Abstract
Background
There is substantial variability in prostate cancer (PCa) mortality rates across Caucasian American (CA), African American (AA), Asian, and Hispanic men; however, these estimates are unable to disentangle race or ethnicity from confounding factors. The current study explores survival differences in long‐term PCa outcomes between self‐reported AA and CA men, and examines clinicopathologic features across self‐reported CA, AA, Asian, and Hispanic men.
Methods
This retrospective cohort study utilized the Center for Prostate Disease Research (CPDR) Multi‐center National Database from 1990 to 2017. Subjects were consented at military treatment facilities nationwide. AA, CA, Asian, or Hispanic men who underwent radical prostatectomy (RP) for localized PCa within the first year of diagnosis were included in the analyses. Time from RP to biochemical recurrence (BCR), BCR to metastasis, and metastasis to overall death were evaluated using Kaplan–Meier unadjusted estimation curves and adjusted Cox proportional hazards regression.
Results
This study included 7067 men, of whom 5155 (73%) were CA, 1468 (21%) were AA, 237 (3%) were Asian, and 207 (3%) were Hispanic. AA men had a significantly decreased time from RP to BCR compared to CA men (HR = 1.25, 95% CI = 1.06–1.48, p = 0.01); however, no difference was observed between AA and CA men for a time from BCR to metastasis (HR = 0.73, 95% CI = 0.39–1.33, p = 0.302) and time from metastasis to overall death (HR = 0.67, 95% CI = 0.36–1.26, p = 0.213).
Conclusions
In an equal access health care setting, AA men had a shorter survival time from RP to BCR, but comparable survival time from BCR to metastasis and metastasis to overall death.
Keywords: healthcare disparities, prostatectomy, prostatic neoplasms
Previous research has demonstrated significant differences in long‐term prostate cancer outcomes in Caucasian American compared to African American men. This study explored whether racial differences in long‐term oncological outcomes exist among men diagnosed with prostate cancer and treated surgically, in equal access health care settings. While African American men had slightly poorer biochemical recurrence‐free survival compared to Caucasian American men, there were no racial differences observed for distant metastasis‐free survival or overall survival, controlling for detailed clinical factors. In a large racially heterogeneous cohort of men within an equal access health care setting, long‐term prostate cancer outcomes were comparable across race.
1. INTRODUCTION
Prostate cancer (PCa) is the most commonly diagnosed non‐skin malignancy and the fifth leading cause of cancer death in men worldwide, with an estimated incidence of 1.3 million new cases and 359,000 deaths in 2018. 1 In the United States, population‐based estimates show that African‐American (AA) men are more likely to be diagnosed with PCa, present with distant metastasis, and 2.5 times more likely to die from PCa compared to Caucasian American (CA) men, while Hispanic and Asian men are more likely to be diagnosed with higher stage disease but appear to have comparable oncologic outcomes compared to CA men. 2 These racial disparities, especially in AA men, are a major health concern and a focus of continual research. 3
Previous studies that adjusted for relevant confounding variables found a moderate significant or non‐significant decrease in time from radical prostatectomy (RP) to biochemical recurrence (BCR), when comparing AA men to CA men. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 Fewer studies have examined the association between race and metastasis‐free survival, prostate cancer‐specific mortality, and overall death. These studies report conflicting results: Some suggest that AA men have a moderate decrease in survival time, 24 , 25 , 26 , 27 , 28 , 29 , 30 whiles others found no difference in survival between AA and CA men. Emerging data from equal access systems and/or adjusting for socioeconomic variables suggest that AA men have non‐inferior oncologic outcomes. 31 , 32 , 33 , 34 , 35 , 36 However, due to the nature of observational study designs, it is difficult to distinguish race from confounding variables such as differences in access to and receipt of healthcare, socioeconomic status, age at diagnosis, and related comorbidities. 37
In order to further address the relationship between race and long‐term PCa outcomes, a racially diverse, surgically treated cohort of men, enrolled over a 28‐year period in an equal access military health care system was examined. The primary aim of this study was to examine the relationship between CA and AA race and survival time from radical prostatectomy (RP) to BCR, BCR to metastasis, and metastasis to overall survival, controlled for a wide array of clinicopathologic relevant variables. A secondary study aim was to compare clinicopathologic variables across CA, AA, Hispanic, and Asian men.
2. METHODS
2.1. Study design and participants
A retrospective cohort study was conducted on patients enrolled in the Center for Prostate Disease Research (CPDR) Multi‐Center National Database in men with biopsy‐confirmed PCa who underwent RP treatment within 12 months of diagnosis between January 1, 1990 and December 31, 2017. Enrollees in the CPDR database are military health care beneficiaries who are eligible for TRICARE‐for‐life health care coverage. Demographic, clinical, treatment, and outcomes data were collected as part of routine patient follow‐up on all CPDR enrollees. Informed consent was obtained at the time of transrectal ultrasound‐guided biopsy (TRUS) for suspicion of PCa, as described previously. 38 Men without self‐reported race, with distant metastasis within a year, local and/or distant metastasis at biopsy, distant metastasis at pathology, or men who underwent neoadjuvant therapy were excluded from the study (Table S1). Institutional Review Board (IRB) approval for data collection and evaluation activities were granted at each participating medical center and the Uniformed Services University of the Health Sciences (USUHS).
2.2. Demographic, clinical, and pathologic variables
Patient characteristics of interest in this study included: Age at diagnosis (years), time from RP to last follow up (years), time from diagnosis to RP (months), self‐reported race or ethnicity (CA, AA, Asian, Hispanic), obesity (BMI < 30, BMI ≥ 30), number of medical comorbidities (chronic obstructive pulmonary disease, cardiovascular disease, cerebral vascular accident, and/or cancer), PSA (ng/ml) at time of diagnosis, pathologic stage (T2a, T3‐T4), 2014 ISUP Gleason score (≤6, 3 + 4, 4 + 3, ≥8–10), surgical margin status (positive, negative), primary treatment year (1990–1994, 1995–1999, 2000–2004, 2005–2009, 2010–2014, 2015–2019), post‐BCR PSA doubling time (PSADT) (<3 months, 3–8.9 months, 9.0–14.9 months, >15 months), any secondary treatment, BCR, metastasis, metastasis after BCR, death from any cause, and death from any cause after metastasis.
2.3. Study endpoints: Biochemical distant metastasis, and overall death
BCR was defined as a PSA value ≥0.2 ng/ml observed at ≥8 weeks post‐operatively, followed by a subsequent confirmatory PSA level ≥0.2 ng/ml or the initiation of salvage therapy. 39 Distant metastasis was present if the patient had a positive imaging study in the setting of a rising PSA or a confirmed biopsy result of a distant lesion. A positive imaging study included retroperitoneal lymphadenopathy on magnetic resonance imaging or computerized tomography, or a bone lesion on technetium‐99 m‐HDP bone scan or CT scan.
BCR, distant metastasis, and overall death were modeled as time‐dependent study endpoints with three possible results: Achieved endpoint, censored, or achieved end of study with no event. Men with <6 months of follow‐up time were excluded from each model. As an endpoint, distant metastasis must have occurred more than 1 year after PCa diagnosis. For the BCR endpoint, men were censored at treatment date between RP and BCR, date of last known medical visit, or date of death. For the distant metastasis and death endpoint, men were censored at the date of last known medical visit or date of death. Exclusions were also made for men who underwent adjuvant treatments between RP and BCR, between BCR and metastasis, or between metastasis and death, due to the variability and strong correlation of receipt of treatment with PSADT.
2.4. Statistical analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Frequencies and distributions of patient features were calculated for the study cohort, and stratified by race/ethnicity (CA, AA, Asian, and Hispanic). The chi‐square test and Mann–Whitney U test was used to compare categorical and continuous variables, respectively.
A Kaplan Meier curve analysis was used to produce 5‐, 10‐, and 15‐year probability estimates for survival from RP to BCR, BCR to metastasis, and metastasis to overall death as a function of race and race by obesity, restricted to AA and CA men. Cox proportional hazards (PH) analysis was used to model RP to BCR, BCR to distant metastasis, and distant metastasis to overall death as a time‐dependent outcome, as a function of race (CA versus AA). The Cox PH model from RP to BCR was controlled for age at RP, time from diagnosis to RP, primary treatment year, diagnostic PSA, obesity, pathologic stage, Gleason score, surgical margin status, and the number of major comorbidities. The BCR to metastasis Cox PH model was controlled for age at BCR, time from RP to BCR, obesity, pathologic stage, Gleason score, surgical margin status, and PSADT. The metastasis to overall death model was adjusted for age at metastasis, time from RP to metastasis, obesity, and the number of major comorbidities. Hazards ratios (HR) and 95% confidence intervals (CI) were reported for Cox PH models. P‐values were computed using two‐sided statistical tests (α = 0.05). PH assumptions were checked using the ASSESS PH statement in SAS, and each variable met the PH assumption. 40
3. RESULTS
A total of 7067 men were eligible for the study, of whom 5155 (73%), 1468 (21%), 237 (3%), and 207 (3%) self‐reported as CA, AA, Asian, and Hispanic, respectively (Figure S1 and Table S1). Median patient age at diagnosis and median follow‐up time following RP were 61.6 and 6.7 years, respectively. Among variables that were significantly different across races, CA men were more likely to be older at diagnosis (62.4 years, p < 0.001), have a longer time from RP to last follow‐up (7.0 years, p < 0.001), have PCa treatment in earlier years (p < 0.001), and have a longer post‐BCR PSADT (p = 0.11). AA men were less likely to have major comorbidity (87.5%, p < 0.001). Hispanic men were more likely to have a lower diagnostic PSA level (5.1 ng/ml, p < 0.001). Asian men were less likely to be obese (89.3%, p < 0.001), and more likely to have a shorter time from diagnosis to RP (2.0 months, p < 0.001), be diagnosed with the lowest pathologic prostate tumor stage and grade (78.4%, T2, p = 0.009 and 55.7% ≤ 6, p = 0.046, respectively) and to receive another treatment after RP (86.5%, p = 0.017) (Table 1). Men with a shorter PSADT also had a shorter time from RP to BCR (p < 0.001, Table 2).
TABLE 1.
Descriptive characteristics of prostate cancer patients overall and by race and ethnicity
| Characteristic | All subjects a N = 7067 | Race | p‐value comparing all races | ||||
|---|---|---|---|---|---|---|---|
| Caucasian American a N = 5155 (73%) | African American a N = 1468 (21%) | p‐value for CA vs. AA | Asian a N = 237 (3%) | Hispanic a N = 207 (3%) | |||
| Age at diagnosis (years), median (range) | 61.6 (26.7, 84.2) | 62.4 (26.7, 84.2) | 59 (34.4, 82.9) | <0.001 | 60.7 (37.9, 77.8) | 61.4 (36.5, 77.6) | <0.001 |
| Time from RP to last follow‐up (years), median (range) | 6.7 (0.5, 28.1) | 7.0 (0.5, 28.1) | 6.4 (0.5, 26.4) | <0.001 | 4.9 (0.6, 22.1) | 5.8 (0.5, 25.4) | <0.001 |
| Time from diagnosis to RP (months), median (range) | 2.3 (0.3, 12) | 2.2 (0.03, 12) | 2.5 (0.03, 12) | <0.001 | 2.0 (0.6, 11.2) | 2.2 (0.5, 11.2) | <0.001 |
| Time from RP to BCR (years), median (range) (N = 5412) b | 4.7 (0.5, 22.8) | 4.9 (0.5, 22.8) | 4.1 (0.5, 21.6) | <0.001 | 3.8 (0.6, 18.4) | 4.6 (0.5, 15.6) | <0.001 |
| Time from BCR to metastasis (years), median (range) (N = 1026) c | 6.6 (0.5, 23.6) | 6.7 (0.5, 22.7) | 6.7(0.7, 23.6) | 0.96 | 5.2 (1.3, 18.7) | 4.7 (0.6, 18.2) | 0.14 |
| Time from metastasis to death (years), median (range) (N = 106) d | 4.1 (0.6, 23.9) | 4.1 (0.6, 23.9) | 3.9 (0.6, 16.1) | 0.88 | – | 2.6 (2.6, 2.6) | 0.84 |
| Obese (N, %) | 1400 (23.1%) | 957 (21.9%) | 370 (28.8%) | 24 (10.7%) | 49 (26.5%) | <0.001 | |
| Number of comorbidities e | <0.001 | <0.001 | |||||
| 0 | 5645 (79.9%) | 3992 (77.4%) | 1285 (87.5%) | 198 (83.5%) | 170 (82.1%) | ||
| 1 | 1226 (17.3%) | 996 (19.3%) | 161 (11%) | 35 (14.8%) | 34 (16.4%) | ||
| ≥2 | 196 (2.8%) | 167 (3.2%) | 22 (1.5%) | 4 (1.7%) | 3 (1.4%) | ||
| Diagnostic PSA (ng/ml), median (range) | 5.5 (0.01, 1060) | 5.4 (0.01, 250) | 5.8 (0.01, 1060) | <0.001 | 5.5 (0.1, 76.1) | 5.1 (0.04, 27.3) | <0.001 |
| Pathologic T stage | 0.61 | 0.009 | |||||
| T2 | 4646 (68.4%) | 3366 (67.9%) | 963 (68.6%) | 182 (78.4%) | 135 (68.5%) | ||
| T3–T4 | 2147 (31.6%) | 1594 (32.1%) | 441 (31.4%) | 50 (21.6%) | 62 (31.5%) | ||
| Pathologic Gleason score | 0.13 | 0.046 | |||||
| Group 1 (≤6) | 3856 (54.6%) | 2823 (55.7%) | 786 (54.2%) | 132 (55.7%) | 115 (56.4%) | ||
| Group 2 (3 + 4) | 2055 (30.1%) | 1507 (29.7%) | 420 (28.9%) | 67 (28.3%) | 61 (29.9%) | ||
| Group 3 (4 + 3) | 564 (8.3%) | 393 (7.8%) | 123 (8.5%) | 29 (12.2%) | 19 (9.3%) | ||
| Groups 4 & 5 (≥8) | 484 (7%) | 344 (6.8%) | 122 (8.4%) | 9 (3.8%) | 9 (4.4%) | ||
| Positive surgical margin status | 1884 (27.8%) | 1363 (27.6%) | 389 (27.8%) | 74 (32%) | 58 (28.9%) | 0.52 | |
| Year of surgical treatment | <0.001 | <0.001 | |||||
| 1990–1994 | 1406 (19.9%) | 1130 (21.9%) | 231 (15.7%) | 19 (8%) | 26 (12.6%) | ||
| 1995–1999 | 1564 (22.1%) | 1134 (22%) | 339 (23.1%) | 45 (19%) | 46 (22.2%) | ||
| 2000–2004 | 1748 (24.7%) | 1216 (23.6%) | 391 (26.6%) | 69 (29.1%) | 72 (34.8%) | ||
| 2005–2009 | 1341 (19%) | 977 (19%) | 263 (17.9%) | 58 (24.5%) | 43 (20.8%) | ||
| 2010–2014 | 776 (11%) | 545 (10.6%) | 170 (11.6%) | 43 (18.1%) | 18 (8.7%) | ||
| 2015–2019 | 232 (3.3%) | 153 (3%) | 74 (5%) | 3 (1.3%) | 2 (1%) | ||
| Post‐BCR PSADT d , e (N = 1020) | |||||||
| <10 months | 193 (18.9%) | 135 (18.3%) | 52 (22.4%) | 0.17 | 4 (15.4%) | 2 (8%) | 0.243 |
| > = 10 months | 827 (81.1%) | 602 (81.7%) | 180 (77.6%) | 22 (84.6%) | 23 (92%) | ||
| Treatment after RP to prevent BCR | 239 (4.2%) | 181 (4.3%) | 47 (4.2%) | 0.79 | 5 (2.7%) | 6 (3.7%) | 0.78 |
| Treatment after BCR to prevent metastasis | 54 (4.8%) | 42 (5.1%) | 11 (4.3%) | 0.60 | 1 (3.6%) | – | 0.79 |
| Treatment after metastasis to prevent death | 79 (39.9%) | 64 (41%) | 13 (34.2%) | 0.44 | 2 (66.7%) | – | 0.62 |
| Biochemical recurrence events (N = 5651) | 1134 (20.1%) | 821 (19.7%) | 256 (22.7%) | 28 (14.8%) | 29 (17.8%) | ||
| Distant metastatic events | 198 (3%) | 156 (3.2%) | 38 (2.8%) | 3 (1.4%) | 1 (0.5%) | – | |
| Distant metastatic events after biochemical recurrence (N = 1134) | 89 (7.8%) | 71 (79.8%) | 16 (18.0%) | 2 (2.2%) | 0 | – | |
| All deaths | 1028 (15.5%) | 824 (16.9%) | 169 (12.4%) | 19 (8.7%) | 16 (8.4%) | – | |
| All deaths after metastasis (N = 198) | 98 (49.5%) | 82 (83.7%) | 14 (14.3%) | 2 (3.7%) | 0 | – |
Note: Abbreviations: AA, African American; BCR, biochemical recurrence; CA, Caucasian American; PSA, prostate‐specific antigen; PSADT, PSA doubling time; RP, radical prostatectomy.
Number (%) of subjects unless stated otherwise.
Men who received treatment between RP and BCR were removed from analysis; N = 3988 for CA men, N = 1083 for AA men, N = 184 for Asian men, and N = 157 for Hispanic men.
Men who received treatment between BCR and metastasis were removed from analysis; N = 741 for CA men, N = 233 for AA men, N = 25 for Asian men, N = 27 for Hispanic men.
Men who received treatment between metastasis and death were removed from analysis; N = 83 for CA men, N = 22 for AA men, N = 1 for Hispanic men.
Comorbidity was defined as chronic obstructive pulmonary disease, cardiovascular disease, cerebral vascular accident, and/or cancer.
TABLE 2.
Median time between RP and BCR, BCR and metastasis, and metastasis and death in men without treatment to prevent endpoint, overall and by PSA doubling time (N = 7067)
| Characteristic | All Subjects a | PSA doubling time | p‐value | |
|---|---|---|---|---|
| <10 months | ≥10 months | |||
| Time from RP to BCR (years), median (range) (N = 956) a | 2.1 (0.5, 17.6) | 1.7 (0.5, 12) | 2.3 (0.5, 17.6) | <0.001 |
| Time from BCR to metastasis (years), median (range) (N = 789) b | 6.7 (0.5, 23.6) | 7.5 (0.5, 22.3) | 6.5 (0.5, 23.6) | 0.61 |
| Time from to metastasis to death (years), median (range) (N = 51) c | 4.4 (0.6, 23.9) | 5.6 (0.6, 23.9) | 3.9 (0.6, 13.7) | 0.23 |
N = 185 for PSA doubling time < 10 months and N = 772 for PSA doubling time ≥ 10 months.
N = 164 for PSA doubling time < 10 months and N = 789 for PSA doubling time ≥ 10 months.
N = 21 for PSA doubling time < 10 months and N = 30 for PSA doubling time ≥ 10 months.
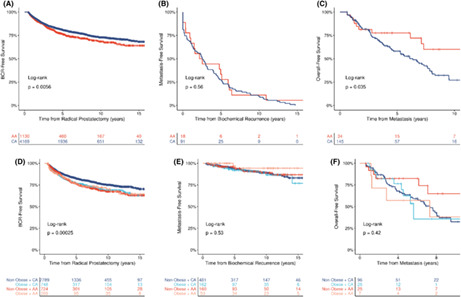
There were 1134 (20%) men who had BCR in the overall cohort: 821 (20%) CA men and 256 (23%) AA men (Table 1). The Kaplan–Meier analysis demonstrated that AA men had a significantly shorter BCR‐free survival time compared to CA men (log‐rank p = 0.005) (Figure 1A). Additionally, compared to non‐obese CA men, obese CA men, non‐obese AA men, and obese AA men all had similar and shorter BCR‐free survival times (log‐rank p = 0.0002). (Figure 1D).
FIGURE 1.

Race‐ and obesity‐stratified Kaplan–Meier estimation curves for 5‐, 10‐, and 15‐year probability estimates of each study outcome. These time to event models were estimated for the following: (A) Radical prostatectomy to biochemical recurrence, (B) Biochemical recurrence to metastasis, (C) Metastasis to all‐cause death, (D) Radical prostatectomy to biochemical recurrence, (E) Biochemical recurrence to metastasis, and (F) Metastasis to overall death, with stratification by race, alone, and race‐by‐obesity subgroups (n = 6623*). Abbreviations: AA, African American; CA, Caucasian American; BCR, biochemical recurrence. *Race comparisons were limited to AA versus CA due to sample size constraints for other racial/ethnic groups.
Of the 1134 men who had BCR, there were 89 (8%) men who had distant PCa metastasis following BCR in the overall cohort: 71 (80%) CA men and 16 (18%) AA men. The Kaplan–Meier analysis demonstrated that there was no difference in survival from BCR to metastasis among CA men compared to AA men (log‐rank p = 0.154) (Figure 1A). There was also no difference in survival from BCR to metastasis when evaluated by race and obesity (log‐rank p = 0.535) (Figure 1E).
Of the 198 men who had metastatic PCa, there were 98 (49%) men who died following their metastatic PCa in the overall cohort: 82 (84%) CA men and 14 (14%) AA men (Table 1). Kaplan–Meier analysis demonstrated that AA men had a significantly longer survival time between metastasis and overall death compared to CA men (log‐rank p = 0.035) (Figure 1A). When evaluated by race and obesity, there was no difference in survival time from metastasis to overall death (log‐rank p = 0.421) (Figure 1E).
Survival time from RP to BCR was shorter for AA men compared to CA men (HR = 1.25, 95% CI = 1.06–1.48, Table 3) However, there was no difference in survival time between AA and CA men from BCR to metastasis (HR = 0.73, 95% CI = 0.39–1.33) and from metastasis to overall death (HR = 0.67, 95% CI = 0.36–1.26).
TABLE 3.
Multivariable Cox proportional hazards models predicting time to event outcomes
| Variable | Radical prostatectomy to biochemical recurrence (N = 3950) a , b | Biochemical recurrence to prostate cancer metastasis (N = 724) a | Prostate cancer metastasis to overall death (N = 154) a | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age at radical prostatectomy (years) | 1.01 (0.998, 1.02) | 0.101 | ||||
| Age at Biochemical recurrence (years) | 0.99 (0.95, 1.03) | 0.513 | ||||
| Age at metastasis (years) | 1.01 (0.93, 1.09) | 0.854 | ||||
| Time from diagnosis to radical prostatectomy (years) | 1.22 (0.59, 2.51) | 0.592 | ||||
| Time from radical prostatectomy to biochemical recurrence (years) | 0.95 (0.83, 1.09) | 0.444 | ||||
| Time from radical prostatectomy to metastasis (years) | 1.12 (0.98, 1.29) | 0.101 | ||||
| Primary treatment year (1990–2017) | 1.01 (0.997, 1.03) | 0.114 | ||||
| Prostate‐specific antigen level at diagnosis (ng/ml) | 1.01 (1.00, 1.02) | 0.040 | ||||
| Race | ||||||
| AA vs. CA | 1.05 (0.88, 1.25) | 0.595 | 0.75 (0.41, 1.38) | 0.363 | 0.68 (0.24, 1.95) | 0.476 |
| Obesity | ||||||
| Yes vs. No | 1.15 (0.97, 1.37) | 0.118 | 0.84 (0.46, 1.51) | 0.550 | 0.53 (0.19, 1.50) | 0.233 |
| Pathologic Gleason score | ||||||
| 3 + 4 vs. 3 + 3 | 0.94 (0.78, 1.12) | 0.476 | 1.44 (0.77, 2.67) | 0.249 | ||
| 4 + 3 vs. 3 + 3 | 1.14 (0.87, 1.49) | 0.342 | 0.83 (0.33, 2.08) | 0.688 | ||
| 8 ~ 10 vs. 3 + 3 | 1.25 (0.98, 1.43) | 0.079 | 2.29 (1.18, 4.42) | 0.014 | ||
| Margin status | ||||||
| Yes vs. No | 1.19 (0.98, 1.43) | 0.078 | 0.87 (0.48, 1.58) | 0.652 | ||
| Pathologic T‐stage | ||||||
| T3‐T4 vs. T2 | 0.95 (0.79, 1.16) | 0.631 | 1.39 (0.74, 2.61) | 0.306 | ||
| Number of comorbidities | ||||||
| 1 vs. 0 | 1.15 (0.95, 1.40) | 0.151 | 1.07 (0.45, 2.52) | 0.880 | ||
| > = 2 vs. 0 | 0.98 (0.68, 1.42) | 0.923 | 6.52 (1.04, 41.08) | 0.046 | ||
| Prostate‐specific antigen doubling time | ||||||
| > = 10 vs. <10 | 0.73 (0.60, 0.89) | 0.002 | 0.35 (0.21, 0.60) | <0.001 | 1.07 (0.46, 2.46) | 0.876 |
Note: Abbreviations: 95% CI, 95% Confidence Interval; HR, Hazard Ratio.
Men with <6 months of follow‐up time after radical prostatectomy were removed from the analysis.
Men who received treatment after radical prostatectomy were censored at treatment.
4. DISCUSSION
The relationship between race and survival time from RP to BCR, BCR to metastasis, and metastasis to overall survival (OS), controlled for a wide array of demographic, clinical, and pathological relevant variables was examined as the primary study aim. Comparison of clinicopathologic variables across CA, AA Hispanic, and Asian race or ethnicity was assessed as a secondary study aim. Both analyses were conducted in a large retrospective cohort of racially diverse PCa patients treated with RP and enrolled in an equal access military health care system over a 28‐year period. This setting reduces the disparity in health care access, treatment, and education, which may influence PCa progression and provides a deeper analysis adjusted for potential confounders. This study supports that there is a positive association between AA race and BCR‐free survival when compared to CA race, but no difference between CA and AA race and survival time between BCR and metastasis, and metastasis and overall death. Furthermore, this analysis revealed that Asian and Hispanic American men have similar or better clinical and pathologic PCa features when compared to CA men. To our knowledge, this is the first study to look at long‐term PCa outcomes on a continuum: From RP to BCR, BCR to metastasis, and metastasis to overall survival, one of the largest RP cohorts comparing long‐term PCa outcomes for CA and AA men, and one of the largest known post‐RP cohorts of Asian and Hispanic American men. 41
Similar to other studies that have examined race and BCR‐free survival, our analysis also revealed a moderate significant decrease in BCR‐free survival time when comparing AA men to CA men, adjusted for confounding variables. When BCR occurs, the treatment team often recommends either androgen‐deprivation (HT) and/or salvage external beam radiation treatment (EBRT) usually happens. In our cohort, there was no significant difference between race and receipt of treatment after BCR (55% AA men vs. 50% CA men, p = 0.18). However, despite a shorter BCR‐free survival time for AA men with a near equal proportion of men receiving treatment after BCR, there was no difference in survival time between BCR and metastasis and metastasis and overall death when comparing AA men to CA men. These data are confirmed by a recent analysis on patients who received HT after BCR in an equal access system, showing that race was not a predictor of DM or other adverse outcomes. Additionally, a recent analysis with the entire Department of Defense database revealed no difference in overall survival between AA and CA men diagnosed with PCa. 42 Together, these findings suggest that AA men may have the less aggressive disease after BCR and/or that AA men may respond better to treatment after BCR conferring improved metastasis‐free survival, prostate cancer‐specific survival, and overall survival for AA men. To this extent, there are a few studies showing that prostate tumors in AA men are more hormonally driven and thus more castrate‐sensitive, possibly leading to better responses to treatment. 43 , 44
Freedland and colleagues 13 were the only other group able to examine AA and CA various long‐term PCa outcomes within one cohort, from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. They found a significant decrease in BCR‐free survival for AA men as compared to CA men on univariable analysis, but not after adjustment for multiple covariates; no difference in aggressive PSA recurrence, metastasis‐free survival, prostate cancer‐specific survival, or overall death for AA men compared to CA men were observed. While their results were comparable to this study's analyses, our data excluded patients who had neoadjuvant therapy, censored all patients who had treatment after RP in the BCR‐free survival models, controlled for comorbidity illness information, and confirmed that treatment after BCR and after metastasis was equal between AA and CA men, ensuring high accuracy of our data, notably in the context of an equal access healthcare setting.
Lee and colleagues 19 evaluated the interaction between obesity and race on BCR‐free survival and found that BCR‐free survival decreased along the following continuum: Non‐obese CA, obese CA, non‐obese AA, and obese AA; however, this association was not significant following adjusted for pathological tumor stage and grade, and age. Obese CA men, non‐obese AA men, and obese‐AA men all had a similar decreased BCR‐free survival time compared to non‐obese CA men. These findings suggest that while obesity is associated with BCR‐free survival in CA men, it may not be for AA men. This relationship merits further investigation to establish the biological basis of the relationship between obesity, race, and prostate cancer outcomes.
Population studies have found different PCa outcomes among subgroups of both Asian men and Hispanic men. 45 , 46 , 47 , 48 Previous reports among Asian men have reported higher stages of disease post‐RP and increased likelihood of presentation with advanced stage disease but generally have equal outcomes. Among Hispanic men, it appears that there is no difference in prostate cancer‐specific mortality when comparing to other races, though outcomes may be slightly different when accounting for specific country of origin. Similar clinicopathologic features were found among post surgically treated Asian and Hispanic men when compared to CA men; however, further research needs to be done to examine clinicopathologic features among sub‐groups of Asian and Hispanic PCa patients and to determine long‐term PCa outcomes in Asian and Hispanic men.
There are important limitations to consider in interpreting these findings. First, this study focused only on men who were eligible for RP opted to treat their disease surgically. Therefore, these men are likely younger and healthier than older men or men with multiple comorbidities. While this fact does provide a more homogenous cohort, results may not be applicable to men who choose other treatment modalities, such as definitive radiation. Another study limitation was that lymph node status was not systematically reported for the vast majority of subjects over the 27 year study period and, therefore, could not be examined in multivariable models. Additionally, prostate cancer‐specific mortality was not collected; however, survival time from PCa metastasis to overall death was modeled, which can serve as a surrogate for prostate cancer‐specific mortality. Furthermore, focusing on OS as the outcome may avoid potential biases and inaccuracies inherent in identifying cause‐specific mortality using death certificates. 42
It is a key strength of the cohort examined in this longitudinal study that all men were enrolled within the military health care system and shared common health care access and insurance providers. All study subjects were military health care beneficiaries with access to TRICARE‐for‐life health care coverage. While we were unable to account for individual‐level education and income levels, this military cohort is fairly homogeneous with respect to SES factors. Also, detailed clinical information was available on study subjects which is a unique attribute to the database from which this study cohort was drawn.
Despite a younger age at diagnosis, increased obesity, higher initial PSA levels, and shorter BCR‐free survival, AA men who underwent RP as primary treatment for PCa had non‐significantly increased survival times between BCR and metastasis and metastasis and OS, compared to CA men in an equal access healthcare system with minimal barriers to healthcare education and access. In concert with the recent results from the numerous studies that have reported only moderate or minimal differences between AA and CA men for long‐term PCa outcomes and highlight the need to decrease racial disparities in access to care.
AUTHOR CONTRIBUTIONS
Nathan Oehrlein: Conceptualization, investigation, methodology, project administration, resources, supervision, validation, writing ‐ original draft, and writing ‐ review and editing. Samantha A. Streicher: Writing ‐ review and editing. Huai‐Ching Kuo: Data curation, formal analysis, methodology, software, visualization, and writing ‐ review and editing Avinash Chaurasia: Writing ‐ review and editing. Jacob McFadden: Writing ‐ review and editing. Darryl Nousome: Formal analysis, writing ‐ review and editing. Yongmei Chen: Data curation, formal analysis, methodology, software, visualization, and writing ‐ review and editing. Sean P. Stroup: conceptualization, investigation, project administration, validation, and writing ‐ review and editing. John Musser: Conceptualization, investigation, project administration, validation, and writing ‐ review and editing. Timothy Brand: Conceptualization, investigation, project administration, validation, and writing ‐ review and editing. Christopher Porter: Conceptualization, investigation, project administration, validation, and writing ‐ review and editing. Inger L. Rosner: Conceptualization, investigation, project administration, validation, and writing ‐ review and editing. Gregory T. Chesnut: Conceptualization, investigation, project administration, validation, and writing ‐ review and editing. Kayla Onofaro: Investigation, writing ‐ review and editing. Timothy R. Rebbeck: Writing ‐ review and editing. Anthony D'Amico: Conceptualization, Funding acquisition, Writing ‐ original draft, Writing ‐ review and editing. Grace Lu‐Yao: Conceptualization, Funding acquisition, Writing ‐ original draft, Writing ‐ review and editing. Jennifer Cullen: Conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, writing ‐ original draft, and writing ‐ review and editing.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
DISCLAIMERS
The contents of this publication are the sole responsibility of the author(s) and do not necessarily reflect the views, opinions or policies of Uniformed Services University of the Health Sciences (USUHS), The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the Department of Defense (DoD), the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
PRECIS
Among men undergoing radical prostatectomy in an equal access health care setting, we identified and described a cohort of African American, Caucasian, Asian, and Hispanic men. While African American men had an increased risk of biochemical recurrence compared to Caucasian American men after controlling for prostate cancer prognostic factors, African American men experienced a similar risk of metastasis and overall death compared to Caucasian American men.
Supporting information
Figure S1
Oehrlein N, Streicher SA, Kuo H‐C, et al.. Race‐specific prostate cancer outcomes in a cohort of military health care beneficiaries undergoing surgery: 1990–2017. Cancer Med. 2022;11:4354‐4365. doi: 10.1002/cam4.4787
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the Center for Prostate Disease Research (CPDR). Restrictions and additional conditions may apply to the availability of these data from the Defense Health Agency (DHA).
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. SEER . Cancer Statistics Review, 1975. ‐2015. https://seer.cancer.gov/csr/1975_2015/. Accessed Oct 30, 2018.
- 3. National Cancer Institute . NCI Center to Reduce Cancer Health Disparities (CRCHD). https://www.cancer.gov/about‐nci/organization/crchd. Accessed June 24, 2019, Published April 5, 2018.
- 4. Moul JW, Douglas T, McCarthy W, McLeod D. Black Race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol. 1996;155:1667‐1673. [PubMed] [Google Scholar]
- 5. Amling CL, Riffenburgh RH, Sun L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22(3):439‐445. [DOI] [PubMed] [Google Scholar]
- 6. Iselin C, Box J, Vollmer R, Layfield L, Robertson J, Paulson D. Surgical control of clinically localized prostate carcinoma is equivalent in African‐American and White males. Cancer. 1998;83(2353–2360):2353‐2360. [DOI] [PubMed] [Google Scholar]
- 7. Schroeck FR, Sun L, Freedland SJ, Jayachandran J, Robertson CN, Moul JW. Race and prostate weight as independent predictors for biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis. 2008;11(4):371‐376. [DOI] [PubMed] [Google Scholar]
- 8. Powell IJ, Banerjee M, Bianco FJ, et al. The effect of race/ethnicity on prostate cancer treatment outcome is conditional: a review of Wayne State University data. J Urol. 2004;171(4):1508‐1512. [DOI] [PubMed] [Google Scholar]
- 9. Freedland SJ, Amling CL, Dorey F, et al. Race as an outcome predictor after radical prostatectomy: results from the shared equal access regional cancer hospital (SEARCH) database. Adult Urology. 2002;60:670‐674. [DOI] [PubMed] [Google Scholar]
- 10. Hamilton RJ, Aronson WJ, Presti JC Jr, et al. Race, biochemical disease recurrence, and prostate‐specific antigen doubling time after radical prostatectomy: results from the SEARCH database. Cancer. 2007;110(10):2202‐2209. [DOI] [PubMed] [Google Scholar]
- 11. Chu DI, Moreira DM, Gerber L, et al. Effect of race and socioeconomic status on surgical margins and biochemical outcomes in an equal‐access health care setting: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Cancer. 2012;118(20):4999‐5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leapman MS, Freedland SJ, Aronson WJ, et al. Pathological and biochemical outcomes among African‐American and caucasian men with low risk prostate cancer in the SEARCH database: implications for active surveillance candidacy. J Urol. 2016;196(5):1408‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freedland SJ, Vidal AC, Howard LE, et al. Race and risk of metastases and survival after radical prostatectomy: Results from the SEARCH database. Cancer. 2017;123(21):4199‐4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grossfeld GD, Latini DM, Downs T, Lubeck DP, Mehta SS, Carroll PR. Is ethnicity an independent predictor of prostate cancer recurrence after radical prostatectomy? J Urol. 2002;168(6):2510‐2515. [DOI] [PubMed] [Google Scholar]
- 15. Latini DM, Elkin EP, Cooperberg MR, Sadetsky N, Duchane J, Carroll PR. Differences in clinical characteristics and disease‐free survival for Latino, African American, and non‐Latino white men with localized prostate cancer: data from CaPSURE. Cancer. 2006;106(4):789‐795. [DOI] [PubMed] [Google Scholar]
- 16. Tewari A, Horninger W, Badani KK, et al. Racial differences in serum prostate‐specific antigen (PSA) doubling time, histopathological variables and long‐term PSA recurrence between African‐American and white American men undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int. 2005;96(1):29‐33. [DOI] [PubMed] [Google Scholar]
- 17. Nielsen ME, Han M, Mangold L, et al. Black race does not independently predict adverse outcome following radical retropubic prostatectomy at a tertiary referral center. J Urol. 2006;176(2):515‐519. [DOI] [PubMed] [Google Scholar]
- 18. Faisal FA, Sundi D, Cooper JL, et al. Racial disparities in oncologic outcomes after radical prostatectomy: long‐term follow‐up. Urology. 2014;84(6):1434‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee DJ, Ritch C, Desai M, Benson MC, McKiernan JM. The interaction of body mass index and race in predicting biochemical failure after radical prostatectomy. BJU Int. 2011;107(11):1741‐1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schreiber D, Levy EB, Schwartz D, et al. Impact of race in a predominantly African‐American population of patients with low/intermediate risk prostate cancer undergoing radical prostatectomy within an equal access care institution. Int Urol Nephrol. 2014;46(10):1941‐1946. [DOI] [PubMed] [Google Scholar]
- 21. Yamoah K, Deville C, Vapiwala N, et al. African American men with low‐grade prostate cancer have increased disease recurrence after prostatectomy compared with Caucasian men. Urol Oncol. 2015;33(2):70.e15‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shahabi A, Satkunasivam R, Gill IS, et al. Predictors of time to biochemical recurrence in a radical prostatectomy cohort within the PSA‐era. Can Urol Assoc J. 2016;10(1–2):E17‐E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dinizo M, Shih W, Kwon Y, et al. Multi‐institution analysis of racial disparity among AfricanAmerican men eligible for prostate cancer active surveillance. Oncotarget. 2018;9:21359‐21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pienta K, Demers R, Hofe M, Kau T, Montie J, Severson R. Effect of age and race on the survival of men with prostate cancer in the metropolitan Detroit tricounty area, 1973 to 1987. Adult Urol. 1995;45:93‐101. [DOI] [PubMed] [Google Scholar]
- 25. Merrill R, Brawley O. Prostate cancer incidence and mortality rates among white and black men. Epidemiology. 1997;8:126‐131. [DOI] [PubMed] [Google Scholar]
- 26. Robbins A, Whittemore A, Van Den Eeden S. Race, prostate cancer survival, and membership in a large Health Maintenance Organization. JNCI. 1998;90:986‐990. [DOI] [PubMed] [Google Scholar]
- 27. Godley PA, Schenck AP, Amamoo MA, et al. Racial differences in mortality among medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst. 2003;95(22):1702‐1710. [DOI] [PubMed] [Google Scholar]
- 28. Freeman V, Durazo‐Arvizu R, Arozullah A, Keys L. Determinants of mortality following a diagnosis of prostate cancer in veterans affairs and private sector health care systems. AJPH. 2003;93:1706‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tyson MD 2nd, Castle EP. Racial disparities in survival for patients with clinically localized prostate cancer adjusted for treatment effects. Mayo Clin Proc. 2014;89(3):300‐307. [DOI] [PubMed] [Google Scholar]
- 30. Williams VL, Awasthi S, Fink AK, et al. African‐American men and prostate cancer‐specific mortality: a competing risk analysis of a large institutional cohort, 1989‐2015. Cancer Med. 2018;7(5):2160‐2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Optenberg S, Thompson I, Friedrichs P, Wojcik B, Stein C, Kramer B. Race, treatment, and long‐term survival from prostate cancer in an equal‐access medical care delivery system. JAMA. 1995;274:1599‐1605. [PubMed] [Google Scholar]
- 32. Graham‐Steed T, Uchio E, Wells CK, Aslan M, Ko J, Concato J. 'Race' and prostate cancer mortality in equal‐access healthcare systems. Am J Med. 2013;126(12):1084‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmid M, Meyer CP, Reznor G, et al. Racial differences in the surgical care of medicare beneficiaries with localized prostate cancer. JAMA Oncol. 2016;2(1):85‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ellis L, Canchola A, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. JCO. 2018;36:25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwartz K, Powell IJ, Underwood W 3rd, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology. 2009;74(6):1296‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer‐specific and other‐cause mortality. JAMA Oncol. 2019;5:975‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate. 2005;62(3):243‐252. [DOI] [PubMed] [Google Scholar]
- 38. Brassell SA, Dobi A, Petrovics G, Srivastava S, McLeod D. The Center for Prostate Disease Research (CPDR): a multidisciplinary approach to translational research. Urol Oncol. 2009;27(5):562‐569. [DOI] [PubMed] [Google Scholar]
- 39. Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24(24):3973‐3978. [DOI] [PubMed] [Google Scholar]
- 40. Lin D, Wei L, Ying Z. Checking the Cox model with cumulative sums of martingale‐based residuals. Biometrika. 1993;80:557‐572. [Google Scholar]
- 41. Amini E, Palmer TC, Cai J, Lieskovsky G, Daneshmand S, Djaladat H. Association between race and oncologic outcome following radical prostatectomy for clinically organ‐confined prostate cancer: a long‐term follow‐up study. World J Urol. 2018;36(8):1233‐1239. [DOI] [PubMed] [Google Scholar]
- 42. Alexander M, Zhu K, Cullen J, et al. Race and overall survival in men diagnosed with prostate cancer in the Department of Defense Military Health System, 1990‐2010. Cancer Causes Control. 2019;30(6):627‐635. [DOI] [PubMed] [Google Scholar]
- 43. Kim HS, Moreira DM, Jayachandran J, et al. Prostate biopsies from black men express higher levels of aggressive disease biomarkers than prostate biopsies from white men. Prostate Cancer Prostatic Dis. 2011;14(3):262‐265. [DOI] [PubMed] [Google Scholar]
- 44. Koochekpour S, Buckles E, Shourideh M, et al. Androgen receptor mutations and polymorphisms in African American prostate cancer. Int J Biol Sci. 2014;10(6):643‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robbins AS, Koppie TM, Gomez SL, Parikh‐Patel A, Mills PK. Differences in prognostic factors and survival among white and Asian men with prostate cancer, California, 1995‐2004. Cancer. 2007;110(6):1255‐1263. [DOI] [PubMed] [Google Scholar]
- 46. Chao GF, Krishna N, Aizer AA, et al. Asian Americans and prostate cancer: a nationwide population‐based analysis. Urol Oncol. 2016;34(5):e237‐e215. [DOI] [PubMed] [Google Scholar]
- 47. Stern MC, Fejerman L, Das R, et al. Variability in cancer risk and outcomes within US latinos by national origin and genetic ancestry. Curr Epidemiol Rep. 2016;3:181‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pinheiro PS, Callahan KE, Siegel RL, et al. Cancer mortality in hispanic ethnic groups. Cancer Epidemiol Biomarkers Prev. 2017;26(3):376‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Data Availability Statement
The data supporting the findings of this study are available from the Center for Prostate Disease Research (CPDR). Restrictions and additional conditions may apply to the availability of these data from the Defense Health Agency (DHA).


