INTRODUCTION:
Non-cardiac surgery in patients with coronary stents is associated with increased risk for major adverse cardiac events (MACE), particularly when the surgery closely follows the stent procedure. Drivers of this risk are myriad, and include stent, surgical, and patient cardiac risk factors. Furthermore, the decrease in peri-operative major adverse cardiac events that occurs as the time from stent placement to surgery increases suggests that the incremental risk of stents on post-operative outcomes decreases over time. However, the optimal time to delay elective surgery following coronary stent placement remains controversial. While risk does decreases over time, the contribution of the stent versus other cardiac and surgical risk factors to this decline is uncertain as most reports lack a control surgical population without stents for comparison.1–3
In light of the findings above, questions remain about whether coronary stenting is beneficial in reducing post-operative MACE and if so, at what time point following the stent procedure. Two randomized controlled trials provide insight into this question by examining whether prophylactic revascularization prior to elective high-risk vascular surgery decreased post-operative MACE.4,5 Neither trial demonstrated a significant difference in event rates with re-vascularization, suggesting that the underlying surgical and cardiac factors, rather than stent intervention, were the predominant drivers of MACE. However, in both trials, patients underwent surgery within a median of 6 weeks following revascularization, a time period known from observational studies to be at an increased for MACE1,3,6. Additionally, perioperative continuation of dual antiplatelet therapy in those with recent stent implantation reduces the risk of stent thrombosis and myocardial infarction but may increase the bleeding risk. Thus the question of whether revascularization leads to improved outcomes when timing from stent to surgery is different remains unanswered.
In order to shed further light on these questions, we assessed MACE rates in patients with coronary stents undergoing non-cardiac surgery in the two years following stent placement matched to a cohort of matched patients with similar cardiac risk undergoing a similar surgery, but without a coronary stent. We hypothesize that prior revascularization with a coronary stent is associated with a higher risk of MACE than similar patients without a stent and this risks decreases as time from stent to surgery increases. These data will better inform the timing of surgery associated with lowest risk after stent placement.
METHODS:
Study Overview
In order to assess the risk of a coronary stent on postoperative outcomes after non-cardiac surgery we conducted a matched retrospective cohort study of patients undergoing non-cardiac surgery within the VA between October 1999 and September 2011. The exposure of interest was the preoperative presence of a coronary stent and the primary outcome was the occurrence of a major adverse cardiac event (MACE), a composite outcome of myocardial infarction, coronary revascularization by percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG), and/or death within 30 days following the index surgery. The study protocol, including a waiver of informed consent, was reviewed and approved by the local VA Institutional Review Board of each co-author.
Cohort Creation
Figure 1 describes the construction of the study cohorts. Patients with a coronary stent were identified by ICD-9 procedure codes (bare-metal stent (BMS) placement: 36.06, drug-eluting stent (DES) placement: 37.07) in the VA Medical SAS Datasets (MedSAS) along with the VA Clinical Assessment, Reporting, and Tracking Program (CART) datasets. Among the subset of 12,080 stented patients who underwent non-cardiac surgery in the 2 years following stent placement, surgical data was collected from the VA Surgical Quality Improvement Program (VASQIP). Each of these patients was then matched to two patients undergoing non-cardiac surgery with no prior coronary stent. Pairs were matched on year of surgery, patient age, surgical complexity, surgeon specialty, renal disease, history of coronary artery disease, congestive heart failure, stroke, insulin-dependent diabetes, and high-risk surgery. Age was categorized in 5-year increments. Surgical complexity was measured using surgical Relative Value Units (RVU) recorded in the VASQIP data and categorized into 5-unit increments. Surgical specialties included gastrointestinal, otolaryngology, ophthalmology, urology, plastic, orthopedic, neurosurgery, thoracic, and vascular. Renal disease was defined as a pre-operative creatinine >2mg/dL. High-risk surgery was defined as supra-inguinal vascular, intra-thoracic, or intra-peritoneal operations, as per the revised Cardiac Risk Index (RCRI)7. Ultimately, 9,391 (77.7%) stent/surgery patients were matched to 18,782 surgery-only patients.
Figure 1. Creation of the Matched Cohort.
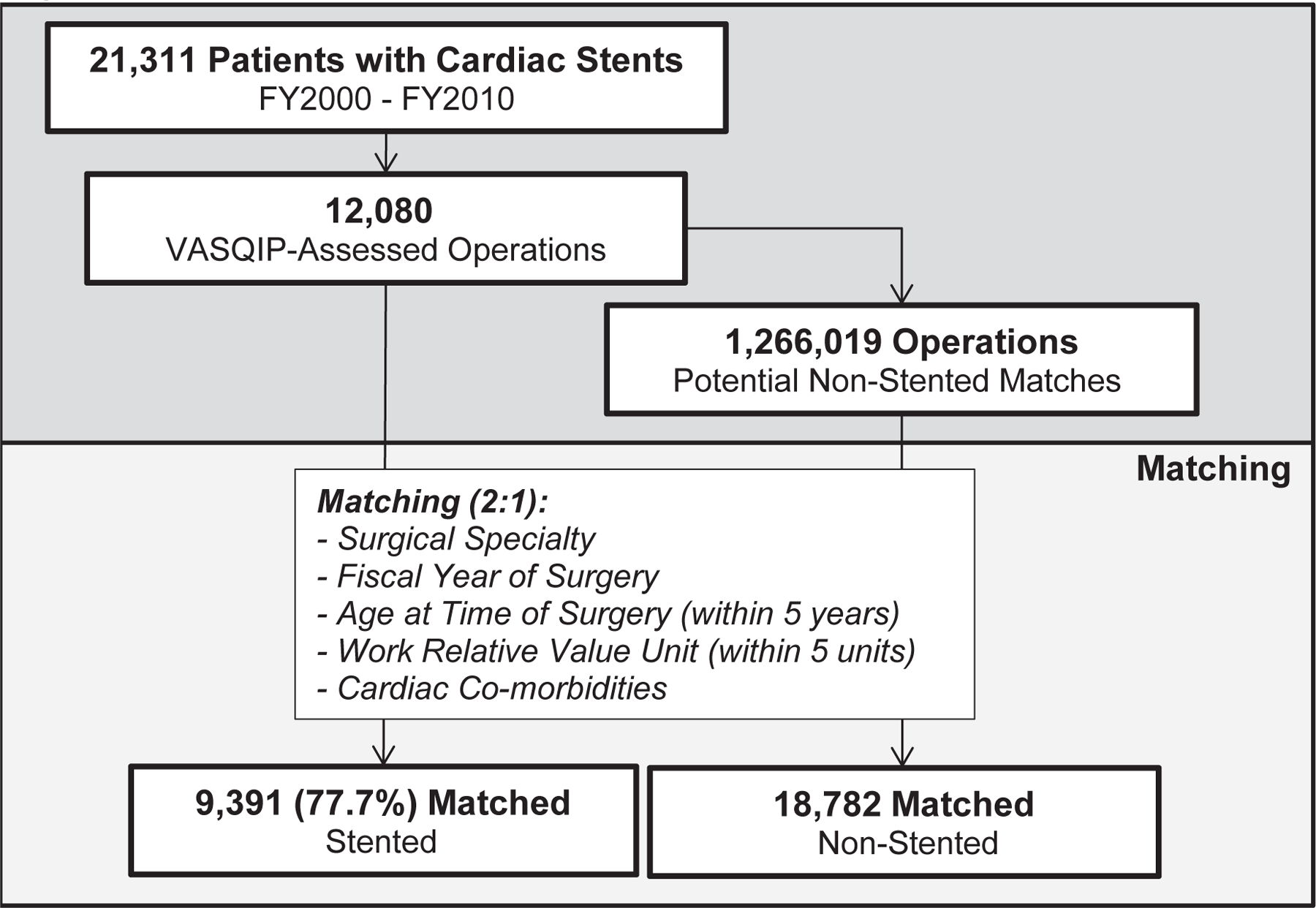
Diagram of the construction of the study cohort using ICD-9 diagnosis codes for coronary stents and VA Surgical Quality Improvement Project Data
Study Variables
The main outcome of interest was the occurrence of a postoperative major adverse cardiac event (MACE) within 30 days following non-cardiac surgery. This was defined as either a nurse-abstracted myocardial infarction (MI) report in the VASQIP dataset, an ICD-9 diagnosis code for MI occurring in the inpatient MedSAS datasets, an ICD-9 procedure code for a coronary revascularization procedure (PCI and/or CABG) in the inpatient MedSAS datasets, or death from the Vital Status file. Secondary outcomes of interest included return to the operating room, postoperative length of stay, transfused intraoperative RBCs, and postoperative RBC transfusion of more than 4 units within 72 hours. All secondary outcomes were documented in VASQIP data. Finally, any readmission to a VA hospital within 30 days post-discharge from the index surgery was identified using MedSAS data. Covariates of interest included demographics, social factors, preoperative comorbidities, and operative characteristics as defined in the VASQIP data.
Statistical Analyses
Univariate and bivariate frequencies of study covariates and outcomes were used to compare stent/surgery patients to their matched surgery-only controls. Differences between stent/surgery and surgery-only patients were assessed using Chi-square tests for categorical variables and Wilcoxon Rank Sums for continuous variables. Unadjusted and adjusted odds ratios for each outcome were calculated using logistic regression with generalized estimating equations to account for the matching. The adjusted model for each outcome included smoking history, ASA classification, recent MI, recent CHF, dyspnea, COPD history, admission status, and emergency case status.
In order to assess the change in risk difference across time since cardiac stent placement, direct adjustment of the risk of each outcome in the surgery-only cohort was performed for each 60-day interval of time between stent and surgery for the matched stent/surgery patients. Once the surgery-only risk was adjusted to represent a similar distribution of characteristics of the stent/surgery patients during that time interval, the adjusted risk difference was calculated by subtracting the observed rate among the stent/surgery patients from the adjusted rate among their matched surgery-only patients. Emergency case status, admission status, ASA classification, and preoperative dyspnea were used to adjust rates as they were the most important factors in the final adjusted model of postoperative MACE. Finally, smoothed plots of the final calculated adjusted risk difference across days since coronary stent were generated with R GGPLOT2.8
The utility of postoperative troponin surveillance in patients at high risk for MACE is uncertain9. As a secondary aim, we performed a sensitivity analysis to identify differences in postoperative troponin surveillance and MI rates among stented versus non-stented patients. Serum troponin levels were obtained from the VA Decision Support System (DSS). Frequency of troponin screening within 72 hours of surgery and postoperative MI were calculated both overall and stratified by stent type and time between stent and surgery. To understand differences in postoperative screening by cohort, frequencies of postoperative troponin levels and MI were stratified by cohort and a Chi-square test was used to examine statistically significant differences. All above analyses were performed using SAS version 9.3. (Ref SAS Software)
RESULTS:
Cohort Description
In the study cohort, 9,391 patients with a coronary stent matched to 18,782 controls on the components of the revised cardiac risk index and surgical characteristics (Table 1). Gastrointestinal, orthopedic, and vascular procedures comprised the majority of operations. The stents in the study cohort consisted of BMS (52.5%) and DES (46.3%) and the median time to surgery following stent was 332 days. The demographics and characteristics of the cohort are shown in Table 2. The stent cohort had a larger proportion of patients with a myocardial infarction (MI) in the last 6 months (20.9% vs 4.8%), emergent surgery (6.1% vs 5.1%), inpatient surgery (62.4% vs 60.7%) and ASA class 4–5 (20.8% vs 16.3%) compared to matched controls. Other differences in comorbidities are presented in Table 2.
Table 1.
Description of Study Population in Terms of Matching Variables and Demographics
| Stented | Non-Stented | |||||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | p-value | ||
|
| ||||||
| OVERALL | 9391 | (33.3) | 18782 | (66.7) | ||
| Demographics | ||||||
| Age | Median (IQR) | 65 | (59–73) | 65 | (59–73) | 0.87 |
| Comorbidities | ||||||
| History of CAD | Yes | 9391 | (100.0) | 18782 | (100.0) | 1.00 |
| No | 0 | (0.0) | 0 | (0.0) | ||
| History of CHF | Yes | 2089 | (22.2) | 4178 | (22.2) | 1.00 |
| No | 7302 | (77.8) | 14604 | (77.8) | ||
| History of Stroke | Yes | 448 | (4.8) | 896 | (4.8) | 1.00 |
| No | 8943 | (95.2) | 17886 | (95.2) | ||
| Insulin-Dependent | Yes | 1432 | (15.3) | 2864 | (15.3) | 1.00 |
| No | 7959 | (84.8) | 15918 | (84.8) | ||
| Creatinine >2 mg/dl | Yes | 511 | (5.4) | 1022 | (5.4) | 1.00 |
| No | 8880 | (94.6) | 17760 | (94.6) | ||
| Surgical Characteristics | ||||||
| High Risk Surgery* | Yes | 2151 | (22.9) | 4302 | (22.9) | 1.00 |
| No | 7240 | (77.1) | 14480 | (77.1) | ||
| Work Relative Value Unit | Median (IQR) | 15.13 | (8.9–21.0) | 16.13 | (8.8–21.0) | 0.97 |
| Surgery Type** | Digestive | 2608 | (27.8) | 5268 | (28.1) | 0.98 |
| Eye/Ear | 6 | (0.1) | 10 | (0.1) | ||
| Genital/Urinary | 1264 | (13.5) | 2515 | (13.4) | ||
| Integumentary | 167 | (1.8) | 322 | (1.7) | ||
| Musculoskeletal | 2078 | (22.1) | 4196 | (22.3) | ||
| Nervous | 333 | (3.6) | 658 | (3.5) | ||
| Other | 235 | (2.5) | 429 | (2.3) | ||
| Respiratory | 297 | (3.2) | 606 | (3.2) | ||
| Vascular | 2403 | (25.6) | 4778 | (25.4) | ||
| Stent Characteristics | ||||||
| Stent Type | BMS | 4929 | (52.5) | |||
| DES | 4350 | (46.3) | ||||
| Both | 112 | (1.2) | ||||
| Stent Indication | ACS / MI | 2803 | (29.9) | |||
| ACS / Non-MI | 3058 | (32.6) | ||||
| Non-ACS | 3530 | (37.6) | ||||
| Days to Surgery | Median (IQR) | 332 | (150–518) | |||
High risk surgery includes vascular suprainguinal procedures, intrathoracic, or intraperitoneal operations.
Controls were also matched by fiscal year of surgery
CAD=Coronary Artery Disease; CHF=Congestive Heart Failure; BMS=Bare Metal Stent; DES=Drug-Eluting Stent; IQR=Interquartile Range
Table 2.
Preoperative Comorbidities and Operative Characteristics of Matched Study Cohort
| Stented | Non-Stented | |||||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | p-value | ||
|
| ||||||
| OVERALL | 9391 | (33.3) | 18782 | (66.7) | ||
| Demographics | ||||||
| Sex | Male | 9240 | (98.4) | 18340 | (97.7) | <0.001 |
| Female | 151 | (1.6) | 442 | (2.4) | ||
| Race | White | 8320 | (90.2) | 15596 | (86.1) | <0.001 |
| Black | 812 | (8.8) | 2309 | (12.8) | ||
| Other | 97 | (1.1) | 203 | (1.1) | ||
| Social Factors | ||||||
| Current Smoker | Yes | 3179 | (33.9) | 6628 | (35.3) | 0.02 |
| No | 6211 | (66.1) | 12152 | (64.7) | ||
| >2 Drinks/Day | Yes | 529 | (5.6) | 1285 | (6.9) | <0.001 |
| No | 8843 | (94.4) | 17450 | (93.1) | ||
| Preoperative Comorbidities | ||||||
| Recent MI Episodes | <6 Months | 1964 | (20.9) | 903 | (4.8) | <0.001 |
| 6–12 Months | 1560 | (16.6) | 575 | (3.1) | ||
| 12–24 Months | 2638 | (28.1) | 888 | (4.7) | ||
| No Prior or Older than 24 Months | 3229 | (34.4) | 16416 | (87.4) | ||
| Recent CHF Episodes | <6 Months | 344 | (3.7) | 579 | (3.1) | 0.01 |
| None within 6 months | 9047 | (96.3) | 18203 | (96.9) | ||
| COPD | Yes | 1856 | (19.8) | 3963 | (21.1) | 0.01 |
| No | 7535 | (80.2) | 14819 | (78.9) | ||
| PVD | Yes | 2726 | (29.0) | 4909 | (26.1) | <0.001 |
| No | 6665 | (71.0) | 13873 | (73.9) | ||
| Hyperlipidemia | Yes | 8492 | (90.4) | 14551 | (77.5) | <0.001 |
| No | 5130 | (18.2) | 4231 | (22.5) | ||
| Functional Status | Independent | 8692 | (92.6) | 17271 | (92.0) | 0.04 |
| Partially Dependent | 626 | (6.7) | 1317 | (7.0) | ||
| Totally Dependent | 70 | (0.8) | 192 | (1.0) | ||
| Dialysis | Yes | 214 | (2.3) | 393 | (2.1) | 0.31 |
| No | 9175 | (97.7) | 18389 | (97.9) | ||
| Dyspnea | None Present | 7309 | (78.4) | 14869 | (79.7) | 0.03 |
| At Minimal Exertion | 1846 | (19.8) | 3489 | (18.7) | ||
| At Rest | 170 | (1.8) | 300 | (1.6) | ||
| Operation Characteristics | ||||||
| Emergent Surgery | Yes | 568 | (6.1) | 964 | (5.1) | 0.001 |
| No | 8823 | (94.0) | 17818 | (94.9) | ||
| Surgery Stay | Inpatient | 5855 | (62.4) | 11371 | (60.7) | 0.004 |
| Outpatient | 3525 | (37.6) | 7373 | (39.3) | ||
| ASA Classification | 1–2 | 396 | (4.2) | 1870 | (10.0) | <0.001 |
| 3 | 7042 | (75.0) | 13833 | (73.7) | ||
| 4–5 | 1947 | (20.8) | 3060 | (16.3) | ||
MI=Myocardial Infarction; COPD=Chronic Obstructive Pulmonary Disease; ASA=American Society of Anesthesiologists
Adverse Events Occurring in the 30 days following Surgery
We assessed adverse outcomes within the 30 days following surgery. Overall, rates of MACE (5.7% vs 3.6%, p<0.001), MI (2.7% vs 1.2%, p<0.001), and revascularization (2.3% vs 1.0%, p<0.001) were higher in the stent cohort compared to controls, but there was no significant difference in mortality (1.9% vs 1.8%, p=0.64) (Table 3). In the entire cohort, 410 (1.5%) underwent a coronary revascularization procedure within the 30 days following surgery, comprised of 376 PCIs and 34 CABGs. In adjusted models, 30-day post-operative rates of MI (OR 1.90, 95% C.I. 1.57–2.30) and revascularization (OR 2.03, 95% CI 1.65–2.50) were higher in the stent cohort compared to controls, but there was no significant difference in mortality (OR 0.84, 95% C.I. 0.69–1.02). The number of major bleeding events, characterized by a postoperative transfusion of greater than 4 units of packed red blood cells, occurred in less than 1% of both cohorts. However, stented patients were more likely to experience a major bleeding episode (OR 1.74, 95% C.I. 1.20–2.52) within 72 hours of surgery than non-stented patients.
Table 3.
Postoperative Outcomes
| Stented | Non-Stented | Unadjusted OR | Adjusted OR* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N / Med. | (% / IQR) | N / Med. | (% / IQR) | p-value | OR | (95% CI) | OR | (95% CI) | |
| Cardiac Outcomes within 30 days | |||||||||
| MACE | 531 | (5.7) | 680 | (3.6) | <0.001 | 1.60 | (1.42–1.79) | 1.35 | (1.19–1.54) |
| MI | 250 | (2.7) | 233 | (1.2) | <0.001 | 2.18 | (1.82–2.61) | 1.90 | (1.57–2.30) |
| Revascularization | 220 | (2.3) | 190 | (1.0) | <0.001 | 2.35 | (1.93–2.85) | 2.03 | (1.65–2.50) |
| Cardiac Death | 47 | (0.5) | 59 | (0.3) | 0.02 | 1.60 | (1.09–2.34) | 1.22 | (0.80–1.87) |
| Death | 179 | (1.9) | 343 | (1.8) | 0.64 | 1.04 | (0.87–1.25) | 0.84 | (0.69–1.02) |
| Other Outcomes within 30 days | |||||||||
| Any Complication | 1194 | (12.7) | 2079 | (11.1) | <0.001 | 1.17 | (1.09–1.26) | 1.08 | (0.99–1.17) |
| Return to OR | 691 | (7.4) | 1375 | (7.3) | 0.91 | 1.00 | (0.92–1.10) | 0.91 | (0.83–1.01) |
| Length of Post Op Stay, Days | 4 | (2–7) | 4 | (2–7) | 0.27 | ||||
| Readmission (30 days Post-Discharge) | 1240 | (13.2) | 2024 | (10.8) | <0.001 | 1.26 | (1.17–1.36) | 1.14 | (1.05–1.23) |
| Bleeding Complications within 72 hours | |||||||||
| Any Intraoperative RBCs | 718 | (7.7) | 1242 | (6.6) | 0.001 | 1.17 | (1.07–1.27) | 1.05 | (0.95–1.15) |
| Number of RBC Units Transfused Intraoperative | 2 | (1–3) | 2 | (1–3) | 0.55 | ||||
| Postoperative Bleeding Requiring >4 Units | 55 | (0.6) | 58 | (0.3) | <0.001 | 1.90 | (1.32–2.75) | 1.74 | (1.20–2.52) |
Adjusted models include smoking history, ASA classification, recent MI, recent CHF, dyspnea, COPD history, admission status, and emergency case status.
MACE=Major Adverse Cardiac Event; MI=Myocardial Infarction; OR=Operating Room; RBC=Red Blood Cells; MI=Myocardial Infarction; CHF=Congestive Heart Failure; COPD=Chronic Obstructed Pulmonary Disease
Differences in MACE rates by the time from stent placement to surgery are shown in Figure 2. When stratified by stent type, the overall MACE rate was not significantly different for BMS (6.0%) versus DES (5.3%) (p=0.30). The highest event rates were observed during the first 60 days from stent placement. MACE rates between the stented and non-stented cohort level off around 4–5% at approximately 1 year.
Figure 2. Postoperative MACE by Stent Type Compared to Non-Stented Cohort.
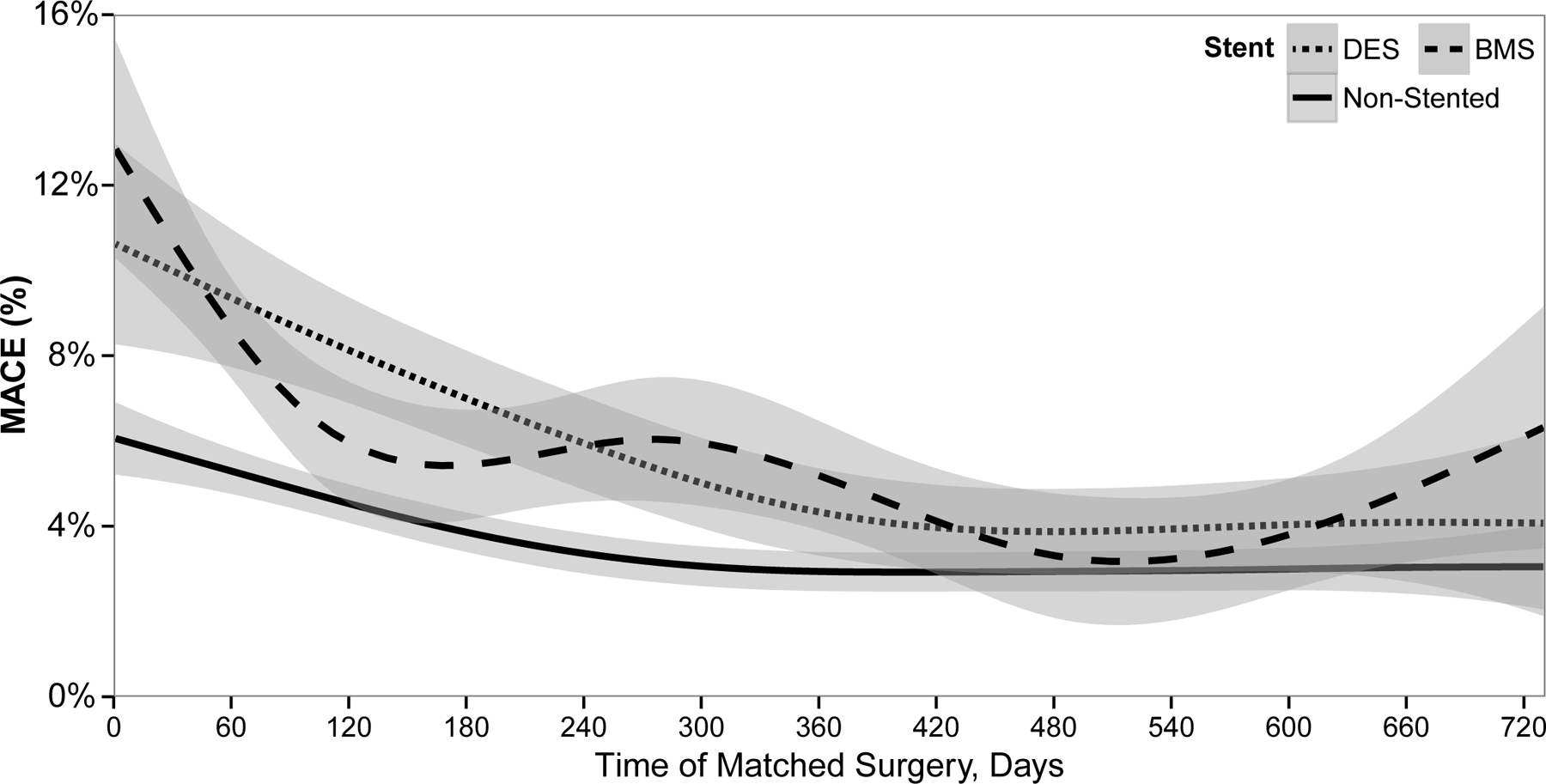
Major adverse cardiac event (MACE) rates within the 30 days following surgery tracked from time since coronary stent placement of the stented cohort (dashed lines) compared to their matched pair within the non-stented cohort (solid line) over a 2 year period. The stented cohort is stratified by type of stent, bare-metal or drug-eluting.
MACE = Major Adverse Cardiac Events; DES = Drug-Eluting Stent; BMS = Bare Metal Stent.
Shaded area represents 95% confidence interval around the risk.
To examine the attributable risk of a coronary stent on MACE and its components of MI, revascularization and death, we plotted 30-day adjusted risk differences by time from stent to surgery (Figure 3). Stented patients had an approximately 2% increased risk for MACE, MI, and revascularization for the 1st year following stent placement compared to the non-stented patients. After 1 year following stent placement, the difference was no longer statistically significant. Patients with coronary stents were not at an increased risk of mortality for surgery occurring at any time in the 2 years following stent placement.
Figure 3. Adjusted Risk Difference of Postoperative Outcomes Between Stented and Non-Stented Surgical Cohorts.
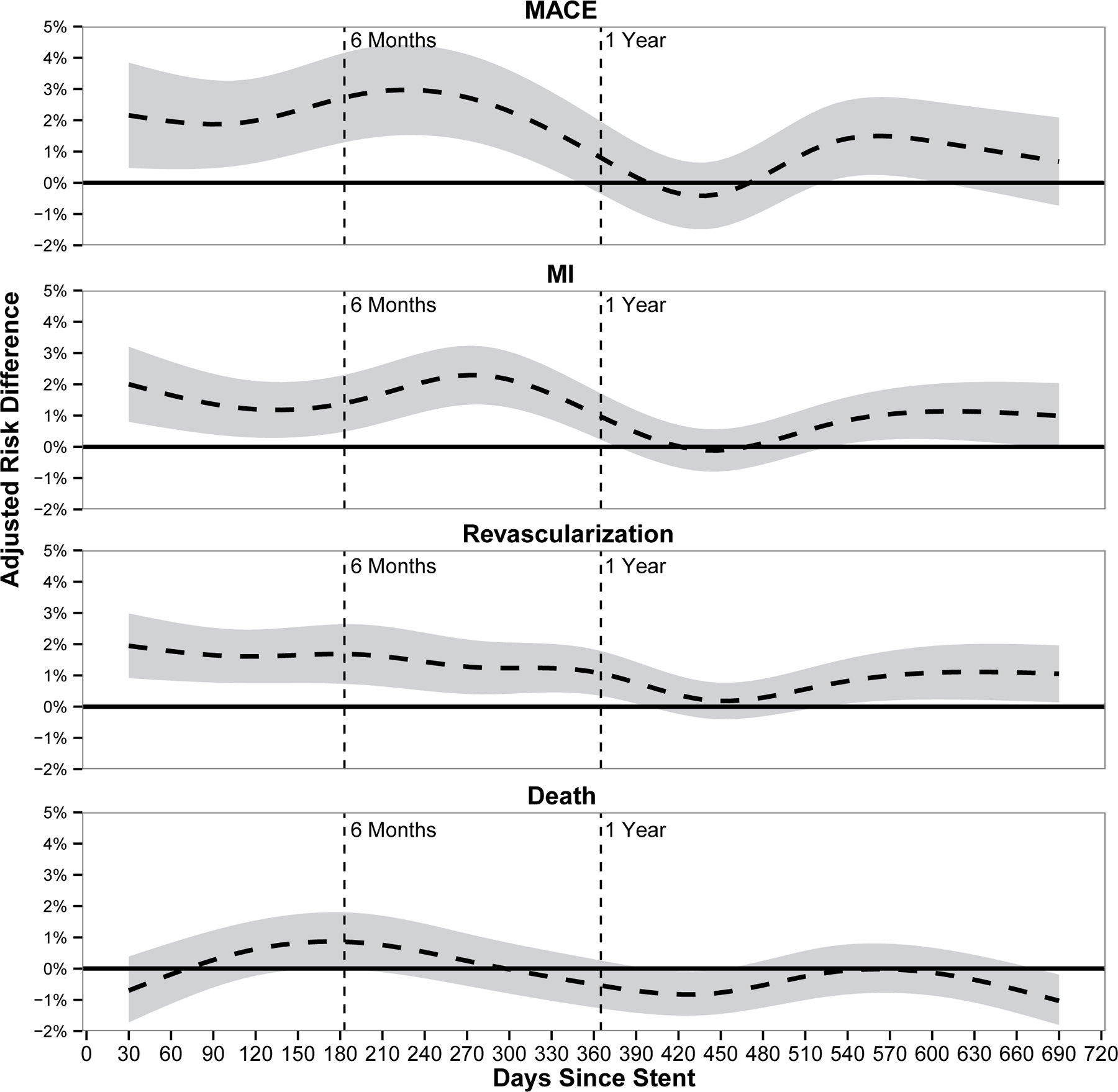
Adjusted risk difference for the composite variable of major adverse cardiac events (MACE) and its components: myocardial infarction (MI), revascularization, and death occurring in the 30 days following surgery by time from stent placement as compared to the non-stented cohort. Risk adjusted for emergency case status, admission status, ASA classification, and preoperative dyspnea.
MACE = Major Adverse Cardiac Events; MI = Myocardial Infarctions.
Shaded area represents 95% confidence interval around adjusted risk difference.
Sensitivity Analysis of Un-Matched Variables
To assess for potential confounding of our results by the imbalance of un-matched variables, we performed two separate sensitivity analyses of the cohort excluding matched pairs with a recent history of MI and those undergoing emergent surgery. The overall adjusted OR for MACE in the stented cohort from the original analysis was 1.35 (95% CI 1.19–1.54) (Table 3). When we excluded matched pairs with a recent MI, the adjusted OR for MACE increased slightly to 1.367 (95% CI 1.174–1.592). When excluding emergent surgeries, the adjusted OR for MACE increased to 1.52 (95% CI 1.28–1.82).
Sensitivity Analysis of Post-Operative Troponin Surveillance and MI
We assessed the prevalence of troponin surveillance and associated MI rates over time from stent as well as by stent type compared to controls (Table 4). Overall, 18.3% of the stented cohort vs 13.1% of the non-stented cohort had evidence of troponin surveillance in the first 72 hours following surgery (p<0.001). Regardless of time from stent, the proportion of troponin surveillance tests was approximately 1.5 times greater in the stented cohort and the proportion of MIs was approximately 2 fold in the stented versus non-stented cohort. Following the occurrence of MI, stented patients had higher median peak troponin levels compared to non-stented patients (4.9 ng/ml (IQR 1.4–18.1) vs 2.6ng/ml (IQR 0.9–8.8) p<0.001).
Table 4.
Prevalence of Postoperative Troponin Surveillance in the 72 Hours After Surgery and the Outcome of MI within 30 Days
| Overall | Stented | Non-Stented | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening | MI | Screening | MI | Screening | MI | Screening p-value |
||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |||
| Overall | 4176 | (14.8) | 483 | (1.7) | 1718 | (18.3) | 250 | (2.7) | 2458 | (13.1) | 233 | (1.2) | ||
| Stent Time | <6 Weeks | 429 | (24.7) | 61 | (3.5) | 181 | (31.3) | 33 | (5.7) | 248 | (21.5) | 28 | (2.4) | <0.001 |
| 6 Weeks to 3 Months | 551 | (19.8) | 74 | (2.7) | 242 | (26.1) | 37 | (4.0) | 309 | (16.7) | 37 | (2.0) | ||
| 3 to 6 Months | 648 | (17.6) | 79 | (2.1) | 242 | (19.7) | 40 | (3.3) | 406 | (16.5) | 39 | (1.6) | ||
| 6 to 12 Months | 932 | (13.9) | 88 | (1.3) | 390 | (17.5) | 55 | (2.5) | 542 | (12.2) | 33 | (0.7) | ||
| >12 Months | 1616 | (12.2) | 181 | (1.4) | 663 | (15.0) | 85 | (1.9) | 953 | (10.8) | 96 | (1.1) | ||
| Stent Type | BMS | 2309 | (15.6) | 266 | (1.8) | 933 | (18.9) | 132 | (2.7) | 1376 | (14.0) | 134 | (1.4) | 0.16 |
| DES | 1824 | (14.0) | 210 | (1.6) | 769 | (17.7) | 115 | (2.6) | 1055 | (12.1) | 95 | (1.1) | ||
| Both | 43 | (12.8) | 7 | (2.1) | 16 | (14.3) | 3 | (2.7) | 27 | (12.1) | 4 | (1.8) | ||
BMS=Bare Metal Stent; DES=Drug-Eluting Stent
DISCUSSION:
This analysis assessed the attributable risk of a preceding coronary stent on post-operative MACE in patients undergoing non-cardiac surgery. Compared to surgery patients without a preceding stent, stented patients had a 2% increased risk for MI and revascularization during the year following stent placement. In contrast, there was no increased risk of mortality between patient groups. The increased risk for MI did not vary by stent type (bare-metal or drug-eluting). Finally, the use of cardiac troponin tests in the immediate postoperative period was significantly higher among stented patients compared to the non-stented patients with similar cardiac risk and surgical procedures.
The findings from this study suggest that patients with a history of coronary stenting prior to an elective surgery are at higher risk for post-operative MACE than would be suggested by the revised cardiac risk index, which incorporates underlying cardiac and surgical factors into its calculation. Furthermore, this higher risk persists for a full year following stent placement. This risk appears limited to post-operative MI and subsequent revascularization, without an increase in mortality. The mechanism behind this elevated risk is not clear. The stent, with its attendant risks for thrombosis, may be directly causative of the higher MACE rates, or it may serve as a marker of more severe CAD and higher cardiac risk than is accounted for by the rCRI. Given that DES and BMS differ in their risk for thrombosis based on time to stent endothelialization and that MACE rates did not differ by stent type would suggest the latter. Additionally, a significant proportion of PCIs fail to achieve complete coronary revascularization10–12 leaving a large territory of myocardium at risk for infarction. Incidentally, in our study, the stented cohort had higher peak troponin levels in association with postoperative MI compared to the non-stented cohort. The findings from this study also support the concept that prophylactic revascularization with coronary stenting prior to high-risk surgery is not beneficial.4,5 Furthermore, they build upon this concept by suggesting this lack of benefit extends for a full year following revascularization, even after adjusting for factors shown to be important predictors of postoperative adverse cardiac events.3
In addition to the cardiac and surgical contributions to post-operative MACE, acute blood loss anemia and subsequent low flow circulatory states can also be significant contributors13,14. In this study, both the requirement of blood transfusions and postoperative MI were higher in the stented cohort as compared to the non-stented cohort. One explanation for this finding is the difference in antiplatelet therapy usage between stented and non-stented patients. In this study, documentation of antiplatelet therapy management in the peri-operative period was not reliable given the inability to determine when and if therapy was held and reinstated. Morbidity and mortality associated with antiplatelet therapy vary by which agent is discontinued and for how long.15–17 However, recent studies have shown that temporary cessation versus continued antiplatelet therapy perioperatively does not significantly alter the risk for MI or mortality and may potentially lead to increased major bleeding events.18,19
Elevated postoperative troponin levels are non-specific for predicting cardiac events but have been shown to be associated with mortality20–23 and are viewed as a marker for poor outcomes. The real question lies in how to react to the elevation in postoperative troponins to mitigate the associated mortality.24 If nothing else, the elevation in post-operative troponins may lead to increased attentiveness and escalation of patient care. We found that much of the cardiac risk in the stent cohort appears to be non-ST elevation MI and may be attributable to the significantly higher use of cardiac biomarker tests in the early postoperative period among stented patients, which would increase MI rates but essentially decrease the risk of MI-associated death for the study cohort. Unfortunately, this observational study cannot determine whether surveillance led to ‘over diagnosis’ or early diagnosis and intervention for post-operative MI. Regardless, the utility of this practice remains unclear without a uniform response strategy to elevated postoperative troponin levels and a measurement of its effect on surgical outcomes.
LIMITATIONS:
There are several limitations to our study. Our cohort is composed entirely of VA patients comprised of mainly white, older males and may not be generalizable to a more diverse population. All of the patients in both cohorts had documented cardiac risk factors for adverse surgical outcomes but we do not have information on their underlying coronary anatomy or the extent of their coronary disease. Thus patients with a history of revascularization may have more extensive underlying atherosclerotic disease. Regardless it does not appear that coronary stenting conveys a protective effect. Examining MI rates in patients who had a plasma troponin level assessed in the early postoperative period may have resulted in an ascertainment bias, as discussed above.
While we were able to control for many variables that were different between the two cohorts, residual confounding could account for the differences we observed. Specifically, confounding by indication exists for those patients selected to receive a revascularization procedure versus those who do not. Patients with coronary stenting likely experienced recent symptomatic cardiac disease compared to their non-stented controls. This is evident from the significantly higher rates of recent MI in the stented compared to the non-stented cohort. However, we recognize that it would be extremely difficult to match on the variable of MI within 6 months because so few would not have undergone a coronary revascularization and we did feel this approach would restrict the population in such a way that it would not be very generalizable. The results of our sensitivity analyses suggest that matching on the other important cardiac and surgical risk factors absorbed this potential source of confounding.
CONCLUSIONS:
Within the first year following coronary stent placement, stented patients undergoing surgery have an approximately 2% higher risk for post-operative MI and revascularization compared to non-stented, matched controls. There were no differences in mortality. Stent type, BMS or DES, did not confer a difference in MACE rates over time from stent placement. Recent coronary stent placement appears to be a marker of advanced coronary artery disease and the risk of adverse cardiac events following surgery remains elevated in the first year following PCI compared to non-stented patients with similar cardiac risk factors.
Acknowledgements
The VA National Surgery Office has approved the manuscript for adherence to the data use agreements. The opinions expressed are those of the authors and not necessarily those of the Department of Veterans Affairs or the United States Government. The authors would like to acknowledge The VA Surgical Quality Data Use Group (SQDUG) for its role as scientific advisors and for the critical review of data use and analysis presented in this manuscript. None of the authors have relevant financial disclosures pertaining to this manuscript.
Source of Funding
This study is supported by a VA Health Services Research & Development Grant (#IIR 09-347). Drs. Maddox and Richman are supported by VA Career Development Awards. Dr. Holcomb is supported by grant T32 HS013852-11 from the Agency for Healthcare Research and Quality, Rockville, MD, USA.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
REFERENCES:
- 1.Wijeysundera DN, Wijeysundera HC, Yun L, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population-based study. Circulation 2012;126(11):1355–1362. [DOI] [PubMed] [Google Scholar]
- 2.van Kuijk JP, Flu WJ, Schouten O, et al. Timing of noncardiac surgery after coronary artery stenting with bare metal or drug-eluting stents. The American journal of cardiology 2009;104(9):1229–1234. [DOI] [PubMed] [Google Scholar]
- 3.Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. Jama 2013;310(14):1462–1472. [DOI] [PubMed] [Google Scholar]
- 4.McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004;351(27):2795–2804. [DOI] [PubMed] [Google Scholar]
- 5.Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V Pilot Study. J Am Coll Cardiol 2007;49(17):1763–1769. [DOI] [PubMed] [Google Scholar]
- 6.Nuttall GA, Brown MJ, Stombaugh JW, et al. Time and cardiac risk of surgery after bare-metal stent percutaneous coronary intervention. Anesthesiology 2008;109(4):588–595. [DOI] [PubMed] [Google Scholar]
- 7.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100(10):1043–1049. [DOI] [PubMed] [Google Scholar]
- 8.Wickham H GGPLOT2: Elegant Graphics for Data Anlaysis New York: Springer; 2009. [Google Scholar]
- 9.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2014. [DOI] [PubMed]
- 10.Kim YH, Park DW, Lee JY, et al. Impact of angiographic complete revascularization after drug-eluting stent implantation or coronary artery bypass graft surgery for multivessel coronary artery disease. Circulation 2011;123(21):2373–2381. [DOI] [PubMed] [Google Scholar]
- 11.Wu C, Dyer AM, Walford G, et al. Incomplete revascularization is associated with greater risk of long-term mortality after stenting in the era of first generation drug-eluting stents. The American journal of cardiology 2013;112(6):775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Head SJ, Mack MJ, Holmes DR Jr., et al. Incidence, predictors and outcomes of incomplete revascularization after percutaneous coronary intervention and coronary artery bypass grafting: a subgroup analysis of 3-year SYNTAX data. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery 2012;41(3):535–541. [DOI] [PubMed] [Google Scholar]
- 13.Henke PK, Zamora-Berridi G, Englesbe MJ, et al. A case-cohort study of postoperative myocardial infarction: impact of anemia and cardioprotective medications. Surgery 2014;156(4):1018–1026, 1029. [DOI] [PubMed] [Google Scholar]
- 14.Walsh M, Devereaux PJ, Garg AX, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology 2013;119(3):507–515. [DOI] [PubMed] [Google Scholar]
- 15.Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation 2006;113(24):2803–2809. [DOI] [PubMed] [Google Scholar]
- 16.Patel PA, Fleisher LA. Aspirin, clopidogrel, and the surgeon. Advances in surgery 2014;48:211–222. [DOI] [PubMed] [Google Scholar]
- 17.Schouten O, van Domburg RT, Bax JJ, et al. Noncardiac surgery after coronary stenting: early surgery and interruption of antiplatelet therapy are associated with an increase in major adverse cardiac events. Journal of the American College of Cardiology 2007;49(1):122–124. [DOI] [PubMed] [Google Scholar]
- 18.Devereaux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. The New England journal of medicine 2014;370(16):1494–1503. [DOI] [PubMed] [Google Scholar]
- 19.Mantz J, Samama CM, Tubach F, et al. Impact of preoperative maintenance or interruption of aspirin on thrombotic and bleeding events after elective non-cardiac surgery: the multicentre, randomized, blinded, placebo-controlled, STRATAGEM trial. British journal of anaesthesia 2011;107(6):899–910. [DOI] [PubMed] [Google Scholar]
- 20.Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014;120(3):564–578. [DOI] [PubMed] [Google Scholar]
- 21.van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation 2013;127(23):2264–2271. [DOI] [PubMed] [Google Scholar]
- 22.Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA : the journal of the American Medical Association 2012;307(21):2295–2304. [DOI] [PubMed] [Google Scholar]
- 23.Weber M, Luchner A, Seeberger M, et al. Incremental value of high-sensitive troponin T in addition to the revised cardiac index for peri-operative risk stratification in non-cardiac surgery. European heart journal 2013;34(11):853–862. [DOI] [PubMed] [Google Scholar]
- 24.Beckman JA. Postoperative troponin screening: a cardiac Cassandra? Circulation 2013;127(23):2253–2256. [DOI] [PubMed] [Google Scholar]


