Abstract
Background
There is increasing evidence that radiation therapy (RT) can be omitted for select older patients who are compliant with hormonal blockade, but there is no recent claim-based analysis for determining patterns of care and guiding possible treatment recommendations.
Methods
Medicare beneficiaries who were 65 years old or older and were diagnosed with breast cancer at 1 of 12 cancer centers affiliated with an academic center in the southeastern United States were analyzed. Stage 0 or I patients treated with lumpectomy from 2012 to 2014 were identified. Patient, treatment, and center characteristics were analyzed for the utilization of RT.
Results
Among 800 women treated with lumpectomy, 64% received adjuvant radiation. The median age was 74 years. The omission of RT was more likely in older patients, stage 0 patients, and patients with more comorbidities (P < .01). Hormonal blockade was used in 41% of the patients who did not receive RT. The utilization of hormonal blockade with the omission of RT was more likely in patients with fewer comorbidities (P < .01).
Conclusions
In an older cohort of patients who otherwise would have qualified for the omission of radiation, two-thirds were treated with radiation. Future guideline recommendations should address omission in the context of hormonal blockade compliance because only 41% of the patients used hormonal blockade when radiation was not delivered.
INTRODUCTION
There is increasing evidence supporting a paradigm shift toward the de-escalation of treatment in older breast cancer patients. For many years, the standard treatment for early-stage (stage 0-II) hormone (estrogen or progesterone) receptor–positive breast cancer was lumpectomy followed by conventional whole-breast radiation and 5 years of hormonal blockade. Many of the data supporting breast-conservation therapy with conventional or hypofractionated radiation were based on multiple clinical trials of younger women.1 The implications of such treatment in an older population were not known because less than 10% of patients were older than 65 years in a large analysis of breast cancer clinical trials.2 This was particularly important because of the increase in the number of early-stage, estrogen receptor–positive breast cancers expected to be diagnosed on account of the increasing longevity of the general population.
Over the last decade, 2 key randomized studies have reported outcomes opening the discussion for further de-escalation of care, beyond hypofractionation, to the consideration of the omission of radiation therapy (RT) in older women with small, early-stage, hormone receptor–positive disease. When the results of the first study were published in 2004 and showed only a 3% reduction in the risk of locoregional control with RT in women older than 70 years, the National Comprehensive Cancer Network amended its treatment algorithm to offer the omission of RT as an option for older women with invasive disease.3, 4 Despite this, 2 subsequent analyses of Surveillance, Epidemiology, and End Results (SEER)–Medicare data from 2001 to 2007 and from 2000 to 2009 reported minimal impact, with more than two-thirds to three-quarters of patients older than 70 years continuing to receive RT.5, 6 Another SEER analysis of women treated from 2000 to 2008 revealed that the majority of omissions occurred from 2003 to 2005, and the rate subsequently stabilized at 29%; a National Cancer Data Base analysis of women treated with hormonal blockade revealed a decrease in the utilization of radiation to 75% in 2011.7, 8 The subsequent 10-year update of the landmark study of radiation omission again showed low overall rates of ipsilateral recurrence without RT, but a subsequent population-based analysis indicated that more than one-third of women older than 85 years continued to receive RT.9, 10 More recently, the Post-operative Radiotherapy In Minimum-risk Elderly - Phase II (PRIME II) study has reported similar findings in a younger subset of older patients (65 years old or older) who were not treated with radiation.11 However, the decision to omit radiation is based on the presumption of compliance with and adherence to hormonal blockade. Several studies have shown that compliance with hormonal blockade is complex and multifactorial in etiology.12–14 Despite the plethora of literature, there is little recent analysis of hormonal blockade utilization in the setting of radiation omission. This is relevant and important because the decision to omit radiation is made under the assumption that there will be adherence to hormonal blockade. Given the lack of evidence describing recent trends of radiation and endocrine therapy use, we sought to describe patterns of adjuvant radiation and hormonal blockade utilization in a cohort of older women and to evaluate patient demographics, clinical factors, and center characteristics associated with patterns of care.
MATERIALS AND METHODS
Data obtained as part of a 2012 Center for Medicare and Medicaid Innovation award concerning patients with cancer within the University of Alabama at Birmingham (UAB) Health System’s Cancer Community Network (CCN) were analyzed. The CCN is a mix of academic and community cancer centers serving a diverse population in the 5 southeastern states: Alabama, Florida, Georgia, Mississippi, and Tennessee (Supporting Table 1 [see online supporting information]).15 A database included pertinent patient information from each institution’s local tumor registry. This database was linked to claim data for outpatient, inpatient, and medication utilization obtained from the Chronic Condition Data Warehouse of the Centers for Medicare and Medicaid Services. Institutional review board approval was obtained at UAB and other main sites.
Population
In the primary analysis, female patients who were 65 years old or older and had stage 0 or I breast cancer (according to the 7th edition of the American Joint Committee on Cancer staging) treated with lumpectomy from the first quarter of 2012 to the fourth quarter of 2014 were identified from each UAB CCN site. To ensure the accurate capture of all medical encounters identified in this analysis, only patients with continuous part A and B data and no health maintenance organization coverage were included.
Outcomes
The receipt of lumpectomy and adjuvant RT (defined as external-beam RT or brachytherapy) was identified with International Classification of Diseases, Ninth Revision, procedure codes and/or Current Procedural Terminology/Healthcare Common Procedure Coding System codes (Supporting Table 2 [see online supporting information]). Patients were considered to have received RT if the treatment was initiated within 6 months of lumpectomy and at least 2 fractions of RT were given. Patients were treated with RT according to individual center policies. For patients continuously enrolled in Part D, hormonal blockade use after diagnosis was identified with prescription drug names.16, 17 Current Procedural Terminology/Healthcare Common Procedure Coding System codes were additionally used to increase the capture of appropriate events.
Variables
The patient’s race (white vs other), Charlson comorbidity score (0, 1, or ≥2), adjuvant hormonal blockade (any used after lumpectomy), and year of treatment (2012–2014) were abstracted from claim data. The patient’s age (65–70, 71–80, or >80 years), stage (0 vs I), and treatment center volume (high vs low) were obtained from each UAB CCN site. Four sites were considered to be high-volume centers (more than 4000 cancer cases per year).
Analysis
Patient demographics, clinical factors, and center characteristics were analyzed with 2-sample t tests (continuous measures) and with chi-square tests of independence (categorical measures). Between-group differences were assessed between the radiation status (adjuvant radiation vs none) and hormonal blockade usage (any vs none). Multivariate logistic regression was used to obtain adjusted odds ratios and 95% confidence limits evaluating the association between the omission of RT and patient demographics (age and race), clinical factors (stage, estrogen and progesterone receptor status, and Charlson comorbidity score), and center characteristics (treatment center volume). Both the variables chosen for the model and the final model itself were selected on the basis of a priori hypotheses. The model fit was checked with the c statistic (0.67). All analyses were performed with SAS software (version 9.4; SAS Institute, Cary, North Carolina). Results were considered statistically significant if the P value was <.05.
RESULTS
Patient Demographics, Clinical Factors, and Center Characteristics
Eight hundred patients with stage 0 or I breast cancer who underwent lumpectomy were identified within the linked database (Fig. 1). The mean age at lumpectomy was 74 years for the entire cohort (Table 1). Most patients were white (87%) and had stage I breast cancer (75%). The Charlson comorbidity score was 0 to 1 for 69% of the patients. The hormone receptor status was available for 77% of the patients, and 90% of these were positive for estrogen or progesterone. Larger volume centers treated 74% of the patients.
Figure 1.
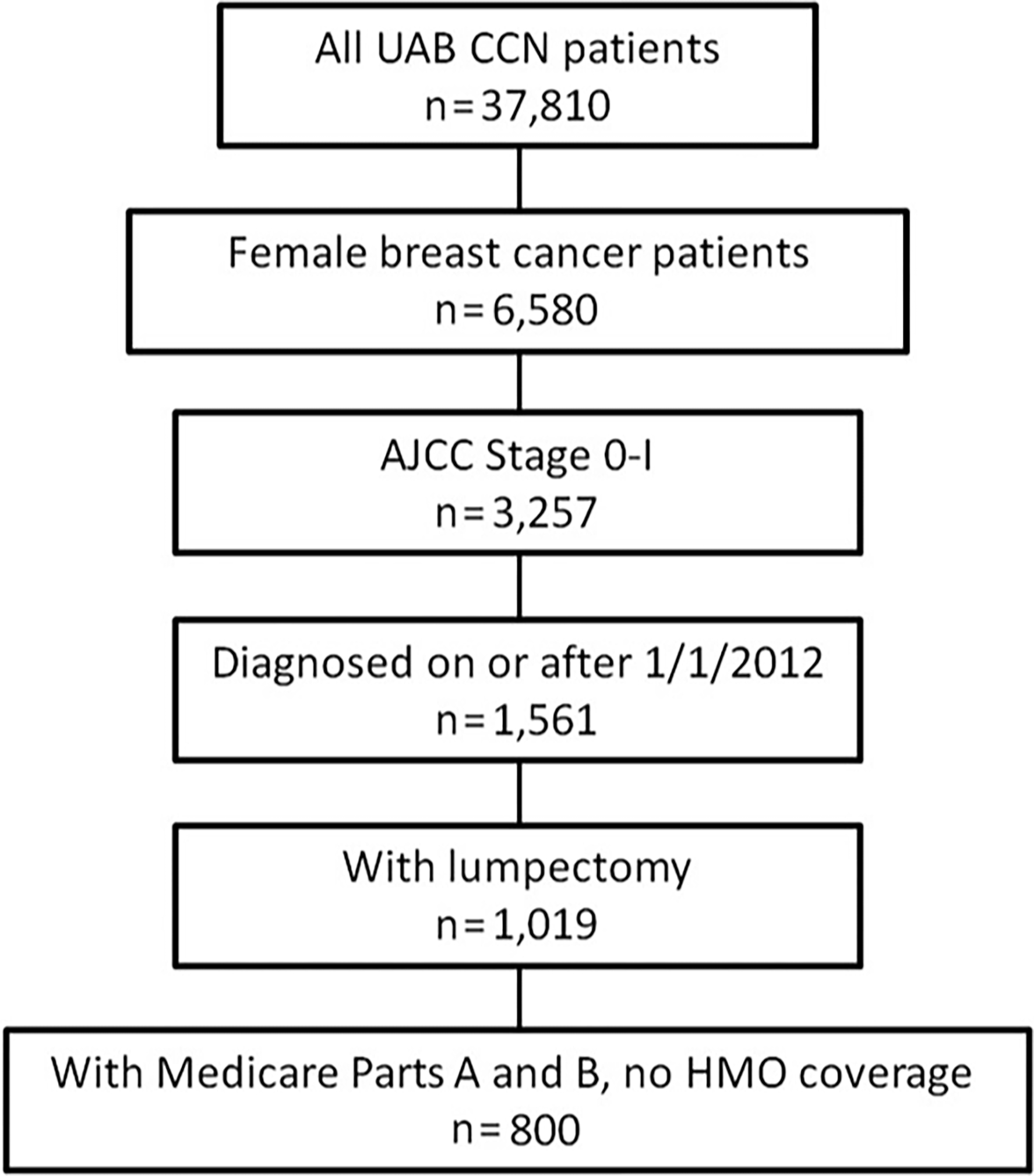
Study population exclusion cascade (n = 800). AJCC indicates American Joint Committee on Cancer; CCN, Cancer Community Network; HMO, health maintenance organization; UAB, University of Alabama at Birmingham.
Table 1.
Characteristics of Women Diagnosed With Stage 0 or I Breast Cancer and Treated With Lumpectomy: Radiation Therapy Versus No Radiation (n = 800)
| Characteristic | Total (n = 800) | Radiation (n = 508) | No Radiation (n = 292) | P |
|---|---|---|---|---|
| Age at lumpectomy, mean (SD), y | 73.6 (5.8) | 72.4 (5.1) | 75.8 (6.4) | <.001 |
| Age category, No. (%) | <.001 | |||
| 65–70 y | 318 (39.8) | 239 (47.1) | 79 (27.1) | |
| 71–80 y | 381 (47.6) | 231 (45.5) | 150 (51.4) | |
| >80 y | 101 (12.6) | 38 (7.5) | 63 (21.6) | |
| Race, No. (%) | .77 | |||
| White | 695 (86.9) | 440 (86.6) | 255 (87.3) | |
| Other | 105 (13.1) | 68 (13.4) | 37 (12.7) | |
| Charlson comorbidity score, No. (%) | .002 | |||
| 0 | 320 (40.0) | 223 (43.9) | 97 (33.2) | |
| 1 | 229 (28.6) | 146 (28.7) | 83 (28.4) | |
| ≥2 | 251 (31.4) | 139 (27.4) | 112 (38.4) | |
| Stage, No. (%) | <.001 | |||
| 0 | 199 (24.9) | 106 (20.9) | 93 (31.9) | |
| I | 601 (75.1) | 402 (79.1) | 199 (68.2) | |
| Positive ER/PR status, No. (%) | .57 | |||
| Yes | 554 (69.3) | 356 (70.1) | 198 (67.8) | |
| No | 62 (7.8) | 41 (8.1) | 21 (7.2) | |
| Unknown | 184 (23.0) | 111 (21.8) | 73 (25.0) | |
| Treatment center volume, No. (%) | .58 | |||
| High | 590 (73.8) | 378 (74.4) | 212 (72.6) | |
| Low | 210 (26.3) | 130 (25.6) | 80 (27.4) |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; SD, standard deviation.
Utilization of Radiation
Within this population, 508 (64%) were treated with adjuvant RT. Patients receiving RT were more likely to be younger, to have higher stage disease, and to have fewer comorbidities in comparison with patients who did not receive RT (all P values < .01). The volume of the treating center did not influence the omission of radiation. In a multivariate analysis, an older age (odds ratio, 5.10; confidence interval, 3.11–8.38), pre-invasive disease (odds ratio, 2.05; confidence interval, 1.45–2.90), and a higher comorbidity status (odds ratio, 1.57; confidence interval, 1.09–2.26) were significantly associated with the omission of RT (Table 2).
Table 2.
Multivariate Logistic Regression Model Estimating the Association Between the Omission of Radiation Therapy and Patient Demographics, Clinical Factors, and Center Characteristics (n = 800)
| Characteristic | OR (95% CI) | P |
|---|---|---|
| Age category | ||
| 65–70 y | 1.00 | |
| 71–80 y | 1.97 (1.41–2.76) | <.001 |
| >80 y | 5.10 (3.11–8.38) | <.001 |
| Race | ||
| White | 1.00 | |
| Other | 0.93 (0.59–1.48) | .77 |
| Stage | ||
| I | 1.00 | |
| 0 | 2.05 (1.45–2.90) | <.001 |
| Positive ER/PR status | ||
| No | 1.00 | |
| Yes | 1.21 (0.67–2.18) | .57 |
| Charlson comorbidity score | ||
| 0 | 1.00 | |
| 1 | 1.08 (0.74–1.58) | .70 |
| ≥2 | 1.57 (1.09–2.26) | .02 |
| Treatment center volume | ||
| High | 1.00 | |
| Low | 0.76 (0.41–1.40) | .35 |
Abbreviations: CI, confidence interval; ER, estrogen receptor; OR, odds ratio; PR, progesterone receptor.
Utilization of Hormonal Blockade
Among patients with complete Medicare Part D data who received RT (n = 235), 67% had at least 1 episode of hormonal blockade. Among patients who did not receive RT (n = 132), only 41% showed any utilization of hormonal blockade (Table 3). A sensitivity analysis revealed that the rates of hormonal blockade utilization were similar in patients with invasive disease at 37%. Patients who used hormonal blockade but omitted RT were more likely to have fewer comorbidities (P < .01). These patients were also younger and had higher stage disease, although these trends were not statistically significant.
Table 3.
Characteristics of Patients With Omission of Radiation: Hormonal Blockade Versus No Hormonal Blockade (n = 132)
| Characteristic | Hormonal Blockade (n = 78) | No Hormonal Blockade (n = 54) | P |
|---|---|---|---|
| Age at lumpectomy, mean (SD), y | 74.9 (5.0) | 76.3 (7.0) | .20 |
| Age category, No. (%) | .36 | ||
| 65–70 y | —a | —a | |
| 71–80 y | —a | —a | |
| >80 y | —a | —a | |
| Race, No. (%) | .46 | ||
| White | —a | —a | |
| Other | —a | —a | |
| Stage, No. (%) | .14 | ||
| 0 | 17 (21.8) | 18 (33.3) | |
| I | 61 (78.2) | 36 (66.7) | |
| Charlson comorbidity score, No. (%) | .006 | ||
| 0 | 28 (35.9) | —a | |
| 1 | 25 (32.1) | —a | |
| ≥2 | 25 (32.1) | —a | |
| Treatment center volume, No. (%) | .81 | ||
| High | 55 (70.5) | 37 (68.5) | |
| Low | 23 (29.5) | 17 (31.5) |
Abbreviation: SD, standard deviation.
The n value is too small to report.
DISCUSSION
Despite the growing body of level 1 evidence supporting the omission of radiation in select older breast cancer patients in the setting of adjuvant hormonal blockade, no formal guidelines have been issued by specialty organizations. This may in part explain why nearly two-thirds of patients in a population with a median age of 74 years were treated with radiation. The omission of radiation is still an area of controversy at national meetings, and the data in this analysis have the potential to contribute to the discussion of treatment options for older women in the setting of noncompliance or nonadherence with hormonal blockade. The possible impact of formal guidelines on increasing the omission of RT in this setting can be extrapolated from results seen after the issuance of guidelines for the utilization of hypofractionation by the American Society for Radiation Oncology. Various population-based analyses have shown an increase in hypofractionation from 5.4% in 2004 to 22.8% in 2011 and to 34.5% in 2013.18, 19 This is reflective of an overall trend toward the increasing utilization of hypofractionation over the last decade, albeit at a slow and subpar pace. There are many factors used to determine the choice of fractionation offered to women, such as body habitus. Similarly, there are likely additional patient and physician factors at play in the decision to omit radiation.
One such factor may be the difficulty in defining the older breast cancer patient because age alone may not be the best solo arbiter of treatment decisions on account of the complexity and heterogeneity of health status in this population. In the current analysis, there was decreasing utilization of RT with both increasing comorbidity status and the age category. This becomes important because the most common causes of death were not breast cancer–related events in both the C9343 and PRIME II trials.3, 9, 11 The consideration of age and comorbidities should be balanced with the risk of locoregional recurrence as 35% of patients treated with lumpectomy alone had ipsilateral recurrence at 12 years in the era before the use of routine hormonal blockade.20 The decision to omit radiation as a component of breast-conservation therapy must be made carefully because good outcomes are dependent on compliance with hormonal blockade.
In the current study, the omission of RT was accompanied by similar omissions of hormonal blockade in almost 60% of the patients. Although this number may appear high, it is similar to the findings of a National Cancer Data Base analysis of older women in which the omission of RT was accompanied by a 46% rate of omission of hormonal blockade.10 More recently, older women who underwent lumpectomy for in situ disease were also more likely to omit hormonal blockade when no adjuvant radiation was delivered.17 They found that women were more likely to initiate hormonal blockade if they received RT, which is in line with our findings.
As part of the treatment discussion for the older breast cancer patient, one alternative in the setting of difficulty with adhering to an oral regimen for 5 years or longer may be a short course of RT. Evidence for this can be found in the British Association of Surgical Oncology II trial, in which a combination of hormonal blockade and RT provided the largest decrease in the risk of locoregional recurrence, but the use of either provided a smaller but acceptable decreased risk in a population with a mean age of 57 years.21 These data can be extrapolated to the current population of older patients because of the importance of minimizing treatment modalities while respecting the impact of recurrence on morbidity. The burden of 3 to 4 weeks of daily RT should be weighed against the inconvenience of daily oral hormonal blockade for 5 years or more. Those with the shortest life expectancy may benefit from a candid discussion about radiation or hormonal blockade. Hence, the issuance of any guideline recommendations for the omission of radiation should include recommendations for a realistic discussion of expected compliance with hormonal therapy or efforts to ensure compliance.
Despite having an excellent prognosis, patients with in situ disease were not included in the clinical trials evaluating the omission of radiation in older women. Thus, there remains controversy surrounding the optimal management of pre-invasive disease. RT has been shown to decrease the annual risk of local recurrence by more than 50%; however, the absolute benefit is likely low. Attempts by 4 prospective studies to identify subgroups for which radiation could be omitted have not been successful, and they have demonstrated a 20% to 30% risk of local recurrence at 15 years. However, the median age in 3 of these studies was 58 to 60 years, and only 2.8% of these women were 70 years of age or older in the fourth study.22–25 Because typically only half of recurrences are invasive, the risk of recurrence should be weighed against the expected benefit in the older cohort. Social Security Administration actuarial tables show life expectancies of 12.8, 9.64, and 6.92 years for women aged 75, 80, and 85 years, respectively.26 Although pre-invasive disease continues to have a late and ongoing risk of recurrence well past a decade of follow-up, there are likely higher competing causes of mortality in this cohort. Hence, although the major studies looking at the omission of radiation did not directly address the question in pre-invasive disease, it may be reasonable to have a discussion about the omission of radiation in older women with competing causes of mortality.
Finally, attention should also be paid to the location of landmark studies and the research milieu when one is assessing patterns of care. Although breast cancer clinical trials have focused on the omission of RT in older women or the utilization of hypofractionation in Canada and Europe over the last 2 decades, the primary research focus in the United States has been a better understanding of partial breast irradiation. In a 2008 survey of 363 mostly private-practice physicians in the United States, 82% reported using conventional fractionation in more than two-thirds of women, 56% reported never using hypofractionation, and 36% reported utilization of partial breast irradiation.27 This is reflective of the trend toward the de-escalation of care through fractionation or treatment volume reduction but not toward the omission of radiation.
Limitations
Our study has several limitations. First is the lack of specific disease characteristics, such as histology, grade, margin, and complete receptor status for the entire cohort. Despite this, it is unlikely that more than 20% of the patients presented with high-risk features such as human epidermal growth factor receptor 2–positive or triple-negative invasive disease. Second, as with any claim-based study, patient preferences for specific treatments are not known. Third, this study reports results from sites in the southeastern United States, which represent states with larger low-income, low-education populations, and it may not reflect changes in other parts of the country. However, our study reflects a population that is typically not reported because many of the sites do not fall into the SEER geographic domain. Fourth, it is difficult to discern accelerated partial breast irradiation from hypofractionated radiation, but anecdotal evidence from the 2 largest sites indicates that utilization was low. Finally, this study lacks detailed information about physician factors that may have influenced practice patterns.
In conclusion, in a patient population that would otherwise have qualified for the omission of RT on the basis of level 1 evidence, 64% received RT. Although patient factors likely play a role in this trend, the impact of physician-directed treatment decisions should be further investigated. Formal guideline recommendations may serve as a stepping stone for initiating a change toward evidence-based patterns of practice, but they should also address the importance of radiation in the setting of low utilization of hormonal blockade in older patients.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
This publication was made possible by grant 1C1CMS331023 from the Centers for Medicare and Medicaid Services (US Department of Health and Human Services). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Health and Human Services or any of its agencies.
We acknowledge the Patient Care Connect Group for its contribution to this project and article. The Patient Care Connect Group includes the following: Lee Jackson, MD, and Zoe Scott, RN (Memorial Hospital, Chattanooga, Tennessee); Guilherme Cantuaria, MD, PhD, Debbie Bickes, RN, and Tina Berry, BS (Northside Hospital Cancer Institute, Atlanta, Georgia); George Reiss, MD, and Hang Mai, RN (Gulf Coast Regional Medical Center, Panama City, Florida); Ming Chang, MD, Louiz Gomez, RN, and Rhonda Meeker, RN, MSN (Fort Walton Beach Medical Center, Fort Walton Beach, Florida); James Clarkson, MD, and Maggie Clarkson, RN (Singing River Health System, Pascagoula, Mississippi); Steven Stokes, MD, and Tina Newman, RN (Southeast Alabama Medical Center, Dothan, Alabama); Mary Sheffield, MD (Russell Medical Center, Alexander City, Alabama); Ellen Spremulli, MD, and Wendy Watson, RN (Northeast Alabama Regional Medical Center, Anniston, Alabama); Tom Payne, MD, Hanna Bright, RN, and Stacey Holman, CNP (Marshall Medical Center, Albertville, Alabama); Thomas Butler, MD, and Cathy Tinnea, LPN (Mitchell Cancer Institute, Mobile, Alabama); Fred Schnell, MD, and Cyndi Pyle, RN (Medical Center Navicent Health, Macon, Georgia); and Gabrielle B. Rocque, MD, Richard A. Taylor, DNP, CRNP, ANP-BC, Aras Acemgil, MBA, Xuelin Li, PhD, Kelly M. Kenzik, PhD, Bradford E. Jackson, PhD, Courtney P. Williams, MPH, Karina I. Halilova, MD, Maria Pisu, PhD, Wendy Demark-Wahnefried, PhD, RD, Karen Meneses, PhD, RN, Yufeng Li, PhD, Michelle Y. Martin, PhD, Carol Chambless, BA, Nedra Lisovicz, PhD, Valeria Pacheco-Rubi, MBA, Terri L. Salter, MBA, MSN, Warren Smedley, MSHA, Mona Fouad, MD, MPH, Elizabeth A. Kvale, MD, and Edward E. Partridge, MD (University of Alabama at Birmingham Comprehensive Cancer Center, Birmingham, Alabama).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Audrey S. Wallace reports stock in Baxter, Shire PLC, Baxalta, Johnson & Johnson and Edward’s Life Science from her husband’s former employment with those companies and her husband’s pension fund from his former employment with Johnson and Johnson. Gabrielle B. Rocque reports her receipt of the Walter B. Frommeyer Jr Fellowship in Investigative Medicine; honoraria, research funding, travel expenses, and accommodations from Medscape; and grants from Pack Health, Genentech, and Carevive outside the submitted work.
REFERENCES
- 1.Benda RK, Mendenhall NP, Lind DS, et al. Breast-conserving therapy (BCT) for early-stage breast cancer. J Surg Oncol. 2004; 85: 14– 27. [DOI] [PubMed] [Google Scholar]
- 2.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999; 341: 2061– 2067. [DOI] [PubMed] [Google Scholar]
- 3.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004; 351: 971– 977. [DOI] [PubMed] [Google Scholar]
- 4.Carlson RW, McCormick B. Update: NCCN breast cancer clinical practice guidelines. J Natl Compr Canc Netw. 2005; 3(suppl 1): S7– S11. [PubMed] [Google Scholar]
- 5.Soulos PR, Yu JB, Roberts KB, et al. Assessing the impact of a cooperative group trial on breast cancer care in the Medicare population. J Clin Oncol. 2012; 30: 1601– 1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palta M, Palta P, Bhavsar NA, et al. The use of adjuvant radiotherapy in elderly patients with early-stage breast cancer: changes in practice patterns after publication of Cancer and Leukemia Group B 9343. Cancer. 2015; 121: 188– 193. [DOI] [PubMed] [Google Scholar]
- 7.Shen X, Anne PR, Keith SW, et al. Radiation therapy use and outcomes among older women with ER-positive and ER-negative stage I breast cancer. Am J Clin Oncol. 2014; 37: 241– 247. [DOI] [PubMed] [Google Scholar]
- 8.Rhieu BH, Rajagopalan MS, Sukumvanich P, et al. Patterns of care for omission of radiation therapy for elderly women with early-stage breast cancer receiving hormonal therapy. Pract Radiat Oncol. 2015; 5: e267– e273. [DOI] [PubMed] [Google Scholar]
- 9.Hughes KS, Schnaper LA, Bellon JR. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013; 31: 2382– 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu QD, Medeiros KL, Zhou M, et al. Impact of cooperative trial and sociodemographic variation on adjuvant radiation therapy usage in elderly women (≥70 years) with stage I, estrogen receptor–positive breast cancer: analysis of the National Dancer Data Base. J Am Coll Surg. 2016; 222: 667– 678. [DOI] [PubMed] [Google Scholar]
- 11.Kunkler IH, Williams LJ, Jack WJ, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015; 16: 266– 273. [DOI] [PubMed] [Google Scholar]
- 12.Hershman DL, Tsui J, Meyer J, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst. 2014; 106: dju319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hershman DL, Tsui J, Wright JD, et al. Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol. 2015; 33: 1053– 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010; 28: 4120– 4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocque GB, Partridge EE, Pisu M, et al. The Patient Care Connect Program: transforming health care through lay navigation. J Oncol Pract. 2016; 12: e633– e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farias AJ, Du XL. Racial differences in adjuvant endocrine therapy use and discontinuation in association with mortality among Medicare breast cancer patients by receptor status. Cancer Epidemiol Biomarkers Prev. 2017; 26: 1266– 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao H, Hei N, Wu Y, et al. Initiation of and adherence to tamoxifen and aromatase inhibitor therapy among elderly women with ductal carcinoma in situ. Cancer. 2017; 123: 940– 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bekelman JE, Sylwestrzak G, Barron J, et al. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008–2013. JAMA. 2014; 312: 2542– 2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang EH, Mougalian S, Soulos PR, et al. Adoption of hypofractionated whole-breast irradiation for early-stage breast cancer: a National Cancer Data Base analysis. Int J Radiat Oncol Biol Phys. 2014; 90: 993– 1000. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Anderson S, Redmond CK, et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995; 333: 1456– 1461. [DOI] [PubMed] [Google Scholar]
- 21.Blamey RW, Bates T, Chetty U, et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer. 2013; 49: 2294– 2302. [DOI] [PubMed] [Google Scholar]
- 22.Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: randomised controlled trial. Lancet. 2003; 362: 95– 102. [DOI] [PubMed] [Google Scholar]
- 23.Wong JS, Chen YH, Gadd MA, et al. Eight-year update of a prospective study of wide excision alone for small low- or intermediate-grade ductal carcinoma in situ (DCIS). Breast Cancer Res Treat. 2014; 143: 343– 350. [DOI] [PubMed] [Google Scholar]
- 24.McCormick B, Winter K, Hudis C, et al. RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol. 2015; 33: 709– 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solin LJ, Gray R, Hughes LL, et al. Surgical excision without radiation for ductal carcinoma in situ of the breast: 12-year results from the ECOG-ACRIN E5194 study. J Clin Oncol. 2015; 33: 3938– 3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Social Security Administration. 2014. actuarial life table. https://www.ssa.gov/oact/STATS/table4c6.html. Accessed May 8, 2017.
- 27.Hoopes DJ, Kaziska D, Chapin P, et al. Patient preferences and physician practice patterns regarding breast radiotherapy. Int J Radiat Oncol Biol Phys. 2012; 82: 674– 681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


