Abstract
Background
Frailty assessment in the Swedish health system relies on the Clinical Frailty Scale (CFS), but it requires training, in-person evaluation, and is often missing in medical records. We aimed to develop an electronic frailty index (eFI) from routinely collected electronic health records (EHRs) and assess its association with adverse outcomes in hospitalized older adults.
Methods
EHRs were extracted for 18 225 patients with unplanned admissions between 1 March 2020 and 17 June 2021 from 9 geriatric clinics in Stockholm, Sweden. A 48-item eFI was constructed using diagnostic codes, functioning and other health indicators, and laboratory data. The CFS, Hospital Frailty Risk Score, and Charlson Comorbidity Index were used for comparative assessment of the eFI. We modeled in-hospital mortality and 30-day readmission using logistic regression; 30-day and 6-month mortality using Cox regression; and length of stay using linear regression.
Results
Thirteen thousand one hundred and eighty-eight patients were included in analyses (mean age 83.1 years). A 0.03 increment in the eFI was associated with higher risks of in-hospital (odds ratio: 1.65; 95% confidence interval: 1.54–1.78), 30-day (hazard ratio [HR]: 1.43; 1.38–1.48), and 6-month mortality (HR: 1.34; 1.31–1.37) adjusted for age and sex. Of the frailty and comorbidity measures, the eFI had the highest area under receiver operating characteristic curve for in-hospital mortality of 0.813. Higher eFI was associated with longer length of stay, but had a rather poor discrimination for 30-day readmission.
Conclusions
An EHR-based eFI has robust associations with adverse outcomes, suggesting that it can be used in risk stratification in hospitalized older adults.
Keywords: Comorbidity, Electronic frailty index, Frailty, Geriatrics
With the aging of the global population, it becomes increasingly important to identify and support older adults who are at the greatest risk of adverse outcomes. Frailty, characterized by reduced physiological reserve and increased vulnerability to stressors (1), is a measure that captures such risk. Frailty has consistently been linked to negative health outcomes, such as mortality (2,3), hospitalization (4), and increased health care costs (5,6). Despite the lack of a universal consensus on how to best assess frailty, the most widely used and validated frailty models are the physical frailty phenotype (characterized by weight loss, weakness, slowness, inactivity, and exhaustion) (7) and the frailty index (FI; multidimensional deficit accumulation) (8). Nevertheless, these measures are time- and resource-consuming, which limits their incorporation into routine clinical practice (9). One of the most frequently adopted frailty measure in clinical settings is the Clinical Frailty Scale (CFS) (10), which is a quick and simple screening tool and often has high accuracy and feasibility (11). However, as an instrument that requires in-person assessment, it may be subject to interrater error and may not always be available at hospital admission (12–14).
Automated frailty scores, based on routinely collected electronic health records (EHRs) or administrative claims data, have recently been developed in certain countries such as the United Kingdom (15), the United States (16,17), and Canada (18). One of the first models was the electronic frailty index (eFI) proposed by Clegg et al. (15), who created it using the UK primary care Read codes on the basis of the Rockwood deficit accumulation approach (8). Several variations of the eFI, such as claims-based frailty indices (16,18), or an eFI incorporating diagnosis codes, functional impairments, and laboratory measures (17), have later been developed. While these tools are commonly used on data retrieved from primary care, recent studies have shown that such eFIs show good predictive performance for all-cause mortality also in-hospital settings (19–21). Another database-derived frailty measure, the Hospital Frailty Risk Score (HFRS), is calculated based on the International Classification of Diseases, Tenth Revision (ICD-10) codes (22), and has been validated for its ability to predict mortality and prolonged length of stay in hospitalized older patients (23–25). However, its composition of ICD-10 diagnoses makes it more similar to a comorbidity measure, possibly missing out other frailty aspects such as functioning (22).
To date, frailty is not yet routinely assessed in all older adults in Sweden, but the CFS has started to be implemented in geriatric clinics in Stockholm. Compared with other cities in Sweden or the Nordic countries, Stockholm has a relatively large number of geriatric clinics, and patients admitted to these clinics are usually more frail and need more comprehensive care. Routine frailty screening can help planning of resource allocation and identifying patients who would benefit most from a Comprehensive Geriatric Assessment (CGA) (26). However, not all hospitals in Sweden perform systematic CFS assessment. There is also no standardized frailty measure in Sweden that can be used in communication between different healthcare providers. To reduce the burden of bedside frailty assessment, aid in risk stratification, and potentially lead to a systematic and unified frailty screening across different healthcare providers in Sweden, there is an increasing need to analyze whether an eFI can be adapted to Swedish context. The objective of this study was therefore to develop an eFI using routinely collected EHRs in geriatric clinics in Stockholm, Sweden. For validation, we compared its discriminative ability to other validated frailty and comorbidity measures, the CFS, HFRS, and the Charlson Comorbidity Index (CCI) (27), considering mortality, readmission, and the length of stay as outcomes.
Method
Study Design and Sample
We conducted a retrospective cohort study using electronic medical records in the Stockholm Region. Between 1 March 2020 and 17 June 2021, patients with unplanned admissions to 9 geriatric clinics treated for any causes, except COVID-19, were included. The geriatric clinics in the Stockholm Region are specialized in inpatient geriatric care and are standalone geriatric hospitals or situated in larger emergency hospitals. They generally enroll older patients who have reduced physical and/or cognitive function, have multiple comorbidities, and in need of geriatric medical care and/or rehabilitation. COVID-19 patients (defined based on ICD-10 codes of U07.1, U07.2, U08.9, U09.9, or U10.9) often have different characteristics and prognosis and were analyzed in a separate paper (28). Exclusion criteria included admissions without discharge information or with a length of stay <24 h. Most patients had only one admission during the study period (73.0%); for those with multiple admissions (17.5% had 2 admissions, 9.5% had 3 or more admissions), the first available admission was considered in the analyses. In total, 18 225 non-COVID-19 patients were included, of whom 13 188 (72.4%) had sufficient data for calculation of the eFI. A flowchart of sample selection is presented in Supplementary Figure 1. This study was approved by the Swedish Ethical Review Authority (Dnr 2020-02146, 2020-03345, 2021-00595, 2021-02096).
Construction of the eFI
Following the eFI model developed by Pajewski et al. (17), we selected a total of 48 items for the construction of the eFI. The items consisted of deficits in 3 categories: (i) disease diagnoses, extracted from ICD-10 codes assigned during the current admission (29 items), (ii) functional data and other health indicators, such as falls, neuropsychological problems, and oral health (10 items), and (iii) laboratory measures (9 items). The full list of the eFI items and the coding methods are shown in Supplementary Table 1. Consistent with the original deficit accumulation model (8), we calculated the eFI as the sum of deficit items divided by the total number, providing that the patient had ≥30 non-missing items (8). For instance, a patient with 10 deficit points out of 45 non-missing items would receive an eFI of 10/45 = 0.22. As 60% (29 of 48) of the eFI items were based on ICD-10 codes (which were non-missing for all participants), we required that at least half of the functioning and/or laboratory-based deficit items were non-missing in each individual. This was to avoid the lack of such items that are necessary in reaching the multidimensional definition of frailty and prevent the eFI from having an overrepresentation of comorbidity items. We presented a clinically meaningful change of eFI per 0.03 increase (29), as well as per 0.1 increase, in the association analyses. Based on both the distribution of the eFI in our sample and a previously defined cut-off of 0.25 (30,31), we categorized patients into 4 groups: fit (eFI ≤ 0.15), mild frailty (0.15 > eFI ≥ 0.2), moderate frailty (0.2 > eFI ≥ 0.25), and severe frailty (eFI > 0.25).
Other Frailty and Comorbidity Measures
Other frailty measures, the CFS and HFRS, and the CCI, which is a measure of comorbidity, were used for comparative performance assessment and validation of the eFI. The CFS was scored by a physician or a trained nurse during first day of admission, using a chart review and face-to-face assessments. The CFS ranges from 1 (“very fit”) to 9 (“terminally ill”), and was categorized in 3 groups: CFS 1–3, CFS 4–5, and CFS 6–9. Both the HFRS and CCI were calculated based on ICD-10 codes. The HFRS is based on 109 frailty-associated and differently weighted ICD-10 codes and categorizes the individuals into low-risk (<5), intermediate-risk (5–15), and high-risk (>15) frailty groups (22). The CCI was computed using an algorithm adapted for Swedish settings (32), and was considered as a continuous variable in all analyses.
Outcomes
Primary outcomes were in-hospital, 30-day, and 6-month all-cause mortality, calculated from the date of admission. Dates of death were extracted from the Population System in Sweden. Secondary outcomes were 30-day readmission to any of the 9 included clinics, and the length of stay. Only patients discharged to home were included in the 30-day readmission analysis, that is, excluding those who were transferred to another unit or care facility after the stay at the geriatric unit.
Statistical Analysis
Patients’ characteristics were summarized and compared by eFI categories using one-way analysis of variance or Kruskal–Wallis tests for continuous variables, and Pearson χ 2 tests for categorical variables. Spearman’s correlations (ρ) were calculated between continuous frailty and comorbidity measures.
We fitted multivariable regression models to compare the eFI to the CFS, HFRS, and CCI in associations with different outcomes. In-hospital mortality and 30-day readmission were modeled using logistic regression, with odds ratios (ORs) and 95% confidence intervals (CI) presented. The diagnostic accuracies (discrimination) of the logistic regression models including continuous frailty and comorbidity measures, in addition to age and sex, was examined using area under the receiver operating characteristic curve (AUC). Hazard ratios (HRs) and 95% CI for 30-day and 6-month mortality were estimated using Cox proportional-hazards models. Harrell’s C-statistics were calculated for assessing the discriminative abilities of the Cox models, with 95% CI calculated through 1 000 bootstrapping resampling. Linear regression models were performed for the length of stay, and coefficients of determination (R2) were calculated to assess the proportion of variation explained by the independent variables. All models were first adjusted for age and sex (model 1), and additionally accounting for the clustering of patients in the geriatric clinics (model 2) using the generalized estimating equation methods or stratified Cox models.
We further stratified the analysis of the association between the eFI and in-hospital mortality by (1) age and sex to examine whether the associations differ by demographic characteristics; (2) the 9 admitting clinics to assess the variation by hospitals; (3) the main diagnosis leading to admission of a patient to test robustness of the eFI in different patient groups; and (4) categories of the CFS to investigate whether the eFI could complement the CFS in identifying high-risk patients.
As a sensitivity analysis, we considered the last available admission of patients (instead of the first admission) and tested whether inclusion of ICD-10 codes from multiple admissions could have changed the distribution of the eFI and its association with mortality. Due to the high missingness of the CFS (~60%), we also performed multiple imputation by chained equations and calculated the pooled estimates for the association between the CFS and in-hospital mortality in the imputed data.
All analyses were performed using R version 4.0.5.
Results
Sample Characteristics
Among the 13 188 patients who had sufficient data for calculation of the eFI, the mean age was 83.1 years and 60.2% were women; the in-hospital mortality rate was 1.4% and the median length of stay was 6.7 days (Table 1). The most common causes of admission were fragility fracture (18.9%), congestive heart failure (6.4%), dementia (4.7%), stroke or transient ischemic attack (3.9%), and urinary system disease (3.4%; Supplementary Figure 2).
Table 1.
Sample Characteristics by Categories of the Electronic Frailty Index (n = 13 188)a
| Characteristic | All Patients (n = 13 188) | Fit (n = 3 860) | Mild Frailty (n = 4 366) | Moderate Frailty (n = 3 220) | Severe Frailty (n = 1 742) | p b |
|---|---|---|---|---|---|---|
| Age, y, mean ± SD | 83.1 ± 8.4 | 81.5 ± 8.3 | 83.2 ± 8.4 | 84.0 ± 8.4 | 84.9 ± 8.2 | <.001 |
| Age category, n (%) | <.001 | |||||
| <65 years | 215 (1.6) | 92 (2.4) | 65 (1.5) | 41 (1.3) | 17 (1.0) | |
| 65–74 years | 1 917 (14.5) | 178 (4.6) | 349 (8.0) | 327 (10.2) | 190 (10.9) | |
| 75–84 years | 4 909 (37.2) | 718 (18.6) | 629 (14.4) | 381 (11.8) | 189 (10.8) | |
| 85–94 years | 5 103 (38.7) | 1 558 (40.4) | 1 638 (37.5) | 1 144 (35.5) | 569 (32.7) | |
| ≥95 years | 1 044 (7.9) | 1 314 (34.0) | 1 685 (38.6) | 1 327 (41.2) | 777 (44.6) | |
| Men, n (%) | 5 245 (39.8) | 1 438 (37.3) | 1 716 (39.3) | 1 324 (41.1) | 767 (44.0) | <.001 |
| CFS score, median [IQR] | 5 [4, 6] | 4 [3, 5] | 5 [4, 6] | 6 [5, 7] | 6 [5, 7] | <.001 |
| CFS category, n (%) | <.001 | |||||
| 1–3 | 830 (6.3) | 445 (11.5) | 262 (6.0) | 96 (3.0) | 27 (1.5) | |
| 4–5 | 1 869 (14.2) | 620 (16.1) | 681 (15.6) | 415 (12.9) | 153 (8.8) | |
| 6–9 | 2 246 (17.0) | 333 (8.6) | 676 (15.5) | 729 (22.6) | 508 (29.2) | |
| Missing | 8 243 (62.5) | 2 462 (63.8) | 2 747 (62.9) | 1 980 (61.5) | 1 054 (60.5) | |
| HFRS, median [IQR] | 2.8 [1.4, 5.0] | 1.8 [0.0, 3.6] | 2.6 [1.4, 4.7] | 3.3 [1.6, 5.8] | 4.4 [2.3, 6.8] | <.001 |
| HFRS category, n (%) | <.001 | |||||
| Low risk (<5) | 9 811 (74.4) | 3 256 (84.4) | 3 342 (76.5) | 2 195 (68.2) | 1 018 (58.4) | |
| Intermediate risk (5–15) | 3 316 (25.1) | 598 (15.5) | 1 005 (23.0) | 1 008 (31.3) | 705 (40.5) | |
| High risk (>15) | 61 (0.5) | 6 (0.2) | 19 (0.4) | 17 (0.5) | 19 (1.1) | |
| CCI, median [IQR] | 1 [0, 2] | 0 [0, 1] | 1 [0, 2] | 1 [1, 3] | 2 [1, 3] | <.001 |
| In-hospital mortality, n (%) | 183 (1.4) | 5 (0.1) | 29 (0.7) | 61 (1.9) | 88 (5.1) | <.001 |
| Discharged to home, n (%) | 10 448 (79.2) | 3 418 (88.5) | 3 626 (83.1) | 2 353 (73.1) | 1 051 (60.4) | <.001 |
| 30-day readmissionc, n (%) | 1 114 (10.7) | 266 (7.8) | 369 (10.2) | 331 (14.1) | 148 (14.1) | <.001 |
| Length of stay, day, median (IQR) | 6.7 [4.7, 8.9] | 5.7 [3.7, 7.1] | 6.6 [4.8, 8.5] | 6.9 [5.2, 9.9] | 7.9 [5.8, 11.8] | <.001 |
Note: CCI = Charlson Comorbidity Index; CFS = Clinical Frailty Scale; eFI = electronic frailty index; HFRS = Hospital Frailty Risk Score; IQR = interquartile range.
aThe electronic frailty index was categorized into 4 groups in the main analysis: fit (≤0.15); mild frailty (>0.15–0.2); moderate frailty (>0.2–0.25); severe frailty (>0.25).
b P-values for comparison between the 4 eFI categories, based on one-way analysis of variance or Kruskal–Wallis tests for continuous variables, and χ 2 tests for categorical variables.
cOnly patients discharged to home after the first admission were considered for 30-d readmission.
The patients without sufficient data for calculation of the eFI were those with <30 available eFI items (n = 9) or those missing >9 of the 18 functioning and/or laboratory eFI items (n = 5 028). These patients were similar to those with sufficient data for the eFI, with regards to age, sex, frailty level according to CFS and HFRS, and in-hospital death rate. Missing data on the eFI seemed to be mostly dependent on the clinic rather than patient characteristics (Supplementary Table 2). Similarly, the admitting clinic was the main factor associated with missing data on the CFS (Supplementary Table 3).
The eFI was approximately normally distributed, with a median of 0.181 (interquartile range: 0.143–0.222; range: 0–0.432); the distribution of the eFI was similar in the subgroup for 30-day readmission analysis (ie, patients who were discharged to home; Supplementary Figure 3). The proportions of patients classified as fit, mildly frail, moderately frail, and severely frail were 29.3%, 33.1%, 24.4%, and 13.2%, respectively (Table 1). Although women were on average older than men (mean age: 84.1 vs. 81.6), men had significantly higher frailty scores than women when defined by the eFI (severe frailty: 14.6% vs. 12.3%) and the HFRS (intermediate or high risk: 28.0% vs. 24.1%), but not by the CFS (Supplementary Table 4). The eFI was moderately correlated with the CFS (ρ = 0.420), and to a lesser extent with the HFRS (ρ = 0.289) and CCI (ρ = 0.368) (Supplementary Figure 4 and Supplementary Table 5).
Associations With In-hospital, 30-Day, and 6-Month Mortality
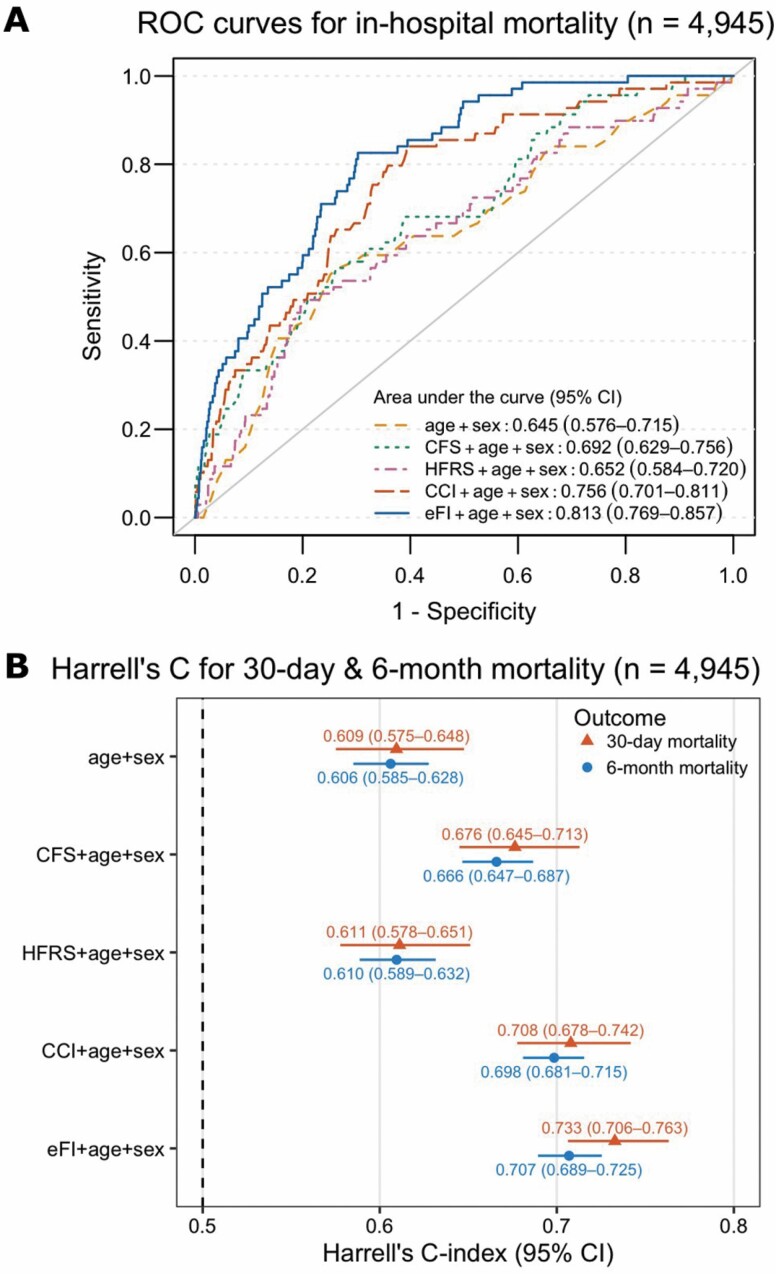
After adjusting for age and sex, the eFI was strongly associated with in-hospital mortality (OR per 0.03 increase: 1.65, 95% CI: 1.54–1.78); the results were largely consistent when additionally accounted for the clustering by the clinics (Table 2). The CFS, HFRS, and CCI also had positive associations with in-hospital mortality; however, among all the measures, the eFI had the greatest discriminative ability for in-hospital mortality when added to a model with age and sex, yielding an AUC of 0.813 (95% CI: 0.769–0.857; Figure 1A). In the subgroup analysis, the association between the eFI and in-hospital mortality was essentially unchanged when stratified by age groups, sex, clinics, main diagnosis of the admission, and CFS categories (Supplementary Figure 5). However, as the number of deaths was small in some of the groups, these results should be interpreted with caution.
Table 2.
Associations Between Frailty and Comorbidity Measures and Mortality Outcomes (n = 13 188)
| Model | In-Hospital Mortality, OR (95% CI) | 30-d Mortality, HR (95% CI) | 6-mo Mortality, HR (95% CI) | |||
|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | Model 2b | Model 1a | Model 2b | |
| eFI | ||||||
| Continuous, per 0.03 increase | 1.65 (1.54, 1.78)* | 1.63 (1.54, 1.72)* | 1.43 (1.38, 1.48)* | 1.43 (1.38, 1.48)* | 1.34 (1.31, 1.37)* | 1.34 (1.31, 1.37)* |
| Continuous, per 0.1 increase | 5.34 (4.20, 6.82)* | 5.07 (4.23, 6.09)* | 3.28 (2.91, 3.69)* | 3.26 (2.90, 3.67)* | 2.70 (2.52, 2.90)* | 2.66 (2.47, 2.85)* |
| Categorical | ||||||
| Fit (≤0.15) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Mild frailty (>0.15–0.2) | 4.60 (1.94, 13.5)* | 4.64 (2.5, 8.8)* | 2.60 (1.90, 3.55)* | 2.63 (1.93, 3.60)* | 1.91 (1.63, 2.22)* | 1.94 (1.66, 2.26)* |
| Moderate frailty (>0.2–0.25) | 12.5 (5.51, 35.7)* | 12.1 (5.3, 27.7)* | 4.79 (3.54, 6.49)* | 4.83 (3.57, 6.54)* | 3.42 (2.95, 3.97)* | 3.45 (2.97, 4.01)* |
| Severe frailty (>0.25) | 32.8 (14.7, 93.3)* | 31.5 (14.3, 69.5)* | 9.81 (7.27, 13.2)* | 9.87 (7.31, 13.3)* | 5.81 (4.99, 6.77)* | 5.78 (4.96, 6.74)* |
| CFS (n = 4 945)c | ||||||
| Continuous, per point increase | 1.63 (1.34, 2.00)* | 1.86 (1.29, 2.69)* | 1.57 (1.42, 1.74)* | 1.78 (1.60, 1.98)* | 1.44 (1.36, 1.52)* | 1.60 (1.51, 1.70)* |
| Categorical | ||||||
| 1–3 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 4–5 | 4.33 (1.26, 27.1)* | 4.19 (1.37, 12.9)* | 3.74 (1.88, 7.46)* | 3.99 (2.00, 7.98)* | 1.98 (1.45, 2.72)* | 2.10 (1.53, 2.89)* |
| 6–9 | 7.22 (2.21, 44.4)* | 9.74 (3.44, 27.6)* | 6.11 (3.12, 12.0)* | 8.42 (4.24, 16.7)* | 3.68 (2.72, 4.97)* | 4.86 (3.56, 6.64)* |
| HFRS | ||||||
| Continuous, per point increase | 1.06 (1.02, 1.11)* | 1.04 (1.01, 1.07)* | 1.04 (1.02, 1.07)* | 1.04 (1.02, 1.06)* | 1.04 (1.02, 1.05)* | 1.03 (1.02, 1.05)* |
| Categorical | ||||||
| Low risk (<5) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Intermediate risk (5–15) | 1.63 (1.19, 2.20)* | 1.48 (1.00, 2.18) | 1.26 (1.08, 1.48)* | 1.23 (1.04 1.44)* | 1.22 (1.11, 1.34)* | 1.20 (1.09, 1.32)* |
| High risk (>15) | Not estimable | Not estimable | 1.21 (0.45, 3.25) | 1.04 (0.39, 2.80) | 0.70 (0.33, 1.48) | 0.64 (0.30, 1.35) |
| CCI | ||||||
| Continuous, per point increase | 1.40 (1.31, 1.50)* | 1.41 (1.33, 1.50)* | 1.34 (1.29, 1.39)* | 1.34 (1.30, 1.39)* | 1.33 (1.31, 1.36)* | 1.33 (1.30, 1.36)* |
Note: CCI = Charlson Comorbidity Index; CFS = Clinical Frailty Scale; CI = confidence interval; eFI = electronic frailty index; HFRS = Hospital Frailty Risk Score; HR = hazard ratio; OR = odds ratio. The eFI, CFS, and HFRS were used as both continuous and categorical variables in separate models, while the CCI was used as continuous variable only.
aModel 1 was adjusted for age and sex. Odds ratios for in-hospital mortality were estimated by logistic regression models, and hazard ratios for 30-day and 6-month mortality were estimated by Cox models.
bModel 2 was adjusted for age and sex, and additionally accounted for clustering of patients in the 9 geriatric clinics. Odds ratios for in-hospital mortality were estimated by generalized estimating equation with the logit link, and hazard ratios for 30-day and 6-month mortality were estimated by stratified Cox regression models.
cSample size was smaller in analysis of CFS due to missing data.
*p < .05.
Figure 1.
Diagnostic performance of frailty and comorbidity measures for mortality outcomes in patients with all the measures available (n = 4 945). (A) Receiver operating characteristics (ROC) curves for in-hospital mortality; (B) Harrell’s C-statistics from Cox models for 30-day and 6-month mortality. CFS, HFRS, CCI, and eFI were considered as continuous variables in all the models. CFS = Clinical Frailty Scale; HFRS = Hospital Frailty Risk Score; CCI = Charlson Comorbidity Index; eFI = electronic frailty index.
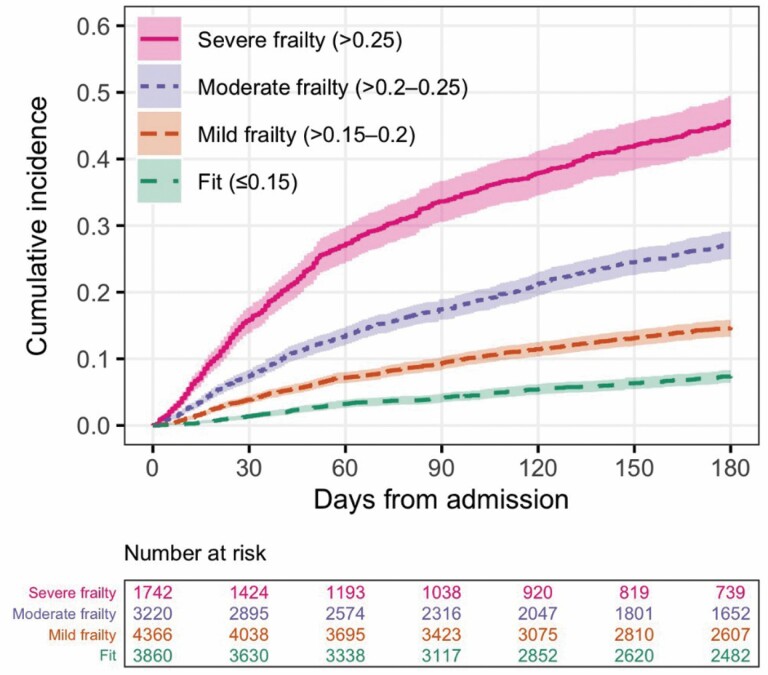
We observed a gradient of an increasing risk of mortality in the higher eFI categories compared with the lowest category (fit) in 6 months from admission (Figure 2). Similar to in-hospital mortality, a 3% rise in the eFI score was significantly associated with elevated risks of 30-day (HR: 1.43, 95% CI: 1.38–1.48) and 6-month mortality (HR: 1.34, 95% CI: 1.31–1.37) after adjusting for age and sex (Table 2). The eFI models had the highest Harrell’s C-statistics (0.733 for 30-day mortality and 0.707 for 6-month mortality) in comparison to the other frailty and comorbidity measures, indicating that the eFI had good discrimination for 30-day and 6-month mortality in the Cox models (Figure 1B).
Figure 2.
Kaplan–Meier curves for all-cause mortality by categories of the electronic frailty index (n = 13 188).
Associations With 30-Day Readmission and Length of Stay
Despite the significant associations between the eFI and 30-day readmission (OR per 0.03 increase: 1.13, 95% CI: 1.09–1.17), and likewise between the CFS, HFRS, and CCI, and 30-day readmission, the AUCs for these models were <0.6, indicating poor discrimination for readmission (Supplementary Table 5).
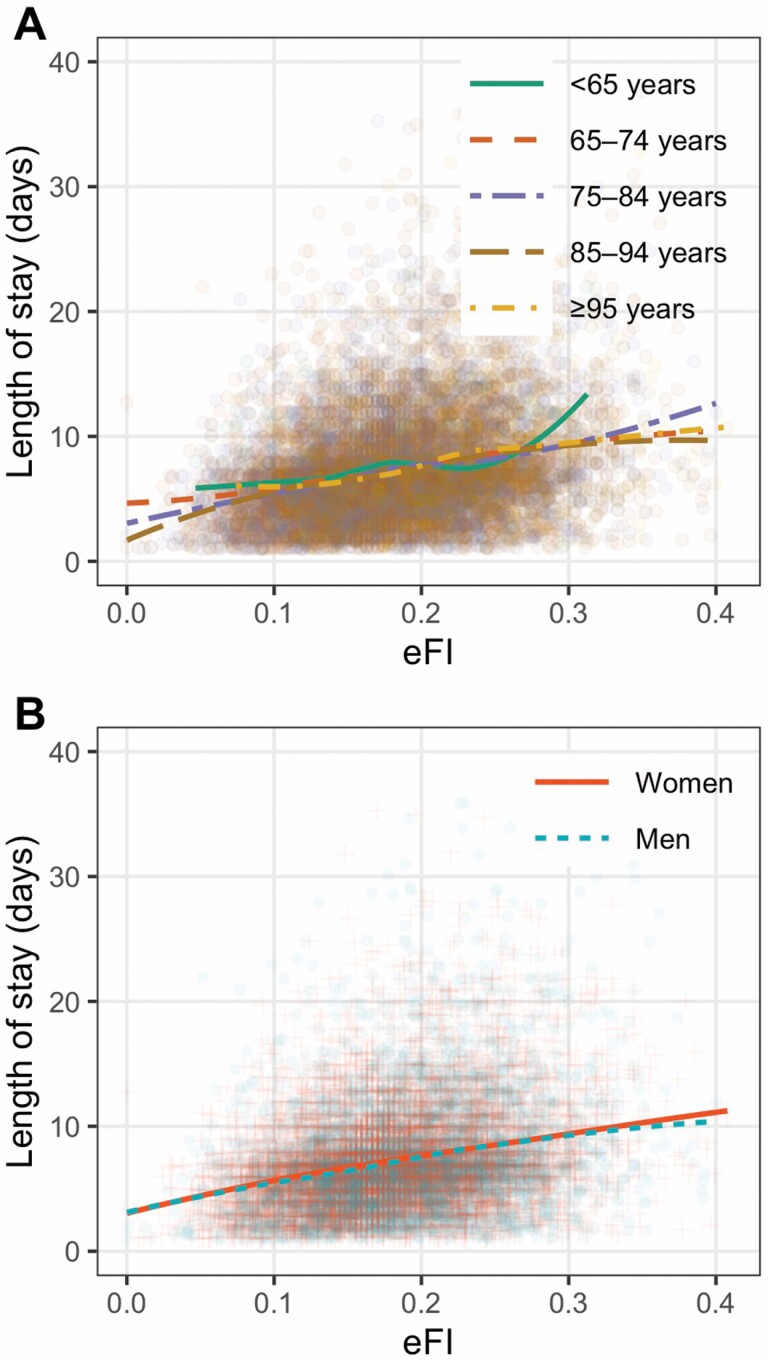
There was an approximately linear relationship between the eFI and the length of stay, regardless of age and sex (Figure 3). Similarly, after adjusting for age and sex, a 3% increment in the eFI was on average associated with a 0.6 day longer hospital stay (95% CI: 0.56–0.64). The model including eFI, age, and sex explained 7.21% of the variation in the length of stay (Supplementary Table 6).
Figure 3.
Scatter plots of the electronic frailty index and the length of stay (n = 13 188). (A) stratified by age; (B) stratified by sex. The colored fit lines represent the fitted locally estimated scatterplot smoothing curves (LOESS).
Sensitivity Analysis
For patients with multiple admissions during the study period, we performed a sensitivity analysis to test whether using data from only a single admission would have underestimated the eFI score. Compared with the eFI constructed using only data from the last admission, an eFI calculated using ICD-10 codes from all available admissions had higher median (0.230 vs. 0.202) and maximum (0.522 vs. 0.422) values (Supplementary Figure 6). These 2 eFIs had similar associations with in-hospital mortality. The association between the CFS and in-hospital mortality was also largely consistent after performing multiple imputations (OR per point increase in CFS: 1.60; 95% CI: 1.39–1.84).
Discussion
Although it is well-known that frailty predicts adverse outcomes, traditional frailty measurements often require in-person assessment and may not always be feasible or available in clinical settings. There is a growing interest of deriving frailty measures from routinely collected health data to facilitate frailty screening in clinical practice. Several such eFI models have been developed for primary care, and inpatient and outpatient settings using diagnostic codes, health service codes, and clinical information (33). Here we adapted the U.S. eFI model (17) to Swedish EHR data, that is, retrieved from geriatric clinics in the Stockholm Region. We found that the eFI outperformed the currently available frailty and comorbidity scales (ie, CFS, HFRS, and CCI) in discriminating in-hospital mortality, yielding an AUC of 0.813 when added to a model with age and sex. It also had better accuracy for 30-day and 6-month mortality than CFS and HFRS and was significantly associated with longer length of stay. Nevertheless, all the frailty scales and the CCI had a poor discrimination for 30-day readmission.
Previous work has shown the association between an eFI and adverse outcomes in several countries (15–21). However, there has been no EHR-based frailty measure developed and implemented in Sweden or other Nordic countries so far. For a country like Sweden with a high life expectancy (83 years in 2019) (34) and increasing healthcare needs among the older adults, it is important to assess whether an eFI can be used to identify patients that are at a high risk of adverse outcomes before such a tool can be implemented into routine clinical practice. Based on data availability and following the model developed by Pajewski et al. (17), we included ICD-10 codes, functional assessment scales and other health indicators, and laboratory measurements in the eFI. It had a moderate correlation with a clinical frailty measure (ie, CFS, ρ = 0.420), but a weaker correlation with a comorbidity measure (ie, CCI, ρ = 0.368) and a frailty measure based on ICD-10 codes (ie, HFRS, ρ = 0.289). These findings suggest that the eFI captures frailty rather than multimorbidity. This is also on par with previous studies showing a moderate correlation for the eFI with the CFS (35), but a low correlation with the HFRS (36).
Although a data-driven or machine learning approach can be used in claims-based frailty indices (33), we applied the conventional deficit accumulation model (8)—a generalizable approach to frailty, in which a wide range of deficits, for example, signs, symptoms, diseases, functional limitations, laboratory measures, can be included as long as it includes at least 30 age-related deficits (8,37). We noticed, however, some differences in the characteristics of our eFI compared with a survey-based Rockwood FI. First, instead of a right-skewed distribution characteristic to the Rockwood FI, our eFI was approximately normally distributed, possibly due to a homogenous sample of less healthy hospitalized older adults (38,39), and inclusion of laboratory tests (representing subclinical and cellular deficits) (40,41). Second, our eFI had a relatively low maximum value of 0.432. One reason is that for assessing the diagnosis-based ICD-code items (29 of 48), we used only those codes that were recorded during the current visit, possibly leading to underreporting of certain diseases. Third, we observed slightly, but significantly higher eFI scores in men than in women. Although FI scores are generally higher in women in community populations (42), no sex difference (39,43) or higher scores in men (18) have been found in hospitalized patients. The sex differences may also be influenced by whether self-reported (44) and laboratory deficits (40) are included. Indeed, more studies are needed to understand the mechanisms regarding sex differences in frailty (42).
Our finding of a robust association of the eFI with mortality and the length of stay is consistent with previous studies (18–21). Our observed Harrell’s C-statistics of 0.70 for 6-month mortality was also comparable to the US eFI model that has a C-statistics of 0.75 (19). Importantly, our eFI performed better than the CFS in predicting adverse outcomes, hence providing important insights on its potential use in risk stratification in hospitalized older adults to ease personnel burden in-hospital settings. However, all the frailty and comorbidity measures had a poor discrimination for 30-day readmission. This may partly be explained by the potential misclassification of readmission, since we did not have data on patients who were admitted to other clinics than the 9 geriatric clinics. Besides, a relatively low AUC or c-statistic for frailty in predicting readmission (around 0.5–0.6) is also frequently reported in the literature (19,22,23,36,45,46). Hospital readmission is often described as a complex outcome not merely related to the health status of patients, but also social factors such as access to care, social support, and drug abuse (47,48), which are factors not captured in the frailty measures. We may speculate that many of the more frail individuals living in residential care facilities have better access to care and thus a less pronounced tendency for readmission to hospital, compared with less frail individuals living in their own homes.
This study has a relatively large sample size from multiple hospitals, allowing us to assess the association of the eFI with adverse outcomes across the 9 clinics. We also had data on other validated frailty and comorbidity measures for validation and comparative assessment of the eFI. Nevertheless, some limitations should be considered. First, as mentioned above, the use of ICD-10 codes from a single admission may have caused an underreporting of the diseases, and the same applies to the HFRS and CCI. However, when we have more data over several years, we will assess the effect of including diagnoses from previous admissions too, and identify the optimal look-back period for the eFI. Second, as we only analyzed patients in geriatric clinics, our results cannot be applied to older patients in other units. It would be of interest to investigate whether the eFI performs equally well in other older in-patient groups. Third, there was an overall 6% decrease in-hospital admissions in Sweden in 2020 compared with 2019, with the most notable decreases seen in hospitalizations due to influenza and pneumonia (49). The influenza and pneumonia-related decreases are likely due to restrictions that were issued to prevent the spread of COVID-19, whereas for the other diseases, it may be that people were less active in seeking help for their health conditions during the pandemic. Given that the overall decrease was relatively small, it is unlikely to have a major impact on the results. However, generalizability to patients hospitalized for influenza or pneumonia may be decreased. Fourth, we were also unable to adjust for other demographic data as they were not available in the EHRs. Finally, the eFI could not be calculated for 27.6% of our sample due to missing data on functioning and laboratory measures. Our rate of missing data is nevertheless comparable to the ~30% missing data in the U.S. eFI by Pajewski et al. (17). Instead of the patients’ demographic characteristics and health status being associated with the rate of missing data in the U.S. eFI, our missing data were more related to variations in the data collection practice between hospitals. The COVID-19 pandemic might have affected the data collection routines for the functional and other health assessments, leading to more missing data than usual. Future work may reveal which eFI items are the most decisive for predicting adverse outcomes, as well as which alternative available items from the EHRs could be included to complement the current eFI. Furthermore, it may be relevant to use other outcome measures than mortality in coming research efforts, although measures related to physical function and cognition are inherent in the FI. Crucial is also to show whether the eFI is able to identify patients that will benefit from CGA (26), and that will respond to treatment tailored from the outcome of the CGA. When implementing these results, it is also essential to include patients’ perspectives and to understand their feelings on frailty (50).
The Swedish eFI can be used for several purposes: (i) to complement the CFS in identifying patients who have high and complex care needs and would benefit from a CGA; (ii) to describe health status of an individual and communicate it to other care providers (eg, planning of medical procedure and treatment among cardiologists and neurosurgeons); (iii) to describe group-level health trends for administrative and research purposes. When automated, the Swedish eFI will provide a tool that does not require extra resources for data collection. As the majority of the healthcare providers within primary, secondary, and tertiary care use the same EHR system in the Stockholm Region, the eFI—that is updated at each visit—can be made available to all healthcare units treating the patient. We envision that the eFI can also pave the way towards a unified model of frailty assessment in Sweden.
To conclude, we developed an eFI based on routinely collected EHRs for Swedish hospitalized older adults, and showed that it correlates with the CFS and has robust associations with short- and long-term mortality and the length of stay. This work provides evidence that an eFI is feasible for risk stratification among hospitalized older adults in Stockholm, Sweden, and may as well be applicable to countries with similar health system.
Supplementary Material
Acknowledgments
We acknowledge all patients, caregivers, and staff who contributed to this study. This study was accomplished within the context of the Swedish National Graduate School for Competitive Science on Ageing and Health (SWEAH) funded by the Swedish Research Council.
Contributor Information
Jonathan K L Mak, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Sara Hägg, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Maria Eriksdotter, Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden; Theme Inflammation and Aging, Karolinska University Hospital, Huddinge, Sweden.
Martin Annetorp, Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden; Theme Inflammation and Aging, Karolinska University Hospital, Huddinge, Sweden.
Ralf Kuja-Halkola, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden.
Laura Kananen, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; Faculty of Social Sciences (Health Sciences) and Gerontology Research Center (GEREC), University of Tampere, Tampere, Finland.
Anne-Marie Boström, Theme Inflammation and Aging, Karolinska University Hospital, Huddinge, Sweden; Division of Nursing, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden.
Miia Kivipelto, Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden; Theme Inflammation and Aging, Karolinska University Hospital, Huddinge, Sweden.
Carina Metzner, Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden; Theme Inflammation and Aging, Karolinska University Hospital, Huddinge, Sweden.
Viktoria Bäck Jerlardtz, Department of Geriatric Medicine, Jakobsbergsgeriatriken, Stockholm, Sweden.
Malin Engström, Department of Geriatric Medicine, Sabbatsbergsgeriatriken, Stockholm, Sweden.
Peter Johnson, Department of Geriatric Medicine, Capio Geriatrik Nacka AB, Nacka, Sweden.
Lars Göran Lundberg, Department of Geriatric Medicine, Dalengeriatriken Aleris Närsjukvård AB, Stockholm, Sweden.
Elisabet Åkesson, Research and Development Unit, Stockholms Sjukhem, Stockholm, Sweden; Division of Neurogeriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden.
Carina Sühl Öberg, Department of Geriatric Medicine, Handengeriatriken, Aleris Närsjukvård AB, Stockholm, Sweden.
Maria Olsson, Department of Geriatric Medicine, Capio Geriatrik Löwet, Stockholm, Sweden; Department of Geriatric Medicine, Capio Geriatrik Sollentuna, Stockholm, Sweden.
Tommy Cederholm, Theme Inflammation and Aging, Karolinska University Hospital, Huddinge, Sweden; Department of Public Health and Caring Sciences, Uppsala University, Uppsala, Sweden.
Juulia Jylhävä, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; Faculty of Social Sciences (Health Sciences) and Gerontology Research Center (GEREC), University of Tampere, Tampere, Finland.
Dorota Religa, Division of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden; Theme Inflammation and Aging, Karolinska University Hospital, Huddinge, Sweden.
Funding
This work was supported financially by the Swedish Research Council grants (2018-02077, 2020-02014, 2020-06101), the regional agreement on medical training and clinical research between the Stockholm County Council and the Karolinska Institutet (ALF), the Karolinska Institutet Strategic Research Program in Epidemiology, the King Gustaf V and Queen Victoria’s Foundation of Freemasons, the Academy of Finland through its funding to the Centre of Excellence in Research of Ageing and Care (CoEAgeCare, grant numbers 335870, 326567, and 336670), Läkarsällskapet and Gösta Miltons Donationsfond grant, and the Stockholm University—Region Stockholm grant.
Conflict of Interest
None declared.
Author Contributions
D.R., J.J., S.H., M.E., and T.C. contributed to the study concept and design. J.K.L.M., R.K.-H., and L.K. performed data analyses. M.E., M.A., A.-M.B., M.K., C.M., V.B.J., M.E., P.J., L.G.L., E.Å., C.S.Ö, M.O., T.C., and D.R. contributed to data acquisition. J.K.L.M. and J.J. wrote the manuscript. All authors contributed to interpretation of the results, and participated in writing and reviewing of the manuscript.
References
- 1. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365–1375. doi: 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 2. Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 2018;47(2):193–200. doi: 10.1093/ageing/afx162 [DOI] [PubMed] [Google Scholar]
- 3. Chong E, Ho E, Baldevarona-Llego J, Chan M, Wu L, Tay L. Frailty and risk of adverse outcomes in hospitalized older adults: a comparison of different frailty measures. J Am Med Dir Assoc. 2017;18(7):638.e7–638.e11. doi: 10.1016/j.jamda.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 4. Chang S-F, Lin H-C, Cheng C-L. The relationship of frailty and hospitalization among older people: evidence from a meta-analysis. J Nurs Scholarsh. 2018;50(4):383–391. doi: 10.1111/jnu.12397 [DOI] [PubMed] [Google Scholar]
- 5. Hajek A, Bock J-O, Saum K-U, et al. . Frailty and healthcare costs—longitudinal results of a prospective cohort study. Age Ageing. 2018;47(2):233–241. doi: 10.1093/ageing/afx157 [DOI] [PubMed] [Google Scholar]
- 6. García-Nogueras I, Aranda-Reneo I, Peña-Longobardo LM, Oliva-Moreno J, Abizanda P. Use of health resources and healthcare costs associated with frailty: The FRADEA study. J Nutr Health Aging. 2017;21(2):207–214. doi: 10.1007/s12603-016-0727-9 [DOI] [PubMed] [Google Scholar]
- 7. Fried LP, Tangen CM, Walston J, et al. . Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 8. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 10. Rockwood K, Song X, MacKnight C, et al. . A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aucoin SD, Hao M, Sohi R, et al. . Accuracy and feasibility of clinically applied frailty instruments before surgery: a systematic review and meta-analysis. Anesthesiology. 2020;133(1):78–95. doi: 10.1097/ALN.0000000000003257 [DOI] [PubMed] [Google Scholar]
- 12. Shrier W, Dewar C, Parrella P, Hunt D, Hodgson LE. Agreement and predictive value of the Rockwood Clinical Frailty Scale at emergency department triage. Emerg Med J. 2020. doi: 10.1136/emermed-2019-208633 [DOI] [PubMed] [Google Scholar]
- 13. Surkan M, Rajabali N, Bagshaw SM, Wang X, Rolfson D. Interrater reliability of the Clinical Frailty Scale by geriatrician and intensivist in patients admitted to the intensive care unit. Can Geriatr J. 2020;23(3):235–241. doi: 10.5770/cgj.23.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pugh RJ, Battle CE, Thorpe C, et al. . Reliability of frailty assessment in the critically ill: a multicentre prospective observational study. Anaesthesia. 2019;74(6):758–764. doi: 10.1111/anae.14596 [DOI] [PubMed] [Google Scholar]
- 15. Clegg A, Bates C, Young J, et al. . Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. doi: 10.1093/ageing/afw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol Ser A Biol Sci Med Sci. 2018;73(7):980–987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pajewski NM, Lenoir K, Wells BJ, Williamson JD, Callahan KE. Frailty screening using the electronic health record within a Medicare accountable care organization. J Gerontol Ser A Biol Sci Med Sci. 2019;74(11):1771–1777. doi: 10.1093/gerona/glz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McIsaac DI, Wong CA, Huang A, Moloo H, van Walraven C. Derivation and validation of a generalizable preoperative frailty index using population-based health administrative data. Ann Surg. 2019;270(1):102–108. doi: 10.1097/SLA.0000000000002769 [DOI] [PubMed] [Google Scholar]
- 19. Callahan KE, Clark CJ, Edwards AF, et al. . Automated frailty screening at-scale for pre-operative risk stratification using the electronic frailty index. J Am Geriatr Soc. 2021;69(5):1357–1362. doi: 10.1111/jgs.17027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tew YY, Chan JH, Keeling P, et al. . Predicting readmission and death after hospital discharge: a comparison of conventional frailty measurement with an electronic health record-based score. Age Ageing. 2021;50(5):1641–1648. doi: 10.1093/ageing/afab043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Darvall JN, Greentree K, Loth J, et al. . Development of a frailty index from routine hospital data in perioperative and critical care. J Am Geriatr Soc. 2020;68(12):2831–2838. doi: 10.1111/jgs.16788 [DOI] [PubMed] [Google Scholar]
- 22. Gilbert T, Neuburger J, Kraindler J, et al. . Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775–1782. doi: 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gilbert T, Cordier Q, Polazzi S, et al. . External validation of the Hospital Frailty Risk Score in France. Age Ageing. 2022;51(1):afab126. doi: 10.1093/ageing/afab126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harvey LA, Toson B, Norris C, Harris IA, Gandy RC, Close JJCT. Does identifying frailty from ICD-10 coded data on hospital admission improve prediction of adverse outcomes in older surgical patients? A population-based study. Age Ageing. 2021;50(3):802–808. doi: 10.1093/ageing/afaa214 [DOI] [PubMed] [Google Scholar]
- 25. Nghiem S, Afoakwah C, Scuffham P, Byrnes J. Hospital frailty risk score and adverse health outcomes: evidence from longitudinal record linkage cardiac data. Age Ageing. 2021;50(5):1778–1784. doi: 10.1093/ageing/afab073 [DOI] [PubMed] [Google Scholar]
- 26. Nord M, Lyth J, Alwin J, Marcusson J. Costs and effects of comprehensive geriatric assessment in primary care for older adults with high risk for hospitalisation. BMC Geriatr. 2021;21(1):263. doi: 10.1186/s12877-021-02166-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 28. Mak JKL, Eriksdotter M, Annetorp M, et al. . Using an electronic frailty index to predict adverse outcomes in geriatric COVID-19 patients: data from the Stockholm GeroCovid study. medRxiv 2021. doi: 10.1101/2021.12.03.21267254 [DOI] [Google Scholar]
- 29. Theou O, van der Valk AM, Godin J, et al. . Exploring clinically meaningful changes for the frailty index in a longitudinal cohort of hospitalized older patients. J Gerontol Ser A Biol Sci Med Sci. 2020;75(10):1928–1934. doi: 10.1093/gerona/glaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol Ser A Biol Sci Med Sci. 2007;62(7):738–743. doi: 10.1093/gerona/62.7.738 [DOI] [PubMed] [Google Scholar]
- 31. Aliberti MJR, Szlejf C, Avelino-Silva VI, et al. . COVID-19 is not over and age is not enough: using frailty for prognostication in hospitalized patients. J Am Geriatr Soc. 2021;69(5):1116–1127. doi: 10.1111/jgs.17146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ludvigsson JF, Appelros P, Askling J, et al. . Adaptation of the Charlson Comorbidity Index for register-based research in Sweden. Clin Epidemiol. 2021;13:21–41. doi: 10.2147/CLEP.S282475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim DH. Measuring frailty in health care databases for clinical care and research. Ann Geriatr Med Res. 2020;24(2):62–74. doi: 10.4235/agmr.20.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. The World Bank. Life Expectancy at Birth, Total (Years)—Sweden. 2019. https://data.worldbank.org/indicator/SP.DYN.TO65.FE.ZS?locations=SE
- 35. Brundle C, Heaven A, Brown L, et al. . Convergent validity of the electronic frailty index. Age Ageing. 2019;48(1):152–156. doi: 10.1093/ageing/afy162 [DOI] [PubMed] [Google Scholar]
- 36. Hollinghurst J, Housley G, Watkins A, Clegg A, Gilbert T, Conroy SP. A comparison of two national frailty scoring systems. Age Ageing. 2021;50(4):1208–1214. doi: 10.1093/ageing/afaa252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Howlett SE, Rutenberg AD, Rockwood K. The degree of frailty as a translational measure of health in aging. Nat Aging. 2021;1(8):651–665. doi: 10.1038/s43587-021-00099-3 [DOI] [PubMed] [Google Scholar]
- 38. Singh I, Gallacher J, Davis K, Johansen A, Eeles E, Hubbard RE. Predictors of adverse outcomes on an acute geriatric rehabilitation ward. Age Ageing. 2012;41(2):242–246. doi: 10.1093/ageing/afr179 [DOI] [PubMed] [Google Scholar]
- 39. Gordon EH, Peel NM, Hubbard RE. The male–female health-survival paradox in hospitalised older adults. Maturitas. 2018;107:13–18. doi: 10.1016/j.maturitas.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 40. Howlett SE, Rockwood MRH, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12(1):171. doi: 10.1186/s12916-014-0171-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blodgett JM, Theou O, Howlett SE, Wu FCW, Rockwood K. A frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomes. Age Ageing. 2016;45(4):463–468. doi: 10.1093/ageing/afw054 [DOI] [PubMed] [Google Scholar]
- 42. Hägg S, Jylhävä J. Sex differences in biological aging with a focus on human studies. Elife. 2021;10:e63425. doi: 10.7554/eLife.63425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hao Q, Zhou L, Dong B, Yang M, Dong B, Weil Y. The role of frailty in predicting mortality and readmission in older adults in acute care wards: a prospective study. Sci Rep. 2019;9(1):1207. doi: 10.1038/s41598-018-38072-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Theou O, O’Connell MDL, King-Kallimanis BL, O’Halloran AM, Rockwood K, Kenny RA. Measuring frailty using self-report and test-based health measures. Age Ageing. 2015;44(3):471–477. doi: 10.1093/ageing/afv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hubbard RE, Peel NM, Samanta M, Gray LC, Mitnitski A, Rockwood K. Frailty status at admission to hospital predicts multiple adverse outcomes. Age Ageing. 2017;46(5):801–806. doi: 10.1093/ageing/afx081 [DOI] [PubMed] [Google Scholar]
- 46. Wou F, Gladman JRF, Bradshaw L, Franklin M, Edmans J, Conroy SP. The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing. 2013;42(6):776–781. doi: 10.1093/ageing/aft055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kansagara D, Englander H, Salanitro A, et al. . Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698. doi: 10.1001/jama.2011.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Navathe AS, Zhong F, Lei VJ, et al. . Hospital readmission and social risk factors identified from physician notes. Health Serv Res. 2018;53(2):1110–1136. doi: 10.1111/1475-6773.12670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. The Swedish National Board of Health and Welfare. Minskat antal patienter på sjukhus under pandemin. https://www.socialstyrelsen.se/om-socialstyrelsen/pressrum/press/minskat-antal-patienter-pa-sjukhus-under-pandemin/. Published September 22, 2021.
- 50. Grundberg Å, Ebbeskog B, Abrandt Dahlgren M, Religa D. How community-dwelling seniors with multimorbidity conceive the concept of mental health and factors that may influence it: a phenomenographic study. Int J Qual Stud Health Well-Being. 2012;7(1):19716. doi: 10.3402/qhw.v7i0.19716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.