≤ or >1000 mg/g). After randomization, we conducted in-person study visits after 2 weeks; at 2, 4, and 8 months; and at 4-month intervals thereafter. At each follow-up visit, study personnel recorded vital signs, obtained blood and urine samples, and recorded information on potential study end points, AEs, concomitant therapies, and study drug adherence. Participants and all study personnel (except the independent data) were masked to treatment allocation.
Kidney Function and Thresholds of Acute eGFR Change
We calculated eGFR using the CKD Epidemiology Collaboration (CKD-EPI) and incorporated results from the equation as originally defined, including a term for self-reported race (Black versus non-Black). We included all participants with eGFR measurements at baseline and week 2 in our analyses. We defined the acute change in eGFR as the percentage or absolute change in eGFR at week 2. The 2-week period was chosen because it was the first time point at which follow-up eGFR measurements were available and prior studies have shown that the acute effect of dapagliflozin on eGFR is fully manifested after 2 weeks. We categorized participants by percentage decline in eGFR at week 2 as follows: greater than 10% decline (defined as acute eGFR drop); between 0% and 10% decline (acute modest eGFR drop); and no decline (acute eGFR increase). These cutoffs were chosen post hoc with the aim of providing easily understandable thresholds and approximately equal sample sizes for each category. In an additional analysis, we defined the acute change in eGFR as an absolute change in eGFR at week 2. We stratified patients in the following categories: >3 ml/min per 1.73 m2 decline; between 0 and 3 ml/min per 1.73 m2 decline; and no decline. We also used finer categories of acute change in eGFR (>30% decrease, >20%–30% decrease, >10%–20% decrease, >0%–10% decrease, or ≤0% increase) to allow for a direct comparison with a prior study. We defined our subgroups by a postrandomization variable (eGFR change at week 2), and therefore these results do not represent randomized comparisons.
Outcomes
The primary outcome of the DAPA-CKD trial was composite end point of sustained ≥50% decline in eGFR (confirmed by a second serum creatinine after at least 28 days), onset of ESKD (defined as maintenance dialysis for more than 28 days, kidney transplantation, or eGFR<15 ml/min per 1.73 m2 confirmed by a second measurement after at least 28 days), or death from kidney or cardiovascular cause. Prespecified secondary end points were (1) a kidney-specific end point defined as the primary composite end point with exclusion of cardiovascular death, (2) a composite end point of cardiovascular death or hospitalization for heart failure, and (3) all-cause mortality. These efficacy end points were adjudicated by a masked, independent event adjudication committee. An exploratory end point in DAPA-CKD was the rate of eGFR decline. In this study, we calculated the rate of eGFR decline from month 2 until the end of treatment. Because we categorized patients on the basis of their initial eGFR change, we used the month 2 value as baseline to avoid potential bias in the chronic eGFR slope due to regression to the mean. Regression to the mean could be apparent in the more extreme eGFR change categories (>10% acute reduction in eGFR and no acute reduction in eGFR) with the consequence that a proportion of patients are allocated to these categories due to an unusually high or low eGFR value at week 2. The week-2 value may be followed by a more representative value at the subsequent visit (month 2) which potentially leads to an overestimation of the chronic eGFR slope for the >10% acute reduction in eGFR category and an underestimation of the chronic eGFR slope for the no acute reduction in eGFR category.
Because of extensive clinical experience with dapagliflozin, safety ascertainment was limited to assessment of serious AEs, AEs resulting in the discontinuation of the study drug, and AEs of special interest (symptoms of volume depletion, kidney-related AEs, major hypoglycemia, bone fractures, amputations, and diabetic ketoacidosis). All safety outcomes were investigator reported.
Statistical Analyses
We summarized baseline characteristics by acute percentage reduction in eGFR as follows: >10%, modest acute decline (0% until 10%), and increase in eGFR (>0%). Within these categories and treatment allocation, we described numeric variables with an approximate symmetric distribution by their mean and SD. Variables with skewed distributions were reported by calculating their median (25th, 75th percentiles) or geometric mean; categorical variables were reported as counts and proportions.
We calculated the likelihood of experiencing an acute reduction in eGFR>10% at week 2 with dapagliflozin compared with placebo using logistic regression. Subsequently, we assessed if patient characteristics at baseline modified the treatment effect of dapagliflozin versus placebo on this end point by adding an interaction between treatment and the respective patient subgroup to the model. The model also included diabetes status, baseline UACR (≤ or >1000 mg/g), and eGFR as covariates. We explored subgroups defined by the following patient characteristics: age, sex, self-reported race, Quételet body mass index, systolic BP, baseline eGFR, baseline UACR, type 2 diabetes, cardiovascular disease history, heart failure history, and use of diuretics, ACEis, or ARBs.
We analyzed the association between acute reductions in eGFR on the mean on-treatment eGFR slope by fitting a three-slope mixed effects linear spline model, with a knot at 2 weeks and 2 months, with correlated random intercepts and slopes for each participant over time, incorporating an unstructured covariance matrix. A second knot was added at 2 months to account for potential regression to the mean between the week 2 and month 2 visits, as above. We excluded eGFR determinations after treatment discontinuation from the eGFR slope analyses to avoid bias in eGFR slope estimates from hemodynamic changes in eGFR after discontinuation of dapagliflozin. The mixed effects model included fixed effects for categories of acute change in eGFR, time, an interaction term for acute change in eGFR category by time, and the following baseline covariates and an interaction term between these covariates and time: age, sex, cardiovascular disease history, baseline eGFR, log-transformed UACR, systolic BP, hemoglobin, HbA1c, and change in systolic BP at week 2. With this model, we calculated the acute slope as the mean change in eGFR from baseline to week 2 and the chronic eGFR slope as the mean rate of change after month 2 until the last on-treatment visit. We fitted models separately for each treatment arm.
In order to visualize trajectories and estimate the mean eGFR for each visit without presupposing a linear decline, we fitted a separate set of longitudinal models in which follow-up time was represented by visit as a categorical variable. We fitted these models for the placebo and dapagliflozin arms separately and included fixed effects for category of acute change in eGFR, visit (as a categorical factor), baseline covariates as used in the two-slope model, acute change in eGFR by visit interaction, and an interaction between visit and each baseline covariate.
To determine the association between an acute change in eGFR and the primary and secondary end points, we estimated the relative hazard of a large acute reduction in eGFR versus a less pronounced reduction or no reduction (0% until 10%) and acute eGFR decline (>10%) relative to an acute eGFR increase. We used a landmark approach and included only clinical events occurring after week 2 in the analysis. We calculated hazard ratios (HRs) and 95% confidence intervals (95% CIs) using Cox proportional hazards regression adjusting for the following baseline covariates: age, sex, cardiovascular disease history, eGFR, log-transformed UACR, and type 2 diabetes status. We performed the analyses separately for the dapagliflozin and placebo groups. We calculated a P value for interaction (P-interaction value) between treatment and acute change in eGFR for each clinical end point using a combined (dapagliflozin and placebo) model. This model also included interaction terms between category of acute change in eGFR and the above-mentioned baseline covariates. To visualize the association between acute change in eGFR at 2 weeks and clinical outcomes, we repeated the Cox proportional hazards regression analyses in each treatment group separately and fitted acute change in eGFR at week 2 as a continuous variable. In this model, we calculated HR relative to 0% acute eGFR decline using a restricted cubic spline with three knots (at the 25th, 50th, and 75th percentiles of the data). We used a Wald test to calculate a P-interaction value for interaction between acute change in eGFR (with a restricted cubic spline) and treatment using the estimates from the placebo and dapagliflozin model separately. We plotted the treatment effect of dapagliflozin versus placebo for each clinical outcome as a function of acute change in eGFR.
Finally, we summarized safety data according to category of acute percentage and absolute change in eGFR for dapagliflozin- and placebo-treated participants. Safety outcomes were described by counts, proportions, and odds ratios (ORs). We calculated ORs and 95% CIs for each safety event using a logistic regression model and included fixed effects for category of acute change in eGFR and baseline covariates mentioned above. We calculated the P-interaction value using the same approach as described above for estimation of HRs. We performed all analyses with R version 4.1.0 (R Foundation).
Results
Of the 4304 patients included in the DAPA-CKD trial, a total of 4157 (96.6%) had eGFR measurements at baseline and at week 2. Mean (SD) baseline eGFR was 43.2 (12.4) ml/min per 1.73 m2. At week 2, the mean eGFR change was −4.0 (6.7) ml/min per 1.73 m2 in the dapagliflozin group and −0.8 (6.8) ml/min per 1.73 m2 in the placebo group, corresponding to −9.3% (14.3%) and −1.7% (15.0%) decline, respectively. An acute reduction in eGFR>10% occurred more frequently in the dapagliflozin group (1026 [49.4%]) compared with the placebo group (494 [23.7%]; Figure 1). Few patients experienced an acute reduction in eGFR>30% at week 2: 97 (4.7%) in the dapagliflozin and 47 (2.3%) in the placebo group. The distribution was similar when acute reductions in eGFR were categorized by absolute rather than relative changes (Figure 1).
Figure 1.

Proportional distribution of eGFR decline by treatment arm.
Clinical Correlates of Acute Reduction in eGFR>10% at Week 2
Patients with an acute reduction in eGFR>10% assigned to dapagliflozin were more likely to be older, had a higher body mass index, higher systolic BP, and lower hemoglobin, and were more likely to smoke and to use diuretics at baseline (Table 1). In the placebo group, patients with an acute reduction in eGFR>10% were more likely to be female, had higher systolic BP, higher UACR, and lower hemoglobin. Baseline characteristics according to subgroups defined by acute absolute changes in eGFR are presented in Supplemental Table 1 and showed a similar pattern.
Table 1.
Baseline characteristics by percentage decline in eGFR between baseline and week 2 in DAPA-CKD
| Dapagliflozin (n=2075) | Placebo (n=2082) | |||||||
|---|---|---|---|---|---|---|---|---|
| >10% Decline n=1026 | 10%–0% Decline n=601 | Increase n=448 | P Value | >10% Decline n=494 | 10%–0% Decline n=603 | Increase n=985 | P Value | |
| Age, yr | 62.9 (11.5) | 61.1 (12.1) | 61.1 (12.7) | 0.003 | 61.7 (11.9) | 62.1 (12.7) | 61.9 (11.8) | 0.887 |
| Female sex, n (%) | 349 (34.0) | 182 (30.3) | 153 (34.2) | 0.252 | 194 (39.3) | 206 (34.2) | 297 (30.2) | 0.002 |
| Race,a n (%) | 0.005 | 0.068 | ||||||
| White | 570 (55.6) | 302 (50.2) | 226 (50.4) | 254 (51.4) | 324 (53.7) | 560 (56.9) | ||
| Black or African American | 63 (6.1) | 28 (4.7) | 13 (2.9) | 25 (5.1) | 25 (4.1) | 35 (3.6) | ||
| Asian | 308 (30.0) | 224 (37.3) | 171 (38.2) | 161 (32.6) | 214 (35.5) | 309 (31.4) | ||
| Other | 85 (8.3) | 47 (7.8) | 38 (8.5) | 54 (10.9) | 40 (6.6) | 81 (8.2) | ||
| Current smoker, n (%) | 115 (11.2) | 92 (15.3) | 64 (14.3) | 0.042 | 59 (11.9) | 90 (15.0) | 141 (14.3) | 0.321 |
| Body mass index (kg/m2) | 29.8 (6.4) | 29.3 (5.7) | 28.7 (5.5) | 0.003 | 29.6 (6.3) | 29.4 (6.6) | 30.0 (6.1) | 0.222 |
| Blood pressure, mm Hg | ||||||||
| Systolic | 138.1 (18.4) | 136.4 (16.3) | 134.7 (16.5) | 0.002 | 139.1 (17.7) | 138.5 (17.5) | 136.1 (16.9) | 0.001 |
| Diastolic | 77.2 (10.7) | 77.8 (11.2) | 77.6 (10.0) | 0.607 | 77.8 (10.1) | 77.7 (10.3) | 77.3 (10.4) | 0.570 |
| HbA1c, % | 7.1 (1.7) | 6.9 (1.6) | 7.2 (1.9) | 0.001 | 7.1 (1.6) | 7.0 (1.8) | 7.0 (1.7) | 0.479 |
| Median urinary albumin-creatinine ratiob (Q1, Q3) | 999 (492, 1921) | 959 (467, 1846) | 893 (443, 1819) | 0.110 | 1098 (525, 2181) | 925 (489, 1771) | 859 (448, 1690) | <0.001 |
| eGFR (ml/min per 1.73 m2) | 43.0 (12.3) | 43.4 (12.3) | 43.9 (12.5) | 0.380 | 43.1 (12.6) | 43.2 (12.0) | 42.9 (12.6) | 0.846 |
| Type 2 diabetes, n (%) | 737 (71.8) | 378 (62.9) | 292 (65.2) | <0.001 | 351 (71.0) | 403 (66.8) | 657 (66.7) | 0.202 |
| Cardiovascular disease,c n (%) | 404 (39.4) | 222 (36.9) | 164 (36.6) | 0.478 | 175 (35.4) | 228 (37.8) | 367 (37.3) | 0.696 |
| Heart failure, n (%) | 109 (10.6) | 59 (9.8) | 60 (13.4) | 0.163 | 53 (10.7) | 55 (9.1) | 117 (11.9) | 0.228 |
| Prior medication, n (%) | ||||||||
| ACE inhibitor | 333 (32.5) | 171 (28.5) | 149 (33.3) | 0.160 | 153 (31.0) | 202 (33.5) | 299 (30.4) | 0.412 |
| ARB | 681 (66.4) | 421 (70.0) | 289 (64.5) | 0.137 | 334 (67.6) | 387 (64.2) | 664 (67.4) | 0.350 |
| Diuretic | 490 (47.8) | 250 (41.6) | 161 (35.9) | <0.001 | 245 (49.6) | 259 (43.0) | 421 (42.7) | 0.030 |
Q1, quartile 1; Q3, quartile 3; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Race was reported by the investigators; “Other” includes Native Hawaiian or other Pacific Islander, American Indian or Alaska Native, and other.
The albumin-creatinine ratio was calculated with albumin measured in milligrams and creatinine measured in grams.
Cardiovascular disease was defined as a history of peripheral artery disease, angina pectoris, myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, heart failure, valvular heart disease, abdominal aorta aneurysm, atrial fibrillation, atrial flutter, ischemic stroke, transient ischemic attack, hemorrhagic stroke, carotid artery stenosis, cardiac pacemaker insertion, vascular stent, coronary artery stenosis, ventricular arrhythmia, implantable cardioverter-defibrillator, noncoronary revascularization, or surgical amputation.
The odds of an acute reduction in eGFR >10% was more than three-fold higher among patients randomized to dapagliflozin relative to placebo (OR, 3.2; 95% CI, 2.8 to 3.6; P<0.001). Results were similar for an absolute decline in eGFR>3 ml/min per 1.73 m2 (OR, 3.3; 95% CI, 2.9 to 3.7; P<0.001). The effects of dapagliflozin versus placebo on the acute reduction in eGFR>10% were generally consistent in patients with and without type 2 diabetes and in most other subgroups (Figure 2), although the effect was more pronounced among older patients (P-interaction=0.02) and patients who self-identified as White (P-interaction=0.02; Figure 2). Subgroup findings were similar among patients who experienced an acute absolute reduction in eGFR >3 ml/min per 1.73 m2, with the exception of more pronounced effects in men (P-interaction=0.006) and patients with a history of cardiovascular disease(P-interaction=0.02).
Figure 2.

Odds ratios and 95% confidence intervals (95% CIs) of dapagliflozin versus placebo for the risk of an acute decline (>10% or >3 ml/min per 1.73 m2) in eGFR across participant subgroups defined by baseline characteristics.
eGFR Slope over Time by Acute Change Categories
Within the dapagliflozin group, there was a statistically significant difference in the mean decline in eGFR (eGFR slope), across categories of an acute change in eGFR (P-interaction<0.001). Specifically, after multivariable adjustment, the mean annual decline in eGFR from month 2 to the end of treatment was attenuated in patients with an acute reduction in eGFR>10% (eGFR decline, −1.58 ml/min per 1.73 m2 per year; 95% CI, −2.07 to −1.08) compared with those with a modest acute reduction in eGFR (−2.44 ml/min per 1.73 m2 per year; 95% CI, −3.09 to −1.80; P=0.04 versus >10% decline) or no reduction in eGFR (−2.48 ml/min per 1.73 m2 per year; 95% CI, −3.24 to −1.73; P=0.05 versus >10% decline; Figure 3A). There was no heterogeneity among the association between randomization to dapagliflozin and chronic eGFR slope by category of acute reduction in eGFR when comparing patients with or without type 2 diabetes (P-interaction=0.47). Within the placebo group, there was no statistically significant difference in the mean annual decline in eGFR across categories of acute change in eGFR (P-interaction=0.48; Figure 3B). Notably, the rates of eGFR decline were slower in dapagliflozin- than in placebo-treated patients across the three categories of acute change in eGFR with changes of −3.27 (CI, −4.01 to −2.52), −3.84 (CI, −4.50 to −3.17), and −3.77 (CI, −4.29 to −3.24) ml/min per 1.73 m2 per year, respectively, for eGFR>10%, modest, and no reduction. Modeling acute changes in eGFR on a continuous scale, we observed attenuated progression of CKD in patients with larger acute reductions in eGFR in the dapagliflozin and placebo group with no evidence that the association was different between the two groups (P-interaction=0.38; Figure 3C). Among the 97 participants with an acute reduction in eGFR >30% on dapagliflozin initiation, the long-term eGFR decline was similar compared with those who did not experience an acute reduction in eGFR≥30% (Supplemental Figure 1). Results were similar when acute eGFR changes were categorized as absolute changes (Supplemental Figure 2).
Figure 3.
eGFR by acute percentage decline categories for dapagliflozin and placebo. The annual eGFR decline from week 2 to the end of treatment for (A) the dapagliflozin group and (B) the placebo group. (C) The annual eGFR decline from week 2 to the end of treatment as a function of acute changes in eGFR in the placebo and dapagliflozin group.
Primary and Secondary Composite End Points Associated with Acute Change in eGFR
During a median of 2.3 (interquartile range, 2.0–2.6) years after the 2-week period during which acute eGFR declines were determined, 494 patients developed the primary composite end point. There were 377 kidney-specific end points, 228 heart failure hospitalization or cardiovascular deaths composite end points, and 235 deaths. In the dapagliflozin group, the multivariable adjusted HR for the primary and three secondary outcomes were similar among patients with an acute reduction in eGFR>10% and an acute reduction in eGFR between 0% and ≤10% or those with no acute reduction in eGFR (Figure 4). In contrast, in the placebo group, a larger acute reduction in eGFR was associated with a higher risk of the primary outcome; the multivariable adjusted HR for moderate eGFR decline and large eGFR decline were 1.26 (95% CI, 0.95 to 1.68) and 1.82 (95% CI, 1.38 to 2.39), respectively, with evidence of a positive log-linear trend (P<0.001; Figure 4, Supplemental Figure 3). A similar pattern was observed for the kidney-specific outcome and all-cause mortality.
Figure 4.
Risk of primary and secondary outcomes according to initial change in eGFR in the dapagliflozin and placebo groups separately. Patients with an increase in eGFR (eGFR decline ≤0%) are used as reference group for the subgroups of patients with modest acute decline (>0 to ≤10%) and acute decline (>10%).
Modeling the acute eGFR change as a continuous variable with a restricted cubic splines model, we observed that a larger acute reduction in eGFR was independently associated with a higher risk of the primary composite and secondary composite outcomes in patients randomized to placebo (Figure 5). In contrast, larger acute reductions in eGFR were not associated with a higher risk of the primary composite or secondary composite kidney end point in patients randomized to dapagliflozin (Figure 5). There was a statistically significant interaction when comparing associations between the acute reduction in eGFR and the treatment effect on the primary composite (P-interaction=0.02; Supplemental Figure 4A) and secondary composite kidney end point (P-interaction=0.04; Supplemental Figure 4B) in the placebo and dapagliflozin groups.
Figure 5.

Hazard ratio as function of acute eGFR slope fitted as a continuous variable with a restricted cubic spline model. Graphs showing acute eGFR change for (A) primary composite outcome; (B) secondary kidney outcome; (C) composite of ESKD and kidney death; and (D) all-cause death.
Safety and Study Drug Discontinuation
In the overall dapagliflozin and placebo groups included in this analysis, drug discontinuation due to AEs occurred in 115 (5.5%) and 118 (5.7%) patients, serious AEs in 618 (29.8%) and 711 (34.1%) patients, kidney-related AEs in 152 (7.3%) and 183 (8.8%) patients, and volume depletion events in 122 (5.9%) and 89 (4.3%) patients, respectively. The frequency of serious AEs was similar for dapagliflozin-treated patients regardless of the degree of acute reduction in eGFR (Figure 6), whereas the rates of serious AEs increased in the placebo group with larger declines in eGFR. For placebo-treated patients with a decline in eGFR≥30%, there was a suggestion of a higher frequency of kidney-related AEs compared with patients with no eGFR decline, but this was not observed in the dapagliflozin group. Results were similar when we analyzed acute eGFR change as a continuous parameter (Figure 7).
Figure 6.

Safety events according to acute eGFR change in the dapagliflozin and placebo groups separately.
Figure 7.

Safety events according to acute eGFR change fitted as a continuous variable in the dapagliflozin and placebo groups separately. Graphs showing acute eGFR change for (A) any AE leading to discontinuation of study drug, (B) any serious AE (SAE), (C) any kidney AE, and (D) symptoms of volume depletion.
Discussion
In this study of patients with CKD and albuminuria enrolled in the DAPA-CKD trial, we demonstrated that an acute reduction in eGFR is more common in patients receiving dapagliflozin compared with placebo. Among dapagliflozin-treated patients, a larger acute reduction in eGFR was associated with an attenuated chronic eGFR slope and was not associated with increased risk of the primary composite end point or the secondary composite kidney end point.
SGLT2 inhibitors frequently induce an acute reversible reduction in eGFR of approximately 3–8 ml/min per 1.73 m2 by increasing sodium chloride transport to the distal tubule and augmenting tubulo-glomerular feedback.14 In patients with CKD participating in the DAPA-CKD trial, the mean acute reduction in eGFR was similar to that observed in other randomized placebo-controlled trials in patients with diabetic kidney disease such as the DELIGHT trial (−4.8 ml/min per 1.73 m2) and the CREDENCE trial (−3.2 ml/min per 1.73 m2).3,15 Understanding clinical characteristics associated with a decline in eGFR can help guide optimal use of SGLT2 inhibitors in clinical practice. Within the dapagliflozin group, older patients, those with a higher BP or body mass index or lower hemoglobin, and patients who were using diuretics were more likely to experience a more pronounced reduction in eGFR. Several of these characteristics were also associated with the acute reduction in eGFR in the placebo group.
The acute reduction in eGFR after initiation of dapagliflozin is reminiscent of experience with ACEis and ARBs. In previous studies in patients with CKD, the magnitude of the acute reduction in eGFR after ACEi or ARB initiation was associated with the degree of preservation of kidney function in the long term.11,12 A post hoc analysis from the CREDENCE trial in patients with type 2 diabetes and CKD demonstrated that within the canagliflozin group, long-term eGFR trajectories were similar regardless of the acute change in eGFR. In the DAPA-CKD trial, we observed an attenuated chronic eGFR slope in patients with an acute reduction in eGFR>10%.10 These data suggest that the acute reduction in eGFR could reflect a dapagliflozin-induced reduction in glomerular hyperfiltration. Differences in patient characteristics between the DAPA-CKD and CREDENCE trials may explain these disparate findings. The lower baseline eGFR in DAPA-CKD is noteworthy (43 ml/min per 1.73 m2 compared with 56 ml/min per 1.73 m2 in CREDENCE). Indeed, within the subgroup of patients in CREDENCE with baseline eGFR similar to the DAPA-CKD cohort, those with an acute reduction in eGFR >10% experienced a less pronounced longer-term eGFR decline (i.e., slower progression) compared with those with a modest or no acute decrease in eGFR upon initiation of canagliflozin.10
Other observations about patients exhibiting an acute reduction in eGFR are also clinically relevant. We demonstrated that there was no excess of drug discontinuation due to AEs among patients with an acute decline in eGFR>10%. Moreover, the risk of AEs or serious AEs were not increased in this subgroup. When we stratified the population in finer subgroups of acute declines in eGFR, the proportion of patients with AEs related to hypoglycemia, kidney events, and volume depletion was higher in those with more pronounced acute declines in eGFR. However, this was true for both the placebo and dapagliflozin groups indicating that the higher AEs rates cannot be attributed to dapagliflozin initiation. These reassuring efficacy and safety data suggest that it seems reasonable that for the majority of patients there is no need to routinely check electrolytes or kidney function shortly after initiating dapagliflozin unless there is clinical concern for volume depletion such as in elderly patients who are treated with high doses of diuretics.
There are limitations which should be considered when interpreting the findings. First, this was a post hoc analysis and we stratified the population on the basis of a postrandomization variable which may have introduced confounding. Second, the intraindividual variability over time in serum creatinine and eGFR is high, which may have resulted in misclassification of acute changes in eGFR. Third, although the data on eGFR decline support the contention that treatment with dapagliflozin may be interpreted as a marker of therapeutic response, we were unable to distinguish whether the acute reduction in the dapagliflozin group represents an acute “hemodynamic-induced” reduction in eGFR or an acute reduction in eGFR due to disease progression. This may explain why within the dapagliflozin group we did not observe an association between a larger acute reduction in eGFR and lower risk of clinical end points. That said, we observed no safety concerns related to or associated with larger relative or absolute acute eGFR declines, and suggest that a reversible decline in eGFR after initiation of dapagliflozin therapy should not be a reason to discontinue the drug. Fourth, only 144 (3.5%) participants experienced an acute reduction in eGFR>30% decline. This study is therefore not powered to assess the consequences of large acute reductions in eGFR. This study can also not assess if SGLT2 inhibitors should be discontinued after a large acute reduction in eGFR. Finally, we did not collect eGFR after the completion of the trial, which might have accentuated the differences observed among the dapagliflozin-treated patient groups defined by the acute reduction in eGFR, particularly if the “rebound” in eGFR matched the magnitude of the initial, reversible reduction in eGFR. Other studies with dapagliflozin have, however, shown that in patients with CKD with and without diabetes the acute decline in eGFR is completely reversible.15,16
In conclusion, among patients with CKD and albuminuria treated with dapagliflozin, acute reductions in eGFR (from baseline to week 2) are not associated with higher rates of CKD progression.
Disclosures
G. Chertow has received fees from AstraZeneca for the DAPA-CKD trial steering committee; has received research grants from Amgen, National Institute of Allergy and Infectious Diseases, and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); is on the board of directors for Satellite Healthcare; has received fees for advisory boards from Baxter, Cricket, DiaMedica, and Reata; holds stock options for Ardelyx, CloudCath, Durect, DxNow, and Outset; has received fees from Akebia, Sanifit, and Vertex for trial steering committees; has received fees for data and safety monitoring board service from Angion, Bayer, Gilead, Mineralys, NIDDK, Palladio, and ReCor; reports consultancy fees from Akebia, Ardelyx, AstraZeneca, Cricket, DiaMedica, Gilead, Miromatrix, Reata, Sanifit, Unicycive, and Vertex; reports ownership interest with Eliaz Therapeutics, Physiowave, PuraCath, Rénibus, and Unicycive; and is co-editor of Brenner & Rector's The Kidney (Elsevier). R. Correa-Rotter has received honoraria from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Medtronic, and Sanofi; has lectured for AbbVie, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, Sanofi, and Takeda; has received research support from AstraZeneca, Bayer, GlaxoSmithKline, and Novo Nordisk; reports consultancy fees from AstraZeneca, Bayer, Boehringer Ingelheim, and Novo Nordisk; has an advisory or leadership role with DAPA-CKD AstraZeneca (member of steering committee), GlaxoSmithKline (national leader of the ASCEND study), and Novo Nordisk (national leader of the FLOW study); is on the editorial board of Current Opinions Nephrology and Hypertension, Nefrologia Latinoamericana, Revista de Investigación Clinica, American Journal of Kidney Diseases, Frontiers in Nephrology (associate editor), and Blood Purification (associate editor); and is a member of American Society of Nephrology, International Society of Nephrology, National Kidney Foundation, Mexican Institute for Research in Nephrology, Latin American Society of Nephrology and Hypertension, and European Dialysis and Transplant Association/European Renal Association. T. Greene has received grants for statistical consulting from AstraZeneca, Boehringer Ingelheim, and CSL; has received personal fees from Durect, Janssen, and Pfizer for statistical consulting; has received consultancy fees from AstraZeneca, Invokana, Janssen, Novartis, and Pfizer; and reports research funding from Vertex. H. Heerspink is a consultant for AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL, Dimerix, Eli Lilly, Gilead, Goldfinch, Janssen, Merck, Mundipharma, Mitsubishi Tanabe, Novo Nordisk, Retrophin, and Travere Therapeutics; has received research support from AbbVie, AstraZeneca, Boehringer Ingelheim, Janssen, and Novo Nordisk; and is on the speakers bureau for AstraZeneca. N. Kashihara has received honoraria research grants from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, Otsuka, and Takeda; has received consultancy fees from AstraZeneca, Kyowa Hakko Kirin, and Novartis; reports research funding from Astellas, Daiichi-Sankyo, Otsuka, and Takeda; reports an advisory or leadership role with AstraZeneca, Kyowa Hakko Kirin, and Novartis; and is a member of Japan Kidney Association and Japanese Society of Nephrology. A.M. Langkilde is an employee and stockholder of AstraZeneca. J. McMurray has received payments to his employer, Glasgow University, for his work on clinical trials, consulting, advisory or leadership role, and other activities from AbbVie Alnylam, Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Cardurion, Cytokinetics, Dal-Cor, GlaxoSmithKline, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos; has received personal lecture fees from Abbott, Alkem Metabolics, The Corpus, Eris Lifesciences, Hikma, Lupin, Medsca, Medscape/theheart.org, Pro-AdWise Communications, Radcliffe Cardiology, Servier, and Sun Pharmaceuticals; and is director of Global Clinical Trial Partners Ltd. P. Rossing has received honoraria to Steno Diabetes Center Copenhagen for consultancy from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Novo Nordisk, Merck, Mundipharma, Sanofi, and Vifor Pharma; and has received research support from AstraZeneca, Bayer, and Novo Nordisk. C.D. Sjöström is an employee and stockholder of AstraZeneca. B. Stefánsson is an employee and stockholder of AstraZeneca and is also an employee of Vifor Pharma. R. Toto is a consultant for ACI Pharmaceuticals, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Medscape, Otsuka, Reata, Relypsa, and Vifor Pharma; has received honoraria from ACI Pharmaceuticals, Akebia, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Medscape, Otsuka, Reata Pharmaceuticals, Relypsa, and Vifor Pharma; and reports advisory or leadership role with ACI Pharmaceuticals, Akebia, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Medscape, Otsuka, Reata Pharmaceuticals, Relypsa, and Vifor Pharma. D. Wheeler provides ongoing consultancy services to AstraZeneca; has received honoraria and/or consultancy fees from Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Bayer, Gilead, GlaxoSmithKline, Janssen, Medscape, Merck Sharp & Dohme, Mundipharma, Napp, Pharmacosmos, Reata, Takeda, Tricida, Vifor Fresenius, and Zydus; reports advisory or leadership role with AstraZeneca; is on the speakers bureau for Amgen, Astellas, AstraZeneca, Janssen, Mundipharma, Napp, Merck Sharp & Dohme, and Vifor Fresenius; and is an honorary professorial fellow of George Institute for Global Health. All remaining authors have nothing to declare.
Funding
The DAPA-CKD study was funded by AstraZeneca.
Supplementary Material
Acknowledgments
The authors thank all investigators, trial teams, and patients for their participation in the trial. The authors would also like to acknowledge Parita Sheth, inScience Communications, London, UK, for assistance in editing and preparation of figures. H. Heerspink is supported by the BEAt-DKD project. The BEAt-DKD project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement 115974. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and the European Federation of Pharmaceutical Industries and Associations. R. Toto’s contribution to this manuscript was supported by endowments from the Mary M. Conroy Professorship in Kidney Disease and the Houston J. and Florence A. Doswell Center for the Development of New Approaches for the Treatment of Hypertension.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Members of the DAPA-CKD Trial Committees and Investigators, Hiddo J.L. Heerspink, David C. Wheeler, Glenn Chertow, Ricardo Correa-Rotter, Tom Greene, Fan Fan Hou, John McMurray, Peter Rossing, Robert Toto, Bergur Stefánsson, and Anna Maria Langkilde
Author Contributions
H. Heerspink, B. Stefánsson, and D. Wheeler conceptualized the study; H. Heerspink and N. Jongs were responsible for formal analysis and wrote the original draft; H. Heerspink, A.M. Langkilde, and B. Stefánsson were responsible for project administration; D. Wheeler was responsible for visualization; G. Chertow, R. Correa-Rotter, T. Greene, H. Heerspink, N. Jongs, N. Kashihara, A.M. Langkilde, J. McMurray, P. Rossing, C.D. Sjöström, B. Stefánsson, and D. Wheeler were responsible for methodology; all authors were responsible for investigation; and all authors reviewed and edited the manuscript.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2022030306/-/DCSupplemental.
Supplemental Table 1. Baseline characteristics by absolute decline in eGFR between baseline and week 2 in DAPA-CKD (n=4157).
Supplemental Figure 1. Acute decline in eGFR ≥30% or <30% in the dapagliflozin group (panel A) and placebo group (panel B).
Supplemental Figure 2. eGFR by acute absolute decline categories for dapagliflozin and placebo. Panel A shows the dapagliflozin group; panel B the placebo group, and panel C the annual eGFR decline from week 2 to end of treatment as a function of acute changes in eGFR in the placebo and dapagliflozin groups.
Supplemental Figure 3. Risk of primary and secondary outcomes with acute increase in eGFR in the dapagliflozin group
Supplemental Figure 4. Treatment effect of dapagliflozin on primary and secondary outcomes as function of change in eGFR.
References
- 1.Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. : Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: An analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 7: 606–617, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. ; EMPA-REG OUTCOME Investigators : Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. ; CREDENCE Trial Investigators : Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. ; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, et al. : SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 7: 845–854, 2019 [DOI] [PubMed] [Google Scholar]
- 6.van Bommel EJM, Muskiet MHA, van Baar MJB, Tonneijck L, Smits MM, Emanuel AL, et al. : The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int 97: 202–212, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Wanner C, Heerspink HJL, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, et al. ; EMPA-REG OUTCOME Investigators : Empagliflozin and kidney function decline in patients with type 2 diabetes: A slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol 29: 2755–2769, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. : Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 6: 691–704, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Kraus BJ, Weir MR, Bakris GL, Mattheus M, Cherney DZI, Sattar N, et al. : Characterization and implications of the initial estimated glomerular filtration rate “dip” upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int 99: 750–762, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Oshima M, Jardine MJ, Agarwal R, Bakris G, Cannon CP, Charytan DM, et al. : Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int 99: 999–1009, 2021 [DOI] [PubMed] [Google Scholar]
- 11.Apperloo AJ, de Zeeuw D, de Jong PE: A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int 51: 793–797, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJ, et al. : An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int 80: 282–287, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R, Greene T, Hou FF, et al. ; DAPA-CKD Investigators : Rationale and protocol of the Dapagliflozin And Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 35: 274–282, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZ: Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134: 752–772, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Pollock C, Stefánsson B, Reyner D, Rossing P, Sjöström CD, Wheeler DC, et al. : Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 7: 429–441, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, Greasley PJ, et al. ; DIAMOND investigators : Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): A randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol 8: 582–593, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.