Abstract
Five years ago, the National Institute of Health (NIH) introduced a mandate to revolutionize the way sex as a biological variable (SABV) is considered in NIH-funded preclinical research. Given the known effects of sex on aging physiology, pathology, treatment response, and the effectiveness of interventions it is particularly important that SABV be considered in basic biology of aging research. Five years after this mandate, a significant amount of published work funded by the National Institute on Aging (NIA) is still not including mice of both sexes and/or not considering sex differences or comparisons in preclinical studies. Here we review a cross-section of recently published NIA-funded research to determine adherence to this mandate. We discuss the state of the preclinical aging field in terms of SABV and suggest strategies for improving adherence to the NIH mandate. It is imperative that we consider SABV and include males and females in all aspects of aging biology research to improve health outcomes for all.
Keywords: Aging research, Female, Male, Preclinical, Sex
NIH Mandate: Preclinical Research Must Consider Sex as a Biological Variable (SABV)
In 2016, the National Institute on Aging (NIA) enacted a policy, following solicitation of feedback from National Institute of Health (NIH) stakeholders and the broader scientific community, to require grant applications for NIH funding on vertebrates and humans to consider sex as a biological variable (SABV) (1). This mandate requires the consideration of both sexes in the design, analysis and reporting of all preclinical research funded by the NIA. This is particularly important as animal models provide opportunities to understand the complex mechanisms of sex differences in many pathologies that cannot be easily studied in humans (2). Single-sex applications are possible, but require a strong justification, for example, sex-specific disease indications like ovarian or prostate cancer. The SABV Policy complements and extends the NIH’s requirement, implemented in 1993 as the NIH Revitalization Act, for inclusion of women and underrepresented minorities in clinical research. Today we see approximately half the participants in NIH clinical studies are now women, although there is ample scope for improving the integration of sex (a biological variable, defined genetically) and gender (a person’s self-identity) into research. NIH also created a scientific interest group on Sex and Gender in Health and Disease (SGHD) with a goal of advancing the understanding of intra- and extramural scientists about the ways that SABV applies across disciplines (3).
SABV Beyond the USA
Beyond the United States, SABV is also gaining traction in other countries. In Canada, the Canadian Institutes of Health Research (CIHR) has implemented similar policies to the NIH requiring both sexes to be included, as well as developing standardized training on SABV for researchers (5, 8) (3). The European Commission has been asking recipients to include sex and gender in their analysis since 2013 (4). Not surprisingly, fewer researchers than expected implemented these suggestions. The Commission recently published a series of case studies as examples of good practice to help researchers to better address sex and gender analysis (5). However, it is encouraging to see that organizations, such as the European Society for Medical Oncology have taken initiatives to launch tasks forces to raise awareness of the presence of potential sex and gender differences in the biology and treatment outcomes of nonsex related cancers (6). When we think about implementation of these policies it is important to communicate to researchers that SABV does not require researchers to study sex-specific differences but rather encourages them to understand the role that sex could have as a variable in basic scientific studies (3). It is important for researchers, funders, reviewers, and journals to recognize when and how consideration of SABV is implemented in basic and clinical research (3).
Current Research: Why it Is Important to Consider SABV in Preclinical Aging Research
The importance of considering SABV in preclinical studies is highlighted in the study by Tran et al., in the present issue of The Journal of Gerontology, Series A: Biological Sciences and Medical Sciences. The authors highlight the importance of sex on the effects of age on functional performance measures in mice (7). They show that old age reduces exploratory activities and increases grooming in mice and that age-related declines vary between the sexes but tend to be greater in males. This, of course, has implications for the interpretation of the functional outcomes of intervention studies, many of which are carried out only in males, as well as potential translational implications. As the authors so astutely state, this important study contributes to the emerging yet still limited body of evidence of sex differences in biomedical research. Using both sexes in preclinical studies are crucial to allow for comprehensive scientific evaluations which will ultimately improve translation into clinical applications. Importantly, as this study highlights, the results of studies using single sexes are not always generalizable beyond that sex.
The study by Tran et al. (2021) supports other work showing clear sex differences in the biology of aging. Clinically, we know that there are sex differences in mortality risk, susceptibility to diseases (8) and frailty (9). These sex differences are observed not just in humans but also in preclinical studies. Female UM-HET3 mice in the NIA interventions testing program (ITP) live longer than males (10) and differences are observed in frailty between male and female C57BL/6 mice (11). Additionally, preclinical studies, including those conducted by the ITP, have shown that interventions that increase lifespan in mice often do so in a sex-specific manner (10,12–14). There is also evidence for sex differences in the biology of aging at the molecular and cellular level from both human and preclinical studies, including differences in gene expression (15), epigenetics (16,17), the immune system (18), and senescence (19). In addition, sex can affect treatment response, as it impacts both the pharmacokinetics and pharmacodynamics of drugs (20). Further details on sex differences in aging biology can be found in several recent and thorough reviews (17,21). Overall, growing work in this area shows that what is true in aging for 1 sex is not necessarily the same for the other and highlights the need for consideration of SABV in both clinical and preclinical research. Including both sexes in ongoing and future aging research will allow us to identify sex differences, understand the mechanisms of these differences and ultimately optimize aging for all older people.
State of the Field: Is SABV Currently Considered in Preclinical Aging Research?
In order to understand why moving forward with SABV is so important, we must consider the past. Historically, preclinical biological sciences research has predominantly included only males (22). More recently, although inclusion of both sexes has increased somewhat, most papers still do not include analysis by sex (23). This means that findings related to biological mechanisms, biomarkers, and interventions are often only known to be relevant and correct for males, and data for females or on biologically relevant sex differences is missing. This has negative impacts on the rigor of scientific inquiry (for example important scientific discoveries may be missed because they are not observed in males) and on the translation of findings to the clinic (24). Historically, the lack of consideration of SABV has been especially true in research on the biology of aging, where the majority of studies have used only males or, even worse, not even specified the sex studied (25). Anecdotal evidence indicates that reasons for this bias include that aging studies are long and expensive so only a single-sex can be used, the incorrect interpretation that the estrous cycle makes females too “variable”, or that males have historically been used so should continue to be used. These are similar to the “myths” outlined, and debunked, in a recent Nature Neuroscience article (26). Given the overall aim of the aging research field is ultimately to understand the aging process to improve healthspan and lifespan for all older people―not just males―it is clearly important that we study both sexes.
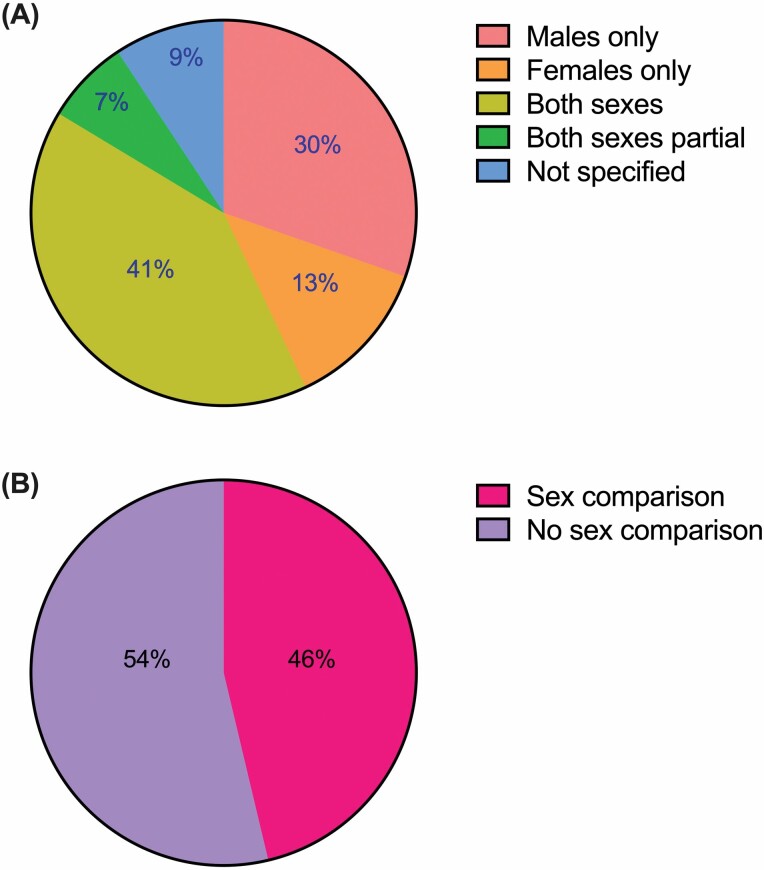
We sought to understand whether things have changed since the introduction of the NIH SABV mandate 5 years ago. Are more preclinical aging studies including both sexes, and considering possible sex differences? In order to answer this question we completed an informal review of PubMed-listed research articles that reported NIH extramural research support, published during the period between January 1, 2020 and May 31, 2021, which included the search terms “NIA” and “mice”. Of the approximately 509 articles returned using this search criteria, 2 were removed as they did not include mouse studies (Supplementary Table S1). Of the remaining 507 articles, approximately half incorporated both sexes in the design of all or a subset of experiments (40.6% and 7.1%, respectively), while 43% reported single-sex data (30.4% male-only; 12.6% female-only), and 9.3% failed to specify sex at all (Figure 1A). Although some articles acknowledged single-sex study designs as a limitation, a mere 17.4% of male- or female-only experiments offered any justification as to why. Even fewer publications (6.9%) were actually studying a sex-specific phenomenon (such as ovarian cancer, prostate cancer, or menopause), which we would define as a “strong justification” for the purpose of this article. Of the articles that included both sexes in some or all experiments, only 46.3% reported comparisons between males and females (Figure 1B). Clearly this argues for a stronger enforcement of SABV. While there are some obvious limitations to this type of informal review, such as the inclusion of only mouse studies completed within a specific time frame with funding from 1 institute, this data shows that more than 50% of NIA-funded studies published in the last 2 years are not considering SABV, despite the NIH mandate being in place since 2016.
Figure 1.
Results of an informal review of NIA-funded research in mice published from 2020 to 2021 and whether sex as a biological variable was considered. Five hundred and nine research articles were included from PubMed that reported NIH extramural research support, were published between January 1, 2020 and May 31, 2021, and included the search terms “NIA” and “mice”. (A) A pie graph indicating whether studies included male, female, both, or did not specify the sex of mice, and (B) of those that included both sexes, was data compared by sex. NIA = National Institute on Aging; NIH = National Institute of Health.
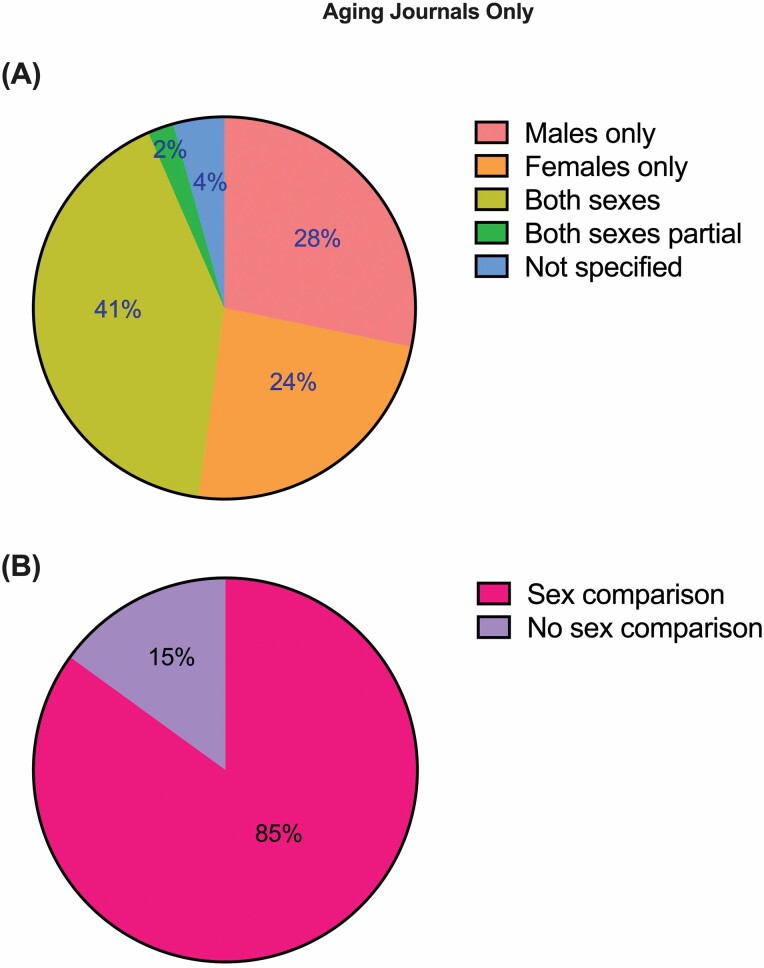
We also filtered our results (Figure 2) to include only work published in subject-specific journals dedicated to the biology of aging (Aging, Aging Cell, Experimental Gerontology, Geroscience, Journals of Gerontology Series A, and Neurobiology of Aging). Interestingly although the number of studies including either both sexes (41.3%) or males only (28.3%) stayed consistent with the larger data set (Figure 2A), more female-only studies (23.9%) were included in the aging journals. Additionally, in this subset of manuscripts, 20.8% of male- or female-only experiments were studying sex-specific phenomena, and 85% of the articles that included both sexes in some or all experiments reported comparisons between males and females (Figure 2B). Although this was a small subset of articles (46 total) and there is still a long way to go, this data provides encouraging evidence that journals focused on the biology of aging may be starting to focus more on SABV.
Figure 2.
Results of an informal review of NIA-funded research in mice published in subject specific journals dedicated to the biology of aging (Aging, Aging Cell, Experimental Gerontology, Geroscience, Journals of Gerontology Series A, and Neurobiology of Aging), from 2020 to 2021 and whether sex as a biological variable was considered. (A) A pie graph indicating whether studies included male, female, both, or did not specify the sex of mice, and (B) of those that included both sexes, was data compared by sex. NIA = National Institute on Aging.
Moving Forward: Strategies to Improve Adherence to SABV Guidelines
As researchers interested in sex differences, it is encouraging to us to see that inclusion of SABV in preclinical research is no longer optional for NIH-funded investigators. The lack of adherence to this mandate, however, is disheartening. Inclusion of SABV in biomedical research is critical to ensure the most rigorous science. As demonstrated by our review (Figures 1 and 2), we clearly need more strictly enforced measures to ensure compliance by the greater research community. Funding bodies could consider following the example of the CIHR, which has implemented into their grant application process mandatory questions addressing whether and how applicants are taking sex/gender into account (27). This kind of transparency in animal studies is clearly essential if these studies are to add to the knowledge base and inform future research, policy, and clinical practice. Such checklists could be implemented into NIH funding proposals and grant applications with relative ease and would significantly increase the transparency and translatability of animal studies.
As journal editors and reviewers, we are the gatekeepers to rigorous science. The responsibility lies with us to ensure that critical SABV requirements are implemented at the journals on which we serve as editors and reviewers. We are beginning to see that journals are recognizing the importance of SABV and requiring the acknowledgment of this within publications. The Journals of Gerontology, for example, encourages all authors and reviewers to take into account the need to report the sex of all experimental models including cell lines and to report whether there are any sex differences in outcomes (25). While other journals such as Arteriosclerosis, Thrombosis, and Vascular Biology have published statements recommending this to their authors (2), there are many which have yet to adopt these recommendations. Many journals encourage the use of the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for manuscripts (journal list available here: https://arriveguidelines.org/supporters/journals), which require reporting of the sex of experimental animals. A new revised version of these guidelines published in 2020 (ARRIVE guidelines 2.0 (28)) recommends that the sex of animal models used is even listed in the abstract, as a response to male bias in biomedical research. We hope that requirements by journals to adhere to these, or similar, guidelines will inspire researchers to make consideration of SABV the norm. As peer-reviewers, we can require authors to give their rationale for studying only 1 sex and describe the potential implications for not studying the other. If we, as editors and reviewers promote sex- and gender-specific analysis of research data as a matter of routine, then we will fulfill our role in advancing the quality and transparency of reported data. Moreover, we will educate the new generation of junior researchers who come through labs on the importance of SABV, and the proper way to incorporate SABV into research. By doing so we raise a new generation of biomedical scientists who have never known SABV to not be the norm.
Conclusion
Sex is an important biological variable, and especially so in aging research. Preclinical research studies that incorporate both sexes are crucial to recognizing the applicability of study findings and to informing the translation of research from basic scientific discovery to drug development and testing of therapeutics. Including both sexes in preclinical studies and experimental designs that appropriately account for sex as a biological variable promotes understanding of experimental outcomes for both males and females. Although here we have focused on SABV in the preclinical context, when we think about sex differences in the broader research community, we need scientists, funding bodies, and journals to embrace not only SABV but sex and gender-based analysis, and Sex and Gender Equity in Research. Translation of such results to clinical testing advances us 1 step closer to evidence-based appropriate treatments for all people. Ultimately it comes down to the fact that aging science is better, and more impactful when we consider sex and gender.
Supplementary Material
Acknowledgments
The authors would like to thank the Editor-in-Chief Rozalyn Anderson for the opportunity to prepare this perspective.
Contributor Information
Colleen Carmody, Department of Genetics, Blavatnik Institute, Paul F. Glenn Center for Biology of Aging Research at Harvard Medical School, Boston, Massachusetts, USA.
Charlotte G Duesing, Department of Genetics, Blavatnik Institute, Paul F. Glenn Center for Biology of Aging Research at Harvard Medical School, Boston, Massachusetts, USA.
Alice E Kane, Department of Genetics, Blavatnik Institute, Paul F. Glenn Center for Biology of Aging Research at Harvard Medical School, Boston, Massachusetts, USA.
Sarah J Mitchell, Department of Health Sciences and Technology, Swiss Federal Institute of Technology ETH Zürich, Zürich, Switzerland.
Funding
S.J.M. was supported by the NIA/NIH (P01AG055369). A.E.K. was supported by a Diamond/AFAR Postdoctoral Transition Award in Aging (DIAMOND19036) and is now supported by the NIA (K99 AG070102).
Conflict of Interest
None declared.
References
- 1. Miller LR, Marks C, Becker JB, et al. . Considering sex as a biological variable in preclinical research. FASEB J. 2017;31:29–34. doi: 10.1096/fj.201600781R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies-statement from ATVB council. Arterioscler Thromb Vasc Biol. 2018;38:292–303. doi: 10.1161/ATVBAHA.117.309524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnegard ME, Whitten LA, Hunter C, Clayton JA. Sex as a biological variable: a 5-year progress report and call to action. J Women Health (2002). 2020;29:858–864. doi: 10.1089/jwh.2019.8247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nature. Accounting for sex and gender makes for better science. Nature. 2020;588:196. doi: 10.1038/d41586-020-03459-y [DOI] [PubMed] [Google Scholar]
- 5. Commission E. How inclusive analysis contributes to research and innovation: policy review 2020. https://op.europa.eu/en/publication-detail/-/publication/33b4c99f-2e66-11eb-b27b-01aa75ed71a1/language-en/format-PDF/source-search# The DOI is: 10.2777/316197
- 6. ESMO. ESMO gender medicine task force. 2021. Accessed December 20, 2021. https://www.esmo.org/about-esmo/organisational-structure/esmo-task-forces/esmo-gender-medicine-task-force
- 7. Tran T, Mach J, Gemikonakli G, et al. . Male–female differences in the effects of age on performance measures recorded for 23 hours in mice. J Gerontol A Biol Sci Med Sci 2021;76:2141–2146. doi: 10.1093/gerona/glab182 [DOI] [PubMed] [Google Scholar]
- 8. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, et al. . Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396:565–582. doi: 10.1016/S0140-6736(20)31561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gordon EH, Hubbard RE. Differences in frailty in older men and women. Med J Aust. 2020;212:183–188. doi: 10.5694/mja2.50466 [DOI] [PubMed] [Google Scholar]
- 10. Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab. 2016;23:1022–1033. doi: 10.1016/j.cmet.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kane AE, Howlett SE. Sex differences in frailty: comparisons between humans and preclinical models. Mech Ageing Dev. 2021;198:111546. doi: 10.1016/j.mad.2021.111546 [DOI] [PubMed] [Google Scholar]
- 12. Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, et al. . Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 2016;23:1093–1112. doi: 10.1016/j.cmet.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strong R, Miller RA, Bogue M, et al. . Rapamycin-mediated mouse lifespan extension: late-life dosage regimes with sex-specific effects. Aging Cell. 2020;19:e13269. doi: 10.1111/acel.13269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrison DE, Strong R, Allison DB, et al. . Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berchtold NC, Cribbs DH, Coleman PD, et al. . Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc Natl Acad Sci USA. 2008;105:15605–15610. doi: 10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horvath S, Gurven M, Levine ME, et al. . An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17:171. doi: 10.1186/s13059-016-1030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sampathkumar NK, Bravo JI, Chen Y, et al. . Widespread sex dimorphism in aging and age-related diseases. Hum Genet. 2020;139:333–356. doi: 10.1007/s00439-019-02082-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Márquez EJ, Chung C-h, Marches R, et al. . Sexual-dimorphism in human immune system aging. Nat Commun. 2020;11:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yousefzadeh MJ, Zhao J, Bukata C, et al. . Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell. 2020;19:e13094. doi: 10.1038/s41467-020-14396-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rubinow DR, Moore M. Sex-dependent modulation of treatment response. Dialogues Clin Neurosci. 2004;6:39–51. doi: 10.31887/DCNS.2004.6.1/drubinow [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ganz J, Shacham T, Kramer M, et al. . A novel specific PERK activator reduces toxicity and extends survival in Huntington’s disease models. Sci Rep. 2020;10. doi: 10.1038/s41598-020-63899-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamaza H, Komatsu T, Wakita S, et al. . FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell. 2010;9:372–382. doi: 10.1111/j.1474-9726.2010.00563.x [DOI] [PubMed] [Google Scholar]
- 23. Woitowich NC, Beery A, Woodruff T. A 10-year follow-up study of sex inclusion in the biological sciences. eLife. 2020;9:e56344. doi: 10.7554/eLife.56344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tannenbaum C, Ellis RP, Eyssel F, Zou J, Schiebinger L. Sex and gender analysis improves science and engineering. Nature. 2019;575:137–146. doi: 10.1038/s41586-019-1657-6 [DOI] [PubMed] [Google Scholar]
- 25. Le Couteur DG, Anderson RM, de Cabo R. Sex and aging. J Gerontol A Biol Sci Med Sci. 2018;73:139–140. doi: 10.1093/gerona/glx221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shansky RM, Murphy AZ. Considering sex as a biological variable will require a global shift in science culture. Nat Neurosci. 2021;24:457–464. doi: 10.1038/s41593-021-00806-8 [DOI] [PubMed] [Google Scholar]
- 27. Hankivsky O, Springer KW, Hunting G. Beyond sex and gender difference in funding and reporting of health research. Res Integr Peer Rev. 2018;3:6. doi: 10.1186/s41073-018-0050-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Percie du Sert N, Hurst V, Ahluwalia A, et al. . The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.