Abstract
Background
People with a spinal cord injury (SCI) have a high rate of bowel-related morbidity, even compared with people with other neurological disorders. These complications lower quality of life and place a financial burden on the health system. A noninvasive intervention that improves the bowel function of people with an SCI should reduce morbidity, improve quality of life, and lead to cost savings for health care providers.
Objectives
To investigate the effectiveness of noninvasive abdominal functional electrical stimulation (FES) for improving bowel function in people with a chronic SCI.
Methods
A prospective, double-blinded, 1:1 randomized, placebo-controlled intervention trial will be conducted with 80 adults with chronic SCI (>12 months since injury) above T8 single neurological level. The intervention will be a 45-minute abdominal FES (or placebo) session, 3 days per week, for 6 weeks.
Main Study Parameters/Endpoints
Primary endpoint is whole gut transit time before and after 6 weeks of abdominal FES. Secondary endpoints measured before and after 6 weeks of abdominal FES are (1) colonic transit time; (2) quality of life (EQ-5D-5L); (3) participant-reported bowel function (International SCI Bowel Function Basic Data Set Questionnaire and visual analogue scale); (4) respiratory function (forced vital capacity, forced expiratory volume in 1 second, peak expiratory flow, maximal inspiratory pressure, and maximal expiratory pressure); (5) bladder symptoms (Neurogenic Bladder Symptom Score); (6) daily bowel management diary; and (7) unplanned hospital visits.
Conclusion
Safety data will be collected, and a cost utility analysis using quality of life scores will be performed.
Trial registration
Australian New Zealand Clinical Trials Registry (ANZCTR): ACTRN12621000386831.
Keywords: abdominal functional electrical stimulation, bowel function, respiratory function, spinal cord injury, whole gut transit time
Introduction
Background and rationale
Colorectal, anal, and pelvic floor problems are common in people with spinal cord injury (SCI),1,2 with few having normal anorectal sensation, anal sphincter control, and bowel motility.3 The SCI-related alterations in autonomic, sensory, and motor function lead to high rates of bowel-related morbidity, even compared to those with other neurological disorders.4,5 Complications including abdominal pain, constipation, fecal incontinence, and bloating can lower the quality of life of people with an SCI and place a financial burden on the health system.6 As a result, the improvement of bowel function is considered a high priority for people with an SCI.7
Bowel management strategies for people with SCI are traditionally comprised of manual and pharmacological interventions, such as dietary supplements, digital rectal stimulation, enemas, and suppositories.5 These solutions are often only partially effective for people with SCI, thus research has turned to novel uses of electrical stimulation for bowel management. When used with an additional method of bowel management to aid evacuation, implanted electrical stimulation of anterior sacral nerve roots can reduce constipation for people with SCI.8 Additionally, DiMarco et al.9 implanted a spinal cord stimulation system that activated abdominal muscles to restore cough, and this improved bowel management time when also applied during a bowel routine for five people with an SCI. The authors attributed the significant reduction in bowel routine time to the changes in intraabdominal pressure created by the activation of stimulated abdominal musculature.9 However, implantable systems are costly and carry with them the potential risk of complications tied to invasive surgical procedures, such as infection but also autonomic dysreflexia in some people with SCI.9 Alternatively, noninvasive, surface (transcutaneous) functional electrical stimulation (FES) of the abdominal muscles can increase intraabdominal pressure10,11 and has the potential to improve bowel management in people with SCI.9,12 Currently, there is a lack of data from randomized controlled trials,9,12 and there is no standard surface abdominal FES paradigm to improve bowel function.
Objectives
The aim of this study is to investigate the effects of noninvasive surface abdominal FES on bowel function in people with an SCI as measured by whole gut transit time (WGTT). Based on abdominal FES paradigms used in previous studies10,13 and anecdotal evidence, we hypothesize that participants receiving the active intervention of abdominal FES will have improved bowel function and, in turn, improved quality of life as compared to participants who receive the placebo intervention.
Methods
This is a prospective, randomized, placebo-controlled double-blind (participant and assessor) clinical trial. This report of the details of the protocol is structured according to the SPIRIT guidelines.14 The study will be conducted at participant’s homes or at a clinical research institute in Australia. Eligibility criteria for the study are listed in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
|
|
| Spinal cord injury of at least 12 months duration |
| Neurological injury above T843 |
| ≥18 years of age |
| Able to eat and drink normally |
| Able to breathe independently |
|
|
| Exclusion criteria |
|
|
| AIS score D or E 42 |
| A history of other bowel conditions such as irritable bowel syndrome, gastroesophageal reflux, organic bowel obstruction |
| Presence of physical obstacles that prevent abdominal FES (e.g., pregnancy, abdominal trauma, cardiac pacemaker, or other implanted electromedical devices) |
| Lack of response to abdominal FES, (e.g., lower motor neuron impairment) [Assessed by the inability to elicit a muscular contraction via surface abdominal FES when tested by a member of the research team during trial screening procedures] |
| Additional contraindications for use of the SmartPill such as a history of gastric bezoars, suspected or known strictures, fistulas, or physiological/mechanical gastrointestinal obstruction, a history of gastrointestinal surgery within the past 3 months, Crohn’s disease, or diverticulitis |
| Severely obese participants (>40 BMI) |
| A lack of understanding of English |
| A scheduled MRI scan within 14 weeks of start of the study |
| A previous history of recurrent episodes of autonomic dysreflexia |
Note: AIS = American Spinal Injury Associaion Impairment Scale; BMI = body mass index; FES = functional electrical stimulation.
Interventions
The intervention in this study is a 45-minute session of abdominal FES, delivered three times per week for 6 weeks. The intervention device used is the VentFree™ (Liberate Medical, KY) electrical muscle stimulator. VentFree is an electrotherapy device that monitors the user’s voluntary breathing activity using an airflow sensor. This device sends a trigger signal for the application of surface FES to the abdominal muscles over two stimulation channels during the expiratory phase of breathing when the abdominal muscles are active. VentFree is designed to prevent or retard disuse atrophy of the abdominal muscles. The setup consists of a Conformite Europeenne (CE) marked commercially available stimulation and trigger system developed by Liberate Medical (VentFree), a 7-in. nasal cannula (ProFlow, Phillips, Netherlands), and surface stimulation electrodes (Liberate Medical, KY).
Previous studies of the effects of abdominal FES on respiratory muscle function10,13,15 and bowel function16 have performed the abdominal FES for up to an hour for 5 days per week; in this study, the intervention is delivered three times per week for 45 minutes. This frequency and duration of treatment is based on results from studies of strength training in people with an SCI that show an improvement in voluntary strength when training three times per week17,18 and is also based on the standard recommendations for exercises intended to increased muscle strength.19
Active abdominal FES
The active group will receive abdominal FES for a 45-minute session, 3 days per week, for 6 weeks. Electrodes will be placed posterolaterally on the torso20 to activate the transversus abdominis and internal and external oblique muscles. A stimulation frequency of 30 Hz and a pulse width of 350 μs will be used for all participants. For the active group, electrical current will be adjusted until a strong visible symmetrical abdominal muscle contraction is observed (typically below 100 mA).10 The current will be adjusted throughout each session to account for fatigue. We have used similar training protocols in people with tetraplegia.15,21,22
Placebo abdominal FES
Participants allocated to the placebo group will receive sub-motor threshold abdominal FES. Stimulation will be applied for a 45-minute session, 3 days per week, for 6 weeks. Although the setup will be the same as the active group, current will be limited to 10 mA. This is below the threshold for activating the abdominal motor axons, thus preventing any muscle contraction.
Outcomes
Primary outcome
The primary outcome is the group difference in the postintervention WGTT controlling for preintervention WGTT. WGTT will be measured before and after the intervention using the SmartPill motility system.23–25 The SmartPill is a small (26.8 × 11.7 mm) ingestible capsule containing sensors that continuously measure pressure, pH, and temperature as it passes through the gastrointestinal tract. Based on the large changes in pH in the gastrointestinal tract, the SmartPill can quantify the transit times for gastric emptying and movement through the small bowel and the colon. The sum of these transit times gives the WGTT, the time from ingestion of the SmartPill until its exit from the body.23 A concomitant estimate of WGTT will be made with a 125 g tin of corn kernels ingested immediately after the SmartPill to independently assess the reliability of the WGTT measured using the SmartPill. The corn will also provide a guide for the participant for when to expect expulsion of the SmartPill, if the corn is observed in the feces.
WGTT and colonic transit time (CTT) will be measured using the SmartPill before and after 6 weeks of abdominal FES. On Study Assessment Visits 1 and 3 (see Figure 1), participants will fast for 4 hours before ingesting a standardized meal (cereal bar, Smartbar) and tin of corn and swallowing the SmartPill under supervision of the investigators. Corn will also be ingested at Study Assessment Visit 4. Data recordings will be sent from the SmartPill to a receiver kept near the participant from the time of ingestion until the SmartPill is expelled. Participants will make note of whether or not they detect corn in their feces in their daily Bowel Diary (see later section). The SmartPill has been shown to be a safe, noninvasive diagnostic tool for use in people with SCI.26 The within-subject variability of measuring WGTT and CTT have been assessed in healthy subjects.27 The coefficients of variation for WGTT measured in 10 subjects tested 2 weeks apart and 9 subjects tested 4 weeks apart were 26.4% and 35.1%, respectively. The corresponding results for CTT measured 2 and 4 weeks apart were 31.8% and 42.4%, respectively.27 Measurements of WGTT and CTT can be performed in the participant’s home.26
Figure 1.
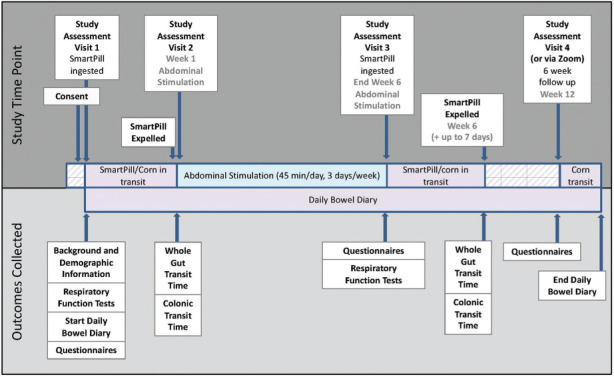
Timeline of study assessments and outcome measurements.
Secondary Outcomes
Colonic transit time
CTT will be measured in conjunction with WGTT (see earlier sections). The CTT is defined as the time between the ileocecal junction (indicated by an abrupt decrease in pH after an abrupt rise in pH) and the exit of the SmartPill from the body, indicated by an abrupt decrease in the measured temperature and/or a loss of signal from the SmartPill that occurs at the same time as a bowel movement.23,26
Quality of life
Quality of life will be measured using the EuroQol-five dimension-five level (EQ-5D-5L) questionnaire.28 This questionnaire will be administered before and after 6 weeks of abdominal FES on Study Assessment Visits 1 and 3 and 6 weeks after the abdominal FES on Study Assessment Visit 4 (see Figure 1). This survey allows estimation of utilities for cost-utility analysis, has been validated in people who have suffered a stroke,29 and is a preferred quality of life questionnaire for SCI research.30
Participant-reported bowel function
Participant-reported bowel function will be measured using the International SCI Bowel Function Basic Data Set Questionnaire31 (including a neurogenic bowel dysfunction score validated in people with an SCI32) and a simple visual analogue scale (VAS). The questionnaire and VAS assessment will be administered on the same days as the EQ-5D-5L.
Bowel management strategy/Bowel Diary
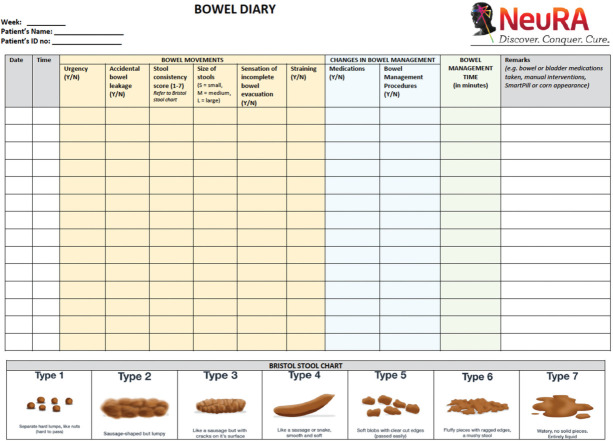
Bowel management strategy will be measured via analysis of daily diary entries regarding bowel movements (time taken and frequency), Bristol Stool Form Scale,33,34 use of laxatives, and manual procedures. The Bowel Diary will be completed daily starting on Study Assessment Visit 1 and will conclude when the corn has been expelled after Study Assessment Visit 4 (Appendix A).
Respiratory function
Respiratory function will be assessed using tests of lung function before and after the 6 weeks of abdominal FES on Study Assessment Visits 1 and 3 (see Figure 1). Measurements of forced vital capacity (FVC), forced expiratory volume in one second (FEV1), peak expiratory flow (PEF), maximum inspiratory pressure (MIP), and maximal expiratory pressure (MEP) will be obtained using the Micro I Spirometer (CareFusion via Stark Medical Australia, Sydney, NSW, Australia) and the Micro RPM Mouth Pressure Meter (Micro Medical via Stark Medical Australia, Sydney, NSW, Australia). Data will be recorded with the participant seated upright and breathing through a mouthpiece. In accordance with American Thoracic Society and European Respiratory Society standards, each measurement will be repeated until three acceptable results within 10% (or within 0.150 L for FEV1 and FVC) are registered, and the greatest value will be used for analysis.35,36 These validated measures form part of the International Spinal Cord Society core pulmonary function data set.37
Bladder symptoms
Bladder symptoms will be assessed using the Neurogenic Bladder Symptom Score (NBSS)38,39 before and after the 6 weeks of abdominal FES and at a follow-up visit 6 weeks after the abdominal FES (Study Assessment Visits 1, 3, and 4). The NBSS is a reliable measure of bladder symptoms validated in a large cohort of people with an SCI.38
Unplanned hospital visits
Any unplanned hospital visits during the study will be recorded in the daily Bowel Diary.
Other data
Background and demographic information
On Study Assessment Visit 1, participant demographic profile and medical history will be recorded by the assessor using the standardized International Spinal Cord Society Core Data Set Questionnaire,40,41 the gold standard data set for SCI. Information collected will include date and cause of injury, neurological level of injury, American Spinal Injury Association Impairment Scale (AIS) score,42,43 participant age and gender, history of respiratory complications (both pre and post injury, including chronic conditions such as asthma), and history of mechanical ventilation (both pre and post injury).40,41 Additionally, current medications will be recorded. Changes to medications during the study will be recorded in the Bowel Diary.
Economic analysis
The data from the quality of life surveys and estimations of resource utilization will be used to perform a cost-utility analysis to determine whether there is a financial benefit resulting from use of abdominal FES to improve bowel function from a health care provider perspective. The costs and health outcomes of abdominal FES versus current practice will be compared at the 6-week follow-up from a health care provider perspective. Health system resource use for all participants will be collected during the trial and valued using the appropriate costs (such as the National Weighted Activity Units).44 Total health care cost per participant per group will be compared (cost analysis). The economic outcomes will include hospital readmissions (cost effectiveness analysis) and quality-adjusted survival, calculated by combined length of life with quality of life weights, using the EQ-5D-5L instrument (cost-utility analysis).
Safety
The participant will be monitored during SmartPill ingestion, assessments, and the abdominal FES sessions, and their skin will be checked at the beginning, during, and at the end of each abdominal FES session. The study team member performing the intervention will also check with the participant before each abdominal FES session to ensure that no adverse events have occurred. In addition, each participant will be provided with a card with the study member contact details and emergency contact details and is advised to contact a member of the study team if they experience any adverse events, except in the case of an emergency, when they should go to their nearest emergency department. Any adverse events associated with the intervention, including adverse sensations or skin reactions, will be reported; these will be reviewed by the principal investigator and a clinician on a regular basis. All adverse events will be summarized to determine the safety of using abdominal FES to improve bowel function. An elective hospital admission will not be considered a serious adverse event (SAE). The sponsor will report all SAEs to the relevant ethics committee within 3 days of first knowledge of the event.
As abdominal FES may cause an increase in blood pressure, the participant’s blood pressure will be measured before participants begin abdominal FES sessions to ensure systolic blood pressure is below 140 mm Hg using an electronic device (Omron HEM-7322 Automatic Blood Pressure Monitor, Omron Healthcare Co., Limited Kyoto, Japan). Blood pressure will be assessed every 10 minutes during the abdominal FES session to reduce the risk of autonomic dysreflexia remaining undetected. The stimulation will be stopped if the participant experiences a sustained increase of more than 40 mm Hg in systolic pressure. The stimulation will be restarted when systolic blood pressure lowers to within 40 mm Hg of baseline. The abdominal FES will also be stopped at any time if the participant does not wish to continue with the session. The electrodes may be moved or the intensity of the stimulation adjusted if necessary.
Participant Timeline
Each participant will be involved in the study for approximately 13 weeks. Approximately 1 week is required for SmartPill clearance preintervention, the intervention will be delivered for 6 weeks, and the last follow-up occurs 6 weeks after the intervention (Figure 1). The target is to recruit all participants within 3 years.
Sample Size
Using the SmartPill, the WGTT has been recorded in people with a SCI as 3.3 ± 2.5 days, compared with 1.0 ± 0.7 days in able-bodied people.26 A 25% reduction in WGTT would require 70 participants (based on a general linear model, p = .05, α = 0.9) when using the coefficient of variation of 31.8% for repeated measures of WGTT in able-bodied participants.27 Allowing for 5% noncompliance and 5% drop out, we will require 80 participants for this study.
Recruitment
Eighty eligible participants will be recruited through an institution-based volunteer registry and community advertisements.
Assignment of Interventions
Sequence generation
The randomisation sequence is allocated using computer-generated random numbers stratified in blocks of 10.
Allocation
Participants will be randomly assigned to receive either active or placebo abdominal FES in a 1:1 ratio using a secure, web-based program (REDCap). Randomization will occur after Assessment Visit 1 (see Figure 1) but before the abdominal FES session commences. Participants will be instructed not to discuss their perception of randomized allocation group with anyone.
Blinding
The allocation procedure will ensure concealment of the allocation schedule from the participant and assessor. The study team member that applies the intervention is unblinded.
Data Collection, Management, and Analysis
Data are collected on paper case report forms. A study member is present during each intervention session to promote adherence. This study member is able to encourage the participant to continue in the study during each session. If a participant decides that they wish to discontinue the study, they will be asked to complete the study endpoint assessment but may choose not to complete any further study assessments, as per the participant information and consent form.
A secure web-based data management system (REDCap) will be established on a server at the host research institute, enabling data from participants to be collected in a central database. REDCap allows the designation of roles for data entry, verification, validation, and study management. Investigators who have not assessed outcomes will validate the data using the paper case report forms before data is finalized.
All data will be stored for 15 years after the completion of the project. Once the 15 years have elapsed, all information relating to this study will be disposed of via confidential destruction by the appropriate research staff.
Statistical methods
Participant variables will be presented using means and standard deviations for continuous, normally distributed variables, medians and interquartile ranges for continuous non-normally distributed variables, and proportions and absolute numbers for categorical variables. All adverse events will be recorded and summarized with descriptive statistics.
A generalized linear model will be used to assess the effect of abdominal FES (active vs. placebo) on the primary outcome of WGTT after the intervention, when controlling for preintervention WGTT, injury level, severity of injury (AIS), and age as covariates. Generalized linear models will also be used to assess secondary postintervention outcomes including CTT, respiratory function (as % predicted), and bowel and bladder symptoms between groups, when controlling for the respective preintervention assessment, injury level, severity of injury (AIS), and age. An exploratory analysis will be used to investigate the effect of abdominal FES on quality of life and associations between bowel management (and medications) on WGTT and CTT. Missing data will be handled with multiple imputations, generating 30 imputed datasets for analysis.
An incremental cost-effectiveness ratio (ICER) of abdominal FES will be calculated to determine the cost per reduction in health care utilization and the additional cost per QALY gained. A detailed sensitivity analysis, including nonparametric bootstrapping methods, will be undertaken to identify the areas of uncertainty.
Monitoring
All adverse advents will be reported, and these will be reviewed by the principal investigator and a study clinician on a regular basis. Therefore, there is no separate data monitoring committee for this study.
There will be random checks of the correct usage of the selection criteria and informed consent by an independent researcher not directly involved in the study. Monitoring of 15% of case report forms and all consent forms will be performed at the time the 40th participant is recruited. This monitoring will be performed by a researcher who has experience of both clinical trials and abdominal FES.
Ethics and Dissemination
Research ethics approval
This clinical trial was approved by the independent local ethics committee of the University of New South Wales (HC210106) and is registered at the Australian New Zealand Clinical Trials Registry (ANZCTR): ACTRN12621000386831.
Consent
If a person indicates that they are interested in taking part in the study, a member of the research team will provide that person with information relating to the study and, after a period of 24 hours, will ask the person whether they want to take part in the study. If the person does want to take part in the study, written, informed consent will be sought.
Confidentiality
Identifying data will be handwritten and stored in a locked data storage room with limited access and will not be transmitted. An anonymous identification number will be used to connect data in the system with individuals. Electronic data (consisting of nonidentifiable participant information) will be stored in a secure, password-protected network at the host research institution. Access to these files is limited to the research team, with the password for these files only distributed to direct members of the team.
Access to data
The nonidentifiable datasets used for the current study may be requested from the corresponding author on reasonable request, once the data is published.
Data dissemination
Data obtained from the current study will be submitted for publication to an international peer reviewed journal. Data may also be used for conference publications and as part of an undergraduate or postgraduate thesis. No participants will be identified in the dissemination of any research results. Publications will only include nonidentifiable, aggregated data. Study sites will also not be identified in publications or presentations. Participants who state in the consent form that they would like a copy of the results of this study will be provided with a copy of any journal publications.
COVID-19 considerations
The COVID-19 pandemic has led to the alteration or cessation of many research studies. Rather than postponing the study outlined here, it has been adapted since conception to maintain participant safety and sound methodology in the face of the changing landscape of the pandemic. Specifically, protocol amendments were made to enable both the intervention and assessments to be delivered at the research institute, or via Zoom, should the pandemic restrict visits to a participant’s home.
APPENDIX A
Bowel Diary
Funding Statement
Financial Support This study is supported by New South Wales Health and through three National Health and Research Council of Australia fellowships (J.B., S.G., E.M.).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Registration
Australian New Zealand Clinical Trials Registry (ANZCTR): ACTRN12621000386831.
Device Status
The VentFree™ electrical muscle stimulator, an electrotherapy device developed by Liberate Medical LLC, USA. The VentFree™ and has Conformite Europeenne (CE) mark approval.
REFERENCES
- 1.Krogh K, Mosdal C, Laurberg S. Gastrointestinal and segmental colonic transit times in patients with acute and chronic spinal cord lesions. Spinal Cord . 2000;38(10):615–621. doi: 10.1038/sj.sc.3101066. [DOI] [PubMed] [Google Scholar]
- 2.Coggrave M, Norton C, Wilson-Barnett J. Management of neurogenic bowel dysfunction in the community after spinal cord injury: A postal survey in the United Kingdom. Spinal Cord . 2009;47(4):323–330. doi: 10.1038/sc.2008.137. quiz 331–333. [DOI] [PubMed] [Google Scholar]
- 3.Vallès M, Mearin F. Pathophysiology of bowel dysfunction in patients with motor incomplete spinal cord injury: Comparison with patients with motor complete spinal cord injury. Dis Colon Rectum . 2009;52(9):1589–1597. doi: 10.1007/DCR.0b013e3181a873f3. [DOI] [PubMed] [Google Scholar]
- 4.Benevento BT, Sipski ML. Neurogenic bladder, neurogenic bowel, and sexual dysfunction in people with spinal cord injury. Phys Ther . 2002;82(6):601–612. [PubMed] [Google Scholar]
- 5.Krassioukov A, Eng JJ, Claxton G, Sakakibara BM, Shum S. Neurogenic bowel management after spinal cord injury: A systematic review of the evidence. Spinal Cord . 2010;48(10):718–733. doi: 10.1038/sc.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roach MJ, Frost FS, Creasey G. Social and personal consequences of acquired bowel dysfunction for persons with spinal cord injury. J Spinal Cord Med . 2000;23(4):263–9. doi: 10.1080/10790268.2000.11753535. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KD. Targeting recovery: Priorities of the spinal cord-injured population. J Neurotrauma . 2004;21(10):1371–83. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 8.Valles M, Rodriguez A, Borau A, Mearin F. Effect of sacral anterior root stimulator on bowel dysfunction in patients with spinal cord injury. Dis Colon Rectum . 2009;52(5):986–92. doi: 10.1007/DCR.0b013e31819ed459. [DOI] [PubMed] [Google Scholar]
- 9.DiMarco AF, Geertman RT, Tabbaa K, Nemunaitis GA, Kowalski KE. Effects of lower thoracic spinal cord stimulation on bowel management in individuals with spinal cord injury. Arch Phys Med Rehabil . 2021;102(6):1155–1164. doi: 10.1016/j.apmr.2020.09.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McBain RA, Boswell-Ruys CL, Lee BB, Gandevia SC, Butler JE. Abdominal muscle training can enhance cough after spinal cord injury. Neurorehabil Neural Repair . 2013;27(9):834–43. doi: 10.1177/1545968313496324. [DOI] [PubMed] [Google Scholar]
- 11.McBain RA, Boswell-Ruys CL, Lee BB, Gandevia SC, Butler JE. Electrical stimulation of abdominal muscles to produce cough in spinal cord injury: Effect of stimulus intensity. Neurorehabil Neural Repair . 2015;29(4):362–369. doi: 10.1177/1545968314552527. [DOI] [PubMed] [Google Scholar]
- 12.Hascakova-Bartova R, Dinant JF, Parent A, Ventura M. Neuromuscular electrical stimulation of completely paralyzed abdominal muscles in spinal cord-injured patients: A pilot study. Spinal Cord . 2008;46(6):445–50. doi: 10.1038/sj.sc.3102166. [DOI] [PubMed] [Google Scholar]
- 13.McCaughey EJ, Borotkanics RJ, Gollee H, Folz RJ, McLachlan AJ. Abdominal functional electrical stimulation to improve respiratory function after spinal cord injury: A systematic review and meta-analysis. Spinal Cord . 2016;54(9):628–39. doi: 10.1038/sc.2016.31. [DOI] [PubMed] [Google Scholar]
- 14.Chan AW, Tetzlaff JM, Gøtzsche PC et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. Br Med J . 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaughey EJ, Berry HR, McLean AN, Allan DB, Gollee H. Abdominal functional electrical stimulation to assist ventilator weaning in acute tetraplegia: A cohort study. PLoS One . 2015;10(6):e0128589. doi: 10.1371/journal.pone.0128589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin SD BJ, Boswell-Ruys CL, Hoang PD, Jarvis T, Gandevia SC, McCaughey AE. The effect of abdominal functional electrical stimulation on bowel function in multiple sclerosis: A cohort study. Multiple Sclerosis J Exp Trans Clin 2020. 2020;6(3):1–9. doi: 10.1177/2055217320941530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bye EA, Harvey LA, Gambhir A et al. Strength training for partially paralysed muscles in people with recent spinal cord injury: A within-participant randomised controlled trial. Spinal Cord . 2017;55(5):460–465. doi: 10.1038/sc.2016.162. [DOI] [PubMed] [Google Scholar]
- 18.Harvey LA, Fornusek C, Bowden JL et al. Electrical stimulation plus progressive resistance training for leg strength in spinal cord injury: A randomized controlled trial. Spinal Cord . 2010;48(7):570–575. doi: 10.1038/sc.2009.191. [DOI] [PubMed] [Google Scholar]
- 19.American College of Sports Medicine position stand Progression models in resistance training for healthy adults. Med Sci Sports Exerc . 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 20.Butler JE, Lim J, Gorman RB et al. Posterolateral surface electrical stimulation of abdominal expiratory muscles to enhance cough in spinal cord injury. Neurorehabil Neural Repair . 2011;25(2):158–167. doi: 10.1177/1545968310378509. [DOI] [PubMed] [Google Scholar]
- 21.McLachlan AJ, McLean AN, Allan DB, Gollee H. Changes in pulmonary function measures following a passive abdominal functional electrical stimulation training program. J Spinal Cord Med . 2013;36(2):97–103. doi: 10.1179/2045772312y.0000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BB, Boswell-Ruys C, Butler JE, Gandevia SC. Surface functional electrical stimulation of the abdominal muscles to enhance cough and assist tracheostomy decannulation after high-level spinal cord injury. J Spinal Cord Med . 2008;31(1):78–82. doi: 10.1080/10790268.2008.11753985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YY, Erdogan A, Rao SS. How to assess regional and whole gut transit time with wireless motility capsule. J Neurogastroenterol Motil . 2014;20(2):265–270. doi: 10.5056/jnm.2014.20.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YY, Erdogan A, Rao SS. How to perform and assess colonic manometry and barostat study in chronic constipation. J Neurogastroenterol Motil . 2014;20(4):547–552. doi: 10.5056/jnm14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saad RJ, Hasler WL. A technical review and clinical assessment of the wireless motility capsule. Gastroenterol Hepatol . 2011;7(12):795–804. [PMC free article] [PubMed] [Google Scholar]
- 26.Williams RE, 3rd, Bauman WA, Spungen AM et al. SmartPill technology provides safe and effective assessment of gastrointestinal function in persons with spinal cord injury. Spinal Cord . 2012;50(1):81–4. doi: 10.1038/sc.2011.92. [DOI] [PubMed] [Google Scholar]
- 27.Diaz Tartera HO, Webb DL, Al-Saffar AK et al. Validation of SmartPill(®) wireless motility capsule for gastrointestinal transit time: Intra-subject variability, software accuracy and comparison with video capsule endoscopy. Neurogastroenterol Motility . 2017;29(10):1–9. doi: 10.1111/nmo.13107. [DOI] [PubMed] [Google Scholar]
- 28.Herdman M, Gudex C, Lloyd A et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res . 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golicki D, Niewada M, Buczek J et al. Validity of EQ-5D-5L in stroke. Qual Life Res . 2015;24(4):845–50. doi: 10.1007/s11136-014-0834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitehurst DG, Noonan VK, Dvorak MF, Bryan S. A review of preference-based health-related quality of life questionnaires in spinal cord injury research. Spinal Cord . 2012;50(9):646–54. doi: 10.1038/sc.2012.46. [DOI] [PubMed] [Google Scholar]
- 31.Krogh K, Emmanuel A, Perrouin-Verbe B, Korsten MA, Mulcahey MJ, Biering-Sørensen F. International spinal cord injury bowel function basic data set (Version 2.0) Spinal Cord . 2017;55(7):692–698. doi: 10.1038/sc.2016.189. [DOI] [PubMed] [Google Scholar]
- 32.Krogh K, Christensen P, Sabroe S, Laurberg S. Neurogenic bowel dysfunction score. Spinal Cord 2006/10/01. 2006;44(10):625–631. doi: 10.1038/sj.sc.3101887. [DOI] [PubMed] [Google Scholar]
- 33.Lewis SJ, Heaton KW. Stool Form Scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997/01/01. 1997;32(9):920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 34.O’Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. Br Med J . 1990;300(6722):439–40. doi: 10.1136/bmj.300.6722.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham BL, Steenbruggen I, Miller MR et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med . 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laveneziana P, Albuquerque A, Aliverti A et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J . 2019;53(6) doi: 10.1183/13993003.01214-2018. [DOI] [PubMed] [Google Scholar]
- 37.Biering-Sorensen F, Krassioukov A, Alexander MS et al. International spinal cord injury pulmonary function basic data set. Spinal Cord . 2012;50(6):418–21. doi: 10.1038/sc.2011.183. [DOI] [PubMed] [Google Scholar]
- 38.Welk B, Lenherr S, Elliott S et al. The Neurogenic Bladder Symptom Score (NBSS): A secondary assessment of its validity, reliability among people with a spinal cord injury. Spinal Cord . 2018;56(3):259–264. doi: 10.1038/s41393-017-0028-0. [DOI] [PubMed] [Google Scholar]
- 39.Welk B, Lenherr S, Elliott S et al. The creation and validation of a short form of the Neurogenic Bladder Symptom Score. Neurourol Urodynamics . 2020;39(4):1162–1169. doi: 10.1002/nau.24336. [DOI] [PubMed] [Google Scholar]
- 40.Devivo MJ, Biering-Sorensen F, Charlifue S et al. International Spinal Cord Injury Core Data Set. Spinal Cord . 2006;44(9):535–540. doi: 10.1038/sj.sc.3101958. [DOI] [PubMed] [Google Scholar]
- 41.Biering-Sørensen F, Charlifue S, DeVivo MJ et al. Using the Spinal Cord Injury Common Data Elements. Top Spinal Cord Inj Rehabil . 2012;18(1):23–27. doi: 10.1310/sci1801-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirshblum SC, Burns SP, Biering-Sorensen F et al. International standards for neurological classification of spinal cord injury (revised 2011) J Spinal Cord Med . 2011;34(6):535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betz R, Biering-Sørensen F, Burns SP et al. The 2019 revision of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI)—What’s new? Spinal Cord . 2019;57(10):815–817. doi: 10.1038/s41393-019-0350-9. [DOI] [PubMed] [Google Scholar]
- 44. Australian Refined Diagnosis Related Groups Version 10.0. Final Report. 2019.