Abstract
Background:
Central serotonergic system originating from the dorsal raphe nucleus (DR) plays a critical role in anxiety disorders and trauma-related disorders such as posttraumatic stress disorder. Even though selective serotonin reuptake inhibitors are the first line of pharmacological treatment to these conditions, they are not fast-acting and tend to increase anxiety and enhance fear responses initially, with the desired clinical effects arising 2-3 weeks following initiation treatment. Although many studies have investigated the role of serotonin (5-HT) within pro-fear brain regions such as the amygdala, the majority of these studies have utilized non-selective pharmacological approaches or poorly understood lesioning techniques which limit their interpretation.
Aim:
Here we investigated the role of amygdala-projecting 5-HT neurons in the DR in innate anxiety and conditioned fear behaviors.
Methods:
To achieve this goal, we utilized (1) selective 5-HT lesioning with saporin toxin conjugated to anti-serotonin transporter (SERT) injected into the amygdala and (2) optogenetic excitation of amygdala-projecting DR cell bodies with a combination of a retrogradely transported canine adenovirus-expressing Cre-recombinase injected into the amygdala and a Cre-dependent-channelrhodopsin injected into the DR.
Results:
While saporin treatment lesioned both local 5-HT fibers and neurons in the DR and reduced conditioned fear behavior, LED activation of amygdala-projecting DR neurons enhanced anxiety-like behavior and conditioned fear response.
Conclusions:
Collectively, these studies support the hypothesis that amygdala-projecting 5-HT neurons in the DR represent an anxiety and fear-on network.
Funding:
This work was supported with K01 AG044466 to PLJ, R01 MH52619 and MH52619 to AS, and 1R01MH106568-01A1 to WAT.
Keywords: Anxiety, fear, dorsal raphe, 5-HT, SERT, amygdala
1. Introduction
Selective serotonin reuptake inhibitors (SSRIs) are a first line of pharmacological treatment for anxiety disorders, such as generalized anxiety disorder and social anxiety disorder, as well as trauma- and stressor-related disorders, such as posttraumatic stress disorder (PTSD) [see review (Koen and Stein, 2011)]. The anxiolytic effects of SSRI therapy occur 2-3 weeks following daily treatments and there is evidence that they actually increase anxiety initially (Masand and Gupta, 1999, Spigset, 1999, Teicher et al., 1990). For instance, in humans, taking citalopram prior to fear acquisition increases fear-potentiated startle response (Browning et al., 2007, Grillon et al., 2007), but chronically reduces conditioned fear response (Bui et al., 2013). Similarly, in rodents SSRIs initially enhance conditioned fear behavior, but chronically will reduce conditioned fear response [(Burghardt et al., 2007, Burghardt et al., 2004) see review (Burghardt and Bauer, 2013)]. Thus, the mechanism of action appears to be compensatory changes that occur within anxiety and fear networks with repeated use. In light of this, we and others hypothesize that a hyperactive or hyperresponsive central serotonergic system contributes to increases in anxiety sensitivity in anxiety disorders and to exacerbated anxiety/fear in response to SSRIs acutely, but over time becomes insensitized with chronic SSRIs.
A critical part of the innate and learned fear network is the basolateral amygdala (BLA; which includes the basolateral and lateral nuclei). The BLA is highly responsive to various stress-inducing stimuli (Brydges et al., 2013, Butler et al., 2011, Henderson et al., 2012, Johnson et al., 2008, Singewald et al., 2003) and increase in amygdala activity is associated with increase in anxiety in humans (Rauch et al., 2003). The amygdala plays a critical role in fear conditioning in rodents [see reviews (Johansen et al., 2011, Johansen et al., 2012)], and in humans. For instance, humans with bilateral ablation of the amygdala: 1) have normal facial recognition but do not recognize fearful faces (Adolphs et al., 1994); 2) do not display fear in response to normally threatening stimuli (Feinstein et al., 2011); and 3) do not show conditioned fear response, even though declarative learning is intact (Bechara et al., 1995).
Over 2/3 of the serotonergic neurons in the central nervous system are clustered within the raphe system distributed in the midbrain and the medulla. The majority of these raphe serotonergic neurons are localized in the dorsal raphe (DR), with far fewer being present at the median raphe (MR) (Ishimura et al., 1988). Serotonergic fibers are dense in the BLA and their associated soma in the raphe nuclei primarily originate from the midline ventral (DRV) and dorsal (DRD) DR, outnumbering the MR by ~15:1 (Hale et al., 2008). Extracellular levels of 5-HT increase in the BLA during conditioned fear (Zanoveli et al., 2009) and in response to inescapable stress (Amat et al., 1998). Meanwhile, administration of anxiogenic drugs with diverse pharmacological properties, or administering a light cue that was previously paired with a shock increases c-Fos in 5-HT neurons in the BLA-projecting regions of the DRV and DRD (Abrams et al., 2005, Spannuth et al., 2011). Additionally, single systemic injection of an SSRI in rats increases extracellular 5-HT in the amygdala by ~150% (Bosker et al., 2001), enhances freezing responses during acquisition and consolidation [(Ravinder et al., 2013) see review (Burghardt and Bauer, 2013)]. Up to 24 h following exposure to inescapable stress, baseline concentrations of 5-HT in the BLA are increased, and animals show increased response to two brief footshocks, suggesting that inescapable stress can sensitize BLA-projecting serotonergic system (Amat et al., 1998).
The persistent increase in extracellular 5-HT concentration within the amygdala following stress may contribute to a net loss of local GABA inhibition and subsequent increase in excitation of fear-promoting glutamatergic projection neurons (McDonald, 1982). These latter neurons make connections with the central amygdala (CeA), which in turn directly projects to the hypothalamus and ventro-lateral periaqueductal gray, regions underlying cardiovascular and freezing responses, respectively (LeDoux et al., 1988; Tovote et al., 2016). In support of this, serotonin acutely increases GABAergic tone in the BLA by exciting local GABAergic interneurons via the postsynaptic 5-HT2A receptors (Jiang et al., 2009, McDonald and Mascagni, 2007, Rainnie, 1999a), but also facilitates synaptic plasticity of pyramidal neurons via NMDA-dependent mechanisms upon activation of 5-HT2C receptors (Chen et al., 2003). Equally important is that stress can downregulate the 5-HT2A receptor and reduce 5HT’s effects on local GABAergic tone (Jiang et al., 2009), but also enhance cell surface expression of 5-HT2C receptors in the amygdala (Baratta et al., 2016), which may bias toward excitation of 5-HT2C receptors on fear-promoting BLA glutamatergic projection neurons. Overall, these studies suggest that serotonergic projections to the BLA play a role in modulation of animal-specific anxious (hereafter referred to as anxiety, please see Keifer and Summers, 2016) and conditioned fear behaviors.
Yet, these data are correlational and pharmacological methods of understanding the role of 5-HT in fear are complicated in that 5-HT is capable of exciting and inhibiting both GABA and glutamatergic neurons in BLA. In order to test our hypothesis that 5-HT projections from the DRD/DRV region of the DR promote anxiety and fear, in recent studies we injected a serotonergic neurotoxin 5,7-dihydroxytryptamine (5,7-DHT) into the BLA, which reduced local 5-HT concentration (but not dopamine, norepinephrine or epinephrine) and attenuated anxious behavior in the social interaction test and cue-induced conditioned fear response (Johnson et al., 2015). These data supported our hypothesis, but the mechanism of action of 5,7-DHT is largely unknown and requires co-injection of the norepinephrine reuptake inhibitor, desipramine, in order to be selective. In order to further explore our loss-of-function approach, we first injected a selective anti-serotonin transporter (SERT)-saporin (SAP) toxin into the BLA and assessed anxiety and conditioned fear, then injected a retrograde tracer into BLA to confirm that we not only lesioned SERT-immunoreactive fibers in the BLA but also serotonergic neurons in the DRD/DRV. We then utilized a wireless optogenetic approach to excite BLA projecting 5-HT neurons in the DR while assessing anxiety and conditioned fear.
2. Methods and Materials
Animals and housing conditions
All experiments were carried out using adult male Wistar rats acquired from Envigo (Indianapolis, IN, USA), and housed in plastic cages under standard environmental conditions (12/12 light/dark cycle with lights on at 7:00 AM, and 22°C). Animals were tested during the light phase under red dim light (15 lux) conditions for comparison purposes with the literature and our previous work. Food and water were provided ad libitum. Animal caretaking and experimental procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, Eighth Edition (National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. et al., 2011) and the Institutional Animal Care and Use Committee at Indiana University Purdue University at Indianapolis approved all procedures.
Experiments 1-2: Cholera Toxin B and Phaseolus vulgaris leucoagglutinin (Phal) microinjections and analysis of serotoninergic projections using Allen Mouse Brain Connectivity Atlas.
Experiment 1: In order to confirm the serotonergic projections to the BLA, a group of Wistar rats received unilateral microinjections of the retrograde tracer Cholera Toxin B subunit (CTB; 1% w/v in ACSF, 100 nl, List Biological Laboratories, Campbell, CA, USA) into the BLA (all coordinates are expressed in mm from bregma, AP: −1.9; ML: 5.00; DV: − 8.5) in experiment 1. CTB was delivered to the BLA with injectors (33 gauge, C311I, Plastics One, Roanoke, VA, USA) connected to Hamilton syringe and an infusion pump (PHD Ultra, Harvard Apparatus, Holliston, MA, USA). Each microinjection was delivered over 5 min, and the injectors remained in place for another 5 min before being removed. This allowed us not only to compare our BLA tracings to those previously published, but also to determine which rostrocaudal BLA site to target for our posterior gain and loss of function studies.
In experiment 2, we sought to determine how broad was the DR innervation onto the BLA region. For this purpose, a separate group of Wistar rats received iontophoretic injection of the anterograde tracer Phal [(2.5% w/v in artificial cerebrospinal fluid (ACSF), cat. no. L-1110, Vector Laboratories, Burlingame, CA, USA) in the DR (−6.5 to −7.5; ML: 0.00; DV:−5.5, Fig. 1) using positive current pulses of 10 μA (7 s on; 7 s off) for 15 min.
Figure 1:
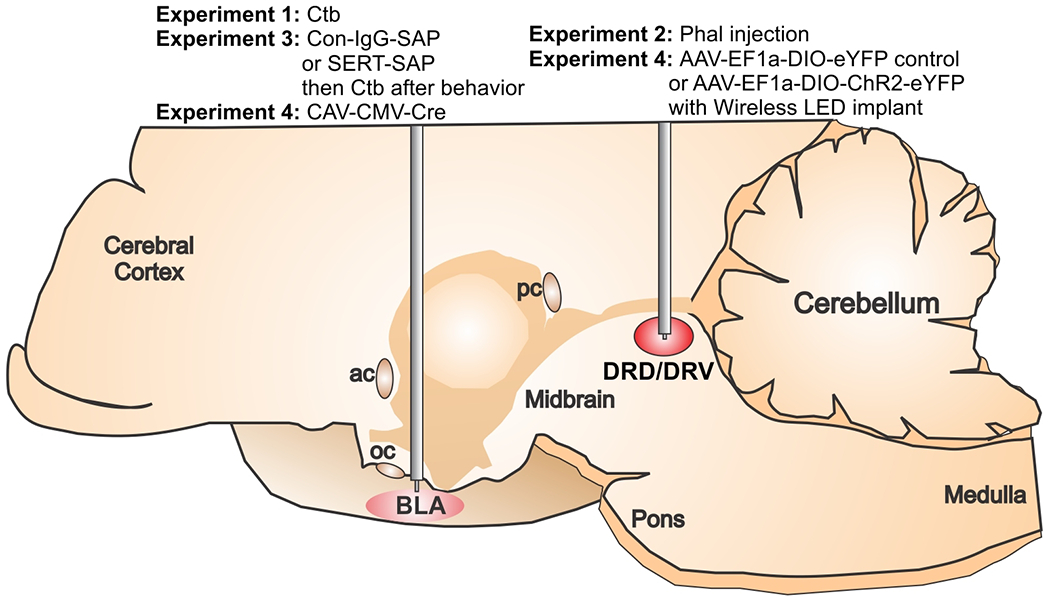
Illustration of a midsagittal section of a rat brain indicating what tracers [i.e., Phaseolus vulgaris leucoagglutinin (Phal) or Cholera Toxin B (CTB)], toxins [i.e., control IgG conjugated to saporin (Con-IgG-SAP) or anti-serotonin transporter conjugated to saporin (SERT-SAP)], or viruses [i.e., CAV-CMV-Cre, AAV-EF1a-DIO-eYFP control, or AAV-EF1a-DIO-ChR2-eYFP] were injected into the dorsal and ventral dorsal raphe nucleus (DRD/DRV) or basolateral amygdala (BLA) from each experiment. Abbreviations: ac, anterior commissure, pc, posterior commissure, oc, optic chiasm.
However, because Phal is not specific for neurochemical systems, in order to confirm that there were serotonergic-specific projections to the BLA we utilized the connectome of the mouse brain in the Allen Brain Atlas (Oh et al., 2014) and found a SERT-Cre (Slc6a4-Cre_ET33) mouse (Gong et al., 2007) that was injected with a Cre-dependent fluorescent reporter in the DR to selectively identify serotonergic neurons and their projections. This tool also helped us determine how conserved the DR projections to the BLA were between rats and mice.
Experiment 3: Lesioning of the BLA-projecting serotonergic neurons with saporin toxin.
In order to deliver intracranial injections of saporin (SAP) toxin and determine the loss of serotonergic neurotransmission in the amygdala, guide-cannulas (26 gauge, C311G, Plastics One, Roanoke, VA, USA) were bilaterally implanted aiming the BLA (AP: −1.9; ML: 5.00; DV: − 8.5, Fig. 1) of adult male Wistar rats (300-350 g) using an ultraprecise stereotaxic Kopf frame. Each rat received two bilateral microinjections per site (100 nl each, 1 μM in ACSF) of either SERT-SAP or the control IgG-SAP (Kit-23, Advanced Targeting Systems, San Diego, CA, USA) via an injector (33 gauge, C311I, Plastics One) that fitted into and extended 1 mm beyond the tip of the guide-cannula as described previously in experiment 1. Upon completion of injections, dummy-cannulas (C311DC, Plastics One) that went the length of the guide were screwed in place. Behavioral assessments (social interaction (SI) and fear conditioning (FC) tests, see below) were carried out 3 weeks post-saporin injection. Volume, concentration, and timeline of SAP injections were based on previous studies (Nattie et al., 2004). In order to confirm the selective lesioning of serotonergic neurons that project to the BLA, IgG- and SERT-SAP-injected rats received 100 nl of CTB (List Biological Laboratories) over 5 min into the BLA following behavior assessments and three weeks prior to perfusion.
Experiment 4: Virus injections and optical fiber implantation
All viral injections were done in post-weening juvenile male Wistar rats (45-55 g) that were group housed (2-3 animals per cage) under standard conditions for 2-3 days prior to the virus injection.
In order to selectively target and stimulate the BLA-projecting DR neurons, we utilized intersectional genetics by combining a retrogradely trafficked Cre-expressing virus and a DR-directed Cre-dependent channelrhodopsin. First, we injected 300 nl of CAV-CMV-Cre virus (Institut de Génétique Moléculaire de Montpellier, France) bilaterally into the BLA (AP: −1.48; ML: 4.25; DV: −8.00) and then 300 nl of a Cre-dependent channelrhodopsin (AAV-EF1a-DIO-ChR2-eYFP) or its control (AAV-EF1a-DIO-eYFP, University of North Carolina Viral Core, Raleigh, NC, USA) unilaterally into the DR (AP: −6.60; ML: 1.40; DV:−5.50, 15 degree oblique to the midsagittal plane, Fig. 1) with a flow rate set at 100 nl/min. The pipette remained at the injection site for 2 min and then removed slowly over 5 min to minimize backflow. This two-virus approach allowed us to specifically target DR neurons projecting to the BLA, similar to the approach used by others (Junyent and Kremer, 2015, Schwarz et al., 2015). Virus titer were between 4 to 6 x 1012 pp/ml for all constructs used. Animals were allowed to recover from virus injections until they reached a body weight of 250-300 g, at which time the unilateral wireless optical fibers (TeleLC-B-8.5-500, TeleOpto, Nagoya, Japan) were implanted aiming the DRD/DRV (AP: −7.50; ML: 1.20; DV: −5.60, 10 degree oblique to the midsagittal plane). Rats were allowed 7 days to recover prior the beginning of the behavior assessments.
All stereotaxic coordinates were selected according to a standard stereotaxic atlas of the adult rat brain (Paxinos and Watson, 1997).
Optogenetic stimulation
All animals implanted with unilateral wireless optical fibers (Fig. 1) were photostimulated (470 nm, 20 Hz, 1.5 mW, 5 ms pulses, cell body stimulation) for 5 min as specified in each experiment. These stimulation parameters were chosen based on in vitro and in vivo DR neuronal firing patterns and serotonin release in rodents (Li et al., 2016, Marcinkiewcz et al., 2016). The simulation pulses were generated by a software (Prizmatix Pulser, Version 2.3.1; Prizmatix, Southfield, MI, USA) connected to a programmable TTL pulse train generator (Prizmatix Pulser, Prizmatix), a stimulator device (Remote Controller, TeleOpto), and an infrared emitter (TELE-EMITTER, TeleOpto). The stimulation pulses were transmitted via infrared signals from an emitter to a wireless optogenetic receiver (TELER-3-P, TeleOpto) connected to the optical fibers implanted on the animal’s head. The infrared emitter was placed approximately 1 m above the apparatus in which the animals were being stimulated.
General methods for Experiments 1-4
Immunohistochemistry
We stained BLA and DR slices against Phal-l and CTB/TPH (Tryptophan hydroxylase), respectively, in Experiments 1 and 2 to determine innervations and projection patterns of the BLA-projecting DR neurons. In Experiment 3, to confirm lesioning of 5-HT system caused by SERT-SAP injections we 1) stained BLA and hypothalamus (control site) against SERT to determine loss of local serotonergic fibers and 2) to verify retrograde lesioning of their associated cell bodies we did TPH/CTB immunofluorescence in DR/MR slices. Next, to investigate indications of cell activity post optogenetic stimulation of the BLA-projecting DR neurons in experiment 4, we performed staining against c-Fos in BLA slices and double staining against c-Fos and TPH in DR/MR slices. Lastly, to confirm colocalization between eYFP signal and local SERT+ fibers or TPH+ cell bodies in experiment 4 we performed immunofluorescence against SERT in the BLA and PeF (control site) or TPH in the DR/MR, respectively.
In the end of experiments, rats were deeply anesthetized with isoflurane, transcardially perfused, and submitted to immunohistochemistry reactions as described previously (Johnson et al., 2005). Briefly, our general procedure was as follows: sections were washed for 30 min in PBS, 20 min in 1% H2O2 in PBS, 30 min in PBS, 10 min in PBS with 0.3% Triton X-100 (PBST), and incubated overnight in primary antibody diluted in PBST. On day 2, sections were washed for 30 min in PBS, incubated for 90 min with the appropriate secondary antibody diluted 1:200 in PBST, and washed in PBST for 30 min. For immunofluorescence, sections used for double labeling underwent an additional overnight incubation with the appropriate primary antibody and were submitted to the same protocol of day 2. For chromogen reactions, slices were incubated for 90 min in avidin-biotin complex kit (cat no. PK-6100, Vector Laboratories) diluted 1:500 in PBST, then washed for 30 min in PBST, and submitted to a 5-10 min chromogen reaction with SG Kit (SK-4700, Vector Laboratories) or 0.01% 3,3’-diaminobenzidine-tetrahydrochloride (DAB; cat no. D-5637, Sigma-Aldrich, St. Louis, MO, USA) in PBS containing 0.003% H2O2. The sections used for double immunostaining underwent an additional overnight incubation with the appropriate primary antibody. On day 3, the protocol was identical to the steps carried out on day 2 followed by a 7-10 min DAB reaction.
Primary and secondary antibodies used immunohistochemistry: rabbit anti-SERT (cat. no. 24330, ImmunoStar, Hudson, WI, USA; 1:1000), rabbit anti-c-Fos (cat. no. SC52, Santa Cruz Biotechnology, Dallas, TX, USA; 1:500), goat anti-Phal antiserum (cat. no. AS-2224, Vector Laboratories; 1:1000), mouse anti-TPH antibody (cat. no. T0678, Sigma-Aldrich; 1:1000), biotinylated goat anti-rabbit, biotinylated rabbit anti-goat, and horse anti-mouse antibody (cat. nos. BA-1000, BA-5000, and BA-2000, respectively, Vector Laboratories).
Primary and secondary antibodies used for immunofluorescence: goat anti-CTB (cat. no. 703, List Biological Laboratories, 1:1000), rabbit anti-SERT (1:500), mouse anti-TPH antibody (1:300), donkey anti-goat Alexa Fluor 488 (cat. no. A-32814, Invitrogen, Rockford, IL, USA) or donkey Cy™3-conjugated anti-rabbit (cat. no. 711-165-152, Jackson Laboratories, Bar Harbor, ME, USA), and goat anti-mouse Alexa Fluor 594 (cat. no. A-11020, Life Technologies, Carlsbad, CA, USA).
Photography and densitometry of SERT+ fibers in the BLA and quantification of c-Fos+ cells
Photomicrographs were obtained with a Leica DMLB microscope connected to a Leica DFC 300 digital camera or a Nikon A1R+ confocal microscope. Densitometry analyses were done on grayscale inverted photographs from the BLA using Adobe Photoshop version 16. The IgG-SAP group mean represented 100% which was compared to SERT-SAP values. The number of c-Fos+ cells were counted in the entire field of view at 400x magnification in the BLA and subdivisions of the DR. All cell counts were done by an observer that was blind to the experimental treatment of each animal.
General methods for Experiment 3 and 4
Social interaction test
To test whether perturbations of the BLA-projecting DR neurons via gain- or loss-of-function would alter animals’ behavior, three weeks after SERT- or IgG-SAP injections (Experiment 3) or one week after optical fiber implantation (Experiment 4), rats underwent the Social Interaction (SI) test. The SI test is a fully validated test of experimental anxious behavior in rats, and was conducted as described previously (File, 1980, Sanders et al., 1995). Briefly, the “experimental” rat and an unfamiliar “partner” rat were placed individually in the center of the open field (90 cm long x 90 cm wide x 30 cm high) 24 hours before the SI test and are allowed to explore the apparatus for 5 min for habituation purposes under red dim light (15 lux). During the SI test (5 min duration), the two rats were placed on opposite sides of the open field, and the total duration of non-aggressive physical contact (grooming, sniffing, and crawling over and under) initiated by the “experimental” rat was manually scored by an experimenter unaware of treatment groups. Rats from experiment 4 were photostimulated for 5 min during the SI test.
Fear conditioning test
To investigate how the gain- or loss-of-function manipulations would alter fear acquisition, recall and extinction, rats from experiments 3 and 4 were exposed to the fear conditioning paradigm as described previously (Johnson et al., 2015) one week after SI testing. Shortly, all rats were handled for 5 min by the experimenter prior to day 1 of the fear conditioning experiment. On day 1 – habituation session – rats were exposed for 10 min to the conditioning box with a grid floor that was connected to a scrambled shock generator (Ugo Basile, Monvale, Italy). The conditioning box was placed in a larger sound-attenuated chamber (background noise and light set at 60 dB and 15-lux) during all sessions. On day 2 – acquisition session – rats were submitted to 5 trials consisting of a 20 s, 4 kHz, 80 dB tone [conditioned stimulus (CS)] that co-terminated with a 0.5 s, 0.8 mA single footshock [unconditioned stimulus (US)] with inter-trial intervals (ITIs) of 100 s. On day3 – consolidation session – rats were exposed to 5 CS, whereas on day 4 – recall/extinction session – rats were exposed to 10 CS. Rats from experiment 4 received 5-min optogenetic stimulations immediately prior to the beginning of acquisition, consolidation, and extinction sessions. Total time freezing during the CS were manually scored by a blind experimenter. Freezing was defined as the absence of all movement except for normal breathing.
Statistical analysis
The following dependent variables were analyzed using a two-tailed independent Student’s t-test (densitometry, SI, and cell counts). The co-localization analysis of SERT and ChR2-eYFP was determined using Pearson’s correlation coefficient with Nikon NIS Elements software. Conditioned fear freezing responses were analyzed using a two-way analysis of variance (ANOVA) with Saporin or AAV injections as between-subjects factors and time as the within-subjects factor. In the presence of significant main effects, between-subjects post hoc tests were conducted using Fisher’s least significant different (LSD) tests. Statistical significance was accepted with p≤0.05. All statistical analyses were carried out using SPSS 22.0 (SPSS Inc., Chicago, IL, USA) and all graphs were generated using GraphPad Prism 7.04 for Windows (GraphPad Software Inc., San Diego, CA, USA) and figure plate illustrations were done using CorelDraw version X8 for Windows (Corel Corp, Ottawa, Canada).
3. Results
Experiments 1 and 2: Effects of retrograde and anterograde tracing of potential DR projections to BLA
The results from experiment 1 indicated that injections of the retrograde tracer CTB into the BLA (Fig. 2a) led to CTB expression in many TPH+ neurons in the DRD and DRV (Fig. 2c–d), but few in the lwDR or MR (Fig. 2e–f, see topography of midbrain TPH+ neurons in Fig. 2b, n=4) with only 7-10% of CTB being expressed in TPH− neurons.
Figure 2.
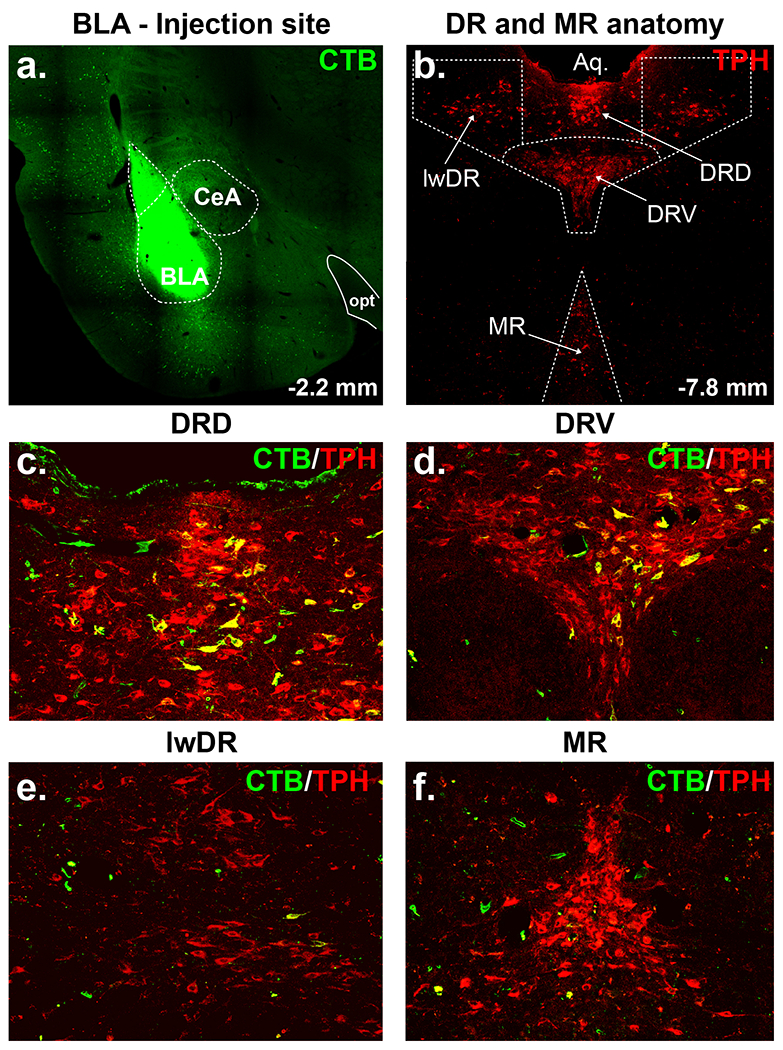
Retrograde tracing from the basolateral amygdala (BLA). a) Injection site illustrating Cholera Toxin b subunit (CTB, Alexa 488, green) injection into the BLA. b) Topography of tryptophan hydroxylase (TPH, Cy3, red) immunoreactive serotonergic neurons in the midbrain raphe nuclei [i.e., dorsal (DRD), ventral (DRV) and lateral wing (lwDR) divisions of the dorsal (DR) and median raphe (MR) nuclei] In panels c) through e) are immunohistochemistry reactions against TPH and CTB highlighting many BLA projecting neurons originating from c) DRD and d) DRV, but only a few from e) lwDR and f) MR. Abbreviations: CeA, central amygdala; opt, optic tract.
Anterograde Phal injections into the midbrain midline DR in the experiment 2 induced expression of Phal within the DR that was most highly expressed in the DRV, then DRD (Fig. 3a). Phal+ fibers were most highly expressed in the BLA, but also in surrounding cortex and CeA (Fig. 3b–c). In experiment 480074702-DR from the Allen Brain Atlas (mouse connectivity), injecting a Cre-dependent AAV-hSyn-EGFP construct into the midline DR of a SERT-Cre mouse led to local expression of EGFP fluorescence in the DR (Fig. 3d), distal EGFP fluorescence in serotonergic fibers in the BLA and CeA and surrounding cortex (Fig. 3e).
Figure 3.
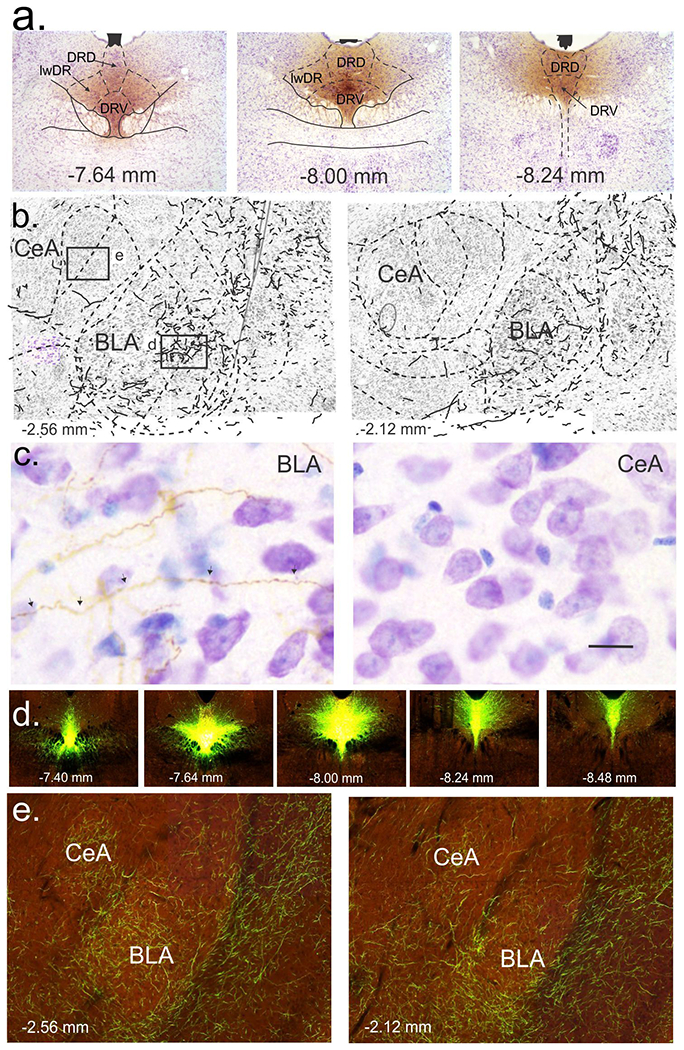
Anterograde tracing from the rat and mouse dorsal raphe nucleus to the basolateral (BLA) and central (CeA) amygdala. a) Coronal sections of the midbrain and pontine dorsal raphe nucleus (DR) illustrating Phaseolus vulgaris leucoagglutinin (Phal)-immunostained cells and fibers following injection of the anterograde tracer into the midline dorsal (DRD) and ventral (DRV), but not lateral wings (lwDR) divisions of the DR [see orange/brown 3,3’-diaminobenzidine tetrahydrochloride (DAB) chromogen immunostaining of Phal with purple cresyl violet staining of cells]. Numbers at bottom of each photograph indicate rostrocaudal level [mm from bregma; (Paxinos and Watson, 1997)]. b) Coronal sections of the BLA and CeA nuclei of the amygdala with dashed line delineating nuclei and solid lines drawn over Phal-immunoreactive anterograde projections from DRD/DRV at two bregma levels indicated at bottom left. Boxes in the illustration on the left indicate where high magnification photos were taken and shown in c) for BLA and CeA (orange/brown Phal-immunoreactive fibers with purple cresyl violet-stained cells. d) Photos represent coronal sections of the DR from a serotonin transporter (SERT)-Cre mouse where a Cre-dependent AAV with a fluorescent reporter was injected into the DR and provided by the connectome of the Brain in the Allen Brain Atlas (Oh et al., 2014). e) Photos are coronal sections of the BLA and CeA from the SERT-Cre mice in d) showing expression of 5-HT terminals from the midline DR. Image credit for parts d) and e): Allen Institute for Brain Science.
Experiment 3: Effects of SERT-SAP injections in the BLA on behavior and immunohistochemical validation of approach
Compared to the control IgG-SAP group, injection of SERT-SAP into the BLA significantly reduced the density of local SERT+ fibers in the BLA by ~90% (t(9)=19.6, p<0.001; Fig. 4a, n=6 IgG-SAP, n=5 SERT-SAP, one section damaged in control IgG-SAP group). In contrast, injection of SERT-SAP into the BLA resulted in approximately 10% reduction in the density of SERT+ fibers in the perifornical hypothalamus (PeF, control site, t(9)=2.4=0.041, data not shown), which receives sparse collateral projections from 5-HT neurons that project to the BLA (Muzerelle et al., 2016). Intra-BLA injections of SERT-SAP had no effect (i.e., no treatment x time interaction or main effect of treatment) on fear acquisition (F(1,10)=0.3, p=0.591; F(1,10)=0.4, p=0.550, Fig. 4b, n=7 IgG-SAP, 5 SERT-SAP) and consolidation (F(4,40)=1.2, p=0.305; F(1,10)=2.3, p=0.161, not shown), but did reduce overall freezing response during fear recall/extinction [i.e., there was a main effect of treatment (line graph F(1,10)=7.52, p=0.02, bar graph t(10)=2.5, p=0.03), Fig. 4c, n=7 IgG-SAP, 5 SERT-SAP, but no treatment x time interaction (F(4,40)=0.6, p=0.645)]. No effects of intra-BLA injection of SERT-SAP on SI were detected (t(10)=1.1, p=0.285, Fig. 4d, n=7 IgG-SAP, 5 SERT-SAP). In control IgG-SAP rats, bilateral injections of CTB into the BLA (only bilateral hits were included) led to CTB expression in many TPH+ neurons in the DRD and DRV, but few in the lwDR or MR (see topography of midbrain TPH+ neurons in Fig. 2a, and co-localization between CTB and TPH in the DRD, DRV, lwDR, and MR in Fig. 4e) with 7-10% of CTB being expressed in TPH− neurons. SERT-SAP injections into the BLA also reduced the total number of TPH+ neurons in the DRD (t(10)=2.1, p<0.05) and DRV (t(10)=1.7, p<0.05), but not in the lwDR (t(10)=0.98, p=0.349) or MR (t(10)=1.34, p=0.123; Fig. 4f, n=7 IgG-SAP, 5 SERT-SAP for all). SERT-SAP injections into the BLA did not change the total number of CTB+/TPH+ neurons in MR (t(8)=2.2, p=0.0581), but led to mild decreases in the lwDR (t(8)=4.6, p<0.005), and greater reductions occurring the DRD (t(8)=5.9, p<0.001) and DRV (t(8)=5.4, p<0.001; Fig. 4g, n=5 each, two control IgG-Sap rats lost cap after behavior so no CTB was able to be injected). SERT-SAP did not alter the number of TPH− neutral red-expressing cells in the DRD (t(10)=3.9, p=0.136, n=7,5) nor in the DRV (t(10)=4.6, p=0.716, n=7 IgG-SAP, 5 SERT-SAP, Fig. 4h).
Figure 4.
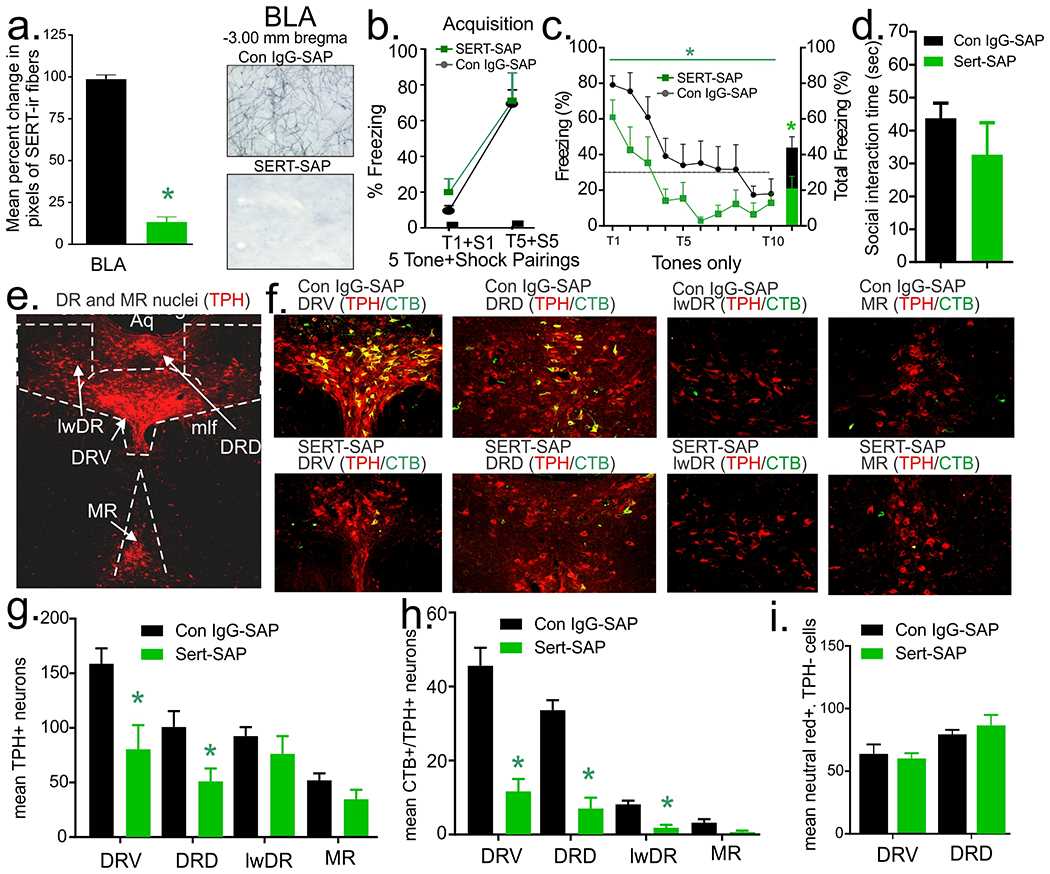
Effects of bilateral injections of anti-serotonin transporter- (SERT-) or control (IgG-) saporin (SAP) into the basolateral amygdala (BLA) on anxiety-related behavior, fear acquisition, and fear recall/extinction. a) reduced local SERT+ fibers in BLA, * indicates p < 0.001 (BLA) in a two-tailed unpaired t-test (see representative photos of BLA to the right); b) Intra-BLA SERT-SAP did not alter conditioned fear freezing following 5 tone (T) + shock (S) pairings during fear acquisition session, but c) did reduce freezing response when presented with the tone (T) only during recall/extinction session (overall treatment effect). d) Intra-BLA SERT-SAP had no effect on social interaction time. Injecting a retrograde tracer (Cholera Toxin B subunit: CTB) bilaterally into the BLA at the end of behavioral experiments induced co-localization of CTB (Alexa fluor 488, green) in midbrain serotonergic neurons (TPH, Cy3, red) of control IgG-SAP injected rats in e), which shows fluorescent photomicrographs from different division of dorsal (DR) and median raphe (MR) nuclei with top row being from control IgG-SAP injected rats and bottom from SERT-SAP injected rats. f) Reduced total number of tryptophan hydroxylase (TPH)+ neurons in the ventral (DRV) and dorsal (DRD), but not in the lateral wings (lwDR) divisions of the DR, nor the MR; g) and reduced number of CTB+/TPH+ neurons in the DRV, DRD, and lwDR, but not MR. h) In contrast, intra-BLA SERT-SAP did not alter the number of neutral red+ cells that were not colocalized with TPH in neither the DRV (p = 0.716) nor DRD (p = 0.398). n’s per group were 7 IgG-SAP and 5 SERT-SAP for all panels except part g, which n=5 each. Bars represent means and standard errors of the means (SEM). Abbreviations: mlf, medial longitudinal fasciculus; Aq., cerebral aqueduct.
Experiment 4: Effects of optogenetic excitation of DR-BLA projections on behavior and neural network
Using an intersectional genetics approach, we injected CAV-CMV-Cre bilaterally into the BLA, then either a Cre-dependent control (AAV-EF1a-DIO-eYFP) or the channelrhodopsin-expressing (AAV-EF1a-DIO-ChR2-eYFP) constructs into the midline DR. In animals injected with control construct we observed strong co-localization of eYFP with TPH+ neuron in DRV and some in DRD (Pearson correlation coefficient 0.85) with almost none in lwDR (Fig. 5a, n=7, photo on the left) or MR (not shown). In the ChR2-injected animals we observed ChR2-eYFP expression within fibers in DRV, DRD (Fig. 5a, n=8, photo to the right), BLA (Fig. 5b, photo on the left, n=8) that co-localized with SERT+ fibers (Fig. 5b, photo on the right, Pearson correlation coefficient 0.95). Wireless optogenetic excitation of the midline DR did not alter fear acquisition [i.e., there was no treatment x time interaction (F(1,13)=0.3, p=0.565), or main effect of treatment (F(1,13)=3.5, p=0.084, Fig. 5c, n=7 eYFP, 8 ChR2), nor consolidation (F(4,52)=0.8, p=0.543 and F(1,13)=3.8, p=0.072, not shown), but it did enhance overall freezing during fear recall/extinction (ChR2 main effect: line graph, F(1,13)=7.8, p=0.015, bar graph t(13)=2.8, p=0.023), but there was no treatment x time interaction (F(4,52)=0.8, p=0.538, Fig. 5d, n=7 eYFP, 8 ChR2). Here, blue light stimulation in the DRV decreased SI time (t(12)=2.59, p=0.023, Fig. 5e, n=7 each), and also increased cellular c-Fos response in TPH+ neurons in the DRD (t(12)=3.2, p=0.0038) and DRV (t(12)=2.9, p=0.0066, Fig. 5f–g), but not in TPH+ neurons in the lwDR (t(12)=0.3, p=0.785) or MR (t(12)=0.3, p=0.763, Fig. 5f, n=7 each, one ChR2 rat was removed due to poor staining) or in the PeF which receives sparse projections from the DRD/DRV (t(13)=0.1, p=0.905, data not shown). The ChR2-expressing group also had increased c-Fos in the BLA (t(13)=3.1, p=0.0042, Fig. 5f, h, n=7 eYFP, 8 ChR2).
Figure 5.
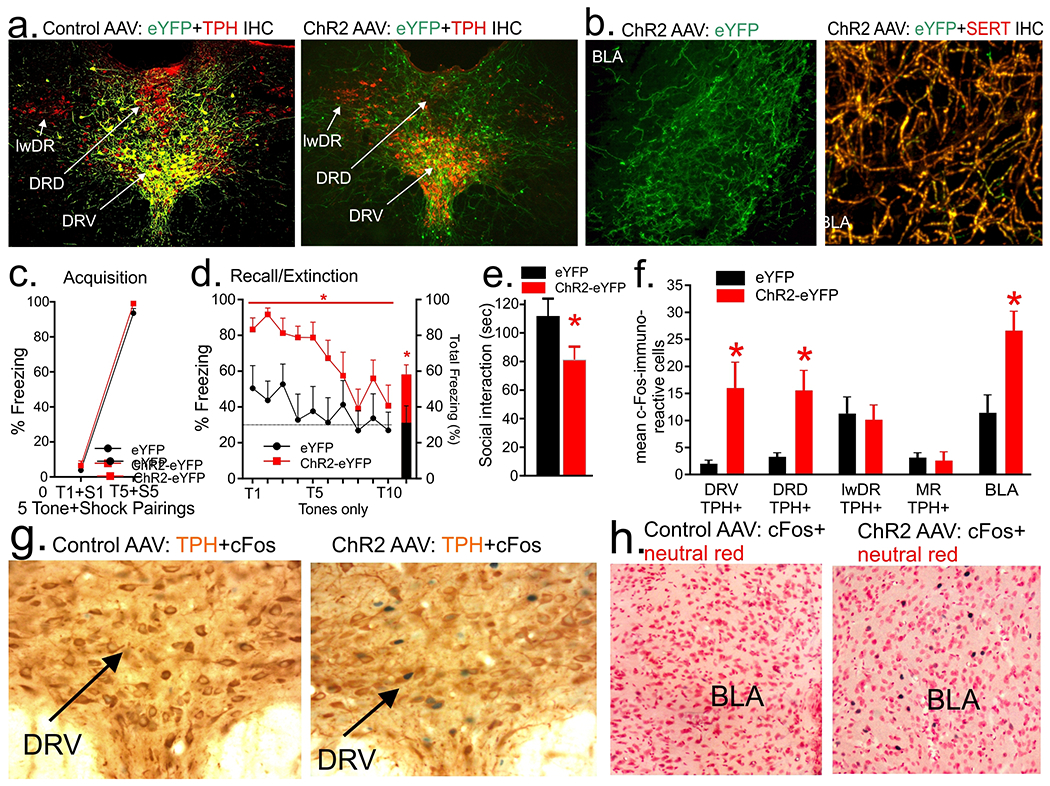
Bilateral injections of CAV-CMV-Cre into the basolateral amygdala (BLA) and unilateral injections of AAV-EF1a-DiO-ChR2-eYFP into the dorsal/ventral divisions (DRD/DRV) of dorsal raphe (DR) and effects of optical stimulation of cell bodies in the DR. a) Cre-dependent expression of eYFP (green) within soma of tryptophan hydroxylase (TPH)+ neurons in the DRD and DRV (Cy3, red, photo on the left) and also ChR2 (eYFP, green) on terminals in the DRD/DRV near TPH+ neurons (Cy3, red, photo on the right). b) Photo represents ChR2-expressing fibers (eYFP, green) in the BLA (photo on the left) that are also co-localized with serotonin transporter (SERT, Cy3, red, photo on the right). Wireless optogenetic excitation of DRD/DRV c) did not alter conditioned fear freezing following 5 tone (T) + shock (S) pairings on fear acquisition session; d) but did increase freezing response when presented with the tone (T) only during recall/extinction session (* indicates p<0.05, ChR2 main effect); e) reduced social interaction time; and f) increased c-Fos within TPH+ neurons in the DRV and DRD, but not lateral wings (lwDR) or median raphe (MR), and increased c-Fos in the BLA (* indicates p<0.05). Line and bar graphs represent means and standard errors of the means (SEM). g) Representative photomicrographs of nuclear c-Fos (blue/black chromogen) and cytoplasmic TPH (orange brown chromogen) immunohistochemistry in the DRV of a control AAV (left) and the ChR2 AAV (right). h) Representative photomicrographs of nuclear c-Fos (blue/black chromogen) and cytoplasmic neutral red staining in the BLA of a control AAV (left) and the ChR2 AAV (right).
4. Discussion
Overall, our major findings support the hypothesis that serotonergic neurons within DRD/DRV that project to the amygdala nuclei may enhance anxiety-related and conditioned fear behaviors.
In our first experiment we confirmed that injections of a retrograde tracer into the BLA resulted in many CTB+/TPH+ neurons in the DRD and DRV, but few in lwDR and MR, with approximately 92% of co-localization between CTB+ and TPH+. Our data are consistent with Hale and colleagues (Hale et al., 2008) that demonstrated substantial co-localization of DRV and DRD serotoninergic neurons after injecting the retrograde tracer CTB into the BLA, and thus reproducible. Our anterograde tracer into the midline DRD/DRV in experiment 2 revealed a high density of fibers in the BLA, and this pattern further confirmed by referencing the connectome of the mouse brain in the Allen Brain Atlas (Oh et al., 2014), where injections of a Cre-dependent AAV with a fluorescent reporter into the midline DR in a SERT-Cre mouse (Gong et al., 2007) resulted in dense fibers within the BLA. Even though it was already expected, the similar pattern of anterograde tracing provided us insight on how conserved is the DR projections to the BLA between these two species, an important issue that should be considered when doing pre-clinical research aiming for translational implications. Collectively, the combination of our anterograde and retrograde tracing studies allowed us to determine not only which site within the BLA to pursue for our following gain and loss of function experiments, but more importantly it showed consistency with the literature, an integral part of research guidelines encouraged by NIH to strengthen published studies.
In our third experiment, we wanted to confirm previous study in which we injected the serotonin neurotoxin 5,7-DHT into the BLA, resulting in reduced local 5-HT concentration and anxious behavior and fear response as assessed by reductions in SI time and in conditioned fear behaviors, respectively (Johnson et al., 2015). In order to do this, we injected a SERT-SAP into the BLA, which did not alter SI behavior but did attenuate freezing during extinction compared to control group injected with IgG-SAP. We confirmed that the SERT-SAP group had ~90% reduction in the density of SERT immunostaining in the BLA and ~10% reduction in the density of SERT immunostaining in the perifornical hypothalamus, a structure that receives less innervation arising from midline DR (Muzerelle et al., 2016), thus a control site. Injection of SERT-SAP into the BLA also reduced the total number of TPH+ neurons in the DRV and DRD, but not in the lwDR or MR. When we assessed CTB+/TPH+ neurons we saw a significant reduction in the DRV and DRD, but also in the few CTB+/TPH+ neurons in the lwDR, but not the MR. We also noted that only ~8% of CTB-expressing cells in the DR and MR were non-serotonergic. Overall, this loss-of-function approach is consistent with our previous work (Johnson et al, 2015) and previous 5,7-DHT study in which reductions of serotonin signaling in the amygdala following contextual fear conditioning reduced recall of fear-associated freezing (Izumi et al., 2012). Although we did not see a significant reduction in SI here, we (Johnson et al., 2015) and others (File et al., 1981) did see significant reductions in SI following 5,7-DHT injections into the amygdala.
In light of our findings that 92% of neurons in the DR that project to the BLA are serotonergic and to further determine this pathway’s role in regulating anxiety and fear, in experiment 4 we utilized intersectional genetics by first injecting into the BLA a retrogradely trafficked CAV that expresses Cre-recombinase (Soudais et al., 2001) which allowed us to selectively introduce a Cre-dependent ChR2 (or its eYFP control) into BLA-projecting DR neurons (DRD/DRV-BLA projections). After immunostaining for TPH in control rats, we determined that all eYFP was co-localized with TPH, with a large expression in the DRV, a few labeled neurons in the DRD, and almost none in the lwDR or the MR. In the animals injected with ChR2 AAV, the eYFP expression was primarily located in fibers in the midline DR and BLA. Altogether, these data further confirmed our anatomical results from our first three experiments, i.e. the tracing studies and the SAP injections. The terminals within the BLA were almost entirely co-localized with SERT, and were, thus, serotonergic. Optogenetic excitation of ChR2-eYFP, but not eYFP control, in the midline DR did not alter fear acquisition nor consolidation, but did increase freezing during initial tone presentation during recall/extinction session. The stimulation also led to reduction in SI time, suggesting an increase in anxiety. We then verified that optogenetic stimulation increased cellular c-Fos response within serotonergic neurons in the DRV and DRD and also within the cells in the BLA.
Overall, while our results did not test the mechanisms of how chronicanically enhancing serotonergic neurotransmission would alter 5-HT receptors locally in the BLA, the aforementioned data support the hypothesis that a hyperactive or hyperresponsive central serotonergic system contributes to exacerbated anxiety/fear, similarly to the response to acute administration of SSRIs. The data revelaed that serotonergic neurons in the DRD/DRV that project to the BLA enhance anxious behavior and conditioned fear response measured in the SI test and fear conditioning, respectively. It is important to re-emphasize that these experiments lesioned or optogenetically excited 5-HT neurons in the midline, of which many are most likely express glutamate as a co-transmitter (Calizo et al., 2011). Along those lines, it has been shown that intra-BLA injections of group 2/3 metabotropic glutamate receptors agonists produce anxiolytic-like effects in rodents (Wierońska et al., 2005) and also long-term depression of synaptic transmission in the rat amygdala (Lin et al., 2000). On the other hand, Baratta and colleagues injected a Cre-dependent Arch-GFP AAV into the midline DR of SERT-Cre mice and observed that optogenetic inhibition of the DR attenuated only stress-enhanced fear (Baratta et al., 2016). The lack of effect on normal cue-induced fear may have been due to use of a reduced shock intensity or species differences. Future studies are needed to understand how exactly lesioning or exciting DRD/DRV 5-HT projections to the BLA is altering electrophysiological activity of BLA projection neurons that control anxiety and fear responses.
In regards to what is known about 5-HT effects in the BLA there are several factors to consider: 1) the amygdala contains all 5-HT receptor subtypes (5-HT1-7); 2) 5-HT induces both excitatory and inhibitory actions depending on the receptor subtype; and 3) 5-HT receptor subtypes are expressed on both GABAergic interneurons and glutamatergic projection neurons (McDonald and Mascagni, 2007). Glutamatergic principal neurons that enhance anxiety and conditioned fear responses have high expression of excitatory 5-HT2C receptors in the dorsal part of the BLA (i.e., lateral amygdala; (Greenwood et al., 2012) and 5-HT excites these neurons via the 5-HT2C receptor (Yamamoto et al., 2014). Additionally, acute injections of 5-HT, an SSRI, or a 5-HT2C receptor agonist into the BLA induce anxiogenic responses (Vicente and Zangrossi, 2012). Yet, application of 5-HT in the BLA region initially produces inhibitory responses by depolarizing GABAergic interneurons (Bocchio et al., 2015, Rainnie, 1999b) which have high expression of excitatory 5-HT2A receptors (McDonald and Mascagni, 2007) and play a role in the depolarization (Bocchio et al., 2015). Amongst the GABAergic interneurons, several of those located in intercalated cell masses can be seen as an ‘off switch’ for the amygdala since they project to the BLA and CeA, inhibiting them (Marowsky et al, 2005, Pare and Smith, 1993; Royer et al, 1999). However, there is previously mentioned evidence that stressful/fearful - and fear-related conditions can produce prolonged release of 5-HT that may lead to loss of local inhibition (Amat et al., 1998, Zanoveli et al., 2009), and there is evidence that this can potentially reduce local GABA inhibition and produce excitation of glutamatergic neurons. For instance, stress exposure can induce downregulation of 5-HT2A receptor expression in the BLA and reduce serotonin’s effects on local GABAergic tone (Jiang et al., 2009). This could lead to excitation of glutamatergic neurons, which play a critical role in enhancing fear conditioning. This hypothesis is supported by previously mentioned studies where initial treatment of rats with SSRIs increases extracellular 5-HT in the amygdala by ~150% (Bosker et al., 2001), enhances initial conditioned fear response in both rodents (Ravinder et al., 2013) and humans (Grillon et al., 2007).
5. Conclusions
These data support the role of DR 5-HT projections to the BLA in the modulation of anxious behavior and conditioned fear freezing response. Furthermore, our data using intersectional genetics approach and SERT-SAP are consistent with previous experiments where increasing or depleting 5-HT levels in the BLA region respectively enhances or diminishes conditioned fear behavior and further support the hypothesis that increased 5-HT activity within the amygdala may be an important mechanism in the pathophysiology of severe anxiety disorders, as well as trauma-related disorders, such as PTSD (Wellman et al., 2007; Zanoveli et al., 2009).
6. Acknowledgements
This work was supported with K01 AG044466 to PLJ, R01 MH52619 and MH52619 to AS, and 1R01MH106568-01A1 to WAT.
Abbreviations
- AAV
adeno-associated virus
- ACSF
artificial cerebrospinal fluid
- 5,7-DHT
5,7-dihydroxytryptamine
- 5-HT
5-hydroxytryptamine; serotonin
- BLA
basolateral amygdala
- CAV
canine adenovirus
- CS
conditioned stimulus
- CTB
cholera toxin B subunit
- DR
dorsal raphe nucleus
- DRD
dorsal raphe nucleus, dorsal part
- DRV
dorsal raphe nucleus, ventral part
- GABA
gamma aminobutyric acid
- ITI
inter-trial interval
- lwDR
dorsal raphe nucleus, lateral wings
- MR
median raphe nucleus
- PB
sodium phosphate buffer
- PBS
phosphate-buffered saline
- Phal
Phaseolus vulgaris leucoagglutinin
- PTSD
posttraumatic stress disorder
- SAP
saporin
- SERT
serotonin transporter
- SSRI
selective serotonin reuptake inhibitor
- US
unconditioned stimulus
Footnotes
Declaration of Conflicting Interests
No author has a conflict of interest for the data presented here.
8. References
- ABRAMS JK, JOHNSON PL, HAY-SCHMIDT A, MIKKELSEN JD, SHEKHAR A & LOWRY CA 2005. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience, 133, 983–97. [DOI] [PubMed] [Google Scholar]
- ADOLPHS R, TRANEL D, DAMASIO H & DAMASIO A 1994. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372, 669–72. [DOI] [PubMed] [Google Scholar]
- AMAT J, MATUS-AMAT P, WATKINS LR & MAIER SF 1998. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res, 812, 113–20. [DOI] [PubMed] [Google Scholar]
- BARATTA MV, KODANDARAMAIAH SB, MONAHAN PE, YAO J, WEBER MD, LIN PA, GISABELLA B, PETROSSIAN N, AMAT J, KIM K, YANG A, FOREST CR, BOYDEN ES & GOOSENS KA 2016. Stress Enables Reinforcement-Elicited Serotonergic Consolidation of Fear Memory. Biol Psychiatry, 79, 814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECHARA A, TRANEL D, DAMASIO H, ADOLPHS R, ROCKLAND C & DAMASIO AR 1995. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science, 269, 1115–8. [DOI] [PubMed] [Google Scholar]
- BOCCHIO M, FUCSINA G, OIKONOMIDIS L, MCHUGH SB, BANNERMAN DM, SHARP T & CAPOGNA M 2015. Increased Serotonin Transporter Expression Reduces Fear and Recruitment of Parvalbumin Interneurons of the Amygdala. Neuropsychopharmacology, 40, 3015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSKER FJ, CREMERS TI, JONGSMA ME, WESTERINK BH, WIKSTROM HV & DEN BOER JA 2001. Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine(1A) receptor-mediated feedback: a microdialysis study in the amygdala. J Neurochem, 76, 1645–53. [DOI] [PubMed] [Google Scholar]
- BROWNING M, REID C, COWEN PJ, GOODWIN GM & HARMER CJ 2007. A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacol, 21, 684–90. [DOI] [PubMed] [Google Scholar]
- BRYDGES NM, WHALLEY HC, JANSEN MA, MERRIFIELD GD, WOOD ER, LAWRIE SM, WYNNE SM, DAY M, FLEETWOOD-WALKER S, STEELE D, MARSHALL I, HALL J & HOLMES MC 2013. Imaging conditioned fear circuitry using awake rodent fMRI. PLoS One, 8, e54197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUI E, ORR SP, JACOBY RJ, KESHAVIAH A, LEBLANC NJ, MILAD MR, POLLACK MH & SIMON NM 2013. Two weeks of pretreatment with escitalopram facilitates extinction learning in healthy individuals. Hum Psychopharmacol, 28, 447–56. [DOI] [PubMed] [Google Scholar]
- BURGHARDT NS & BAUER EP 2013. Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits. Neuroscience, 247, 253–72. [DOI] [PubMed] [Google Scholar]
- BURGHARDT NS, BUSH DE, MCEWEN BS & LEDOUX JE 2007. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry, 62, 1111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGHARDT NS, SULLIVAN GM, MCEWEN BS, GORMAN JM & LEDOUX JE 2004. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry, 55, 1171–8. [DOI] [PubMed] [Google Scholar]
- BUTLER RK, SHARKO AC, OLIVER EM, BRITO-VARGAS P, KAIGLER KF, FADEL JR & WILSON MA 2011. Activation of phenotypically-distinct neuronal subpopulations of the rat amygdala following exposure to predator odor. Neuroscience, 175, 133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALIZO LH, AKANWA A, MA X, PAN YZ, LEMOS JC, CRAIGE C, HEEMSTRA LA & BECK SG 2011. Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology, 61, 524–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN A, HOUGH CJ & LI H 2003. Serotonin type II receptor activation facilitates synaptic plasticity via N-methyl-D-aspartate-mediated mechanism in the rat basolateral amygdala. Neuroscience, 119, 53–63. [DOI] [PubMed] [Google Scholar]
- FEINSTEIN JS, ADOLPHS R, DAMASIO A & TRANEL D 2011. The human amygdala and the induction and experience of fear. Curr Biol, 21, 34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILE SE 1980. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J.Neurosci Methods, 2, 219–38. [DOI] [PubMed] [Google Scholar]
- FILE SE, JAMES TA & MACLEOD NK 1981. Depletion in amygdaloid 5-hydroxytryptamine concentration and changes in social and aggressive behaviour. J Neural Transm, 50, 1–12. [DOI] [PubMed] [Google Scholar]
- GONG S, DOUGHTY M, HARBAUGH CR, CUMMINS A, HATTEN ME, HEINTZ N & GERFEN CR 2007. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci, 27, 9817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD BN, STRONG PV, LOUGHRIDGE AB, DAY HE, CLARK PJ, MIKA A, HELLWINKEL JE, SPENCE KG & FLESHNER M 2012. 5-HT2C receptors in the basolateral amygdala and dorsal striatum are a novel target for the anxiolytic and antidepressant effects of exercise. PLoS One, 7, e46118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRILLON C, LEVENSON J & PINE DS 2007. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology, 32, 225–31. [DOI] [PubMed] [Google Scholar]
- HALE MW, HAY-SCHMIDT A, MIKKELSEN JD, POULSEN B, SHEKHAR A & LOWRY CA 2008. Exposure to an open-field arena increases c-Fos expression in a distributed anxiety-related system projecting to the basolateral amygdaloid complex. Neuroscience, 155, 659–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDERSON LA, STATHIS A, JAMES C, BROWN R, MCDONALD S & MACEFIELD VG 2012. Real-time imaging of cortical areas involved in the generation of increases in skin sympathetic nerve activity when viewing emotionally charged images. Neuroimage, 62, 30–40. [DOI] [PubMed] [Google Scholar]
- ISHIMURA K, TAKEUCHI Y, FUJIWARA K, TOMINAGA M, YOSHIOKA H & SAWADA T 1988. Quantitative analysis of the distribution of serotonin-immunoreactive cell bodies in the mouse brain. Neurosci. Lett 91, 265–70. [DOI] [PubMed] [Google Scholar]
- IZUMI T, OHMURA Y, FUTAMI Y, MATSUZAKI H, KUBO Y, YOSHIDA T & YOSHIOKA M 2012. Effects of serotonergic terminal lesion in the amygdala on conditioned fear and innate fear in rats. Eur J Pharmacol, 696, 89–95. [DOI] [PubMed] [Google Scholar]
- JIANG X, XING G, YANG C, VERMA A, ZHANG L & LI H 2009. Stress impairs 5-HT2A receptor-mediated serotonergic facilitation of GABA release in juvenile rat basolateral amygdala. Neuropsychopharmacology, 34, 410–23. [DOI] [PubMed] [Google Scholar]
- JOHANSEN JP, CAIN CK, OSTROFF LE & LEDOUX JE 2011. Molecular mechanisms of fear learning and memory. Cell, 147, 509–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHANSEN JP, WOLFF SB, LUTHI A & LEDOUX JE 2012. Controlling the elements: an optogenetic approach to understanding the neural circuits of fear. Biol Psychiatry, 71, 1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON PL, HOLLIS JH, MORATALLA R, LIGHTMAN SL & LOWRY CA 2005. Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol, 19, 327–41. [DOI] [PubMed] [Google Scholar]
- JOHNSON PL, MOLOSH A, FITZ SD, ARENDT D, DEEHAN GA, FEDERICI LM, BERNABE C, ENGLEMAN EA, RODD ZA, LOWRY CA & SHEKHAR A 2015. Pharmacological depletion of serotonin in the basolateral amygdala complex reduces anxiety and disrupts fear conditioning. Pharmacol Biochem Behav, 138, 174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON PL, TRUITT WA, FITZ SD, LOWRY CA & SHEKHAR A 2008. Neural pathways underlying lactate-induced panic. Neuropsychopharmacology, 33, 2093–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNYENT F & KREMER EJ 2015. CAV-2--why a canine virus is a neurobiologist’s best friend. Curr Opin Pharmacol, 24, 86–93. [DOI] [PubMed] [Google Scholar]
- KEIFER J & SUMMERS CH 2016. Putting the “Biology” Back into “Neurobiology”: The Strength of Diversity in Animal Model Systems for Neuroscience Research. Front. Syst. Neurosci 10, 1–9.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEN N & STEIN DJ 2011. Pharmacotherapy of anxiety disorders: a critical review. Dialogues Clin Neurosci, 13, 423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDOUX J, IWATA J, CICCHETTI P & REIS D 1988. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci 8, 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Y, ZHONG W, WANG D, FENG Q, LIU Z, ZHOU J, JIA C, HU F, ZENG J, GUO Q, FU L & LUO M 2016. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun, 7, 10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN HC, WANG SJ, LUO MZ & GEAN PW 2000. Activation of group II metabotropic glutamate receptors induces long-term depression of synaptic transmission in the rat amygdala. J. Neurosci 20, 9017–9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCINKIEWCZ CA, MAZZONE CM, D’AGOSTINO G, HALLADAY LR, HARDAWAY JA, DIBERTO JF, NAVARRO M, BURNHAM N, CRISTIANO C, DORRIER CE, TIPTON GJ, RAMAKRISHNAN C, KOZICZ T, DEISSEROTH K, THIELE TE, MCELLIGOTT ZA, HOLMES A, HEISLER LK & KASH TL 2016. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature, 537, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAROWSKY A, YANAGAWA Y, OBATA K & VOGT KE 2005. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron 48 1025–37. [DOI] [PubMed] [Google Scholar]
- MASAND PS & GUPTA S 1999. Selective serotonin-reuptake inhibitors: an update. Harv Rev Psychiatry, 7, 69–84. [PubMed] [Google Scholar]
- MCDONALD AJ 1982. Neurons of the lateral and basolateral amygdaloid nuclei: A golgi study in the rat. J. Comp. Neurol 212, 293–312. [DOI] [PubMed] [Google Scholar]
- MCDONALD AJ & MASCAGNI F 2007. Neuronal localization of 5-HT type 2A receptor immunoreactivity in the rat basolateral amygdala. Neuroscience, 146, 306–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUZERELLE A, SCOTTO-LOMASSESE S, BERNARD JF, SOIZA-REILLY M & GASPAR P 2016. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5-B9) to the forebrain and brainstem. Brain Struct Funct, 221, 535–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATIONAL RESEARCH COUNCIL (U.S.). COMMITTEE FOR THE UPDATE OF THE GUIDE FOR THE CARE AND USE OF LABORATORY ANIMALS., INSTITUTE FOR LABORATORY ANIMAL RESEARCH (U.S.) & NATIONAL ACADEMIES PRESS (U.S.) 2011. Guide for the care and use of laboratory animals. 8th ed. Washington, D.C.: National Academies Press. [Google Scholar]
- NATTIE EE, LI A, RICHERSON GB, RICHERSON G & LAPPI DA 2004. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol, 556, 235–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OH SW, HARRIS JA, NG L, WINSLOW B, CAIN N, MIHALAS S, WANG Q, LAU C, KUAN L, HENRY AM, MORTRUD MT, OUELLETTE B, NGUYEN TN, SORENSEN SA, SLAUGHTERBECK CR, WAKEMAN W, LI Y, FENG D, HO A, NICHOLAS E, HIROKAWA KE, BOHN P, JOINES KM, PENG H, HAWRYLYCZ MJ, PHILLIPS JW, HOHMANN JG, WOHNOUTKA P, GERFEN CR, KOCH C, BERNARD A, DANG C, JONES AR & ZENG H 2014. A mesoscale connectome of the mouse brain. Nature, 508, 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARE D & SMITH Y 1993. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience 57, 1077–90. [DOI] [PubMed] [Google Scholar]
- PAXINOS G & WATSON C 1997. The Rat Brain Stereotaxic Coordinates, San Diego, Academic Press [Google Scholar]
- RAINNIE DG 1999a. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. Journal of Neurophysiology., 82, 69–85. [DOI] [PubMed] [Google Scholar]
- RAINNIE DG 1999b. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol, 82, 69–85. [DOI] [PubMed] [Google Scholar]
- RAUCH SL, SHIN LM & WRIGHT CI 2003. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci, 985, 389–410. [DOI] [PubMed] [Google Scholar]
- RAVINDER S, BURGHARDT NS, BRODSKY R, BAUER EP & CHATTARJI S 2013. A role for the extended amygdala in the fear-enhancing effects of acute selective serotonin reuptake inhibitor treatment. Transl Psychiatry, 3, e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROYER S, MARTINA M & PARE D (1999) An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J. Neurosci 19 10575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDERS SK, MORZORATI SL & SHEKHAR A 1995. Priming of experimental anxiety by repeated subthreshold GABA blockade in the rat amygdala. Brain Res, 699, 250–9. [DOI] [PubMed] [Google Scholar]
- SCHWARZ LA, MIYAMICHI K, GAO XJ, BEIER KT, WEISSBOURD B, DELOACH KE, REN J, IBANES S, MALENKA RC, KREMER EJ & LUO L 2015. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature, 524, 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGEWALD N, SALCHNER P & SHARP T 2003. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol.Psychiatry, 53, 275–283. [DOI] [PubMed] [Google Scholar]
- SOUDAIS C, LAPLACE-BUILHE C, KISSA K & KREMER EJ 2001. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J, 15, 2283–5. [DOI] [PubMed] [Google Scholar]
- SPANNUTH BM, HALE MW, EVANS AK, LUKKES JL, CAMPEAU S & LOWRY CA 2011. Investigation of a central nucleus of the amygdala/dorsal raphe nucleus serotonergic circuit implicated in fear-potentiated startle. Neuroscience, 179, 104–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIGSET O 1999. Adverse reactions of selective serotonin reuptake inhibitors: reports from a spontaneous reporting system. Drug Saf, 20, 277–87. [DOI] [PubMed] [Google Scholar]
- TEICHER MH, GLOD C & COLE JO 1990. Emergence of intense suicidal preoccupation during fluoxetine treatment. Am J Psychiatry, 147, 207–10. [DOI] [PubMed] [Google Scholar]
- TOVOTE P, ESPOSITO MS, BOTTA P, CHAUDUN F, FADOK JP, MARKOVIC M, WOLFF SBE, RAMAKRISHNAN C, FENNO L, DEISSEROTH K, HERRY C, ARBER S & LÜTHI A, 2016. Midbrain circuits for defensive behaviour. Nature 534, 206–12. [DOI] [PubMed] [Google Scholar]
- VICENTE MA & ZANGROSSI H 2012. Serotonin-2C receptors in the basolateral nucleus of the amygdala mediate the anxiogenic effect of acute imipramine and fluoxetine administration. Int J Neuropsychopharmacol, 15, 389–400. [DOI] [PubMed] [Google Scholar]
- WIEROŃSKA JM, SZEWCZYK B, PAŁUCHA A, BRAŃSKI P, ZIEBA B & SMIAŁOWSKA M, 2005. Anxiolytic action of group II and III metabotropic glutamate receptors agonists involves neuropeptide Y in the amygdala. Pharmacol. Reports 57, 734–43. [PubMed] [Google Scholar]
- YAMAMOTO R, HATANO N, SUGAI T & KATO N 2014. Serotonin induces depolarization in lateral amygdala neurons by activation of TRPC-like current and inhibition of GIRK current depending on 5-HT(2C) receptor. Neuropharmacology, 82, 49–58. [DOI] [PubMed] [Google Scholar]
- ZANOVELI JM, CARVALHO MC, CUNHA JM & BRANDAO ML 2009. Extracellular serotonin level in the basolateral nucleus of the amygdala and dorsal periaqueductal gray under unconditioned and conditioned fear states: an in vivo microdialysis study. Brain Res, 1294, 106–15. [DOI] [PubMed] [Google Scholar]


