Abstract
Objective
The Omicron variant of the coronavirus SARS-CoV-2 (COVID-19) had milder clinical impacts than prior variants. This study aimed to describe the impact of COVID-19 on Autoimmune Rheumatic Disease (ARD) patients during the Delta and Omicron variants waves.
Methods
We used data from Clalit Health Services (CHS), the largest health service in Israel. ARD patients diagnosed with COVID-19 between July 1, 2021, to December 1, 2021, were included in the Delta group. Patients diagnosed between December 2, 2021, to March 31, 2022, were included in the Omicron group based on the predominance of COVID-19 in Israel. The study outcomes were COVID-19-related hospitalization or death.
Results
The final study cohort included 8443 actively treated ARD patients diagnosed with COVID-19. 1204 patients were positive during the predefined Delta variant period, and 7249 were positive during the predefined Omicron variant period). Compared to the Delta group, the Omicron group showed a lower rate of COVID-19-related hospitalization (3.9% vs. 1.3% for the Delta Vs. Omicron accordingly, p<0.001) and COVID-19-related death (3.2% vs. 1.1% for the Delta Vs. Omicron accordingly, p<0.001). After applying multivariable regression models, the Omicron group showed a lower risk for COVID-19-related hospitalization (Relative risk 0.4, 95% CI 0.27–0.59) and COVID-19-related mortality (RR 0.48, 95% CI 0.31–0.75).
Conclusion
ARD patients infected with the COVID-19 Omicron variant had a lower risk of developing COVID-19-related adverse outcomes compared to the Delta variant.
Keywords: Autoimmune rheumatic diseases, SARS-COV-2, Delta and Omicron Variants
Introduction
The coronavirus SARS-CoV-2 (COVID-19) caused a worldwide pandemic from the beginning of 2020. This pandemic affected patients with autoimmune rheumatic diseases (ARD) as well. ARD patients suffer from chronic illness and inflammation, while a substantial portion use immune-modulatory and immune-suppression treatments [1]. This predisposition puts ARD patients at higher risk for adverse clinical outcomes after being infected with COVID-19 [2]. Morbidity and mortality from COVID-19 are associated with various factors such as the COVID-19 variant, vaccination status, anti-viral therapy, and background comorbidities [3]. The Omicron variant of COVID-19 was revealed to be more contagious while less violent and causing milder disease than the delta variant [4]. Yet, many other factors such as social distancing, masking policies, and vaccination efficacy influenced the spread of this variant and its effect [5,6]. A recent study, conducted during the Omicron variant wave, included immunocompromised hosts defined after solid organ transplantation, receiving anti-CD-20 therapy, or after hematologic stem cell transplantation. These patients showed a relatively high hospital admission rate (around 20%) but a low mortality rate (0.9%) [7]. The association of ARD patients' background comorbidities and treatments with clinical outcomes has yet to be examined during the Omicron wave. This study aims to assess COVID-19-related hospitalization and death of infected ARD patients with the COVID-19 Omicron variant compared to the Delta variant.
Patients and methods
Study population
We extracted data from Clalit Health Services (CHS), the largest Health Provider Organization in Israel, operating mandatory health insurance, covering over half of the country's population [8]. CHS has a centralized, comprehensive computerized database of electronic healthcare records that has received continuous real-time input from the medical, laboratory, pharmaceutical, vaccination, and hospitalization operating systems, since the beginning of the 2000s. Hence, the CHS dataset represents Israel's general population in Israel and has been previously shown to have provided highly valid and reliable estimates [9]. The CHS central computerized database is subjected to a continuous validation method based on electronic Personal Health Records (PHR) gathered from CHS systems. The diagnoses are inserted into these PHR by CHS physicians. The prescription data is taken from a centralized PHR data warehouse, electronically documenting prescriptions and purchases are electronically documented. (For more details please refer to http://clalitresearch.org/about-us/our-data/_)
The study cohort included all CHS members diagnosed with autoimmune rheumatic diseases (ARD) and COVID-19 during Israel's delta and omicron variants outbreak. We included ARD patients with rheumatoid arthritis (RA), spondyloarthritis (including psoriatic arthritis and ankylosing spondylitis), systemic lupus erythematosus (SLE), and systemic sclerosis. ARD patients were identified retrospectively based on ICD-9 or ICD-10 and related codes before the study period (Supplementary Table 1). To improve our data validity, we combined ARD codes with pulled information on purchases issued for glucocorticoids, conventional synthetic, biological, and targeted synthetic disease-modifying anti-rheumatic drugs (DMARDs) during the three months before the study period (except for six months of Rituximab) based on the CHS prescription system. The Institutional Review Board of the CHS general management approved the study and exempted the requirement of collecting an informed consent form at enrollment.
Data extraction
We included all CHS ARD patients older than 18 with the diagnosis of COVID-19 from July 1, 2021 - to March 31, 2022. The diagnosis of COVID-19 infection was based on at least one of the following: a documented SARS-CoV-2 positive Polymerase Chain Reaction (PCR) test, a documented SARS-CoV-2 antigen test, or an ICD-9 or 10 code of COVID-19 infection from CHS medical diagnosis database. In addition, a subset of the cohort, including only the positive PCR and antigen COVID-19 ARD patients, was used as a sensitivity analysis to validate the findings.
Patients diagnosed with COVID-19 between July 1, 2021 - December 1, 2021, were included in the Delta group. Patients diagnosed between December 2, 2021, to March 31, 2022, were included in the Omicron group. This division was based on the predominance of the SARS-CoV-2 variants in the Israeli population [10]. Additionally, to ascertain a possible co-infection with the Alpha SARS-CoV-2 variant, we also extracted data from all CHS ARD patients and their infection status from March 1, 2020, to June 30, 2021. Patients diagnosed with both Delta and Omicron were excluded from the analysis (a total of 142 subjects, 0.9% of the study population). In addition, patients diagnosed with both Alpha and Delta variants within 90 days were also excluded from the final analysis in 244 subjects (3.0% of positive Alpha and Delta variants).
We extracted information on baseline demographic, medical and pharmaceutical data points, and vaccination status. In Israel, the Pfizer BioNTech (BNT162b2) COVID-19 vaccine was the only available for mass vaccination. The vaccination status was defined as (1) patients vaccinated only with the first two doses (later referred to as "Two doses"; (2) patients vaccinated by booster – for patients who received the third dose (later referred to as "Booster") And (3) patients who received the fourth dose available in Israel since the last week of December 2021 for ARD patients (later referred as "Fourth vaccination").
The study outcomes were COVID-19-related hospitalization or death. COVID-19-related hospitalization was defined as any hospitalization at the COVID-19 unit or during the 14 days after the diagnosis of COVID-19. Similarly, COVID-19-related death was defined as death at the COVID-19 unit or within 30 days following the COVID-19 diagnosis.
All data were extracted from CHS using the Clalit Research Data sharing platform powered by MDClone (https://www.mdclone.com). The study was approved by the Ethics Committee of Emek Medical Center (EMC 220–20) and the Clalit Health Services Research Room Committee.
Statistical analysis
Depending on the variable type, the data points are described by means and standard deviations (SD), medians and interquartile range (IQR), or number and percentage.
To estimate the association of the COVID-19 variant with study outcomes, we used a multivariable negative binomial regression model and calculated the Relative Risk (RR) and the 95% confidence interval (95% CI). Regressions were built separately for each study outcome, COVID-19 hospitalization, or death. In these models, the dependent variable's outcome was regressed over the variant type (Delta vs. Omicron) and a set of covariates (demographic, medical history, pharmaceutical, etc.). The final model was selected based on the clinical relevance of the covariates and a model discriminatory ability. We also considered the statistical significance of the coefficients as a secondary criterion in selecting the final list of covariates. The analysis of COVID-19-related hospitalization was further stratified by the type of ARD, background medical conditions, and selected ARD treatments. Due to a small number of COVID-19-related deaths during the Omicron period, a similar analysis for COVID-19-related death was not feasible.
We conducted a sensitivity analysis by matching the Delta and the Omicron patients at a 1:1 ratio (caliper 0.01, greedy approach) by age, gender, and time of ARD diagnosis, followed by modeling the study outcomes using the original regression on a matched subgroup of patients.
To validate the definition of the study population, we conducted a sensitivity analysis of COVID-19-related hospitalizations and mortality. Specifically, this analysis included only the ARD patients who purchased any immunosuppressant medication, as recorded by the CHS pharmacies within three months before enrollment in the study (within six months window was allowed only for RTX).
To evaluate factors that may be specifically associated with risk for Omicron-related hospitalization, we used a multivariable binomial regression model only during the period of the Omicron outbreak.
Data analysis was performed using SPSS version 26.0.
Results
Overall, there were 73,637 ARD patients (with and without immunomodulatory treatment purchase documentation). We extracted information of 8453 ARD patients with documented treatment purchases, of whom 1204 were diagnosed with COVID-19 during the Delta timeline definitions, and 7249 were diagnosed with COVID-19 during the Omicron timeline definitions. We did not find differences between patients' characteristics using the two datasets (The entire cohort vs. a subset of the cohort, including only the positive PCR and antigen COVID-19 ARD patients). Table 1 presents the baseline characteristics of ARD patients with documented immunomodulatory treatment, according to their COVID-19 variant. Compared to patients with the Delta variant, the patients infected with the Omicron showed a higher rate of females (74.1% vs. 69.9%, p = 0.01 and a lower rate of diabetes (22.1% vs. 25.3%, p = 0.01).Yet, the Charlson comorbidity index score was similar between the groups. The study groups were similar in their distribution of ARD types (see Table 1), malignancy rates (8.1% vs. 8.4%, p = 0.73, for Omicron Vs. Delta accordingly), and documented previous Covid-19 infections (9.7% vs. 10.0%, p = 0.79, for Omicron Vs. Delta accordingly). The Omicron group showed a lower use rate of a prednisone dose of more than 20 mg (34.4% vs. 37.5%, p = 0.04), and a higher use rate of interleukin-17 inhibitors (4.4% vs. 2.7%, p = 0.01). Rituximab and Mycophenolate mofetil did not vary between the study groups (3.6% vs. 3.5%, p = 0.93 and 3.3% vs. 3.1%, p = 0.79, accordingly). Vaccination rates of any kind (first two doses, booster, and fourth dose) were higher among the Omicron group (p<0.001 for all). Compared to the Delta group, the Omicron group showed a lower rate of COVID-19-related hospitalization (1.3% vs. 3.9% for the Omicron Vs. Delta group accordingly, p<0.001) and COVID-19-related death (1.1% vs. 3.2% for the Omicron Vs. Delta group accordingly, p<0.001). Patient characteristics for the whole ARD cohort, regardless of drug purchase documentation showed mainly similar results (see supplementary Table 2).
Table 1.
General characteristics of ARD patients with documented immunomodulatory treatment, infected with Delta and Omicron variants.
| Variable | Delta (n = 1204) | Omicron (n = 7249) | P value |
|---|---|---|---|
| Females (n,%) | 841 (69.9) | 5373 (74.1) | 0.01 |
| Age (mean ±SD) | 53.7 (16.9) | 54.0 (16.4) | 0.46 |
| Body Mass Index (mean ±SD) | 28.4 (6.2) | 27.9 (5.8) | 0.01 |
| Smoking – never | 847 (70.3) | 5014 (69.2) | 0.62 |
| Past | 214 (17.8) | 1372 (18.9) | |
| current | 143 (11.9) | 863 (11.9) | |
| Rheumatoid Arthritis (n,%) | 626 (52.0) | 3674 (50.7) | 0.40 |
| Spondyloarthritis (n,%) | 337 (28.0) | 1980 (27.3) | 0.62 |
| Systemic lupus erythematosus (n,%) | 193 (16.0) | 1266 (17.5) | 0.23 |
| Scleroderma (n,%) | 48 (4.0) | 329 (4.5) | 0.45 |
| Time since ARD diagnosis, years (median, i.q range) | 5.0 (1.0–12.0) | 6.0 (1.0–13.0) | 0.54 |
| Medical History | |||
| Hypertension (n,%) | 430 (35.7) | 2568 (35.4) | 0.84 |
| Myocardial infarction (n,%) | 42 (3.5) | 290 (4.0) | 0.42 |
| Heart failure (n,%) | 90 (7.5) | 488 (6.7) | 0.35 |
| Diabetes (n,%) | 305 (25.3) | 1602 (22.1) | 0.01 |
| Chronic obstructive pulmonary disease (n,%) | 37 (3.1) | 210 (2.9) | 0.71 |
| Cerebro-vascular accident (n,%) | 146 (12.1) | 836 (11.5) | 0.56 |
| Dementia (n,%) | 49 (4.1) | 261 (3.6) | 0.40 |
| Malignancy (n,%) | 101 (8.4) | 589 (8.1) | 0.73 |
| Chronic kidney disease (n,%) | 123 (10.2) | 710 (9.8) | 0.63 |
| Charlson comorbidity index (median, i.q range) | 3.0 (1.0–5.0) | 3.0 (1.0–5.0) | 0.39 |
| Previous COVID-19 infection | 120 (10.0) | 704 (9.7) | 0.79 |
| Medical therapy | |||
| Prednisone ≤5 mg (n,%) | 86 (7.1) | 528 (7.3) | 0.90 |
| 5–20 mg (n,%) | 436 (36.2) | 2435 (33.6) | 0.08 |
| >20 mg (n,%) | 451 (37.5) | 2494 (34.4) | 0.04 |
| Disease-modifying anti rheumatic drugs (n,%) | 683 (56.7) | 4260 (58.8) | 0.18 |
| Azathioprine (n,%) | 44 (3.7) | 247 (3.4) | 0.66 |
| Mycophenolate mofetil (n,%) | 37 (3.1) | 236 (3.3) | 0.79 |
| TNF alpha blockers (n,%) | 263 (21.8) | 1507 (20.8) | 0.40 |
| Rituximab (n,%) | 42 (3.5) | 259 (3.6) | 0.93 |
| IL-6 inhibitors | 39 (3.2) | 261 (3.6) | 0.61 |
| IL-17 inhibitors | 33 (2.7) | 320 (4.4) | 0.01 |
| JAK inhibitors | 64 (5.3) | 367 (5.1) | 0.72 |
| Two doses (n,%) | 910 (75.6) | 6031 (83.2) | <0.001 |
| Booster (n,%) | 354 (29.4) | 5307 (73.2) | <0.001 |
| Fourth vaccination (n,%) | 44 (3.7) | 1022 (14.1) | <0.001 |
| Covid-19 related hospitalization | 47 (3.9) | 96 (1.3) | <0.001 |
| Covid-19 related mortality | 38 (3.2) | 77 (1.1) | <0.001 |
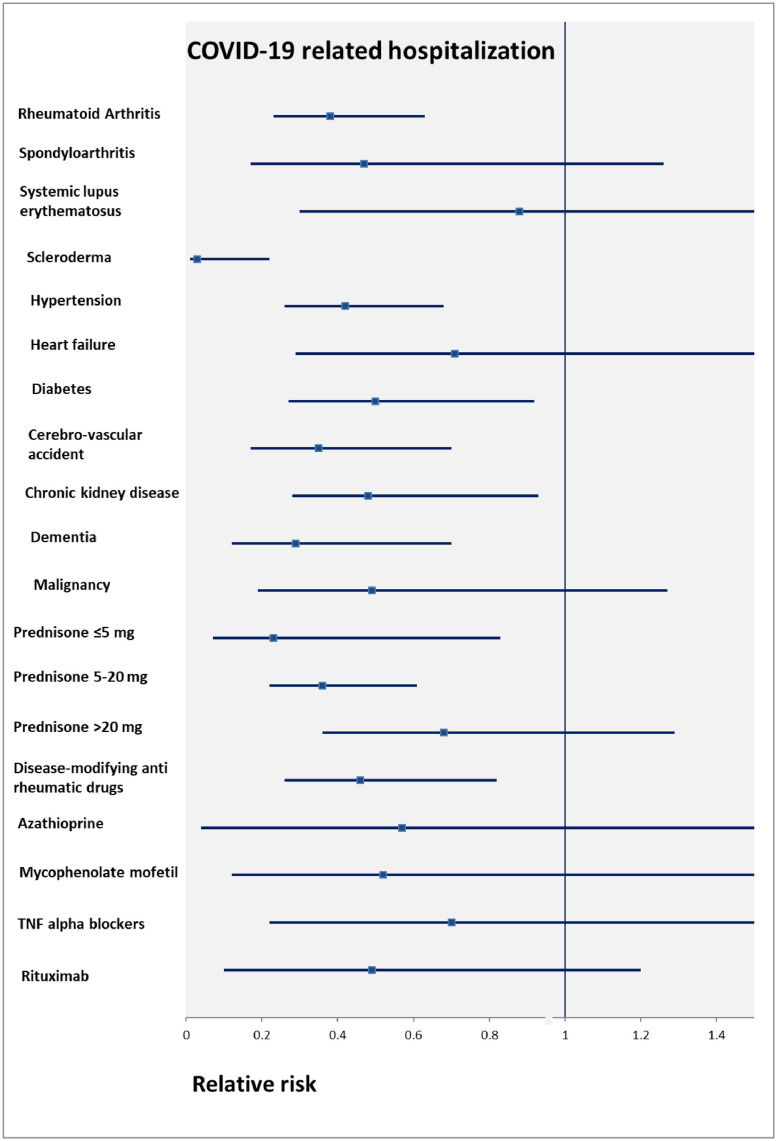
Multivariable logistic regression models for COVID-19-related hospitalization and death among ARD with documented immunomodulatory treatment are presented in Table 2 and Table 3 , respectively. Compared to the Delta group, the Omicron group showed a lower risk for COVID-19-related hospitalization (RR 0.40, 95% CI 0.27–0.59) and COVID-19-related mortality (RR 0.48, 95% CI 0.31–0.75). Sensitivity analysis for the whole ARD cohort, regardless of documented immunomodulatory treatment purchase documentation using a 1:1 Delta-Omicron matched cohort showed similar results for the COVID-19-related hospitalization (RR 0.51, 95% CI 0.32–0.79) (Supplementary Table 2 & 3). However, the matched model did not converge for the mortality analysis. The association between the Omicron variant (vs. Delta is the reference group) and COVID-19-related hospitalization by subgroups, among ARD patients with documented immunomodulatory treatment, is presented in Fig. 1 Factors not associated with COVID-19-related hospitalizations were: spondyloarthropathies; SLE; heart failure; malignancy; prednisone prescription of more than 20 mg; and all immunosuppressants except for DMARDs. The same analysis for the whole ARD cohort, regardless of drug purchase documentation showed similar results (see supplementary Figure 1). The exceptions are that SLE and malignancy subgroups were associated with reduced risk for COVID-19-related hospitalization, while dementia was not.
Table 2.
Multivariable binomial regression matched analysis of COVID-19 related hospitalization for ARD patients with documented immunomodulatory treatment. The relative risk presented for the Omicron group compared to the Delta group.
| Variable | P value | Relative risk | 95% C.I |
|---|---|---|---|
| Female | 0.07 | 0.70 | 0.48–1.020 |
| Age group* | 0.29 | 1.10 | 0.91–1.33 |
| Booster (third vaccination) | 0.05 | 0.68 | 0.47–1.00 |
| Hypertension | 0.01 | 1.76 | 1.12–2.75 |
| Dementia | 0.01 | 2.00 | 1.20–3.31 |
| Charlson index | <0.001 | 1.19 | 1.11–1.28 |
| Prednisone⁎⁎ | 0.01 | 1.26 | 1.09–1.45 |
| Mycophenolate mofetil | 0.01 | 2.98 | 1.58–6.00 |
| Rituximab | 0.01 | 2.35 | 1.31–4.19 |
| Omicron variant | <0.001 | 0.40 | 0.27–0.59 |
Grouped into <50, 50–59, 60–69, 70–79, 80≤.
Grouped into ≤5 mg, 5–20 mg, >20 mg.
Table 3.
Multivariable binomial regression matched analysis of COVID-19 related mortality for ARD patients with documented immunomodulatory treatment only. The relative risk presented for the Omicron group compared to the Delta group.
| Variable | P value | Relative risk | 95% C.I |
|---|---|---|---|
| Female | 0.52 | 0.87 | 0.56–1.33 |
| Age group* | <0.001 | 1.69 | 1.38–2.08 |
| Booster (third vaccination) | <0.001 | 0.43 | 0.29–0.66 |
| Hypertension | 0.01 | 2.02 | 1.16–3.52 |
| Charlson index | <0.001 | 1.26 | 1.17–1.34 |
| Prednisone⁎⁎ | 0.01 | 1.30 | 1.11–1.54 |
| Omicron variant | 0.01 | 0.48 | 0.31–0.75 |
Grouped into <50, 50–59, 60–69, 70–79, 80≤.
Grouped into ≤5 mg, 5–20 mg, >20 mg.
Fig. 1.
The association between Omicron variant (vs. Delta is the reference group) and COVID-19-related hospitalization by subgroups. The estimates were adjusted for age, sex, duration of rheumatic disease, Charlson co-morbidity index, and receiving a booster (third) vaccination. Data presented for ARD patients with documented immunomodulatory treatment purchase.
Supplementary Table 6 shows factors associated with risk for COIVD-19-related hospitalization during the Omicron outbreak. Past infection with COVID-19 and receiving a booster were not associated with Omicron-related hospitalization. However, patients who purchased Mycophenolate (R.R 4.03, 95% CI 1.79–9.08) or Rituximab (RR 3.79, 95% CI 1.80–7.97) had a higher risk for Omicron related hospitalization.
Discussion
Our study found better clinical outcomes for ARD patients infected during the Omicron period than the Delta time frame. In addition, overall COVID-19-related hospitalization and mortality were significantly lower during the Omicron period compared to the Delta period. These outcomes represent important markers of disease severity.
We found differences in ARD patients' background characteristics between the two periods. For instance, we found higher rates of females and a longer duration of ARD disease among Omicron-infected patients. On the other hand, we found lower rates of diabetes (22.1% vs. 25.3%, p = 0.01) in the Omicron Vs. Delta groups. Differences in the prescription of prednisone and anti-Interleukin-17 use were also noted. Although these differences were statistically significant, we believe they stem from our large sample size and had minimal clinical significance. Therefore, we cannot deduce any direct effect of these features on the chance of being infected with COVID-19 at any variant.
Vaccination rates of any kind were higher among the Omicron group. The Israeli Ministry of Health operates an ongoing campaign to encourage Israeli citizens to receive the BNT162b2 mRNA COVID-19 vaccine and booster. Since late December 2021, a fourth dose has been available during the Omicron timeframe [11]. The cumulative protection of COVID-19 vaccines probably has influenced our results. However, this analysis was not intended to assess the association between vaccinated vs. unvaccinated ARD patients and COVID-19 outcomes during each of the Delta/Omicron periods.
The Omicron variant has unique contagiousness, disease severity, and mortality features. A study by Nyberg et al. analyzed clinical outcomes of the Omicron variant compared to the Delta variant in the general population. They investigated more than 1.5 million cases, of which approximately a third were infected with Delta and two-thirds with Omicron. They found significantly lower rates of hospital attendance and hospitalization and lower rates of death in adult patients infected with Omicron compared to the Delta variant [4]. Another study by Menni. Et al. from the United Kingdom UK explored more specifically COVID-19-related symptoms among Omicron Vs. Delta infected patients [6]. They found fewer self-reported hospitalizations in all vaccination groups among Omicron Vs. Delta infected patients. These results go along with our results regarding milder disease course regardless of vaccination status.
Our multivariable models for COVID-19-related hospitalization and death highlight several essential aspects of the differences between the two variants in ARD patients. As previously reported, increased age, hypertension, congestive heart failure, and dementia were associated with the risk for COVID-19-related hospitalization and death. On the other hand, lower hospitalization risk among Omicron variants was not demonstrated within specific subgroups (e.g., spondyloarthropathies; heart failure; dementia; prednisone prescription of more than 20 mg; and all immunosuppressants except DMARDS), most probably explained by the relatively low number of these patients in our cohort. Our results align with prior findings that the risk for poor outcomes in ARD patients with COVID-19 is mediated by the background comorbidities [12,13]. Studies about ARD patients infected with COVID-19 have revealed some essential notions. For instance, an extensive analysis of ARD patients receiving Rituximab found that they have an increased risk of developing severe disease compared to ARD patients without Rituximab treatment [14]. Another study, done during the beginning of the pandemic (March to July 2020), investigating mortality related to COVID-19 among ARD patients found that older age, cardiovascular comorbidities, un-treated rheumatic disease, and the use of several immune-modulatory drugs compared to Methotrexate alone, were associated with higher mortality rates [12]. Vaccination status and the relation to immune-modulatory medications in terms of vaccination efficacy and immunogenicity have also been investigated [15], [16], [17]. These studies found that most ARD patients may benefit from receiving the COVID-19 vaccination and booster.
ARD patients are generally at higher risk for COVID-19-related worse outcomes, probably due to lessened vaccine efficacy and weaker anti-viral immune response of ARD patients receiving these therapies [7,15,[18], [19], [20]]. For instance, a review by Grainger et al. concluded that there is an increased risk of COVID-19-related hospitalization and mortality among ARD patients [3]. Of note, an existing ARD of RA or SLE was associated with a higher risk for hospitalization and death. Furer et al. found that BNT162b2 mRNA vaccination immunogenicity was severely impaired in ARD patients receiving Rituximab and moderately impaired in ARD receiving glucocorticoids, mycophenolate mofetil, and abatacept [16]. Interestingly other biologic drugs such as anti-TNF, anti- Il-6 anti-IL-17, and Janus Kinase inhibitors were not associated with impaired immunogenicity. In this study's primary multivariate analysis, prednisone, Rituximab, and MMF were all associated with an increased relative risk for hospitalization and mortality. However, in subgroup analysis, patients who purchased Rituximab, MMF, Azathioprine, TNF alpha-blockers, and prednisone dose above 20 mg were not associated with a lower risk for COVID-19-related hospitalization during the Omicron outbreak. We believe the most plausible explanation is the relatively low sample size of ARD patients who purchased these medications. Yet, we did find elevated hospitalization risk for other DMARDs.
Our study has some significant limitations. First, this is a retrospective analysis based on extracted data from electronic medical records of ARD patients. CHS policy does not allow researchers to validate the data extracted (such as the ICD −9 and ICD-10 based codes as well as other related codes of rheumatic disease) in a personalized record. Hence, we cannot assess activity level or remission in our cohort. Yet, even if such bias exists, we believe it has equal influence during Delta and Omicron periods.
On the other hand, we found a considerable use of immuno-modulatory treatments in our cohort. Nearly a third of our patients used DMARDS, approximately 11 percent used anti-TNF, and almost 40 percent used different prednisone doses. This data support that our cohort represents a valid sample of ARD patients. Nevertheless, we did not include patients with other rheumatic diseases such as vasculitides in the final analysis. This should be noted when interpreting our results since some immunomodulatory drugs (e.g., Rituximab) are used in vasculitides.
Second, we could not adjust for other confounders influencing COVID-19 disease outcomes, such as comorbidities severity, multiple hospitalizations, ARD treatments compliance, ARD activity level, and patient behavior. Altogether, we assumed that these are not different between Omicron and Delta groups.
Third, another limitation of this study is the definition of Omicron Vs. Delta variant infection. There is no routine identification of SARS-CoV-2 variants in Israel (other than specific tests conducted by the Israeli Ministry of Health). Hence, our variant group's definitions are based on the timetable of the epidemic in Israel. Therefore, some overlap between variant periods, mainly during December 2021, may have occurred [7].
Conclusion
Notwithstanding these limitations, this is the first study that compared COVID-19-related hospitalization and mortality among ARD patients between the Omicron variant to the Delta Variant. Our results suggest that there are better outcomes for ARD patients Who are infected with the Omicron variant. Further studies are needed to investigate specific features of ARD patients and vaccination over COVID-19-related clinical outcomes.
Declarations of Competing Interest
None.
Acknowledgements
We would like to acknowledge Mr. Omri Porat, and the "Clalit Health Services Research Room Team" for their technical support.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.semarthrit.2022.152129.
Appendix. Supplementary materials
References
- 1.Percentage of Rheumatoid Arthritis Patients Taking Disease Modifying Antirheumatic Drug Therapy and/or Biologic Agents(2016-2020) Outcomes | Cleveland Clinic n.d. https://my.clevelandclinic.org/departments/orthopaedics-rheumatology/outcomes/448-percentage-of-rheumatoid-arthritis-patients-taking-disease-modifying-antirheumatic-drug-therapy-andor-biologic-agents2016-2020 (accessed May 5, 2022).
- 2.Conway R., Grimshaw A.A., Konig M.F., Putman M., Duarte-García A., Tseng L.Y., et al. SARS – CoV -2 infection and COVID -19 outcomes in rheumatic diseases: a systematic literature review and meta-analysis. Arthritis Rheumatol. 2022;74:766–775. doi: 10.1002/art.42030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grainger R., Kim A.H.J., Conway R., Yazdany J., Robinson P.C. COVID-19 in people with rheumatic diseases: risks, outcomes, treatment considerations. Nat Rev Rheumatol. 2022;18:191–204. doi: 10.1038/s41584-022-00755-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and Delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/s0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauring A.S., Tenforde M.W., Chappell J.D., Gaglani M., Ginde A.A., Mcneal T., et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376 doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menni C., Valdes A.M., Polidori L., Antonelli M., Penamakuri S., Nogal A., et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dayam R.M., Law J.C., Goetgebuer R.L., Chao G.Y.C., Abe K.T., Sutton M., et al. Accelerated waning of immunity to SARS-CoV-2 mRNA vaccines in patients with immune mediated inflammatory diseases. n.d. [DOI] [PMC free article] [PubMed]
- 8.Miron O., Zeltzer D., Shir T., Balicer R.D., Einav L., Feldman B.S. Rising opioid prescription fulfillment among non-cancer and non-elderly patients—Israel's alarming example. Reg Anesth Pain Med. 2021;46:455–456. doi: 10.1136/RAPM-2020-101924. [DOI] [PubMed] [Google Scholar]
- 9.Shashar S., Ellen M., Codish S., Davidson E., Novack V. Medical practice variation among primary care physicians: 1 decade, 14 health services, and 3,238,498 patient-years. Ann Fam Med. 2021;19:30–37. doi: 10.1370/afm.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Our World in Data n.d. https://ourworldindata.org/(accessed May 4, 2022).
- 11.Magen O., Waxman J.G., Makov-Assif M., Vered R., Dicker D., Hernán M.A., et al. Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2022 doi: 10.1056/NEJMoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strangfeld A., Schäfer M., Gianfrancesco M.A., Lawson-Tovey S., Liew J.W., Ljung L., et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianfrancesco M., Hyrich K.L., Hyrich K.L., Al-Adely S., Al-Adely S., Carmona L., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avouac J., Drumez E., Hachulla E., Seror R., Georgin-Lavialle S., El Mahou S., et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with Rituximab: a cohort study. Lancet Rheumatol. 2021;3:e419–e426. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberman R.H., Herati R.S., Simon D., Samanovic M., Blank R.B., Tuen M., et al. Methotrexate hampers immunogenicity to BNT162B2 mRNA covid-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 17.Bieber A., Sagy I., Novack L., Brikman S., Abuhasira R., Ayalon S., et al. BNT162b2 mRNA COVID-19 vaccine and booster in patients with autoimmune rheumatic diseases: a national cohort study. Ann Rheum Dis. 2022 doi: 10.1136/annrheumdis-2021-221824. annrheumdis-2021-221824. [DOI] [PubMed] [Google Scholar]
- 18.Mageau A., Ferré V.M., Goulenok T., Charpentier C., Delory N., Francois C., et al. Severely impaired humoral response against SARS-CoV-2 variants of concern following two doses of BNT162b2 vaccine in patients with systemic lupus erythematosus (SLE) Ann Rheum Dis. 2022 doi: 10.1136/annrheumdis-2022-222498. annrheumdis-2022-222498. [DOI] [PubMed] [Google Scholar]
- 19.Fragoulis G.E., Karamanakos A., Arida A., Bournia V.K., Evangelatos G., Fanouriakis A., et al. Clinical outcomes of breakthrough COVID-19 after booster vaccination in patients with systemic rheumatic diseases. RMD Open. 2022;8 doi: 10.1136/rmdopen-2022-002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramarič J., Ješe R., Tomšič M., Rotar Ž., Hočevar A. COVID-19 among patients with giant cell arteritis: a single-centre observational study from Slovenia. Clin Rheumatol. 2022 doi: 10.1007/s10067-022-06157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.