Highlights
-
•
Vaccine-hesitant women’s concerns included safety in childbearing and breastfeeding.
-
•
Public health communications must be tailored to address women’s specific concerns.
-
•
Provide robust short- and long-term safety data to address vaccine hesitancy.
-
•
Data linkage infrastructure should track post-vaccination outcomes.
-
•
Vaccine mandates lowered trust in Government and in healthcare providers.
Keywords: COVID-19, Vaccine hesitancy, Women, Pregnancy, Breastfeeding
Abstract
Objective
Women of childbearing age, including pregnant and breastfeeding women, report higher COVID-19 vaccine hesitancy, but reasons for this hesitancy are unknown. We explored factors influencing vaccine decision-making among women of childbearing age in Victoria, Australia to inform strategies to increase COVID-19 vaccine uptake.
Methods
Twenty-four women aged 18–40 years were interviewed July-October 2021. Interview data were analyzed thematically using an inductive, constructivist approach.
Results
Of 24 participants, 14 (57%) were vaccine-hesitant, of whom 10/14 pregnant or breastfeeding. Six key themes were identified: weighing up perceived risks for self and baby; availability of information; change and contradictions; vaccination above everything; practical issues – hurdles of inconvenience. Vaccine-hesitant women’s concerns included safety in pregnancy, breastfeeding and fertility effects. Some participants expressed a loss of trust in healthcare providers following vaccine mandates.
Conclusions
Public health campaigns and communication should be tailored to address specific concerns to increase COVID-19 vaccine uptake and prevent negative COVID-19 outcomes for women of childbearing age. Findings suggest that effective strategies to address hesitancy in this group may include providing robust short- and long-term safety data across fertility, birth outcomes and child development following COVID-19 vaccination. Other supportive strategies may include systemic changes like making childcare available at vaccination points (where practical), and using data linkage infrastructure to track post-vaccination outcomes.
1. Introduction
The long-term solution to the COVID-19 pandemic includes an equitable and safe vaccination program with high vaccine uptake [1]. After Melbourne (Victoria, Australia) experienced among the world’s longest lockdowns in 2020–21 [2], Australia’s COVID-19 vaccine roll-out program commenced in February 2021. Those initially prioritised for vaccination included frontline healthcare workers, border/quarantine workers, adults aged over 70 years and adults with specific underlying health conditions. Most women of childbearing became eligible for vaccination from 25th August 2021 when COVID-19 vaccines become available to all adults aged over 16 years [3].
To increase vaccine uptake, the Victorian Government made vaccination a mandatory requirement for healthcare, essential workers and educational workers [4] and a condition for entry to most public venues [5]. By 3rd December 2021, 90% of the eligible Victorian population (aged ≥12 years) was fully vaccinated, with 93% having received one dose [6]. However, a number of groups have lower vaccine coverage. In particular, women of childbearing age have been found to have higher COVID-19 vaccine hesitancy – defined as being unsure or not intending to be vaccinated - compared to the Australian population overall. As of 19th November 2021, 7.3% of women aged ≥18 years were unsure or not intending to be vaccinated, compared with 5.5% of men [7]. A survey of 326 women in the first half of 2021 (87% of whom were aged 18–40 years) indicated 52% were unsure or not intending to accept a COVID-19 vaccine when it became available to them and over half were definitely/possibly planning pregnancy in the next two years [8]. Among pregnant women, double-dose COVID-19 vaccine coverage was estimated by Victorian maternity care providers as being 30–70% among their patients in mid-November 2021, with 61% first dose coverage at Victoria’s largest maternity service, Monash Health [9].
Pregnant women are at higher risk of severe outcomes from COVID-19 disease, with a 5-times increased risk of hospitalisation and 2–3-times increased risk of requiring invasive ventilation and intensive care unit (ICU) admission compared with non-pregnant women of similar age [10], [11], [12]. The risk of complications for the newborn is also increased, with double the risk of premature delivery and 3-times the risk of requiring admission to the special care nursery [10], [11], [12]. Pregnant women were not included in initial COVID-19 vaccine clinical trials, delaying vaccine safety data. Consequently COVID-19 vaccines were not initially recommended during pregnancy [12]. Following a report of robust observational vaccine safety data during pregnancy from the United States [13], in June 2021 the Australian Technical Advisory Group on Immunisation (ATAGI) and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) recommended the Comirnaty (Pfizer Australia Pty Ltd; ‘Pfizer’) COVID-19 vaccine be routinely offered in pregnancy [11].
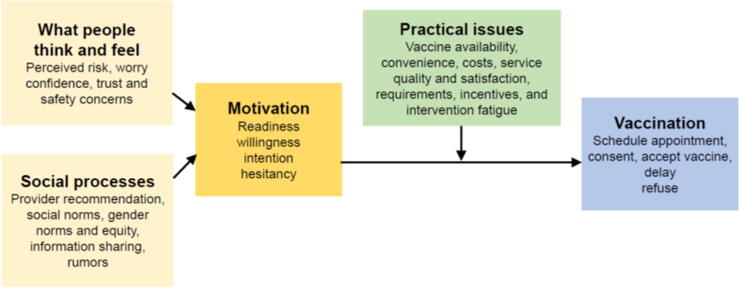
To increase vaccine coverage, it is important to elucidate the reasons why vaccine coverage is low or stagnating. The World Health Organization Behavioural and Social Drivers of Vaccine Uptake framework (BeSD) categorises beliefs and experiences specific to vaccination that are potentially modifiable. Each of the four framework domains driving vaccine uptake and are listed in Fig. 1 [14].
Fig. 1.
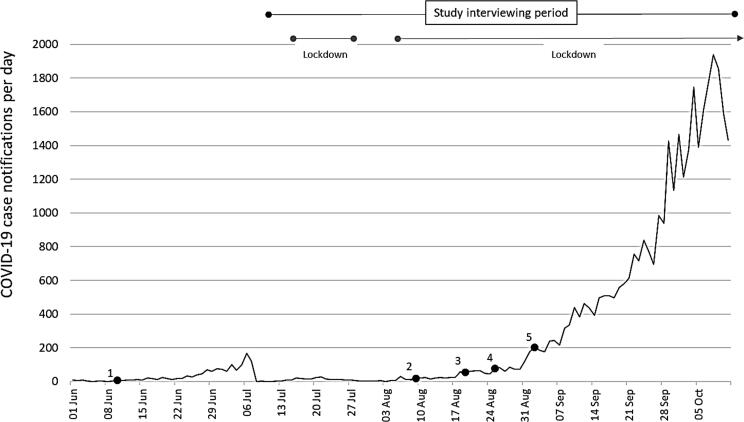
COVID-19 case notifications per day, Victoria, Australia, 1 June to 11 October 2021 [21].
1. 9th June: recommendation that Pfizer is routinely offered in pregnancy. 2. 9th August: Moderna provisionally approved for people aged ≥18 years. 3. 19th August: recommendation Moderna be routinely offered in pregnancy. 4. 25th August: COVID-19 vaccines available to all adults. 5. 3rd September: Moderna provisionally approved for people aged ≥12 years. N.B. COVID-19 vaccines became mandated for workers in some industries, with a requirement that aged care workers receive their first dose 1st October, construction workers by 2nd October, authorised workers by 22nd October, educational workers by 25th October, and healthcare workers by 29th of October 2021. Other essential workers were required to receive their first dose by 22 October 2021.
Despite growing evidence of COVID-19 vaccine safety and the effectiveness of mRNA vaccines for pregnant and non-pregnant women, vaccine hesitancy persists among women of childbearing age. Although prior studies have quantified rates of hesitancy [8], obtaining qualitative data will support understanding the concerns and questions that women of childbearing age have. This knowledge will help inform tailored communications to build trust and confidence in COVID-19 vaccines to increase vaccine uptake. To better understand the factors driving this hesitancy, the aim of this study was to describe the key factors influencing COVID-19 vaccine decision-making among women of childbearing age in Victoria, Australia.
2. Methods
2.1. Study design
We conducted a descriptive qualitative study using one-on-one interviews with women aged 18–40 years to explore their COVID-19 vaccine intentions, information needs, and the attitudinal and behavioural drivers of vaccine uptake. Interviews were undertaken from 8th July to 11th October 2021. We utilised a constructivist approach recognising the pivotal role of experience in the construction of the individuals’ knowledge [15]. This study is presented using the Consolidated Criteria for Reporting Qualitative Research (COREQ) [16].
2.2. Study context
Public health restrictions in Victoria changed frequently throughout 2020 and 2021, with stay-at-home orders, bans on non-essential travel and gatherings, and mandated face masks [17]. Shortly before the study interviewing period, new evidence confirmed an association between the Vaxzevria (AstraZeneca Pty Ltd; ‘AstraZeneca’) COVID-19 vaccine and a rare blood clotting condition, thrombosis with thrombocytopenia (TTS). Consequently, ATAGI recommended Pfizer as the preferred vaccine for all adults aged under 50 years on 8th April 2021 [18]. The recommendation that Pfizer be routinely offered at any stage in pregnancy was made on 9th June 2021 [11]. The Therapeutic Goods Administration (TGA) provisionally approved Spikevax (Moderna Australia Pty Ltd; ‘Moderna’) COVID-19 vaccine for use in Australia on 9th August 2021 for those aged 18 years and older, and for those aged 12 years and older on 3rd September 2021. The recommendation that Moderna be routinely offered in pregnancy (as well as Pfizer) was announced on 19th August 2021 [19]. During the study interviewing period (8th July to 11th October 2021), daily COVID-19 case notifications in Victoria increased, with state-wide transmission becoming widespread [20]. COVID-19 vaccines became available to all adults on 25th August 2021 [17], then were mandated for authorised workers, educational and healthcare workers, with a requirement that their first vaccine dose be received by 22nd, 25th and 29th of October 2021 respectively. Pregnant and breastfeeding women in these roles were not exempted from these mandates [5]. Daily COVID-19 case numbers and changes in vaccine recommendations are described in Fig. 2.
Fig. 2.
World Health Orgnaisation Behavoural and Social Drivers of Vaccine Uptake Framework [14].
2.3. Participant eligibility and recruitment
Women aged 18–40 years living in Victoria were eligible to participate. We aimed to recruit approximately 20 participants; with a mix of pregnant and non-pregnant participants, including both accepting and hesitant views about COVID-19 vaccination. We recruited participants from a number of sources, including the existing Optimise Study cohort (a longitudinal survey-based study investigating how Victorians are experiencing COVID-19 and responding to the measures introduced to stop transmission [22]). Potential participants had provided Optimise Study staff permission to contact them about sub-studies. All eligible Optimise Study participants who indicated they were pregnant were invited to a study interview. In addition, a sample of eligible Optimise Study participants across a range of COVID-19 hesitant and accepting viewpoints as indicated by their response to an Optimise Study survey question were invited to be interviewed. The question asked ‘If a COVID-19 vaccine was to become available to everyone in Australia, do you think you would have it yourself?’ Possible responses were ‘definitely yes’, ‘probably yes’, ‘I am not sure yet’, ‘probably not’ or ‘definitely not’. To supplement the few pregnant/breastfeeding women in the Optimise Study, we also utilised a Facebook advertisement targeting pregnant/breastfeeding women who were unsure or not intending to accept a COVID-19 vaccine. People were invited to leave an expression of interest via an inbuilt link.
A researcher (JO, PhD public health – female, experienced in qualitative interviewing) contacted potential participants to discuss the study. Those interested received a participant information statement and provided informed consent electronically via REDCap. We aimed to achieve thematic saturation, despite the diversity of participants and the range of issues discussed.
2.4. Data collection
The semi-structured interview guide covered a range of topics relating to the COVID-19 pandemic and vaccines (see Supplementary File 1). In brief, topics addressed included the participants’ understanding of their COVID-19 vaccine eligibility, their decision whether to accept COVID-19 vaccines, their views on COVID-19 vaccines during pregnancy/breastfeeding and COVID-19 vaccines for children, their perceptions of the vaccine attitudes of other people in their lives, and their information needs and preferences. Questions were tailored based on participant circumstances (e.g., if pregnant or breastfeeding). A researcher (JO) conducted interviews via Zoom or phone. Minor adaptations were made to the guide for all subsequent interviews during the interviewing period. For the first two interviews, a second researcher (JK) observed and provided input. Interviews were audio-recorded with participant consent, transcribed verbatim and de-identified prior to analysis. Participants were able to review their transcript on request (three did so) and all were offered a digital $30 gift card.
Ethics: Ethics approval was received from the Alfred Hospital Ethics Committee (333/20).
2.5. Analysis
The interviewer performed an initial descriptive thematic analysis using an inductive approach. After reviewing the transcripts, the interviewer categorised data into codes and sub-codes, then grouped codes thematically using NVivo (v12). Three researchers (JO, JK, KB) used a virtual whiteboard (miro.com) to identify common and unique themes and sub-themes, then refined and named themes. Final themes and sub-themes were organised deductively within the BeSD (Fig. 1). Verbatim quotes are provided to support thematic analysis. Some quotes were modified slightly to assist readability.
‘Vaccine-hesitant’ was defined as the participant indicating during the interview that they were unsure or not intending to accept a COVID-19 vaccine when it became available to them, including those delaying vaccination.
‘Vaccine-accepting’ participants indicated during the interview that they intended to get a COVID-19 vaccine when it became available to them.
3. Results
3.1. Description of participants
Twenty-four participants were interviewed. The mean interview duration was 39 min (range: 24 to 66 min). The majority of interviews (N = 22; 92%) occurred on Zoom with the camera on; one participant chose to keep their camera off and one was interviewed over the phone.
Fourteen participants (58%) were vaccine-hesitant and 10 (42%) vaccine-accepting. Seven (29%) had received at least one vaccine dose or were booked to receive this. The majority of participants held university degrees (N = 19; 79%), spoke English at home (N = 23; 96%), resided in metropolitan Melbourne (N = 17; 71%) and had a median annual household income of $100,000 AUD or more (N = 14; 58%). Six participants were pregnant and six were breastfeeding (Table 1).
Table 1.
Characteristics of participants by their stated COVID-19 vaccine intention.
| COVID-19 vaccine-hesitant (N=14) | COVID-19 vaccine-accepting (N=10) | Total (N=24) | |
|---|---|---|---|
| Number (%) | Number (%) | Number (%) | |
| Current reproductive status | |||
| Neither pregnant or breastfeeding | 4 (28) | 8 (80) | 12 (50) |
| Pregnant | 5 (36) | 1 (10) | 6 (25) |
| Breastfeeding | 5 (36) | 1 (10) | 6 (25) |
| Language spoken at home | |||
| Other | 0 (0) | 1 (10) | 1 (4) |
| English | 14 (100) | 9 (90) | 23 (96) |
| Country of birth | |||
| Australia | 11 (79) | 8 (80) | 19 (79) |
| Other (main language not English) | 0 (0) | 2 (2) | 3 (13) |
| Other (main language English) | 3 (21) | 0 (0) | 2 (8) |
| Area of Residence | |||
| Metropolitan Melbourne | 8 (57) | 9 (90) | 17 (71) |
| Regional Victoria | 6 (43) | 1 (10) | 7 (29) |
| Highest education level | |||
| University degree/s | 11 (79) | 8 (80) | 19 (79) |
| TAFE / Trade Certificate | 2 (14) | 1 (10) | 3 (13) |
| High School Certificate or less | 1 (7) | 1 (10) | 2 (8) |
| Received new financial support, Centrelink, rent or mortgage support since 1st March 2020? | |||
| Yes | 4 (29) | 4 (40) | 8 (33) |
| No/Don’t know | 10 (71) | 6 (60) | 16 (66) |
| Chronic health condition that impacts on daily life | |||
| Yes | |||
| No | 2 (14) | 2 (20) | 4 (17) |
| 12 (86) | 8 (80) | 20 (83) | |
| Annual pre-tax household income level (AUD) | |||
| <$50k | |||
| $50-99k | 1 (7) | 0 (0) | 1 (4) |
| $100-149k | 3 (21) | 2 (20) | 5 (21) |
| $149-199k | 3 (21) | 2 (20) | 5 (21) |
| ≥200k | 5 (36) | 2 (20) | 7 (29) |
| Don’t know/decline to answer | 1 (7) | 1 (10) | 2 (8) |
| 1 (7) | 3 (30) | 4 (17) | |
| Total | 14 | 10 | 24 |
3.2. Key themes
Key themes are discussed in detail as follows.
3.2.1. Weighing up perceived risks of COVID-19 vaccination
All participants described weighing up the perceived risks and benefits of vaccination. No-one said they would never get a COVID-19 vaccine, but many had reservations about getting it in the foreseeable future. Pregnant and breastfeeding participants expressed more concern about perceived risk of vaccination to their baby than to themselves. Vaccine-accepting pregnant and breastfeeding participants spoke about wanting to protect their baby from COVID-19. Many vaccine-hesitant pregnant and breastfeeding participants said the vaccine would probably be fine for them, but felt there was a possibility of harm to the baby. In these cases, declining or delaying vaccination was preferred. For example:
“It [COVID-19 vaccine] may not, it probably won’t [harm my baby] and that’s fantastic, but it just feels like an unnecessary risk….” Participant-012, Vaccine-hesitant, pregnant
Vaccine-hesitant participants had safety concerns, especially around the possible long-term effects, including an impact on fertility, as illustrated here:
“Should we be harvesting our eggs before we have the vaccine? Should we be thinking about our fertility a lot more before we have this vaccine?” Participant-016, Vaccine-hesitant, not pregnant or breastfeeding.
Vaccine-hesitant and accepting participants discussed the possibility of severe adverse reactions and feeling unwell after vaccination with some trepidation, but did not usually indicate these as reasons to decline vaccination. For example:
“It’ll [COVID-19 vaccination] get done. …hoping I don’t feel too crappy over the weekend because it’s our wedding anniversary.” Participant-005, Vaccine-accepting, breastfeeding.
There was a perception that as a baby (including an unborn baby) or a child grows older, they become more robust and able to cope with any adverse effects from vaccination. This belief led to participants wanting to delay vaccination until later in pregnancy or after the baby is born, or once breastfeeding and/or all future pregnancies are complete. An example is given by a pregnant participant who was delaying vaccination until the third trimester:
“It’s the third trimester, the baby is not growing any vital organs… So, I sort of feel a bit more comfortable [getting a COVID-19 vaccine] now.” Participant-003, Vaccine-hesitant, pregnant.
Participants acknowledged the increased risk of severe COVID-19 in pregnancy, but hesitant participants (including those who were pregnant and breastfeeding) dismissed this risk, claiming their risk of infection was low as they mostly stayed at home, or because there was little transmission in their locality, or that if they did get COVID-19 they would probably recover without complications.
Hesitant participants felt that vaccination would not prevent COVID-19 transmission, it would only reduce the severity of disease – and they believed they were unlikely to get severely unwell anyway. These participants described vaccination as an unnecessary risk with no realistic benefit for them.
All hesitant participants asserted they were not ‘antivaxxers’ and many spoke derisively about antivaccine misinformation (and sometimes about people they knew who they considered ‘antivaxxers’). Despite this, they saw COVID-19 vaccines as different from the usual recommended vaccines due to the perceived rapid development of COVID-19 vaccines. To illustrate:
“All other vaccinations I am completely in agreement with: I get my children vaccinated, I am vaccinated. I just feel like this one is a bit scary… new is scary.” Participant-011, Vaccine-hesitant, breastfeeding.
3.2.2. The information I want isn’t there
Almost all participants had searched online for information about COVID-19 vaccines. Healthcare providers, government and scientific sources were preferred information sources, even by participants who described having little trust in government. Participants expressed distrust in the mainstream media and described it as being sensationalised. Some hesitant participants felt the media covered up unspecified information. Information from many online sources was widely described as unreliable and biased as this participant described:
“It’s hard to go by the media. They blow everything out of proportion or they cover up a lot of things. …the less you know the better when it comes to the news.” Participant-015, Vaccine-hesitant, not pregnant or breastfeeding.
Many participants felt that the information they wanted was not known to anyone as yet (as opposed to not accessible to them), especially around the long term effects of COVID-19 vaccination for themselves and their infants. Hesitant participants frequently said they perceived doctors and scientists were doing the best they could with the information they had, but this was not enough for participants to feel reassured. Participants wanted robust scientific research on long-term effects, especially in relation to child development, cancer rates, fertility and birth outcomes among vaccinated women. Many hesitant participants spoke of wanting to wait and see what happens to others before getting vaccinated themselves. Hesitant pregnant or breastfeeding women wanted to be reassured by safe outcomes for babies of mothers who had been vaccinated when pregnant, as discussed here:
“I looked at… a study involving over 800 women… only 28 of them had had their baby. I was like, see, it’s still not enough time…. I want to see the development of these children.” Participant-021 Vaccine-hesitant, breastfeeding.
3.2.3. Change and contradictions
Changing COVID-19 information (including vaccine information) was widely discussed by participants, often with concern and frustration by hesitant participants – especially those who did not feel empowered to understand scientific language. Some vaccine-accepting participants felt reassured by the joint ATAGI/RANZCOG recommendation that mRNA vaccines be routinely offered in pregnancy [11], while some hesitant participants regarded this recommendation with suspicion. Some hesitant participants expressed concern that information may change again and show that COVID-19 vaccines are unsafe for them or their baby. Given this possibility, these participants said it was better not to vaccinate yet. This participant asked:
“We [pregnant women] were being told ‘that’ and now we’re being told ‘this’. What if we get told something in a few months’ time that contradicts the action we’ve taken [getting vaccinated]? ….I’m going to wait and find out what it is next.” Participant-012 Vaccine-hesitant, pregnant.
Some participants described confusion and/or concern after being advised by their healthcare providers not to vaccinate earlier in their pregnancy, and now having COVID-19 vaccines recommended for them.
3.2.4. All alone in the crowd
Vaccine-accepting participants often described feeling encouraged to get the vaccine by observing the early phases of the vaccination program in Australia and more advanced COVID-19 vaccine programs overseas proceed with no widespread adverse events. Hesitant pregnant and breastfeeding participants described feeling reassured by others around them becoming vaccinated and being fine, or only experiencing minor side effects. However, these participants also felt as though their own situation was not comparable, usually due to their plans to have a child(ren), as mentioned here:
“I would like to have kids at some point… I was like,’[COVID-19 vaccines] are fine for you, Grandma… there’s nothing to create out of your body’.” Participant-018 Vaccine-hesitant, not pregnant or breastfeeding.
Even when describing other pregnant or breastfeeding woman who had been vaccinated without adverse effects, hesitant pregnant or breastfeeding participants would often dwell on differences between those women’s mindset and their own. Participants generally expressed confidence in their ability to make their own vaccine decisions, but many said talking with others helped them decide. Some participants who were pregnant, breastfeeding or intending to have children in the future reported others they were close to saying that if they were in a similar situation, they would not have had the vaccine.
Several hesitant participants described their views being similar to their partner’s, who did not want them to get the vaccine and often did not want it himself. Some hesitant participants mentioned that talking through their thoughts in the study interview itself gave them more confidence in their views. Participants often discussed a divide in their social or family groups according to whether people were vaccine-accepting or hesitant. A desire to avoid conflict by not discussing their opinions with people who they know would disagree was commonly expressed. Vaccine-hesitant participants often described vaccine-accepting friends responding to them with anger and no longer wanting to be open about their views. To illustrate:
“I haven’t wanted to really voice that I don’t want to get it, maybe because people get very touchy…. I’m just not interested in having to like defend myself to my friends.” Participant-018, Vaccine-hesitant, not pregnant or breastfeeding.
Opinion was split around whether to trust healthcare providers’ vaccine recommendations. Some saw their healthcare provider as a role model. Three hesitant pregnant or breastfeeding participants mentioned their healthcare provider saying they were reluctant to be vaccinated themselves. One vaccine-accepting pregnant participant described initially being hesitant, but changing her mind after talking with her pregnant, vaccinated general practitioner. Some hesitant participants described being influenced by the views of a vaccine-hesitant healthcare provider who was a friend/family member and a perceived expert.
3.2.5. Vaccine mandates above everything
Hesitant participants expressed concerns that the Government’s desire to address COVID-19 through vaccination was being put above everything else – even their baby’s safety. Hesitant participants, including some breastfeeding women, said that they would reluctantly become vaccinated, and some pregnant participants said they would reluctantly accept vaccination once the baby was further along in its development, in order to work, socialise and travel to see family. A desire to delay vaccination was noted, especially among women with fertility concerns who were planning pregnancy. Breastfeeding participants discussed putting off vaccination until maternity leave ended and their workplace vaccine mandates could no longer be avoided. Choice was an important issue as illustrated here:
“I feel like with what restrictions they [the Government] are placing on people that aren’t getting vaccinated, that they might as well be breaking into your homes and holding you down while they vaccinate you. …no matter what it is, having your choice taken away sucks.” Participant-011, Vaccine-hesitant, breastfeeding.
Some described perceived benefits to their own mental health from having fewer restrictions on their day-to-day activities if fully vaccinated as outweighing perceived risks of vaccination. Many participants spoke about vaccine mandates with great upset and anger, expressing fear of stigmatisation and exclusion from ‘normal’ activities and/or their workplace. A loss of trust in Government and COVID-19 vaccines was discussed, for example:
There’s a lot of bitterness towards how the government’s mandated… I absolutely detest the government for it. …I’d be more inclined to trust it [COVID-19 vaccines] if it wasn’t mandated”. Participant-014 Vaccine-hesitant, breastfeeding.
The loss of trust in Government carried through to a loss of trust in healthcare providers, with some hesitant pregnant and breastfeeding participants saying they did not feel their healthcare providers could say what they really thought due to the vaccine being mandated. Some pregnant and breastfeeding participants linked a perceived sudden change in the vaccination advice during pregnancy with underlying Government motives to promote vaccine uptake as illustrated below:
“I feel like because the government is so insistent… people in the medical profession are being told… they have to encourage it [COVID-19 vaccine uptake].” Participant-011, Vaccine-hesitant, breastfeeding.
3.2.6. Practical issues – Hurdles of inconvenience
Some participants thought that accessing vaccination would be straightforward. The only practical barrier which a participant described as preventing vaccination was difficulty booking an appointment at a time she was not required at work. Some hesitant participants dismissed practical barriers completely while others described being inconvenienced by the process of getting vaccinated, as this participant discussed:
“[As] I don’t have any family support, it makes it a bit tricky taking these two wild children to a vaccine hub; it’s going to be challenging.” Participant-004, Vaccine-hesitant, breastfeeding.
Perceived practical barriers (e.g., long wait times at clinics, online booking systems being overwhelmed, and requiring childcare to attend vaccination appointments) were described as inconveniences rather than insurmountable obstacles to vaccine decisions. How participants spoke about perceived practical barriers often depended on how strongly they intended to get vaccinated. Participants who strongly intended to get the vaccine expressed confidence around overcoming practical barriers. Motivated participants (even those who were reluctant to accept the vaccine) provided pragmatic descriptions of barriers and proposed solutions, indicating that they had been thinking through the practicalities and planning ahead. Participants who were less motivated to become vaccinated tended to discuss perceived barriers fairly vaguely, based on what they had seen in the media or heard from others.
4. Discussion
Higher rates of vaccine hesitancy among women of childbearing age are well-documented in Australia and internationally.[8] This is the first Australian study to explore the main factors influencing decision-making for COVID-19 vaccines by focusing on the perspectives of pregnant and breastfeeding women. Vaccine hesitant women were chiefly concerned about vaccine safety, especially potential unrecognised long-term effects of COVID-19 vaccination for themselves and their children. While eager not to be seen as ‘antivaxxers’ who opposed all vaccines, the newness of COVID-19 vaccines underpinned the safety concerns of vaccine hesitant participants. Concerns about vaccine safety continue to be the most commonly cited concerns regarding COVID-19 vaccination in Australia and abroad [23], [24], [25].
A two dose primary series for mRNA COVID-19 vaccines is recommended for pregnant women, which can safely be received in pregnancy [12]. Safety concerns with mRNA COVID-19 vaccines have not been identified during pregnancy, including among a cohort of 35,691 persons who received a COVID-19 vaccine while pregnant or becoming pregnant after vaccination [13]. Long term safety data is still lacking as birth and childhood outcomes following COVID-19 vaccination have not been extensively studied and follow-up beyond 12-months is limited [13]. Continuing to obtain robust safety data from women vaccinated during pregnancy and their babies will inform the use of future vaccines in pregnancy using mRNA technology. To achieve this, the Australian Immunisation Register should be amended to capture pregnancy or breastfeeding status at the time of vaccination, as has been highlighted previously [26]. This modification would support vaccine safety and effectiveness data being linked to health outcomes for mothers, and health and developmental outcomes for babies, to strengthen current safety data and address many questions held by expectant parents. Current large prospective cohort studies should link birth outcomes to maternal vaccination status, as planned by the ‘Observational Maternal COVID-19 Vaccination Study’ [27] and Gen V Maternal Vaccine safety study (Danchin M, unpublished data; follow up of 20,000 mother infant pairs recently funded by the Victorian State Government, Australia).
Practical barriers to maternal vaccination, including concerns around organizing childcare to attend vaccination appointments, were discussed by participants. Inability to access childcare to attend primary healthcare appointments has widely been reported as a barrier to health service uptake [28], [29], [30], including uptake of recommended vaccines [31]. Community outreach campaigns are a recommended public health strategy to increase uptake [32] and enable vaccination to be more accessible for busy parents in conjunction with maximizing their access to primary healthcare clinics.
COVID-19 vaccine mandates have been used around the world to increase vaccine coverage, including for pregnant women as part of essential worker mandates (in healthcare, education, general workplaces etc.). Mandates have been criticized by some as impinging on human rights, promoting stigma and social polarization, and adversely affecting health and well-being [33]. Some studies report a range of unintended negative consequences, including loss of trust in Government, public health authorities and scientific regulatory bodies [33], [34], [35], [36], [37]. Mandates have also been shown in some studies to reduce compliance with other public health measures, including mask wearing and uptake of other, non-COVID-19 vaccines [33], [38], [39]. This loss of trust was echoed in our study, where some participants reported that the vaccine mandates damaged their trust in healthcare providers as they felt the healthcare provider had to encourage COVID-19 vaccination due to the mandate. This could potentially result in pregnant women disengaging from their healthcare providers, being more susceptible to misinformation and having reduced antenatal care leading to greater complications in pregnancy. Furthermore, reducing trust in Government may lead to increases in societal polarization, political instability and extremism [40]. To our knowledge, pregnant women have never before been subjected to vaccine mandates in Australia. COVID-19 vaccine mandates have been the subject of large public protests in Australia [41]. Despite good vaccine efficacy and safety data, the ethics of mandating vaccination continues to be debated, with a legal challenge mounted in the Australian Supreme Court claiming mandated vaccination contravenes the Victorian human rights charter by coercing people into medical treatment without “full, free and informed consent” [42]. Together this highlights the need for transparent, targeted public health communications and ongoing collection of robust vaccine safety data in pregnancy (including from large prospective cohort studies and pregnant women being included in vaccine clinical trials) to provide reassurance, and subsequently promote voluntary vaccine uptake [33].
Separate to COVID-19 vaccines, maternal vaccination is an essential public health intervention which improves maternal and neonatal health outcomes, yet coverage for other maternal vaccines remains low [43]. In Victoria in 2015–2017, maternal vaccine coverage for the pertussis vaccines was 64% and 39% for influenza vaccines [44]. At the time of our study, an estimated 30–70% of pregnant women in Victoria had received two doses of COVID-19 vaccines; 24–64% below the average national Australian COVID-19 vaccine coverage [9]. A number of maternal vaccines are in development and are likely to be recommended in pregnancy, including vaccines for respiratory syncytial virus and Group B streptococcus [45]. Furthermore, future pandemics necessitate rapid vaccine development. Effective communication by healthcare providers with women who are pregnant or breastfeeding to support them to make informed decisions when these vaccines are approved will be critical to their confidence and subsequent uptake [8], [46], [47]. The importance of a trusted healthcare provider’s recommendation in driving maternal vaccine uptake has been noted in a number of studies [48], [49], including Australian research which showed that pregnant women were more likely to decline a COVID-19 vaccine if their healthcare provider had not discussed this with them [50]. It is therefore critical that healthcare providers are equipped to respond to the specific concerns of pregnant and breastfeeding women regarding COVID-19 vaccines to promote vaccine uptake for this group.
The main limitation of our study is that participants were not representative of the Victorian population, with most being highly educated members of the white/Anglo-Saxon culture and earning well above the median household income. Given these demographics, our participants are likely to have better access to resources, including healthcare and information, than many in Australia. Although our sample size may be perceived as small, we achieved thematic saturation during our analyses, so no further interviews were warranted. A strength of this study is that participants were selected to ensure the views of vaccine hesitant women were represented and numerous COVID-19 misconceptions were voiced. Future research is required to consider if different concerns underpin hesitancy of women with different demographics to our sample.
Conclusion
This is the first in-depth study outlining vaccine hesitancy among Australian women of child-bearing age, including pregnant and breastfeeding women. Our results can be used to tailor public health communications to reach COVID-19 vaccine-hesitant women within these high-risk groups to improve vaccine confidence and uptake. Vaccination campaigns should provide robust short- and long-term safety data across fertility and birth outcomes and child development following COVID-19 vaccination in pregnancy. Family-friendly vaccination environments, with increased childcare access, and strategies to build trust with healthcare providers will help to optimize COVID-19 vaccine uptake and improve health outcomes for pregnant women and their infants.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2022.100240.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organisations. COVID-19 Vaccine Delivery Partnership Geneva, Switzerland2022 [updated 20 Jul. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/covid-19-vaccine-delivery-partnership.
- 2.Jose R. Melbourne readies to exit world's longest COVID-19 lockdown. Reuters. Oct 2021;2021:21. [Google Scholar]
- 3.Office of the Premier Victorian Government. Vaccine Eligibility Expanding For Anyone 16 And Over Melbourne, Australia2021 [updated 24 Aug 2021. Available from: https://www.premier.vic.gov.au/vaccine-eligibility-expanding-anyone-16-and-over.
- 4.The Premier of Victoria, The Honourable Dan Andrews, Victorian Government. Vaccination Required To Protect Workers And Victoria Melbourne, Australia2021 [updated 1 Oct 2021; cited 2021 6 Dec]. Available from: https://www.premier.vic.gov.au/vaccination-required-protect-workers-and-victoria.
- 5.Victorian Government. How we live: Information for Victorians Melbourne, Australia2021 [updated 1 Dec. Available from: https://www.coronavirus.vic.gov.au/how-we-live.
- 6.The Age. COVID-19 vaccine tracker Melbourne, Australia: Fairfax Media; 2021 [updated 3 Dec. Available from: https://www.theage.com.au/national/covid-19-global-vaccine-tracker-and-data-centre-20210128-p56xht.html.
- 7.The University of Melbourne – Melbourne Institute: Applied Economic & Social Research. Vaccine Hesitancy Tracker Melbourne, Australia2021 [updated 21 Nov 2021. Available from: https://melbourneinstitute.unimelb.edu.au/publications/research-insights/ttpn/vaccination-report.
- 8.Bradfield Z., Wynter K., Hauck Y., Sweet L., Wilson A.N., Szabo R., et al. COVID-19 vaccination perceptions and intentions of maternity care consumers and providers in Australia. PLoS One. 2021;16(11) doi: 10.1371/journal.pone.0260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll C., Dow A. Vaccination for pregnant women lags national average. The Sydney Morning Herald. Nov 2021;2021:14. [Google Scholar]
- 10.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Australian Government Department of Health. Joint statement between RANZCOG and ATAGI about COVID-19 vaccination for pregnant women Canberra, Australia2021 [updated 9 Jun. Available from: https://www.health.gov.au/news/joint-statement-between-ranzcog-and-atagi-about-covid-19-vaccination-for-pregnant-women.
- 12.Australian Government Department of Health. COVID-19 vaccination decision guide for people who are pregnant, breastfeeding or planning pregnancy. Version 5.1 Canberra, Australia2021 [updated 19 Aug 2021. Available from: https://www.health.gov.au/sites/default/files/documents/2021/08/covid-19-vaccination-shared-decision-making-guide-for-women-who-are-pregnant-breastfeeding-or-planning-pregnancy-covid-19-vaccination-shared-decision-making-guide-for-women-who-are-pregnant-breastfeeding-or-planning-pregna_0.pdf.
- 13.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., et al. Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons. N Engl J Med. 2021;384(24):2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The BeSD expert working group Based on: Brewer NT CG, Rothman AJ, Leask J, and Kempe A, Increasing vaccination: Putting psychological science into action. Psychological Science for the Public Interest. 2017;18(3):149–207. doi: 10.1177/1529100618760521. [DOI] [PubMed] [Google Scholar]
- 15.Braun V, Clarke V. Chapter 4. Thematic Analysis. Cooper H, editor. Washington, United States of America: American Psychological Association; 2012.
- 16.Tong A., Sainsbury P., Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 17.Parliament of Australia. COVID-19: a chronology of state and territory government announcements (up until 30 June 2020) Canberra, Australia2021 [updated 22 Oct 2021. Available from: https://www.aph.gov.au/About_Parliament/Parliamentary_Departments/Parliamentary_Library/pubs/rp/rp2021/Chronologies/COVID-19StateTerritoryGovernmentAnnouncements#_Toc52275800.
- 18.Australian Government Department of Health Therapeutic Goods Association. AstraZeneca ChAdOx1-S COVID-19 vaccine Canberra, Australia2021 [updated 9 Apr. Available from: https://www.tga.gov.au/media-release/astrazeneca-chadox1-s-covid-19-vaccine.
- 19.Australian Technical Advisory Group on Immunisation. Clinical guidance on use of COVID-19 vaccine in Australia in 2021 (v7.0) Australia: health.gov.au; 2021 [updated 17 Aug 2021. Available from: https://www.health.gov.au/sites/default/files/documents/2021/08/covid-19-vaccination-atagi-clinical-guidance-on-covid-19-vaccine-in-australia-in-2021.pdf.
- 20.State Government of Victoria. Victorian COVID-19 data Melbourne, Australia2021 [updated 10 Nov 2021. Available from: https://www.coronavirus.vic.gov.au/victorian-coronavirus-covid-19-data.
- 21.Australian Government Department of Health. Coronavirus (COVID-19) case numbers and statistics Melbourne, Australia2021 [updated 20 Dec. Available from: https://www.health.gov.au/news/health-alerts/novel-coronavirus-2019-ncov-health-alert/coronavirus-covid-19-case-numbers-and-statistics.
- 22.Burnet Institute. The Optimise Study: Optimising Isolation, Quarantine and Distancing for COVID-19 Melbourne, Australia2020 [Available from: https://www.burnet.edu.au/programs/28_behaviours_and_health_risks/projects/459_the_optimise_study_optimising_isolation_quarantine_and_distancing_for_covid_19.
- 23.Kaufman J., Bagot K.L., Tuckerman J., Biezen R., Oliver J., Jos C., et al. Qualitative exploration of intentions, concerns and information needs of vaccine-hesitant adults initially prioritised to receive COVID-19 vaccines in Australia. Aust N Z J Public Health. 2022;46(1):16–24. doi: 10.1111/1753-6405.13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enticott J., Gill J.S., Bacon S.L., Lavoie K.L., Epstein D.S., Dawadi S., et al. Attitudes towards vaccines and intention to vaccinate against COVID-19: a cross-sectional analysis—implications for public health communications in Australia. BMJ Open. 2022;12(1) doi: 10.1136/bmjopen-2021-057127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parental attitudes, beliefs, behaviours and concerns towards childhood vaccinations in Australia: A national online survey. Australian Journal for General Practitioners. 2017;46:145-51. [PubMed]
- 26.Tuckerman J, Blyth CC, Beard FH, Danchin MH. COVID‐19 and changes in the National Immunisation Program: a unique opportunity to optimise the Australian Immunisation Register (AIR). Med J Aust. 2021;214(6):247-9. e1. [DOI] [PMC free article] [PubMed]
- 27.Swamy GK, Weaver KE. Observational Maternal COVID-19 Vaccination Study Durham, United States of America: clinicaltrials.gov; 2021 [updated 23 Nov 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT04826640.
- 28.Gaur P., Kuo M., Kho K.A. Demonstrating Lack of Child Care as a Barrier to Health Care for Women in Parkland Health & Hospital System [04H] Obstet Gynecol. 2020;135:82S. [Google Scholar]
- 29.Zuckerman KE, Perrin JM, Hobrecker K, Donelan K. Barriers to Specialty Care and Specialty Referral Completion in the Community Health Center Setting. The Journal of Pediatrics. 2013;162(2):409-14.e1. [DOI] [PMC free article] [PubMed]
- 30.Judd R.T., Friedman E.E., Schmitt J., Ridgway J.P. Association between patient-reported barriers and HIV clinic appointment attendance: A prospective cohort study. AIDS Care. 2022;34(5):545–553. doi: 10.1080/09540121.2021.1906401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell S., Clarke R., Paterson P., Mounier-Jack S. Parents' and guardians' views and experiences of accessing routine childhood vaccinations during the coronavirus (COVID-19) pandemic: A mixed methods study in England. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0244049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgess R.A., Osborne R.H., Yongabi K.A., Greenhalgh T., Gurdasani D., Kang G., et al. The COVID-19 vaccines rush: participatory community engagement matters more than ever. Lancet. 2021;397(10268):8–10. doi: 10.1016/S0140-6736(20)32642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bardosh K., de Figueiredo A., Gur-Arie R., Jamrozik E., Doidge J., Lemmens T., et al. The unintended consequences of COVID-19 vaccine policy: why mandates, passports and restrictions may cause more harm than good. BMJ Glob. Health. 2022;7(5) doi: 10.1136/bmjgh-2022-008684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuckerman J., Kaufman J., Danchin M. Effective Approaches to Combat Vaccine Hesitancy. Pediatr Infect Dis J. 2022;41(5):e243–e245. doi: 10.1097/INF.0000000000003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vergara R.J.D., Sarmiento P.J.D., Lagman J.D.N. Building public trust: a response to COVID-19 vaccine hesitancy predicament. J Public Health (Oxf) 2021;43(2):e291–e292. doi: 10.1093/pubmed/fdaa282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drew L. Did COVID vaccine mandates work? What the data say. Nature. 2022;607(22-26). [DOI] [PubMed]
- 37.Smith S.E., Sivertsen N., Lines L., De Bellis A. Weighing up the risks—Vaccine decision-making in pregnancy and parenting. Women Birth. 2022 doi: 10.1016/j.wombi.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Sprengholz P., Betsch C., Bohm R. Reactance revisited: Consequences of mandatory and scarce vaccination in the case of COVID-19. Appl Psychol Health Well Being. 2021;13(4):986–995. doi: 10.1111/aphw.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sprengholz P., Felgendreff L., Bohm R., Betsch C. Vaccination policy reactance: Predictors, consequences, and countermeasures. J Health Psychol. 2022;27(6):1394–1407. doi: 10.1177/13591053211044535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casal Bértoa F., Rama J. Polarization: What Do We Know and What Can We Do About It? Frontiers. Pol Sci. 2021;3(56) [Google Scholar]
- 41.Wu D. ‘Freedom’ protesters plan to demonstrate ‘every day’ against COVID-19 vaccination mandates and the Victoria pandemic bill. skynewscomau. 2021;(Dec 4) [Google Scholar]
- 42.Fox K.S. Vaccine mandates contravene Victoria’s human rights charter, lawyers argue. The Age. Nov 2021;2021:3. [Google Scholar]
- 43.Giles M.L., Khai K., Krishnaswamy S., Bellamy K., Angliss M., Smith C., et al. An evaluation of strategies to achieve greater than 90% coverage of maternal influenza and pertussis vaccines including an economic evaluation. BMC Pregnancy Childbirth. 2021;21(1):771. doi: 10.1186/s12884-021-04248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowe S.L., Perrett K.P., Morey R., Stephens N., Cowie B.C., Nolan T.M., et al. Influenza and pertussis vaccination of women during pregnancy in Victoria, 2015–2017. Med J Aust. 2019;210(10):454–462. doi: 10.5694/mja2.50125. [DOI] [PubMed] [Google Scholar]
- 45.Madhi S.A., Polack F.P., Piedra P.A., Munoz F.M., Trenholme A.A., Simoes E.A.F., et al. Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. N Engl J Med. 2020;383(5):426–439. doi: 10.1056/NEJMoa1908380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufman J, Bagot KL, Tuckerman J, Biezen R, Oliver J, Jos C, et al. Qualitative exploration of intentions, concerns and information needs of vaccine-hesitant adults initially prioritised to receive COVID-19 vaccines in Australia. Aust N Z J Public Health. [DOI] [PMC free article] [PubMed]
- 47.Vergallo G.M., Del Rio A., Negro F., Zaami S. COVID-19 vaccine mandates: What are the current European public perspectives. Eur Rev Med Pharmacol Sci. 2022;26:643–652. doi: 10.26355/eurrev_202201_27891. [DOI] [PubMed] [Google Scholar]
- 48.McRaea JE, Deya A, Carlsona S, Sinnd J, McIntyrea P, Bearda F, et al. Influenza vaccination among pregnant women in two hospitals in Sydney, NSW: what we can learn from women who decline vaccination. Public Health Research & Practice. 2022;32(2):31232111-. [DOI] [PubMed]
- 49.Cavaliere A.F., Zaami S., Pallottini M., Perelli F., Vidiri A., Marinelli E., et al. Flu and Tdap Maternal Immunization Hesitancy in Times of COVID-19: An Italian Survey on Multiethnic Sample. Vaccines (Basel) 2021;9(10) doi: 10.3390/vaccines9101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward C., Megaw L., White S., Bradfield Z. COVID-19 vaccination rates in an antenatal population: A survey of women's perceptions, factors influencing vaccine uptake and potential contributors to vaccine hesitancy. Aust N Z J Obstet Gynaecol. 2022 doi: 10.1111/ajo.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.