Abstract
The goal of this study was to evaluate the role of host immunity in gastritis and epithelial damage due to Helicobacter pylori. Splenocytes from H. pylori-infected and uninfected C57BL/6 mice were adoptively transferred to H. pylori-infected and uninfected severe combined immunodeficient (SCID) mice. Transfer was verified by flow cytometry, and all mice were evaluated for the presence of delayed-type hypersensitivity (DTH) by footpad inoculation with sterile H. pylori sonicate and for humoral immunity by enzyme-linked immunosorbent assay. The severity of gastritis and gastric epithelial damage was quantified histologically, epithelial proliferation was determined by proliferating cell nuclear antigen staining, and colonization was quantified by culture. C57BL/6 mice, but not nonrecipient SCID mice, developed moderate gastritis in response to H. pylori. In contrast, recipient SCID mice developed severe gastritis involving 50 to 100% of the gastric mucosa and strong DTH responses not present in C57BL/6 mice. DTH, but not serum anti-H. pylori immunoglobulin G, correlated with adoptive transfer, gastritis, and bacterial clearance. Severe gastritis, but not bacterial colonization, was associated with epithelial metaplasia, erosions, and an elevated labeling index. This study demonstrates that (i) adaptive immunity is essential for development of gastritis due to H. pylori in mice, (ii) T-cell-enriched lymphocytes in SCID mice induce DTH and gastritis, which is more severe than donor gastritis, and (iii) the host inflammatory response, not direct bacterial contact, causes epithelial damage. The greater severity of gastritis in recipient SCID mice than in donor C57BL/6 mice suggests that gastritis is due to specific T-cell subsets and/or the absence of regulatory cell subsets in the transferred splenocytes.
In recent years, studies of the host response to Helicobacter pylori infection have been primarily directed at a protective effect. Most studies have been directed towards the development of vaccination strategies (3, 14–17, 28, 35); only a few studies have examined the role of the host in tissue damage associated with infection by H. pylori (30, 31). In spite of this emphasis on vaccination schemes, a body of evidence regarding the pathogenesis of gastritis is accumulating. Marked differences in the severity of disease in different host species and, more recently, in different strains of the same host species have led to the suggestion that host immune response is important in determining the severity of gastritis. In some hosts, such as humans (26), rodents (12, 19, 27), and cheetahs (13), infection with some Helicobacter species results in severe gastritis with attendant epithelial changes ranging from erosion to ulceration and from metaplasia to neoplasia. In other hosts, such as dogs and cats (9, 21, 33, 34, 41), gastric Helicobacter infection results in little or no inflammation and no epithelial changes. In addition to species differences, host strain differences determine the outcome of infection. C57BL/6 mice are readily susceptible to some species and strains of Helicobacter and develop gastritis in response to infection, whereas BALB/c mice are more resistant to both colonization and inflammation (32, 38). These differences suggest that host response is important in determining if an infected individual develops severe gastritis, ulcers, neoplasia, only mild chronic gastritis, or no disease.
In addition to host response, a large body of data has been generated regarding the potential role of bacterial factors in the damage of host gastric mucosa. A number of bacterial virulence factors, including vacA (1), cagA and the related cag pathogenicity island (37), and iceA1 (36), have been shown to be associated with the increased severity of disease in humans, and there is clear evidence that in vitro H. pylori gene products can cause epithelial damage (6) or induce cytokine secretion potentially leading to inflammation (7). In spite of these correlations, however, studies evaluating the roles of bacterial factors in vivo in experimental models have been limited, and a direct effect of bacterial products causing host epithelial cell damage in vivo is controversial (8, 40). Thus, it is not clear if bacterial products damage host cells directly in vivo, if damage occurs as a by-product of host inflammation, or if both effects are important.
The overall goals of this study were (i) to demonstrate that H. pylori-associated gastritis and gastric epithelial damage are dependent on an intact adaptive immune response, (ii) to determine if such gastritis can be transferred to immunodeficient hosts by splenocytes from infected or uninfected donor mice, and (iii) to determine if gastritis is accompanied by in vivo evidence of a T-cell-mediated immune response (delayed-type hypersensitivity [DTH] response).
MATERIALS AND METHODS
Mice.
Four- to six-week-old female helicobacter-free C57BL/6J mice and C57BL/6J scid/scid (severe combined immunodeficient [SCID]) mice were purchased from Jackson Laboratory (Bar Harbor, Maine). SCID mice are deficient in a DNA-dependent kinase necessary for recombination of both T-cell receptors and antibodies (22). Thus, neither T nor B cells mature in these mice, and they have no adaptive immunity. Mice were housed in microisolator cages and offered sterile Teklad lab chow and water ad libitum. One week following arrival, SCID mice were anesthetized with methoxyflurane, and 100 μl of blood was collected from the periorbital sinus. Serum was separated and stored at −20°C until used for the determination of the immunoglobulin G (IgG) level by enzyme-linked immunosorbent assay (ELISA) (see below). SCID phenotype was verified by the absence of detectable mouse IgG (optical density at 405 nm, <0.1) and/or the absence of a detectable serologic response to antigenic challenge.
For bacterial inoculation, animals were given 0.2 ml of a suspension of broth-cultured H. pylori SS1 containing 109 CFU of bacteria in logarithmic phase per ml. Mice were inoculated three times at 2-day intervals by oral gavage. At the termination of the inoculation phase of the study, mice were anesthetized by injection of phenobarbital and exsanguinated by cardiac puncture. The stomach and spleen were aseptically removed, the stomach was bisected along the greater and lesser curvatures, the squamous portion was removed, and half the stomach was homogenized for the determination of bacterial colonization by plate dilution as previously described (11). The remainder of the stomach was sectioned transversely into 1-mm strips; one strip was frozen in Tissue-Tek OCT compound (Sakura, Inc., Torrance, Calif.) and stored at −70°C, and the remainder was fixed by immersion in 10% neutral buffered formalin, embedded in paraffin, cut into 6-μm sections, and stained with hematoxylin and eosin and Genta (20) stains. Some of the spleens were placed in cold (4°C) Hanks balanced salt solution for the isolation of splenocytes (see below). All procedures involving animals were approved by the Ohio State University laboratory animal care and use committee.
DTH response.
For the preparation of bacterial sonicate, broth cultures containing approximately 109 CFU of H. pylori per ml were washed twice in phosphate-buffered saline (PBS), resuspended in 1/10 of the original volume of PBS, and sonicated with a Bradford sonifier at 4°C for two cycles of four 30-s bursts with 30-s rests. Intact bacteria were removed by centrifugation (5,000 rpm in a Sorvall RC-5B refrigerated superspeed centrifuge for 10 min), and sterility was ascertained by culture. The protein concentration was determined by a microprotein assay (Bio-Rad Laboratories, Hercules, Calif.), and aliquots were stored at −20°C until used. For mouse footpad injection, sonicates were adjusted to 10 μg/30 μl, and each mouse was given 30 μl of sonicate by injection into one hind footpad. Previous evaluation determined that 10 μg of antigen elicited a detectable and specific response (present only in sensitized mice [data not shown]). The opposite footpad received sterile PBS. Footpad thickness was measured with a dial thickness gauge (Swiss Precision Instruments, Los Angeles, Calif.) before injection, 24 h after injection, and, in some cases, 48 h after injection.
Bacterial strains.
H. pylori SS1 (25) was kindly supplied by Adrian Lee. This strain is a cagA- and VacA-positive human isolate which is adapted for the colonization of mice. Bacteria were grown in brucella broth with 10% fetal calf serum or on blood agar plates under microaerobic conditions at 37°C. Broth cultures were enumerated by a hemacytometer and/or plate dilution.
Adoptive transfer.
For the isolation of splenocytes, spleens were disaggregated in cold Hanks balanced salt solution, clumps were allowed to settle out, and the supernatant was sedimented at 1,000 rpm in an IEC PR-600 centrifuge for 10 min. Erythrocytes were removed by hypotonic lysis, and the splenocytes were resuspended in Dulbecco’s PBS (D-PBS). Splenocytes were examined for viability by trypan blue exclusion and adjusted to 107 viable cells/ml, and recipient SCID mice were given 0.1 ml by intraperitoneal injection 4 weeks after bacterial inoculation.
Flow cytometry.
Splenocytes from C57BL/6 and SCID mice were isolated as described above, and 1 × 106 to 2 × 106 cells were resuspended in 100 μl of Fc Block (Pharmingen, San Diego, Calif.) and incubated for 10 min at 4°C. Fluorescein isothiocyanate- or phycoerythrin-labeled anti-CD4, anti-CD8, or anti-CD45R/B220 (Pharmingen) was added, and cells were incubated for 45 min at 4°C. Cells were washed three times in D-PBS with bovine serum albumin and 0.1% NaN3 and resuspended in 100 μl of D-PBS, and 100 μl of 4% paraformaldehyde was added. Cell suspensions were evaluated on an EPICS flow cytometer.
ELISA.
Murine IgG levels in SCID mice and anti-H. pylori IgG levels in all mouse sera taken at termination (herein referred to as terminal sera) were determined by ELISA. ELISA plates (96 wells; Corning) were coated either with 4 μg of unconjugated goat anti-mouse IgG (Pierce) per ml for the determination of circulating IgG in naive SCID mice or with 100 μg of H. pylori SS1 sonicate per ml for the determination of the serologic response to H. pylori. For the determination of mouse IgG levels, plates were coated with IgG for 12 h at 4°C, washed, blocked with 200 μl of 0.1% bovine serum albumin per well, washed, and incubated for 2 h at room temperature with SCID mouse sera diluted 1:20. Plates were washed, incubated with peroxidase-conjugated goat anti-mouse IgG for 90 min at room temperature, and washed again, and peroxidase was detected by incubation with azino-bisthiosulfonate in the dark for 5 to 10 min. The optical density at 405 nm was recorded.
For the determination of anti-H. pylori IgM and IgG levels in terminal sera (29), plates were coated with 100 μg of H. pylori SS1 sonicate per ml for 48 h at 4°C, washed, blocked with blocking buffer (gelatin [1 g/liter] in 0.1 M PBS–0.02% sodium azide), washed again, and incubated for 90 min at 37°C with terminal mouse sera diluted 1:50. Plates were then washed again, incubated with alkaline phosphatase-conjugated goat anti-mouse IgM or IgG (Bio-Rad), and washed, and alkaline phosphatase was detected with an alkaline phosphatase substrate kit (Bio-Rad) according to the manufacturer’s instructions.
Immunohistochemistry for expression of PCNA.
To determine the labeling index, formalin-fixed sections were deparaffinized, antigens were exposed with antigen retrieval solution (Bio Genex), and the presence of proliferating cell nuclear antigen (PCNA) was determined by histochemical staining. Primary antibody was from Pharmingen (PC-10, mouse anti-PCNA), and secondary antibody was from Vector Laboratories (biotinylated goat anti-mouse antibody). Antibody staining was detected with an ABC detection kit from Vector Laboratories, and slides were counterstained with hematoxylin. The labeled and nonlabeled nuclei in three well-oriented glands in three different locations in the gastric fundus were enumerated, and the labeling index was calculated by dividing the number of labeled nuclei by the total number of nuclei counted and then multiplying by 100.
Histologic evaluation.
The severity of gastritis in hematoxylin-and-eosin-stained sections was determined by enumerating the number of 20× microscopic fields containing the following pathologic changes: (i) neutrophilic inflammation (polymorphonuclear leukocytes [PMNs]), (ii) inflammatory infiltrate (lymphocytic, plasmacytic, histiocytic, neutrophilic, or, most commonly, mixed) which was severe enough to displace glands (gastritis), (iii) the destruction of gastric glands or neutrophilic gland abscesses by inflammatory infiltrate (adenitis), and (iv) the loss of normal fundic gland morphology with a replacement of specialized cells by mucus-type epithelium (metaplasia) and epithelial erosions. Genta-stained sections were evaluated to determine the number of fields containing Alcian blue-positive (intestinal-type mucus-containing) epithelial cells (intestinal metaplasia). Each of the categories was expressed as percent affected mucosa (the number of fields containing the lesion divided by the total number of fields). Because mild antral inflammation was present nonspecifically in all mouse groups, only fundic and cardiac mucosae were scored. All sections collected were examined in their entirety, and slides were read blind, i.e., without knowledge of their source.
Statistics.
All experimental groups contained five to eight mice. Group means were compared by nonparametric methods (Mann-Whitney U test). A P value of <0.05 was considered statistically significant.
RESULTS
Adoptive transfer.
Flow cytometric analysis revealed that isolated C57BL/6 splenocytes were 32% T cells (17% CD4 and 14% CD8) and 59% B cells (CD45R/B220) (Table 1). Flow cytometric analysis of cell markers in SCID mice was complicated by low-level staining of large, undifferentiated cells. However, peak populations revealed that cells from recipient SCID mice were enriched with T cells (39%) and depleted of B cells (23%). The CD4-to-CD8 ratio of donor splenocytes (1:1.18) did not differ significantly from that of recipient SCID mice (1:1.22, P > 0.5). Nonrecipient SCID mice did not have a detectable peak in any of the cell marker categories examined.
TABLE 1.
Surface markers of splenocytes from donor and recipient mice
| Mouse strain | % of splenocytesa
|
|||
|---|---|---|---|---|
| CD3 | CD4 | CD8 | CD45R/B220 | |
| C57BL/6 (donors) | 32.3 ± 4.8 | 16.8 ± 2.9 | 13.8 ± 2.2 | 59.3 ± 10.6 |
| SCID (recipients) | 39.3 ± 7.3 (0.014) | 25.1 ± 5.3 (0.009) | 21.2 ± 5.6 (0.001) | 23.4 ± 6.2 (<0.0001) |
Values are means ± standard deviations, with P values in parentheses.
Gastritis in C57BL/6 and SCID mice.
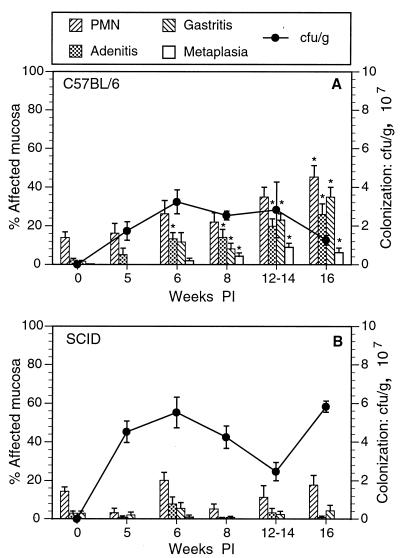
Histologic evaluation of gastritis included four categories of inflammation: PMN, adenitis, gastritis, and mucus metaplasia (see definitions above). These changes are illustrated in Fig. 1. Neutrophils were present in all groups of C57BL/6 mice, infected and uninfected, although in infected mice they increased late in the course of infection, their levels becoming significantly elevated by 16 weeks after inoculation (Fig. 2A). Thus, neutrophils alone correlated only weakly with infection status. In contrast, the levels of inflammation of the other three categories increased markedly in response to bacterial infection. Adenitis, gastritis, and metaplasia were not present in uninfected mice, but their levels significantly increased in infected mice by 6 or 8 weeks after infection. By 16 weeks, all levels were 10- to 20-fold greater than levels in uninfected C57BL/6 mice (Fig. 2A).
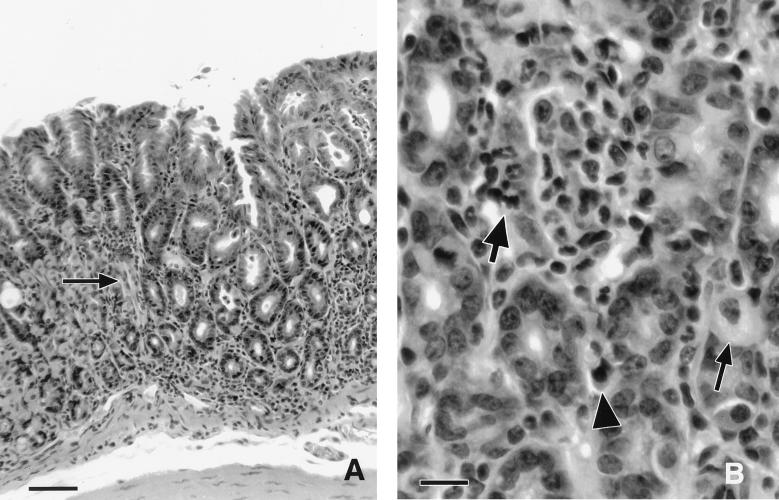
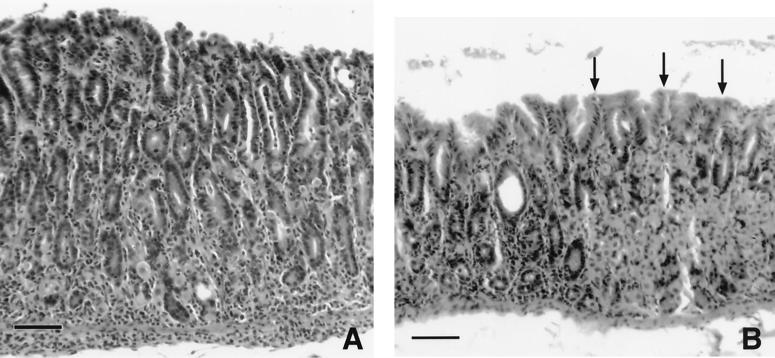
FIG. 1.
Histologic appearance of gastritis in an infected C57BL/6 mouse killed 16 weeks after bacterial inoculation. (A) Junction of normal and abnormal fundic mucosae. The affected area (to the right of the arrow) is characterized by widespread mixed inflammation and the loss of parietal and chief cells, which were replaced by undifferentiated mucus cells (metaplasia). The area to the left of the arrow is normal, characterized by gastric glands containing mostly parietal and chief cells. Bar = 50 μm. (B) Higher magnification demonstrating neutrophilic adenitis with the destruction of glands (large arrow), mixed inflammation including lymphocytes and plasma cells (arrowhead), and the almost complete replacement of gastric glands by undifferentiated mucus cells. A single remaining parietal cell is evident (small arrow). Bar = 14 μm.
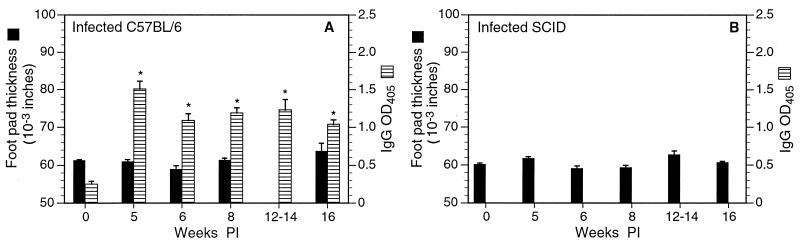
FIG. 2.
Change in gastric inflammation and bacterial colonization in infected C57BL/6 and congenic SCID mice between 5 and 16 weeks postinoculation (PI). (A) In C57BL/6 mice, all four indicators of inflammation increased over the course of infection, but bacterial colonization did not change. (B) Variable numbers of neutrophils were present in infected and uninfected SCID mice at all experimental time intervals. Otherwise, inflammation remained low and did not change over the course of infection. Bacterial colonization was somewhat greater than that in C57BL/6 mice and did not change over the course of infection. ∗, P < 0.05 compared to result for uninfected mice (week 0).
In contrast to infected mice, uninfected mice did not have adenitis, gastritis, or metaplasia (Fig. 2A, week 0). Mild neutrophilic infiltrates were present in some individual uninfected mice (mean, 14%).
In infected SCID mice that did not receive splenocytes, inflammation was minimal or nonexistent (Fig. 2B). Significant adenitis, gastritis, and metaplasia did not occur in infected SCID mice killed 5, 6, 8, 12, or 16 weeks after inoculation, regardless of infection status. Mild neutrophilic infiltrates were present in some infected and uninfected SCID mice, involving up to 30% of the gastric mucosa in individual mice, but there was no relationship between the presence of neutrophils and infection with H. pylori.
Gastritis in recipient SCID mice.
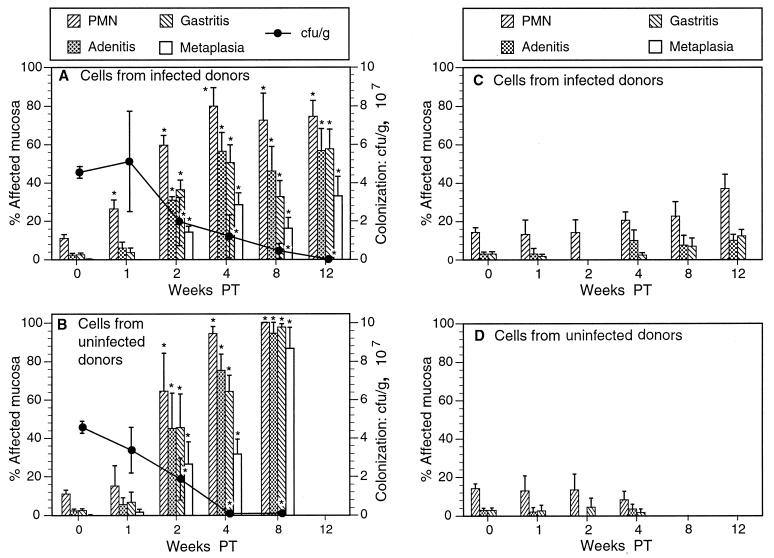
In contrast to nonrecipient SCID mice, H. pylori-infected SCID mice that received splenocytes developed severe gastritis rapidly after adoptive transfer (Fig. 3A and B). Within 2 weeks of the transfer of splenocytes (i.e., 6 weeks after infection) from either infected (Fig. 3A) or uninfected (Fig. 3B) C57BL/6 donors, SCID mice developed widespread neutrophilic infiltrates (up to 100% of the gastric mucosa), adenitis (up to 94%), gastritis (up to 90%), and metaplasia (up to 50%). Mean inflammation of all four categories continued to increase over time both in SCID mice receiving splenocytes from infected donors and in those receiving splenocytes from uninfected donors.
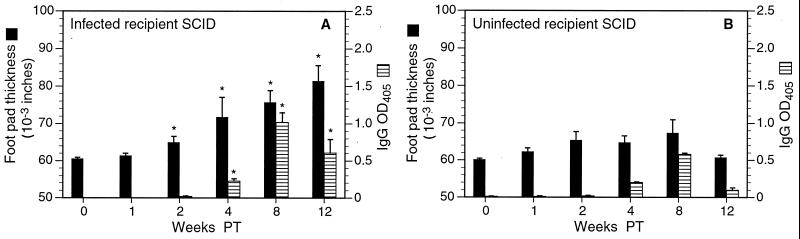
FIG. 3.
Changes in inflammation and colonization in infected and uninfected SCID recipients of splenocytes from infected or uninfected donors. (A) In infected recipients of splenocytes from infected donors, levels of neutrophilic inflammation, adenitis, gastritis, and metaplasia were significantly elevated 2 weeks posttransfer (PT) and continued to increase over the course of the experiment. (B) Infected recipients of cells from uninfected donors responded similarly, but inflammation in all categories was somewhat more pronounced than in the recipients of cells from infected donors. Bacterial colonization decreased precipitously in both groups of mice. Uninfected recipients of splenocytes from infected (C) or uninfected (D) donors developed only minimal, inconsistent gastric inflammation. Slight increases in levels of adenitis and gastritis in recipients of cells from infected donors may have been due to cross-reaction with enteric flora. ∗, P < 0.05 compared to results for nonrecipients (week 0). Note that since adoptive transfer was performed 4 weeks after bacterial infection, the postinoculation interval (Fig. 2) was 4 weeks longer than the PT interval. Thus, recipient SCID mice killed 12 weeks PT had been colonized for as long as nonrecipient SCID mice killed 16 weeks postinoculation (see Materials and Methods).
In recipient SCID mice, the development of gastritis was dependent on H. pylori infection as well as on adoptive transfer. In contrast to infected recipient SCID mice, uninfected SCID mice developed only minimal gastritis whether or not they received splenocytes by adoptive transfer (Fig. 3C and D). Some uninfected SCID mice which received cells from infected donors had neutrophilic infiltrates in up to 30% of the gastric mucosa or adenitis in up to 14% (Fig. 3C), but otherwise no histologic changes were detected.
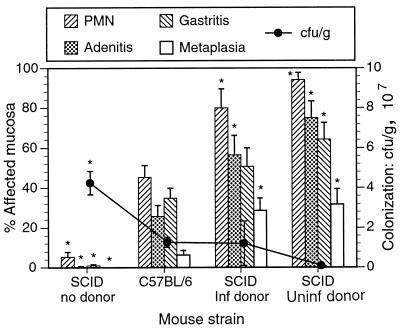
Inflammation in all four categories was significantly more severe in recipient SCID mice than in C57BL/6 mice (Fig. 2 and 3). In the most severely affected group of C57BL/6 mice, those killed 16 weeks after inoculation, inflammation was moderate. Adenitis and gastritis involved less than 35% of the gastric mucosa, and metaplasia involved 6%. In contrast, adenitis and gastritis involved from 50 to 75% of the gastric mucosa in recipient SCID mice killed only 4 weeks after transfer, and metaplasia involved about 30%. Inflammation continued to increase in extent in recipient SCID mice and was somewhat more widespread in recipients of cells from uninfected donors than in recipients of cells from infected donors. Figure 4 compares four groups of infected mice: SCID and C57BL/6 mice, SCID recipients of cells from infected donors, and SCID recipients of cells from immune donors. Data were taken from Fig. 2 and 3. The contrast in the severity of gastritis between recipient SCID and C57BL/6 mice was readily apparent on casual inspection of histologic sections (Fig. 5).
FIG. 4.
Effects of host and donor strains on gastric inflammation and bacterial colonization. SCID mice were killed 4 weeks after transfer (8 weeks after infection), and C57BL/6 mice were killed 16 weeks after infection (the most severely affected C57BL/6 group). Levels of neutrophilic inflammation, adenitis, gastritis, and metaplasia were significantly higher in infected recipient SCID mice than in infected C57BL/6 mice, even though C57BL/6 mice had been infected 8 weeks longer. In SCID recipients of naive cells, inflammation was the greatest and bacterial colonization was the least of any of the groups. Infected nonrecipient SCID mice did not develop inflammation, and bacterial colonization was highest in that group. ∗, P < 0.05 compared to results for C57BL/6 mice.
FIG. 5.
(A) Severe inflammation in an infected recipient SCID mouse infected with H. pylori, given splenocytes from an uninfected donor, and killed 4 weeks after transfer (8 weeks after bacterial inoculation). Note the marked, widespread infiltration of inflammatory cells, loss of parietal cells, destruction of glands, and overall thickening of the mucosa compared to panel B. (B) Moderate, multifocal inflammation in an infected C57BL/6 mouse killed 8 weeks after bacterial inoculation. Inflammation is less extensive and areas of normal mucosa are retained (arrows). Bars = 60 μm.
Epithelial lesions.
In mice killed 3 months after transfer, four morphologic indicators of epithelial damage were evaluated. Mucus metaplasia was defined as described above. The percentages of gastric mucosae with epithelial erosions and intestinal metaplasia were evaluated in Genta-stained sections. Epithelial erosions were defined as the loss of necrosis of surface epithelium accompanied by the flattening or proliferation of adjacent epithelial cells. Intestinal metaplasia was defined as the presence of intestinal-type (Alcian blue-positive) mucus in gastric epithelial cells. Finally, the labeling index was determined in PCNA-stained sections. All four epithelial changes correlated significantly with gastritis and adaptive immune response (Table 2). Mucus metaplasia and erosions were present only in mice with gastritis (infected C57BL/6) or severe gastritis (infected recipient SCID). The group with the most severe gastritis (recipients of splenocytes from uninfected donors) had the most extensive mucus metaplasia, intestinal metaplasia, and epithelial erosions and the highest rate of epithelial proliferation (labeling index). In contrast, the highest degree of bacterial colonization was present in the group with no gastritis and no epithelial lesions (infected nonrecipient SCID mice). In addition to significant differences in mouse groups, there was a statistically significant correlation between the severity of gastritis and epithelial changes. Slopes, significance levels (P), and correlation coefficients (r2) of linear correlations between gastritis and all four epithelial changes are given in Table 3. Gastritis was positively correlated with epithelial changes.
TABLE 2.
Bacterial colonization and epithelial changes in mice killed 3 months after adoptive transfera
| Group | n | % Mucosa with:
|
Labeling index | H. pylori in gastric mucosa (106 CFU/g) | |||
|---|---|---|---|---|---|---|---|
| Gastritis | Mucus metaplasia | Intestinal metaplasia | Erosions | ||||
| Uninfected C57BL/6 | 5 | 0.8 ± 0.8* | 0 | 16.0 ± 2.2 | 0 | 38.6 ± 3.0 | 0 |
| Infected C57BL/6 | 6 | 46.3 ± 6.3 | 6.2 ± 2.8 | 28.7 ± 7.9 | 0 | 46.0 ± 0.0 | 11.6 ± 2.7 |
| Uninfected SCID | 5 | 13.0 ± 3.1* | 0 | 15.2 ± 5.9 | 0 | 40.0 ± 1.7 | 0 |
| Infected SCID | 5 | 0.6 ± 0.6* | 0 | 3.2 ± 2.5 | 0 | 41.6 ± 1.0 | 60.1 ± 3.8* |
| Infected recipient SCID, infected donor | 6 | 69.0 ± 3.8* | 24.3 ± 5.9* | 52.3 ± 11.4 | 3.5 ± 1.6 | 45.8 ± 2.9 | 0.2 ± 0.01* |
| Infected recipient SCID, uninfected donor | 5 | 97.8 ± 2.2* | 94.4 ± 5.6* | 84.6 ± 1.03* | 10.6 ± 6.7 | 52.5 ± 6.0 | 0.1 ± 0.01* |
Values are means ± standard deviations. *, significantly different from infected C57BL/6 mice (P < 0.05).
TABLE 3.
Correlation of gastritis (percent of gastric mucosa) with epithelial changes
| Epithelial change | r2 | Slope of regression line (P) |
|---|---|---|
| Mucus metaplasia | 0.68 | 0.73 (<0.0001) |
| Intestinal metaplasia | 0.76 | 0.71 (<0.0001) |
| Epithelial erosions | 0.26 | 0.10 (0.004) |
| Labeling index | 0.45 | 0.13 (0.0003) |
Bacterial colonization.
Concentrations of H. pylori organisms colonizing C57BL/6 mice ranged from 2 × 107 to 3 × 107 CFU/g of gastric mucosa and did not change significantly over the course of the experiment despite increasing inflammation (Fig. 2A). In SCID mice, however, bacterial colonization was inversely correlated with the severity of gastritis. In nonrecipient SCID mice with no gastritis, concentrations ranged from 2 × 107 to 6 × 107 CFU/g and did not change with time (Fig. 2B). In contrast, in recipient SCID mice, colonization decreased as the severity of inflammation increased (Fig. 3A and B). In SCID mice that received cells from infected donors, the concentration decreased more than 200-fold, from >5 × 107 CFU/g 1 week after transfer to 2 × 105 CFU/g 12 weeks after transfer; in those receiving cells from uninfected donors, the concentration decreased more than 300-fold, from >3 × 107 CFU/g 1 week after transfer to fewer than 9 × 104 CFU/g 8 weeks after transfer (P = 0.055 compared to value for mice given cells from infected donors). Three of five recipient mice given cells from uninfected donors were culture negative 8 weeks after transfer.
Levels of bacterial colonization were reflected in the number of bacteria in Genta-stained sections. Nonrecipient SCID mice often had large numbers of bacteria packed in glands in all areas of the gastric mucosa. In contrast, bacteria were difficult to find in sections from other groups of infected mice and were not apparent in mice with the most severe gastritis (recipient SCID mice). Strikingly, the large numbers of bacteria in nonrecipient SCID mice were closely associated with the gastric mucosa, but no epithelial lesions were present in these mice. As described above (Table 2), epithelial lesions were found only in mice with few bacteria and severe inflammation. Overall, there was a negative correlation between colonization and both gastritis and epithelial lesions (Table 3).
DTH response.
A DTH response, as measured by a marked, statistically significant increase in footpad thickness 24 h after antigen injection, was present only in infected recipient SCID mice. DTH response was absent in both infected C57BL/6 (Fig. 6A) and infected nonrecipient SCID (Fig. 6B) mice. For infected recipient SCID mice, however, mean footpad swelling increased markedly both in groups receiving cells from infected donors (Fig. 7A) and in those receiving cells from uninfected donors (not shown). Slight increases in mean footpad thickness were present in uninfected SCID mice which received cells from infected donors and were killed 2, 4, and 8 weeks after transfer, but these differences were small, were not statistically significant, and did not persist to 12 weeks (Fig. 7B).
FIG. 6.
Change in DTH response (footpad swelling) and serum immune response to H. pylori antigens in infected C57BL/6 and SCID mice. (A) C57BL/6 mice did not develop a DTH response to H. pylori antigens even 16 weeks postinoculation (PI) but did develop a strong serum IgG response by 5 weeks PI (DTH was not measured 12 weeks PI). ∗, P < 0.05 compared to results for uninfected mice. (B) Infected SCID mice developed neither DTH nor serum immune responses (serum IgG was undetectable). OD405, optical density at 405 nm.
FIG. 7.
Change in DTH response (footpad swelling) and serum immune response to H. pylori antigens in infected and uninfected recipient SCID mice. (A) Infected SCID recipients of splenocytes from infected donors developed strong DTH responses by 2 weeks posttransfer (PT). Serum IgG responses developed later and remained consistently lower than those in C57BL/6 mice. Responses of SCID recipients of splenocytes from uninfected donors were similar (not shown). ∗, P < 0.05 compared to results for nonrecipients. (B) DTH and serum immune responses were low and inconsistent in uninfected SCID recipients of splenocytes from infected donors. OD405, optical density at 405 nm.
Immunoglobulin response.
Infected C57BL/6 mice responded to H. pylori infection with strong systemic anti-H. pylori IgG responses that were detectable within 5 weeks of inoculation and reached maximal levels (indicated by optical density, as determined by ELISA) by 6 weeks (Fig. 6A). In contrast, infected SCID mice did not produce any anti-H. pylori IgG (Fig. 6B), and IgG levels in uninfected C57BL/6 mice remained low. Infected recipient SCID mice developed variable levels of anti-H. pylori IgG which gradually increased after transfer but did not reach the levels found in C57BL/6 mice (Fig. 7).
DISCUSSION
This study revealed several novel and important findings. First, we have shown that gastritis and epithelial lesions due to H. pylori are dependent on an intact adaptive immune response and do not develop in T- and B-cell-deficient SCID mice. These results differ from those of a previous study, in which the responses of SCID and wild-type mice to Helicobacter felis did not differ (4). There were a number of differences between that study and the present one which likely explain the different outcomes. Most likely to have contributed to the differences in these studies is the difference in the mouse strains used. Blanchard et al. (4) used C.B-17 mice and congenic SCID mice. These mice are essentially BALB/c mice with a single genetic difference (an IgG isotype) (5). BALB/c mice have been shown in several studies to develop only minimal gastritis in response to both H. felis (32, 38) and H. pylori (38). Based on reported results of these studies as well as our own observations (not shown), BALB/c mice would be expected to have minimal gastritis at 8 weeks after infection (the sacrifice time used in the Blanchard study). Thus, the lack of difference in inflammation and colonization in the Blanchard study may be largely attributable to the failure of the wild-type mice to respond rather than to any significant response by the SCID mice.
A second difference between C.B-17 and C57BL/6 SCID mice is the demonstrated “leakiness” of the former strain. C.B-17 SCID mice are known to produce antibody in increasing concentrations as they age (5, 39), and the possibility that some adaptive immune function remains in these mice must be considered when using this strain. Thus, it is conceivable that the SCID mice in the Blanchard study did develop some specific immune response to H. pylori, resulting in inflammation that approached the gastritis (albeit mild) described for the wild-type mice. In contrast, C57BL/6 mice are not known to be leaky. Furthermore, in the present study, all SCID mice were tested for circulating mouse IgG and shown to be negative at the onset of the study. Mice which did not receive splenocytes remained IgG negative, did not develop H. pylori-specific antibody in response to infection, and did not develop DTH responses, suggesting that they remained immunodeficient throughout the study.
The second important finding of this study is the severity of gastritis in recipient SCID mice. The lesions are notable for two reasons. The most intriguing aspect is the difference between recipient SCID and donor C57BL/6 mice. The marked increases in the severity and rapidity of the onset of gastritis in recipient SCID mice (within 2 weeks of transfer), as well as the induction of DTH not seen in donor mice, indicate a loss of regulation resulting in the derepression of inflammation in recipient SCID mice. It is likely that regulatory cells, cytokines, or both suppress inflammation in normal mice, dampening and delaying gastritis. The absence of these regulatory influences in SCID mice results in the lesions that were produced in this study. While the effector cells are likely T cells (as evidenced by the correlation with DTH and the T-cell enrichment of the engrafted cells), the identification of the regulatory population in donor mice was not addressed by this study.
In addition to providing evidence suggesting the regulation of inflammation due to H. pylori in normal mice, the severe gastritis in recipient SCID mice represents an important addition to the growing number of animal models of disease due to H. pylori. The overall severity and rapidity of the onset of the lesions described here are more extensive and acute than those described for other animals colonized with H. pylori. Lesions of comparable severity and rapid onset (within 4 weeks) have been described for interleukin-10-deficient mice colonized with H. felis (2), and several studies have described severe lesions after prolonged (several months to years) colonization by H. felis (24, 25, 38) or H. pylori (27). However, in most studies, colonization by H. pylori resulted in relatively mild gastritis unaccompanied by epithelial lesions (9, 10, 18, 23).
The third important finding of this study is that epithelial lesions depend on the presence of gastritis and that by themselves, even large numbers of bacteria in close contact with the gastric epithelium do not cause epithelial erosions, metaplasia, or increased epithelial proliferation in vivo in the absence of a host response. These results indicate that if bacterial virulence factors are important in determining the outcome of infection, it is likely that their roles are in the induction of host response rather than in having a direct deleterious effect on host epithelial cells.
In addition to the three important findings described above, several other characteristics of H. pylori gastritis are addressed in this study. This study is the first to describe the effect of adoptive transfer in H. pylori-infected immunodeficient mice. A previous investigation by Mohammadi et al. examined the effect of adoptive transfer between groups of H. felis-infected C57BL/6 mice (31). In that study, findings were similar to those of the present study in that adoptive transfer exacerbated gastritis and suppressed colonization, but the differences in the severity of gastritis between nonrecipient and recipient mice were not as marked as they were in the present study. One interesting difference between the results of Mohammadi et al. and our own was the effect of prior exposure to antigen in donor mice. Mohammadi et al. found that splenocytes from naturally infected or immunized donors induced more severe gastritis than did splenocytes from uninfected donors. In our study, splenocytes from uninfected donors induced more severe gastritis and suppressed bacterial colonization more than splenocytes from infected donors did.
Differences between the study by Mohammadi et al. and the present study are likely due to differences in mouse strain, bacterial strain, and experimental protocol. Probably the most significant difference was that adoptive transfer in that study was done between groups of C57BL/6 mice rather than between C57BL/6 and SCID mice, as was done in the present study. Thus, the recipient mice were immunocompetent, and the contribution of the immune response of the recipients to the outcome could not be determined. Because the recipients in the present study were immunodeficient, endogenous adaptive immunity did not contribute to their responses. Other differences included the number of splenocytes transferred; the bacterial strain (H. felis was used in the study by Mohammadi et al.); and the infection, transfer, and sacrifice times. Finally, Mohammadi et al. did not evaluate DTH responses in their mice.
Other findings of this study are of interest. The strong inverse correlation between gastritis and bacterial colonization is evidence that the host immune response is effective in the suppression of bacterial colonization. This is compatible with other studies showing that increased immunity (e.g., due to vaccination) is accompanied by increased gastritis (postimmunization gastritis) as well as suppressed bacterial colonization (15). The correlation of DTH with severe gastritis is also compatible with previous studies suggesting that T-cell-mediated immunity is necessary for bacterial clearance (and, by extension, for the induction of gastritis) (3, 14, 31, 35). Finally, the development of DTH in infected recipient SCID mice but not infected donors is further evidence of immune regulation in donor mice.
The overall results of this study confirm that host immunity is necessary for both gastritis and the suppression of H. pylori colonization in infected mice. In addition, we demonstrate that epithelial damage is dependent on the host immune response rather than resulting from a direct bacterial effect, and we provide evidence that T-cell-dependent immunity is necessary for both gastritis and bacterial suppression. Finally, we demonstrate that gastritis is derepressed in SCID recipients, suggesting that gastritis is regulated and thus suppressed in normal infected C57BL/6 mice.
ACKNOWLEDGMENTS
This work was supported in part by PHS grants R01 AI43643, CA67498-01, and R29 DK-45340 from the NIH.
We thank Toni Hoepf for excellent technical assistance, Jon Rosenberg for animal care, and Charley Orosz for help in planning and evaluating experiments.
REFERENCES
- 1.Atherton J C, Cao P, Peek R M, Tummuru M K R, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori—association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 2.Berg D J, Lynch N A, Lynch R G, Lauricella D M. Rapid development of severe hyperplastic gastritis with gastric epithelial dedifferentiation in Helicobacter felis-infected IL-10(−/−) mice. Am J Pathol. 1998;152:1377–1386. [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchard T, Czinn S, Redline R, Sigmund N, Harriman G, Nedrud J. Antibody-independent protective mucosal immunity to gastric helicobacter infection in mice. Cell Immunol. 1999;191:74–80. doi: 10.1006/cimm.1998.1421. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard T G, Czinn S J, Nedrud J G, Redline R W. Helicobacter-associated gastritis in SCID mice. Infect Immun. 1995;63:1113–1115. doi: 10.1128/iai.63.3.1113-1115.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosma G C, Custer R P, Bosma M J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 6.Cover T L, Blaser M J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 7.Crabtree J E, Xiang Z, Lindley I J D, Tompkins D S, Rappuoli R, Covacci A. Induction of interleukin-8 secretion from gastric epithelial cells by a CagA negative isogenic mutant of Helicobacter pylori. J Clin Pathol. 1995;48:967–969. doi: 10.1136/jcp.48.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton K A, Cover T L, Tummuru M K R, Blaser M J, Krakowka S. Role of vacuolating cytotoxin in gastritis due to Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1997;65:3462–3464. doi: 10.1128/iai.65.8.3462-3464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton K A, Dewhirst F E, Paster B J, Tzellas N, Coleman B E, Paola J, Sherding R. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: animal and public health implications. J Clin Microbiol. 1996;34:3165–3170. doi: 10.1128/jcm.34.12.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton K A, Morgan D R, Krakowka S. Campylobacter pylori virulence factors in gnotobiotic piglets. Infect Immun. 1989;57:1119–1125. doi: 10.1128/iai.57.4.1119-1125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton K A, Morgan D R, Krakowka S. Motility as a factor in the colonisation of gnotobiotic piglets by Helicobacter pylori. J Med Microbiol. 1992;37:123–127. doi: 10.1099/00222615-37-2-123. [DOI] [PubMed] [Google Scholar]
- 12.Eaton K A, Radin M J, Krakowka S. An animal model of gastric ulcer due to bacterial gastritis in mice. Vet Pathol. 1995;32:489–497. doi: 10.1177/030098589503200506. [DOI] [PubMed] [Google Scholar]
- 13.Eaton K A, Radin M J, Kramer L, Wack R, Sherding R, Krakowka S, Fox J G, Morgan D R. Epizootic gastritis associated with gastric spiral bacilli in cheetahs (Acinonyx jubatus) Vet Pathol. 1993;30:55–63. doi: 10.1177/030098589303000107. [DOI] [PubMed] [Google Scholar]
- 14.Ermak T, Giannasca P, Nichols R, Myers G, Nedrud J, Weltzin R, Lee C, Kleanthous H, Monath T. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–2288. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ermak T H, Ding R, Ekstein B, Hill J, Myers G A, Lee C K, Pappo J, Kleanthous H K, Monath T P. Gastritis in urease-immunized mice after Helicobacter felis challenge may be due to residual bacteria. Gastroenterology. 1997;113:1118–1128. doi: 10.1053/gast.1997.v113.pm9322506. [DOI] [PubMed] [Google Scholar]
- 16.Ferrero R L, Thiberge J M, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrero R L, Thiberge J M, Labigne A. Local immunoglobulin G antibodies in the stomach may contribute to immunity against Helicobacter infection in mice. Gastroenterology. 1997;113:185–194. doi: 10.1016/s0016-5085(97)70094-5. [DOI] [PubMed] [Google Scholar]
- 18.Fox J G, Batchelder M, Marini R, Yan L, Handt L, Li X, Shames B, Hayward A, Campbell J, Murphy J C. Helicobacter pylori-induced gastritis in the domestic cat. Infect Immun. 1995;63:2674–2681. doi: 10.1128/iai.63.7.2674-2681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox J G, Li X T, Cahill R J, Andrutis K, Rustgi A K, Odze R, Wang T C. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996;110:155–166. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 20.Genta R M, Robason G O, Graham D Y. Simultaneous visualization of Helicobacter pylori and gastric morphology: a new stain. Hum Pathol. 1994;25:221–226. doi: 10.1016/0046-8177(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 21.Hermanns W, Kregel K, Breuer W, Lechner J. Helicobacter-like organisms: histopathological examination of gastric biopsies from dogs and cats. J Comp Pathol. 1995;112:307–318. doi: 10.1016/s0021-9975(05)80083-0. [DOI] [PubMed] [Google Scholar]
- 22.Kirchgessner C U, Patil C K, Evans J W, Cuomo C A, Fried L M, Carter T, Oettinger M A, Brown J M. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995;267:1178–1183. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- 23.Krakowka S, Morgan D R, Kraft W G, Leunk R D. Establishment of gastric Campylobacter pylori infection in the neonatal gnotobiotic piglet. Infect Immun. 1987;55:2789–2796. doi: 10.1128/iai.55.11.2789-2796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee A, Fox J G, Otto G, Murphy J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315–1323. doi: 10.1016/0016-5085(90)91156-z. [DOI] [PubMed] [Google Scholar]
- 25.Lee A, O’Rourke J, De Ungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 26.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto S, Washizuka Y, Matsumoto Y, Tawara S, Ikeda F, Yokota Y, Karita M. Induction of ulceration and severe gastritis in Mongolian gerbil by Helicobacter pylori infection. J Med Microbiol. 1997;46:391–397. doi: 10.1099/00222615-46-5-391. [DOI] [PubMed] [Google Scholar]
- 28.Michetti P, Corthesy-Theulaz I, Davin C, Haas R, Vaney A C, Heitz M, Bille J, Kraehenbuhl J P, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell H M, Lee A, Berkowicz J, Borody T. The use of serology to diagnose active Campylobacter pylori infection. Med J Aust. 1988;149:604–609. doi: 10.5694/j.1326-5377.1988.tb120800.x. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 31.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S J. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 32.Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996;64:238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neiger R, Dieterich C, Burnens A, Waldvogel A, Corthésy-Theulaz I, Halter F, Lauterburg B, Schmassmann A. Detection and prevalence of Helicobacter infection in pet cats. J Clin Microbiol. 1998;36:634–637. doi: 10.1128/jcm.36.3.634-637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norris C R, Marks S L, Eaton K A, Torabian S Z, Munn R J, Solnick J V. Healthy cats are commonly colonized with “Helicobacter heilmannii” that is associated with minimal gastritis. J Clin Microbiol. 1999;37:189–194. doi: 10.1128/jcm.37.1.189-194.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pappo J, Torrey D, Castriotta L, Savinainen A, Kabok Z, Ibraghimov A. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect Immun. 1999;67:337–341. doi: 10.1128/iai.67.1.337-341.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peek R M, Jr, Thompson S A, Donahue J P, Tham K T, Atherton J C, Blaser M J, Miller G G. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc Assoc Am Physicians. 1998;110:531–544. [PubMed] [Google Scholar]
- 37.Peek R M, Jr, Miller G G, Tham K T, Perez-Perez G I, Zhao X, Atherton J C, Blaser M J. Heightened inflammatory response and cytokine expression in vivo to cagA(+) Helicobacter pylori strains. Lab Investig. 1995;73:760–770. [PubMed] [Google Scholar]
- 38.Sakagami T, Dixon M, O’Rourke J, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seydel K B, Stanley S L., Jr SCID mice and the study of parasitic disease. Clin Microbiol Rev. 1996;9:126–134. doi: 10.1128/cmr.9.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Telford J L, Ghiara P, Dell’Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce M F, Censini S, Covacci A, Xiang Z. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamasaki K, Suematsu H, Takahashi T. Comparison of gastric lesions in dogs and cats with and without gastric spiral organisms. J Am Vet Med Assoc. 1998;212:529–533. [PubMed] [Google Scholar]