Abstract
Moraxella catarrhalis is an important cause of otitis media in children and lower respiratory tract infections in adults with chronic obstructive pulmonary disease (COPD). Outer membrane protein CD (OMP CD) is a 45-kDa protein which is a potential vaccine antigen to prevent infections caused by M. catarrhalis. Eight monoclonal antibodies were used to study the antigenic structure of the OMP CD molecule by assaying recombinant peptides corresponding to the sequence of the protein. This approach identified two surface-exposed epitopes, including one near the amino terminus (amino acids 25 to 44) and one in the central region of the molecule (amino acids 261 to 331). Assays with serum and sputum supernatants of adults with COPD revealed variable levels of antibodies to OMP CD among individuals. To determine which portions of the OMP CD molecule were recognized by human antibodies, three human serum samples were studied with six recombinant peptides which span the sequence of OMP CD. All three sera contained immunoglobulin G antibodies which recognized exclusively the peptide corresponding to amino acids 203 to 260 by immunoblot assay. Adsorption experiments with whole bacteria established that some of the human antibodies are directed at surface-exposed epitopes on OMP CD. We conclude that OMP CD is a highly conserved molecule which contains at least two separate epitopes which are exposed on the bacterial surface. While individual adults with COPD show variability in the immune response to OMP CD, a specific region of the OMP CD molecule (amino acids 203 to 260) is important as a target of the human immune response.
Moraxella catarrhalis has emerged as an important human respiratory tract pathogen over the past two decades. The bacterium causes 15 to 20% of episodes of otitis media in children (22, 24, 33). M. catarrhalis also causes lower respiratory tract infections in adults with chronic obstructive pulmonary disease (COPD). The natural history of COPD is characterized by intermittent exacerbations, which are associated with substantial morbidity and mortality. Many of these exacerbations are caused by lower respiratory tract infections (4, 30, 39). While it is difficult to assign a microbial etiology to individual episodes, several lines of evidence indicate that M. catarrhalis causes a significant proportion of exacerbations (24, 25, 30, 38). One study estimated that approximately one-third of bacterial exacerbations of COPD are caused by M. catarrhalis (38). In view of the importance of M. catarrhalis as a human respiratory tract pathogen, there is considerable interest in developing a vaccine to prevent these infections.
Several outer membrane proteins (OMPs) of M. catarrhalis have been characterized and studied as potential vaccine antigens (1, 2, 5, 6, 8, 10, 18, 19, 31). One such candidate vaccine antigen is OMP CD, a 45-kDa protein which separates aberrantly (∼60 kDa) upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (28). OMP CD has several characteristics which suggest that it will be an effective vaccine antigen. OMP CD contains abundantly expressed epitopes on the bacterial surface, and the protein is highly conserved among strains of M. catarrhalis (20, 28, 34). Two independent lines of evidence indicate that an immune response to OMP CD may be protective. First, OMP CD is a target of bactericidal antibodies (41). Second, mucosal and systemic immunization with OMP CD each induces enhanced pulmonary clearance of M. catarrhalis in a mouse pulmonary challenge model (29).
The goals of the present study are to begin to elucidate the antigenic structure of the OMP CD molecule and to characterize the human immune response to OMP CD in adults with COPD. The studies are designed to identify epitopes which are present on the surface of the intact bacterium and to characterize the portions of OMP CD which are important targets of human antibodies.
MATERIALS AND METHODS
Bacterial strains.
Five strains of M. catarrhalis were used to immunize mice to develop monoclonal antibodies (MAbs). Strains 4223 and 5191 (provided by Howard Faden) were recovered by tympanocentesis from middle-ear fluid of children with otitis media in Buffalo, N.Y. Strains 56 and 3 (provided by Stephen Berk) were recovered from the sputum of adults with lower respiratory tract infections in Johnson City, Tenn. Strain 25240 is from the American Type Culture Collection.
An additional 47 strains of M. catarrhalis were used to test the strain specificity of the MAbs. These strains were recovered from a variety of clinical sources including middle ear fluid, nasopharynx, sputum, transtracheal aspirate, and conjunctiva. These strains were isolated from patients in several cities in the United States, including Buffalo, N.Y.; Johnson City, Tenn.; Houston, Tex.; and Philadelphia, Pa.
Development of MAbs.
The eight MAbs which are the subject of this study were developed from five separate fusions. MAbs 5E8 and 7D6 were described previously (34). All eight MAbs recognize epitopes on OMP CD.
MAb 3.9D was raised by immunizing 4-week-old BALB/c mice with outer membranes of M. catarrhalis 3 prepared by using the zwittergent extraction method as previously described (26). The following schedule was used: day 0, 50 μg of outer membrane with incomplete Freund adjuvant, intraperitoneally; day 28, 50 μg with incomplete Freund adjuvant, intraperitoneally. The mice were euthanized on day 31 after the initial injection, and the spleens were removed and perfused to collect splenocytes.
MAbs 4G10 and 2D8 were developed by immunizing mice with whole cells of M. catarrhalis 25240, which was grown in iron-deficient medium as described previously (9). Mice were immunized intraperitoneally without adjuvant with approximately 109 cells on day 0 and day 28. Splenocytes were recovered on day 31.
MAb 3.9H was developed by immunizing mice with an extract of strain 25240. A sarcosyl insoluble fraction was extracted with sodium deoxycholate as described previously (23). Mice were immunized on days 0, 14, and 28 without adjuvant, and splenocytes were recovered on day 31.
MAbs 1D3 and 11E2 were developed by immunizing 4-week-old BALB/c mice with outer membranes of M. catarrhalis 5191 prepared by using the zwittergent-extraction method (26). The following schedule was used: day 0, 10 μg of outer membrane with complete Freund adjuvant, subcutaneously; days 7 and 14, 25 μg with incomplete Freund adjuvant, subcutaneously; days 21 and 28, 45 μg with no adjuvant, intraperitoneally. The mice were euthanized on day 32 after the initial injection, and the spleens were removed and perfused to collect splenocytes.
To perform the fusions, splenocytes were fused with SP2/0-Ag-14 plasmacytoma cells by a modification of the procedure of Kennett (21) and our own previously described method (34).
After clones producing antibodies were identified, the hybridomas were cloned by limiting dilution and the isotypes were determined by using the Mouse MonoAB ID Kit (Zymed, San Francisco, Calif.). Immunoglobulin G (IgG) antibodies were purified from tissue culture supernatant by elution from protein A or protein G. IgM antibodies were purified by using the E-Z-SEP System (Pharmacia Biotech, Piscataway, N.J.).
Purification of recombinant OMP CD.
Recombinant OMP CD which contained six histidines on its amino terminus was purified from plasmid clone pCDSA as described previously (29). This clone was derived from M. catarrhalis ATCC 25240.
Cloning of CD gene fragments into pGEX2T.
Peptides corresponding to selected regions of the gene which encodes OMP CD were expressed as fusion proteins by using the pGEX2T plasmid expression system (35, 42). Oligonucleotides were designed with BamHI and EcoRI sites so that DNA fragments from PCR were directionally cloned into pGEX2T which had been cut with BamHI and EcoRI. PCR products corresponding to specific regions of ompcd were ligated to pGEX2T with T4 DNA ligase. Plasmids were electroporated into E. coli HB101 and plated. The resulting colonies were picked and regrown, and plasmids were isolated. The purified plasmids were analyzed by agarose gel electrophoresis to check for the appropriate size insert. The inserts of clones selected for further study were confirmed by sequencing.
Purification of glutathione S-transferase (GST) fusion proteins.
Fusion proteins with GST were purified by elution from glutathione-Sepharose resin essentially as previously described (17).
Determination of DNA sequence.
The nucleotide sequence of inserts of all plasmid constructs were determined. Sequencing was performed by the dideoxy method by using Sequenase (U.S. Biochemicals, Cleveland, Ohio).
Immunodot assays.
Whole-bacterial-cell lysates were prepared by suspending bacteria which were grown overnight on plates in 0.01 M HEPES (pH 7) to an optical density at 600 nm (OD600) of approximately 0.5. The suspension was sonicated on ice three times for 10 s. A volume of 5 μl of this lysate was used.
Whole-bacterial-cell lysates or purified proteins were dotted onto nitrocellulose and allowed to air dry. Unbound sites were blocked by incubating in 3% nonfat dry milk in buffer A (0.01 M Tris, 0.15 M NaCl [pH 7.4]) for 1 h. The nitrocellulose was washed with buffer A, overlaid with an appropriate dilution of MAb, and incubated overnight at room temperature. The nitrocellulose was washed and incubated in goat anti-mouse IgG (or IgM) peroxidase-labeled conjugate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) for 1 h at room temperature. The immunodot assays were developed with horseradish peroxidase color developer as previously described (27). For some experiments, anti-mouse immunoglobulin–alkaline phosphatase-labeled conjugates (Zymed) were used and were developed with AP development reagents fast red and naphthol phosphate (Bio-Rad, Richmond, Calif.).
SDS-PAGE and immunoblot assays.
Samples were subjected to SDS-PAGE on 11% separating gels. Antigens were transferred to nitrocellulose by previously described methods (27). Immunoblot assays were performed as described above for the immunodot assays.
Human serum and sputum supernatant samples.
Human serum samples were obtained from adults with chronic bronchitis who are enrolled in a prospective study in the Chronic Bronchitis Study Clinic of the Department of Veterans Affairs Western New York Healthcare System (Study Clinic). These samples have been described previously (6). Paired samples were obtained from 10 of these patients who experienced purulent exacerbations characterized by an increase in cough and sputum production, and M. catarrhalis was recovered from sputum. Preexacerbation serum was collected approximately 1 month prior to the exacerbation and postexacerbation serum was collected 1 month after the exacerbation.
Paired serum samples from 10 patients whose respiratory tracts have not been colonized by M. catarrhalis were obtained from patients in the Study Clinic. Monthly sputum cultures have documented the absence of M. catarrhalis in the sputum of these patients. A serum sample upon enrollment in the clinic and a second sample obtained a mean of 20.1 months later (range, 15 to 29 months) were tested for antibodies to OMP CD. Ten normal human serum samples obtained from adults who did not have COPD and who did not have an infection were assayed as well. Blood was obtained by venipuncture and was allowed to clot. Serum was obtained by centrifugation and was stored at −80°C.
Paired sputum supernatants from the 20 patients from the Study Clinic were obtained at the same clinic visits as the serum samples described above. The first morning sputum was expectorated and brought by the patient to the clinic. An equal volume of 6.5 mM dithiothreitol in phosphate-buffered saline (PBS) was added to the sputum. The sputum was mixed by vortexing it and incubating it at 37°C for 20 min. The mixture was centrifuged at 27,000 × g for 30 min at 4°C. The supernatants were saved by storing at −80°C. Immunoglobulin levels to OMP CD were assayed in sputum supernatants.
ELISA.
Levels of immunoglobulin to OMP CD in human serum and sputum supernatants were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (6). Wells of a 96-well microtiter plate (Immulon 4; Dynatech) were coated overnight at room temperature with 100 μl of a 1-μg/ml mixture of recombinant, purified OMP CD in 0.1 M sodium carbonate–0.1 M sodium bicarbonate, pH 9.6 (CBC buffer). Wells were washed three times between each step with PBS–0.05% Tween 20 (PBS-Tween). Unbound sites were blocked with 3% nonfat dry milk in PBS-Tween for 1 h at room temperature. Serum or sputum supernatant was diluted in 1% nonfat dry milk in PBS-Tween, added to the wells, and incubated at 37°C for 2 h. At the same time, a microtiter plate for immunoglobulin standards was prepared. The wells were coated with rabbit anti-human IgG (1:2,000), IgM (1:1,000), or IgA (1:1,000) (Kirkegaard & Perry) in CBC buffer and incubated overnight at room temperature. After blocking as described above, decreasing concentrations of human IgG, IgM, or IgA (Cappel/Organon Teknika, Durham, N.C.) were added to the wells and incubated for 2 h at 37°C. Horseradish peroxidase-conjugated rabbit anti-human F(ab′)2 IgG (1:1,000), IgM (1:1,000), or IgA (1:1,000) (Dako, Carpenteria, Calif.) diluted in 3% goat serum was added to the wells with serum, sputum supernatant, and immunoglobulin standards and incubated for 1 h at room temperature. Color was developed by adding 0.1 mg of 3,3′,5,5′-tetramethylbenzidine–dimethyl sulfoxide–0.2% hydrogen peroxide per ml in 0.1 M sodium acetate adjusted to pH 4.5 with citric acid. The reaction was stopped after 15 min by the addition of 4 N H2SO4. The OD450 was then determined.
Samples were run in duplicate. For each sample which was assayed, corresponding wells were “sham coated” with CBC buffer and run with each serum tested. The OD for these wells was subtracted from the value obtained with the serum or sputum supernatant to control for nonspecific background. To control for plate-to-plate and day-to-day variability, duplicate wells were run with OMP CD and a serum sample known to yield an optical density of approximately 1.0 for each peroxidase conjugate. Results from plates which varied more than 15% were eliminated and repeated. Results from samples were standardized to these wells.
The amount of antibody in each sample was calculated from the standard curve run with each experiment, and the result was expressed as micrograms per milliliter. Paired samples from the same patient were always run together.
Adsorption of serum samples.
Aliquots of sera were adsorbed with mid-logarithmic-phase bacterial cells as previously described (26).
Statistical methods.
To compare concentrations of antibodies in serum and sputum supernatant samples, nonparametric statistical methods were used (Statview 5.0 software). For unpaired data, the Kruskall-Wallis and Mann-Whitney U rank tests were used. For paired data, the Wilcoxon signed-rank test was used. A P value of <0.05 was considered significant.
RESULTS
Characterization of MAbs.
Eight MAbs to OMP CD were developed. To assess the antigenic specificity of the antibodies, zwittergent-extracted outer membrane of M. catarrhalis 25240 and purified recombinant OMP CD were subjected to SDS-PAGE and immunoblot assay. All eight MAbs recognize OMP CD in both the non-heat-modified and heat-modified forms. The isotypes and other characteristics of each of the MAbs are noted in Table 1. Antibodies 5E8 and 7D6 have been reported previously (34).
TABLE 1.
Characteristics of MAbs to OMP CD of M. catarrhalis
| Antibody | Immunizing strain | Immunogen | Isotype | Epitope surface-exposeda |
|---|---|---|---|---|
| 3.9H | 25240 | Outer membrane extract | IgG1 | Yes |
| 1D3 | 5191 | Outer membrane | IgG1 | Yes |
| 7D6b | 4223 | Whole bacterial cells | IgG2a | No |
| 11E2 | 5191 | Outer membrane | IgG2b | No |
| 5E8b | 56 | Outer membrane | IgM | Yes |
| 3.9D | 3 | Outer membrane | IgG1 | No |
| 4G10 | 25240 | Whole bacterial cellsc | IgM | Yes |
| 2D8 | 25240 | Whole bacterial cellsc | IgM | Yes |
Result based on flow cytometry.
Previously published (34).
Bacteria were grown in iron-deficient medium.
Strain specificity of MAbs.
Each of the eight MAbs was tested in immunodot assays with 52 strains of M. catarrhalis recovered from a variety of clinical samples from several U.S. cities. Antibodies 3.9D, 5E8, 7D6, 4G10, 11E2, 1D3, and 2D8 were reactive with all 52 strains. Antibody 3.9H was reactive with 49 of 52 strains. It is noteworthy that the intensity of the dots showed some variability among strains even though equal amounts of whole-cell lysates of each strain were tested. This observation indicates that strains expressed various amounts of the epitopes or that the availability of the epitopes for binding in immunodot assay varied somewhat among strains.
Flow cytometry to detect surface-exposed epitopes.
The eight MAbs were subjected to flow cytometry to determine whether the antibodies recognized epitopes which are expressed on the surface of the intact bacterium. In each assay, negative controls included SP2/O-Ag-14 tissue culture supernatant in place of antibody, buffer in place of antibody, or an irrelevant antibody. These controls were consistently negative. A positive control was included in each assay, and this was either polyclonal antiserum or an MAb known to recognize a surface-exposed epitope.
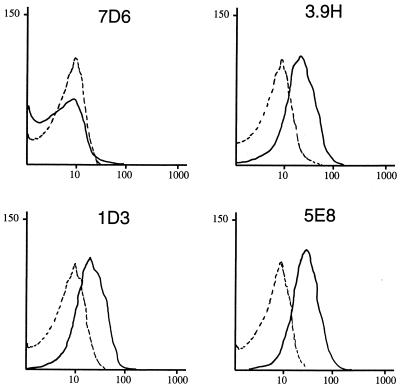
Figure 1 shows the results with four of the MAbs. The broken lines represent the result when, in place of MAbs, bacteria were incubated with SP2/O-Ag-14 tissue culture supernatant followed by fluorescein conjugate. This is a negative control and yields a result identical to that obtained when buffer is used in place of antibody. Figure 1 shows that antibody 7D6 gives a result similar to that observed with SP2/O-Ag-14, indicating that antibody 7D6 recognizes an epitope which is not exposed on the bacterial surface. By contrast, antibodies 3.9H, 1D3, and 5E8 all show a distinct and reproducible shift of the curve to the right, indicating that these antibodies bind to epitopes which are present on the surface of the intact bacterial cell. The presence of a single peak indicates that all cells detected express the epitopes on the bacterial surface. The results of flow cytometry analysis with all eight MAbs are shown in Table 1.
FIG. 1.
Results of flow cytometry with M. catarrhalis 25240. The x axes represent the level of fluorescence, and the y axes represent the particles counted in arbitrary units. The solid lines depict the result with the MAb noted on each graph. The dotted line represents the negative control which is bacterial cells incubated with SP2 tissue culture supernatant in place of antibody. Labeled cells were incubated with peroxidase-conjugated anti-mouse IgG-IgM and analyzed by flow cytometry.
Localization of epitopes on OMP CD.
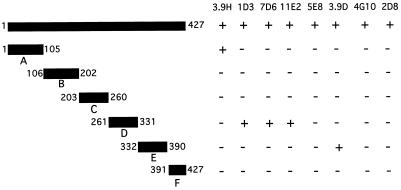
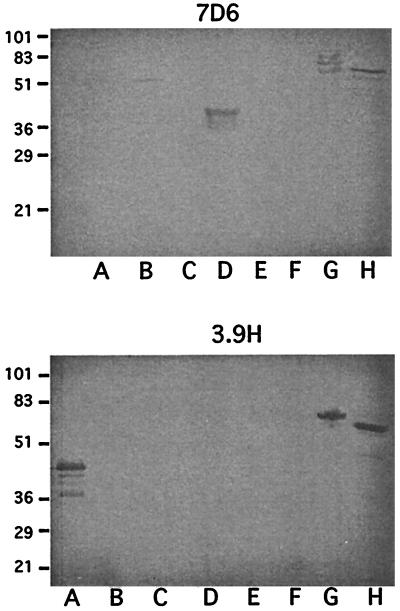
To begin to identify the regions to which the MAbs bind to OMP CD, six peptides which span the sequence corresponding to OMP CD were expressed as fusion proteins with GST (Fig. 2). The purified GST fusion proteins were studied in immunoblot assays with the eight MAbs. The results with MAbs 7D6 and 3.9H are shown in Fig. 3. Figure 2 shows a summary of the results of assays of the six fusion peptides with the eight MAbs.
FIG. 2.
Schematic diagram depicting six fusion protein constructs of OMP CD. Numbers correspond to amino acids in the mature CD protein in each construct. The reactivity of eight MAbs to OMP CD with each fusion protein in immunoblot assay is noted on the right.
FIG. 3.
Immunoblot assays with MAbs 7D6 and 3.9H. Lanes A through F show fusion proteins A through F as noted in Fig. 2. Lane G, purified recombinant OMP CD with a six-histidine tag on its amino terminus; H, purified outer membrane of M. catarrhalis 25240. Molecular mass standards are noted in kilodaltons on the left.
Of the eight MAbs, five bound fusion peptides corresponding to selected regions of the protein. Of particular interest are the two MAbs which recognize surface-exposed epitopes and also bind to peptides corresponding to amino acids 1 to 105 (3.9H) and amino acids 261 to 330 (1D3). These data indicate that surface-exposed epitopes are located within those two regions of the OMP CD molecule.
Three additional MAbs which recognize surface-exposed epitopes (5E8, 4G10, and 2D8) did not bind to any of the six peptide constructs. These results are consistent with two conclusions. (i) The MAbs recognize conformational or discontinuous epitopes which are not preserved in any of the six peptide constructs. (ii) The MAbs recognize epitopes which correspond to regions at or near the ends of the peptide constructs and are not completely expressed on any of the six fusion peptides.
Identification of the epitope recognized by MAb 3.9H.
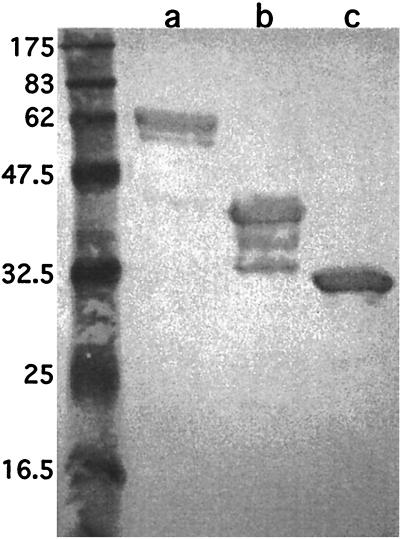
Of 52 isolates of M. catarrhalis, 3 did not express the epitope recognized by antibody 3.9H. In previous work, Hsiao et al. (20) determined the sequences of ompcd of eight clinical isolates. Two of the isolates had minor differences in amino acid sequence corresponding to amino acids 33 to 37 of the mature protein. This observation, along with the observation that antibody 3.9H bound the peptide construct corresponding to amino acids 1 to 105, led to the hypothesis that antibody 3.9H recognizes an epitope in the region of amino acids 33 to 37. A GST fusion peptide corresponding to amino acids 25 to 44 was designed, purified, and assayed with antibody 3.9H. Figure 4 is an immunoblot assay which shows that antibody 3.9H recognizes the peptide. These results indicate that a portion of the sequence corresponding to amino acids 25 to 44 is exposed on the surface of the intact bacterial cell.
FIG. 4.
Immunoblot assay with MAb 3.9H. Lanes: a, purified recombinant OMP CD with a six-histidine tag on its amino terminus; b, GST fusion protein corresponding to amino acids 1 through 105 of OMP CD; c, GST fusion protein corresponding to amino acids 25 through 44 of OMP CD. Molecular mass standards are noted in kilodaltons on the left.
Human antibody response to OMP CD.
To characterize the human antibody response to OMP CD, the IgG, IgA, and IgM to OMP CD were measured in serum and sputum supernatants from adults with COPD. Ten patients who experienced purulent exacerbations associated with the recovery of M. catarrhalis from sputum were identified. Serum and sputum supernatants collected from 1 month before and 1 month after the exacerbation were studied. Serum and sputum supernatants from an additional 10 adults with COPD who had no history of colonization or infection with M. catarrhalis were also studied. Table 2 shows the results of these quantitative assays along with the results for 10 serum samples from healthy adults. Several observations are apparent. (i) The majority of the samples had low or undetectable levels of immunoglobulin to OMP CD. (ii) Nine of ten patients in the exacerbation group had detectable levels of serum IgG. The concentration of serum IgG to OMP CD was significantly higher in the exacerbation group compared to the COPD without colonization group (P = 0.009) and the healthy controls (P = 0.014). (iii) Six of ten patients in the exacerbation group had detectable IgA in sputum supernatant in at least one of the samples tested. (iv) None of the 10 patients with COPD who experienced exacerbations due to M. catarrhalis demonstrated a clearcut rise in antibody titer to OMP CD of any immunoglobulin isotype in serum or sputum supernatant following infection. (v) IgA antibody to OMP CD was the only isotype detected in sputum supernatant; no IgG or IgM to OMP CD was detected in any of the 80 sputum supernatant samples tested. (vi) Serum from 3 of 30 people (two from the exacerbation group and one normal) had serum IgG levels of ≥1 μg/ml.
TABLE 2.
Immunoglobulin levels to OMP CD in serum and sputum samples from adultsa
| Subject | Concn (μg/ml) in serumb
|
Concn (μg/ml) in sputumb
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG
|
IgA
|
IgM
|
IgG
|
IgA
|
IgM
|
|||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| COPD with exacerbation | ||||||||||||
| 1E17/19 | 8.4 | 6.6 | – | – | – | – | – | – | – | 0.30 | – | – |
| 3E10/12 | 0.13 | 0.13 | – | – | – | – | – | – | – | – | – | – |
| 10E10/12 | 16.4 | 15.8 | – | – | – | – | – | – | – | 0.24 | – | – |
| 12E14/16 | 0.82 | 0.95 | 0.22 | 0.30 | – | 0.22 | – | – | – | – | – | – |
| 22E6/11 | 0.38 | 0.14 | – | – | – | – | – | – | 0.25 | 0.24 | – | – |
| 29E1/4 | – | – | – | – | – | 0.38 | – | – | – | 0.23 | – | – |
| 34E4/6 | 0.48 | 0.38 | 0.64 | 0.50 | – | – | – | – | 0.50 | 0.28 | – | – |
| 39E9/11 | 0.12 | 0.12 | – | – | – | – | – | – | – | – | – | – |
| 51E8/10 | – | 0.13 | – | – | – | – | – | – | – | – | – | – |
| 52E/14 | 0.15 | 0.16 | – | – | – | – | – | – | 0.22 | – | – | – |
| COPD without colonization | ||||||||||||
| 6E1/26 | – | – | – | – | – | – | – | – | – | – | – | – |
| 7E1/30 | 0.11 | 0.11 | – | – | – | – | – | – | – | – | – | – |
| 11E1/25 | – | 0.19 | – | – | – | – | – | – | – | – | – | – |
| 24E2/22 | – | – | – | – | 0.80 | 0.79 | – | – | – | – | – | – |
| 27E1/19 | 0.13 | 0.16 | – | – | – | – | – | – | – | – | – | – |
| 28E1/15 | 0.14 | – | – | – | – | – | – | – | 0.25 | 0.33 | – | – |
| 35E1/20 | – | 0.11 | – | – | – | – | – | – | 0.22 | – | – | – |
| 36E1/17 | – | – | – | – | – | – | – | – | – | – | – | – |
| 40E1/16 | – | – | – | – | – | – | – | – | – | – | – | – |
| 44E1/18 | – | – | – | – | – | – | – | – | – | – | – | – |
| Healthy | ||||||||||||
| NHS1 | 1.0 | – | – | |||||||||
| NHS2 | – | – | – | |||||||||
| NHS3 | 0.61 | – | – | |||||||||
| NHS4 | – | – | – | |||||||||
| NHS5 | – | – | – | |||||||||
| NHS6 | – | – | – | |||||||||
| NHS7 | – | – | – | |||||||||
| NHS8 | – | – | 0.30 | |||||||||
| NHS9 | – | – | – | |||||||||
| NHS10 | – | – | – | |||||||||
Ten patients with COPD experienced exacerbations and M. catarrhalis was recovered from sputum. Pre and Post, serum and sputum samples obtained 1 month prior to the exacerbation and 1 month after the exacerbation, respectively, for this group. Ten patients with COPD were free of colonization by M. catarrhalis as confirmed by monthly sputum cultures. Pre refers to serum and sputum samples obtained upon enrollment of the patients in the study clinic, and Post refers to samples obtained an average of 20 months later.
–, Level of <0.1 μg/ml for IgG; level of <0.2 μg/ml for IgA and IgM.
Analysis of epitopes recognized by human antibodies.
To further characterize the human antibody response to OMP CD, serum from three patients in the exacerbation group with the highest levels of IgG to OMP CD (1E19, 10E12, and 12E16) were studied further. The postexacerbation serum IgG levels to OMP CD were 6.6, 15.8, and 0.95 μg/ml in the three samples. To determine what proportion of the antibodies detected by ELISA were directed at epitopes which are on the bacterial surface, adsorption experiments were performed. If adsorption with whole bacterial cells reduces the titer of antibodies to OMP CD, then one can conclude that the adsorbed antibodies were directed toward surface-exposed epitopes on OMP CD. ELISA with purified recombinant OMP CD was used to measure the titer of antibodies to OMP CD. ELISAs were performed at dilutions of sera as determined by the concentration of IgG to OMP CD in the sera. Aliquots of the sera were adsorbed with E. coli as a negative control. The results are shown in Table 3. An additional control was performed. An irrelevant serum sample was adsorbed with M. catarrhalis and tested in an ELISA to an irrelevant antigen (P6 of Haemophilus influenzae) to exclude the possibility that antibodies were binding nonspecifically to M. catarrhalis. These assays revealed no adsorption by M. catarrhalis.
TABLE 3.
Results of adsorption assay with human sera which contain antibodies to OMP CD
| Serum | Adsorption assay results
|
|
|---|---|---|
| OD450 | % Adsorption | |
| 1E19 | ||
| Unadsorbed | 1.074 | |
| M. catarrhalis adsorbed | 0.202 | 81 |
| E. coli adsorbed | 0.947 | 12 |
| 10E12 | ||
| Unadsorbed | 1.668 | |
| M. catarrhalis adsorbed | 1.163 | 30 |
| E. coli adsorbed | 1.529 | 8 |
| 12E16 | ||
| Unadsorbed | 1.183 | |
| M. catarrhalis adsorbed | 0.230 | 81 |
| E. coli adsorbed | 1.434 | 0 |
The results in Table 3 show that adsorption of sera 1E19 and 12E16 with whole M. catarrhalis cells markedly reduced the titer of antibody to OMP CD in ELISA. This result indicates that a large proportion of the antibodies to OMP CD in these sera are directed at epitopes on the bacterial surface. A much smaller proportion of the OMP CD-specific antibodies in serum 10E12 are directed at surface-exposed epitopes because whole cells adsorbed only 30% of the reactivity to OMP CD.
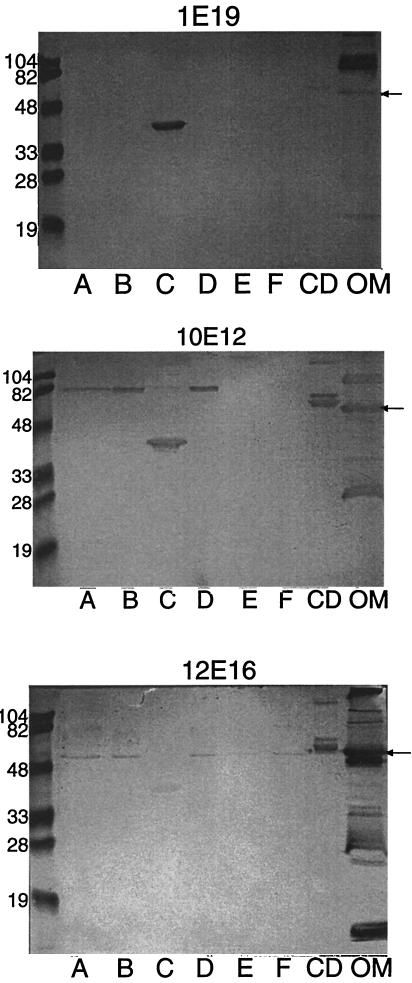
To determine which portions of the OMP CD molecule are recognized by human antibodies, sera 1E19, 10E12, and 12E16 in the exacerbation group were subjected to immunoblot assay with the six fusion proteins corresponding to amino acid sequences which span the molecule. Figure 5 shows the somewhat surprising result that all three sera contain antibodies exclusively to a single construct corresponding to amino acids 203 to 260 of OMP CD. By contrast, serum NHS1 from a healthy adult with 1 μg of IgG to OMP CD per ml showed essentially no reactivity to the six fusion proteins in the immunoblot assay.
FIG. 5.
Immunoblot assays with human serum samples 1E19, 10E12, and 12E16 as noted. Lanes A through F, fusion proteins A through F as noted in Fig. 2; lane CD, purified recombinant OMP CD with a six-histidine tag on its amino terminus; lane OM, purified outer membrane of M. catarrhalis 25240. Immunoblot assays were developed with peroxidase conjugated goat anti-human IgG. Molecular mass standards are noted in kilodaltons on the left. The arrow denotes the OMP CD.
To determine whether adsorption of sera with whole bacteria reduced the amount of antibody to the 203-260 fusion protein, aliquots of adsorbed sera were tested in immunoblot assay. Reactivity with the fusion protein, including amino acids 203 to 260 in the immunoblot assay, was unaffected by adsorption with whole bacteria.
DISCUSSION
Eight MAbs were used to study the antigenic structure of OMP CD and the antigenic conservation of the protein among strains of M. catarrhalis. Three observations led to the mapping of antibody 3.9H to a 20-amino-acid region near the amino terminus of the protein. (i) The epitope recognized by antibody 3.9H was present on OMP CD of 49 of 52 strains of M. catarrhalis, indicating that the sequence of OMP CD of the three nonreactive strains differed from the others. (ii) Antibody 3.9H bound to an epitope in the GST construct corresponding to amino acids 1 to 105 (Fig. 3). (iii) Previous work involving the determination of the nucleotide sequence of the ompcd gene from eight strains identified minor sequence differences in amino acids 33 to 37. Based on these three observations, a peptide corresponding to amino acids 25 to 44 was expressed as a fusion protein and was tested in immunoblot assay with antibody 3.9H. Antibody 3.9H recognized the peptide (Fig. 4). Since this antibody bound whole bacterial cells in flow cytometry, the results indicate that a surface-exposed epitope is located within amino acids 25 to 44 on the OMP CD.
A second surface-exposed epitope is located in the central region of the OMP CD molecule. This conclusion is based on the observation that antibody 1D3 is reactive with whole bacterial cells in flow cytometry and recognizes a peptide composed of amino acids 261 to 331. Three additional MAbs (5E8, 4G10, and 2D8) recognize surface-exposed epitopes on OMP CD but do not bind any of the six peptides which span the sequence of protein. While it is possible that these antibodies recognize linear peptides which are not present in their entirety on the six peptides, the more likely explanation is that these MAbs recognize conformational epitopes on the molecule.
Assays of the antibody response to OMP CD in 20 patients with COPD revealed variability in the antibody response among individuals. Such variability is also observed in the human antibody response to outer membrane proteins of other gram-negative human pathogens (13, 15, 16). Patients who experienced exacerbations associated with the recovery of M. catarrhalis from sputum had a higher prevalence and higher levels of antibodies to OMP CD compared to patients with COPD from whom M. catarrhalis was not recovered (Table 2). However, the levels of antibodies prior to exacerbations and after exacerbations were similar, indicating that no new antibodies to OMP CD were made after infection. Therefore, OMP CD does not appear to be an immunodominant antigen with regard to the human antibody response to natural infection. The increased concentration of antibodies to OMP CD in the exacerbation group may represent a marker for repeated infection with M. catarrhalis.
M. catarrhalis is an exclusively human pathogen. Studies with OMPs have revealed that human antibodies can be directed toward different epitopes compared to those recognized by other mammalian species (3). Therefore, patient samples were used to identify the important portions of the OMP CD molecule recognized by human antibodies. Three human serum samples which contained antibodies to OMP CD were used to begin to elucidate the important parts of the OMP CD molecule with regard to the human immune response. All three sera contained antibodies exclusively to a single region of the OMP CD molecule (amino acids 203 to 260), as detected by immunoblot assay with fusion peptides. Of interest, this is a proline-rich region and likely encompasses the “hinge” region of the OMP CD molecule, which has characteristics of an OMP A-like protein (32, 37, 40).
Adsorption of sera with whole bacterial cells was used to determine the proportion of human serum antibodies which were directed at surface-exposed epitopes. Adsorption of human serum samples with whole bacteria resulted in the removal of most of the OMP CD-specific antibody, as measured by ELISA in serum samples 1E19 and 12E16 (Table 3). This result indicates that a large proportion of the antibodies in sera 1E19 and 12E16 bound to OMP CD epitopes which are exposed on the bacterial surface, whereas serum 10E12 contains predominantly antibodies to non-surface-exposed epitopes. In spite of the adsorption of antibodies in sera 1E19 and 12E16 by whole bacteria as measured by ELISA, the adsorbed serum samples retained reactivity to the fusion protein encompassing amino acids 203 to 260 in an immunoblot assay. These data indicate that ELISA detected human antibodies which were not detected in immunoblot assays with fusion proteins. Therefore, human antibodies to surface-exposed epitopes on OMP CD may recognize conformational epitopes.
The observation in this study that human antibodies preferentially bind to a single region of OMP CD raises the possibility that OMP CD has an immunodominant region. The major OMP (P2) of nontypeable H. influenzae contains an immunodominant region which is the major target of the antibody response after the challenge of experimental animals with whole bacterial cells (43). The observation in this study of human antibodies to a selected region of the OMP CD is somewhat surprising because two lines of evidence indicate that OMP CD does not appear to be an immunodominant antigen in M. catarrhalis: (i) The absence of the formation of new antibodies after infection (Table 2) and (ii) the high degree of sequence conservation of OMP CD among strains and the stability of the sequence in isolates which colonize the human respiratory tract for months (20). One would expect that an immunodominant antigen would be subject to immune selective pressure and therefore demonstrate more sequence variability among strains, as seen with P2 of nontypeable H. influenzae (7, 11, 12, 14, 36).
OMP CD has several characteristics which indicate that the protein may be an effective vaccine antigen. The observation that new antibodies are not made to OMP CD after natural infection indicates that OMP CD does not appear to be an immunodominant antigen when the human host is presented with the whole bacterium. This result does not predict the outcome of immunization with a purified antigen. Active immunization with purified OMP CD induces protective immune responses in mice (29). Future studies are needed to characterize the potential protective effect of immune responses generated by active immunization of humans to purified OMP CD. Such studies are needed to rigorously assess the potential of OMP CD as a vaccine antigen to prevent infections caused by M. catarrhalis.
ACKNOWLEDGMENTS
This work was supported by grant AI28304 from the National Institute of Allergy and Infectious Diseases and the Department of Veterans Affairs.
We thank Adeline Thurston, Karen Muscarella, and Nancy Evans for their work in the COPD Study Clinic.
REFERENCES
- 1.Aebi C, Cope L D, Latimer J L, Thomas S E, Slaughter C A, Jr, McCracken G H, Jr, Hansen E J. Mapping a protective epitope of the CopB outer membrane protein of Moraxella catarrhalis. Infect Immun. 1998;66:540–548. doi: 10.1128/iai.66.2.540-548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi C, Maciver I, Latimer J L, Cope L D, Stevens M K, Thomas S E, McCracken G H, Jr, Hansen E J. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect Immun. 1997;65:4367–4377. doi: 10.1128/iai.65.11.4367-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ala’Aldeen D A A, Stevenson P, Griffiths E, Gorringer A R, Irons L I, Robinson A, Hyde S, Borriello S P. Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect Immun. 1994;62:2984–2990. doi: 10.1128/iai.62.7.2984-2990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball P. Epidemiology and treatment of chronic bronchitis and its exacerbations. Chest. 1995;108:43–52. doi: 10.1378/chest.108.2_Supplement.43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhushan R, Craigie R, Murphy T F. Molecular cloning and characterization of outer membrane protein E of Moraxella (Branhamella) catarrhalis. J Bacteriol. 1994;176:6636–6643. doi: 10.1128/jb.176.21.6636-6643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhushan R, Kirkham C, Sethi S, Murphy T F. Antigenic characterization and analysis of the human immune response to outer membrane protein E of Branhamella catarrhalis. Infect Immun. 1997;65:2668–2675. doi: 10.1128/iai.65.7.2668-2675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunham R C, Plummer F A, Stephens R S. Bacterial antigenic variation, host immune response, and pathogen-host coevolution. Infect Immun. 1993;61:2273–2276. doi: 10.1128/iai.61.6.2273-2276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campagnari A A, Ducey T F, Rebmann C A. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect Immun. 1996;64:3920–3924. doi: 10.1128/iai.64.9.3920-3924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campagnari A A, Shanks K L, Dyer D W. Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production. Infect Immun. 1994;62:4909–4914. doi: 10.1128/iai.62.11.4909-4914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D, McMichael J C, van der Meid K R, Hahn D, Mininni T, Cowell J, Eldridge J. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–1905. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duim B, van Alphen L, Eijk P, Jansen H M, Dankert J. Antigenic drift of non-encapsulated Haemophilus influenzae major outer membrane protein P2 in patients with chronic bronchitis is caused by point mutations. Mol Microbiol. 1994;11:1181–1189. doi: 10.1111/j.1365-2958.1994.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 12.Duim B, Vogel L, Puijk W, Jansen H M, Meloen R H, Dankert J, van Alphen L. Fine mapping of outer membrane protein P2 antigenic sites which vary during persistent infection by Haemophilus influenzae. Infect Immun. 1996;64:4673–4679. doi: 10.1128/iai.64.11.4673-4679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groeneveld K, Eijk P P, van Alphen L, Jansen H M, Zanen H C. Haemophilus influenzae infections in patients with obstructive pulmonary disease despite specific antibodies in serum and sputum. Am Rev Respir Dis. 1990;141:1316–1321. doi: 10.1164/ajrccm/141.5_Pt_1.1316. [DOI] [PubMed] [Google Scholar]
- 14.Groeneveld K, van Alphen L, Voorter C, Eijk P P, Jansen H M, Zanen H C. Antigenic drift of Haemophilus influenzae in patients with chronic obstructive pulmonary disease. Infect Immun. 1989;57:3038–3044. doi: 10.1128/iai.57.10.3038-3044.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guttormsen H-K, Wetzler L M, Solberg C O. Humoral immune response to class 1 outer membrane protein during the course of meningococcal disease. Infect Immun. 1994;62:1437–1443. doi: 10.1128/iai.62.4.1437-1443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guttormsen H-K, Wetzler L M, Naess A. Humoral immune response to the class 3 outer membrane protein during the course of meningococcal disease. Infect Immun. 1993;61:4734–4742. doi: 10.1128/iai.61.11.4734-4742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haase E M, Yi K, Morse G D, Murphy T F. Mapping of bactericidal epitopes on the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1994;62:3712–3722. doi: 10.1128/iai.62.9.3712-3722.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helminen M E, Maciver I, Latimer J L, Cope L D, McCracken G H, Jr, Hansen E J. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect Immun. 1993;61:2003–2010. doi: 10.1128/iai.61.5.2003-2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helminen M E, Maciver I, Latimer J L, Klesney-Tait J, Cope L D, Paris M, McCracken G H, Jr, Hansen E J. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis. 1994;170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 20.Hsiao C B, Sethi S, Murphy T F. Outer membrane protein CD of Branhamella catarrhalis: sequence conservation in strains recovered from the human respiratory tract. Microb Pathog. 1995;19:215–225. doi: 10.1016/s0882-4010(95)90272-4. [DOI] [PubMed] [Google Scholar]
- 21.Kennett R H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- 22.Klein J O. Otitis media. Clin Infect Dis. 1994;19:823–833. doi: 10.1093/clinids/19.5.823. [DOI] [PubMed] [Google Scholar]
- 23.Klingman K L, Murphy T F. Purification and characterization of a high-molecular-weight outer membrane protein of Moraxella (Branhamella) catarrhalis. Infect Immun. 1994;62:1150–1155. doi: 10.1128/iai.62.4.1150-1155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy T F. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol Rev. 1996;60:267–279. doi: 10.1128/mr.60.2.267-279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy T F. Branhamella catarrhalis: epidemiological and clinical aspects of a human respiratory tract pathogen. Thorax. 1998;53:124–128. doi: 10.1136/thx.53.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy T F, Bartos L C. Surface-exposed and antigenically conserved determinants of outer membrane proteins of Branhamella catarrhalis. Infect Immun. 1989;57:2938–2941. doi: 10.1128/iai.57.10.2938-2941.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy T F, Bartos L C, Campagnari A A, Nelson M B, Apicella M A. Antigenic characterization of the P6 protein of nontypable Haemophilus influenzae. Infect Immun. 1986;54:774–779. doi: 10.1128/iai.54.3.774-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy T F, Kirkham C, Lesse A J. The major heat-modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol Microbiol. 1993;10:87–98. doi: 10.1111/j.1365-2958.1993.tb00906.x. [DOI] [PubMed] [Google Scholar]
- 29.Murphy T F, Kyd J M, John A, Kirkham C, Cripps A W. Enhancement of pulmonary clearance of Moraxella (Branhamella) catarrhalis following immunization with outer membrane protein CD in a mouse model. J Infect Dis. 1998;178:1667–1675. doi: 10.1086/314501. [DOI] [PubMed] [Google Scholar]
- 30.Murphy T F, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146:1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 31.Myers L E, Yang Y-P, Du R-P, Wang Q, Harkness R E, Schryvers A B, Klein M H, Loosmore S M. The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen. Infect Immun. 1998;66:4183–4192. doi: 10.1128/iai.66.9.4183-4192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenqvist E, Musacchio A, Aase A, Hoiby E A, Namork E, Kolberg J, Wedege E, Delvig A, Dalseg R, Michaelsen T E, Tommassen J. Functional activities and epitope specificity of human and murine antibodies against the class 4 outer membrane protein (Rmp) of Neisseria meningitidis. Infect Immun. 1999;67:1267–1276. doi: 10.1128/iai.67.3.1267-1276.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruuskanen O, Heikkinen T. Otitis media: etiology and diagnosis. Pediatr Infect Dis J. 1994;13:23–26. [PubMed] [Google Scholar]
- 34.Sarwar J, Campagnari A A, Kirkham C, Murphy T F. Characterization of an antigenically conserved heat-modifiable major outer membrane protein of Branhamella catarrhalis. Infect Immun. 1992;60:804–809. doi: 10.1128/iai.60.3.804-809.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 36.Smith-Vaughan H C, Sriprakash K S, Mathews J D, Kemp D J. Nonencapsulated Haemophilus influenzae in aboriginal infants with otitis media: prolonged carriage of P2 porin variants and evidence for horizontal P2 gene transfer. Infect Immun. 1997;65:1468–1474. doi: 10.1128/iai.65.4.1468-1474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugawara E, Steiert M, Rouhani S, Nikaido H. Secondary structure of the outer membrane proteins OmpA of Escherichia coli and OprF of Pseudomonas aeruginosa. J Bacteriol. 1996;178:6067–6069. doi: 10.1128/jb.178.20.6067-6069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verghese A, Roberson D, Kalbfleisch J H, Sarubbi F. Randomized comparative study of cefixime versus cephalexin in acute bacterial exacerbations of chronic bronchitis. Antimicrob Agents Chemother. 1990;34:1041–1044. doi: 10.1128/aac.34.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson R. The role of infection in COPD. Chest. 1998;113:242–248. doi: 10.1378/chest.113.4_supplement.242s. [DOI] [PubMed] [Google Scholar]
- 40.Wong R S Y, Jost H, Hancock R E W. Linker-insertion mutagenesis of Pseudomonas aeruginosa outer membrane protein OprF. Mol Microbiol. 1993;10:283–292. [PubMed] [Google Scholar]
- 41.Yang Y-P, Myers L E, McGuinness U, Chong P, Kwok Y, Klein M H, Harkness R E. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol Med Microbiol. 1997;17:187–199. doi: 10.1111/j.1574-695X.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 42.Yi K, Murphy T F. Mapping of a strain-specific bactericidal epitope to the surface-exposed loop 5 on the P2 porin protein of nontypeable Haemophilus influenzae. Microb Pathog. 1994;17:277–282. doi: 10.1006/mpat.1994.1073. [DOI] [PubMed] [Google Scholar]
- 43.Yi K, Murphy T F. Importance of an immunodominant surface-exposed loop on outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1997;65:150–155. doi: 10.1128/iai.65.1.150-155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]