Abstract
Emerging evidence suggests that COVID-19 may affect cardiac autonomic function; however, the limited findings in young adults with COVID-19 have been equivocal. Notably, symptomology and time since diagnosis appear to influence vascular health following COVID-19, but this has not been explored in the context of cardiac autonomic regulation. Therefore, we hypothesized that young adults who had persistent symptoms following COVID-19 would have lower heart rate variability (HRV) and cardiac baroreflex sensitivity (BRS) compared with those who had COVID-19 but were asymptomatic at testing and controls who never had COVID-19. Furthermore, we hypothesized that there would be relationships between cardiac autonomic function measures and time since diagnosis. We studied 27 adults who had COVID-19 and were either asymptomatic (ASYM; n = 15, 6 females); 21 ± 4 yr; 8.4 ± 4.0 wk from diagnosis) or symptomatic (SYM; n = 12, 9 females); 24 ± 3 yr; 12.3 ± 6.2 wk from diagnosis) at testing, and 20 adults who reported never having COVID-19 (24 ± 4 yr, 11 females). Heart rate and beat-to-beat blood pressure were continuously recorded during 5 min of rest to assess HRV and cardiac BRS. HRV [root mean square of successive differences between normal heartbeats (RMSSD); control, 73 ± 50 ms; ASYM, 71 ± 47 ms; and SYM, 84 ± 45 ms; P = 0.774] and cardiac BRS (overall gain; control, 22.3 ± 10.1 ms/mmHg; ASYM, 22.7 ± 12.2 ms/mmHg; and SYM, 24.3 ± 10.8 ms/mmHg; P = 0.871) were not different between groups. However, we found correlations with time since diagnosis for HRV (e.g., RMSSD, r = 0.460, P = 0.016) and cardiac BRS (overall gain, r = 0.470, P = 0.014). These data suggest a transient impact of COVID-19 on cardiac autonomic function that appears mild and unrelated to persistent symptoms in young adults.
NEW & NOTEWORTHY The potential role of persistent COVID-19 symptoms on cardiac autonomic function in young adults was investigated. We observed no differences in heart rate variability or cardiac baroreflex sensitivity between controls who never had COVID-19 and those who had COVID-19, regardless of symptomology. However, there were significant relationships between measures of cardiac autonomic function and time since diagnosis, suggesting that COVID-19-related changes in cardiac autonomic function are transient in young, otherwise healthy adults.
Keywords: autonomic nervous system, cardiac baroreflex sensitivity, heart rate variability, SARS-CoV-2
INTRODUCTION
Over 85 million people in the United States are reported to have had the novel coronavirus-19 (COVID-19) illness since the beginning of the pandemic in January 2020; of these, more than 20% are young adults (1). Although most individuals make a full recovery, early reports suggest that COVID-19 may increase the risk for future cardiovascular complications (2). Moreover, there is a growing body of evidence of post-COVID-19 clinical manifestations indicative of blood pressure dysregulation (e.g., orthostatic hypotension) (3, 4), suggesting that COVID-19 may also negatively affect the autonomic nervous system. Much of this information is from patient cohorts with COVID-19 or those experiencing long COVID, with fewer studies in young college-aged adults who had COVID-19. We previously found no effect of breakthrough cases of COVID-19 during the omicron wave on indices of cardiac autonomic function (5); however, studies investigating autonomic function in young adults following earlier variants of COVID-19 (i.e., α and δ variants) are limited, and the results are equivocal.
A recent study by Stute et al. (6) demonstrated early autonomic alterations in young adults (within 8 wk of diagnosis) characterized by elevated resting muscle sympathetic nerve activity and heart rate variability (HRV), indicative of both an increased central sympathetic drive and vagal control of the heart. Conversely, Freire et al. (7) showed reduced cardiac vagal control following COVID-19 in young adults. Although the reason(s) for these conflicting results are unclear, the lack of consideration for COVID-19 symptomology and time since diagnosis may have contributed. Notably, these studies were completed before November 2021, thus representing individuals who contracted the more severe, earlier variants of COVID-19 (i.e., α and δ) where persistent symptoms were more likely, even in young college-aged adults (8). In this regard, we and others have reported impairments in vascular function (e.g., reduced brachial artery flow-mediated dilation) in young adults in the months following these earlier variants of COVID-19 (9, 23, 24), which was related to the continued experience of symptoms (9). However, to our knowledge, the effect of symptomology on indices of cardiac autonomic function has not been considered. Likewise, the time since diagnosis varied among participants with COVID-19 in these previous studies but was also not considered as a potential factor.
With this background in mind, in the present study, we tested the hypothesis that young adults who had COVID-19 and experienced persistent symptoms would exhibit impaired cardiac autonomic function (i.e., HRV and cardiac baroreflex sensitivity; BRS) compared with those who had COVID-19 but were asymptomatic at the time of testing, and to healthy controls who never had COVID-19. Secondarily, we hypothesized that there would be relationships with time since diagnosis in the COVID-19 group such that there would be a greater impact on indices of cardiac autonomic function closer to diagnosis.
METHODS
We studied 27 young adults who previously had COVID-19 and compared them to 20 young, otherwise healthy adults with no history of COVID-19 (control, 24 ± 4 yr, 11 females). Of the COVID-19 group, 15 were asymptomatic at the time of testing [ASYM, 21 ± 4 yr; 6 females; 8.4 ± 4.0 wk (range, 3 to 16 wk) from diagnosis], whereas 12 were still symptomatic [SYM, 24 ± 3 yr old, 9 females; 12.3 ± 6.2 wk (range, 3 to 22 wk) from diagnosis]. All participants with COVID-19 provided documentation of a laboratory-confirmed PCR or antigen test. None of the participants with COVID-19 required hospitalization for their illness. Participants reported their current symptoms on a standardized COVID-19 symptom survey and rated each symptom’s severity on a scale from 1 to 10. Any participant with at least one symptom reported at the time of testing was included in the SYM group (9). All participants with COVID-19 were diagnosed between November 2020 and September 2021 (i.e., before the omicron variant). Participants with COVID-19 and all but two of the control participants were studied during a similar timeframe (COVID-19, March 2021–October 2021; and controls, May 2021– February 2022).
All participants were free from any known cardiovascular, metabolic, or neurological disease. In addition, all participants were nonsmokers and were not taking prescription medications. All experiments were conducted following an overnight fast, with participants instructed to abstain from caffeine for at least 12 h, as well as alcohol and exercise for at least 24 h before the study. Pregnancy tests were administered to all female participants and self-reported last menstrual period was recorded. All experimental procedures conformed to the Declaration of Helsinki and were approved by the University of Texas at Arlington Institutional Review Board (2021-0197). Written, informed consent was obtained following a detailed verbal explanation of the experimental protocol. Participants also completed the International Physical Activity Questionnaire (IPAQ) (10) to assess their physical activity levels during the week before testing. Resting HRV and cardiac BRS data were previously reported for eight participants in the control group (5).
Participants were instrumented with a standard lead II electrocardiogram (model Q710, Quinton, Bothell, WA) to continuously measure heart rate and a pneumobelt (Pneumotrace II 1132, UFI, Morro Bay, CA) to monitor respiratory patterns. Beat-to-beat blood pressure was measured on the left hand following return-to-flow calibration (Finometer PRO, Finapres Medical Systems, Amsterdam, The Netherlands). Automated brachial blood pressures were also obtained on the right arm to confirm Finometer values. Following 20 min of supine rest, heart rate and beat-to-beat blood pressure were continuously recorded during a 10-min resting period and stored for off-line analysis (ADInstruments, LabChart v8). Resting blood pressure was determined from an average of three brachial blood pressure measurements. Beat-to-beat data for systolic blood pressure (SBP) and R-to-R interval (RRI) were exported during a 5-min segment with stable breathing and no irregular cardiac cycles. HRV was measured in the time domain as the root mean square of successive differences between normal heartbeats (RMSSD) and in the frequency domain from the ECG signal sampled at 1,000 Hz (Nevrokard, Izola, Slovenia). Frequency domain measures of HRV were analyzed using fast-Fourier transformation with a Hanning window and determined as high-frequency (HF; 0.15–0.40 Hz) and low-frequency power (LF; 0.04–0.15 Hz) in both absolute (ms2) and normalized units (n.u.) (11). An index of autonomic balance was calculated as LF/HF (12). Spontaneous cardiac BRS was determined using the Sequence Method (Nevrokard, Izola, Slovenia) in which sequences of three or more consecutive beats where SBP and RRI change in the same direction were identified. Sequences were detected using a zero-beat delay, R2 for inclusion set at 0.85, and using 1-mmHg and 5-ms thresholds for SBP and RRI, respectively, as previously described (5, 13). Briefly, cardiac BRS was identified for all sequences combined and separately for up sequences (RRI and SBP increasing) and down sequences (RRI and SBP decreasing). Cardiac baroreflex effectiveness index was also determined using CardioSeries software as the ratio between the number of baroreflex sequences (SBP ramps followed by the respective baroreflex RRI response) and the total number of SBP ramps (14).
Statistical Analysis
All data are presented as means ± SD. Data analysis and graphical representation were completed using GraphPad Prism (v9.4). The sample size was estimated from our previous research on vascular function showing differences between control, ASYM, and SYM groups (9) and previous studies reporting group differences in HRV (6, 15). Ordinary one-way ANOVAs were performed to assess differences between control, ASYM, and SYM groups. Categorical data were compared using a χ2 test. Pearson’s correlations were performed to relate the time since diagnosis to the outcome measures in the COVID-19 group. Statistical significance was set a priori at P < 0.05.
RESULTS
Participant characteristics are shown in Table 1. Briefly, there were no differences in participant demographics between the control, ASYM, and SYM groups (P > 0.05 for all). For female participants, there were no differences between groups for day of menstrual cycle at study visit (P = 0.415). Participants reported no difference in their weekly physical activity on the IPAQ (control, 3,879 ± 3,625 MET min/wk; ASYM, 4,818 ± 4,536 MET min/wk; and SYM, 5,662 ± 5,631 MET min/wk; P = 0.567), and physical activity was not correlated with time since diagnosis in the COVID-19 group (r = 0.267, P = 0.179). For the SYM group, symptoms reported included loss of taste or smell (n = 11), persistent cough (n = 1), fatigue (n = 2), muscle pain (n = 1), and brain fog (n = 1). All but three control participants, eight ASYM, and five SYM had received at least one dose of the vaccine at assessment. There were no differences between those who were vaccinated versus those who were not vaccinated for any outcome (P > 0.05 for all).
Table 1.
Participant characteristics
| Control | ASYM | SYM | P Value | |
|---|---|---|---|---|
| Anthropometrics | ||||
| n | 20 | 15 | 12 | |
| Age, yr | 24 ± 4 | 21 ± 4 | 24 ± 3 | 0.102 |
| Sex, females/males | 11/9 | 6/9 | 9/3 | 0.192 |
| Height, cm | 170 ± 7 | 166 ± 9 | 169 ± 12 | 0.367 |
| Weight, kg | 72 ± 11 | 67 ± 13 | 72 ± 11 | 0.398 |
| Body mass index, kg/m2 | 25 ± 4 | 24 ± 3 | 25 ± 3 | 0.649 |
| Race and ethnicity, n (%) | ||||
| White | 13 (65) | 7 (47) | 7 (58) | 0.553 |
| Black/African American | 1 (5) | 2 (13) | 1 (8) | |
| Asian | 5 (25) | 4 (27) | 0 (0) | |
| Other/multiracial | 1 (5) | 2 (13) | 4 (33) | |
| Non-Hispanic/Non-Latino | 18 (90) | 11 (73) | 9 (75) | 0.388 |
| Hispanic/Latino | 2 (10) | 4 (27) | 3 (25) | |
| Resting cardiovascular measures | ||||
| Heart rate, beats/min | 59 ± 8 | 61 ± 11 | 63 ± 11 | 0.634 |
| SBP, mmHg | 112 ± 7 | 111 ± 7 | 113 ± 7 | 0.718 |
| DBP, mmHg | 67 ± 8 | 69 ± 4 | 70 ± 6 | 0.492 |
| MAP, mmHg | 82 ± 7 | 83 ± 5 | 84 ± 6 | 0.655 |
Values are means ± SD and n (%); n, number of participants. ASYM, asymptomatic; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure; SYM, symptomatic. Data were compared using an ordinary one-way ANOVA or χ2 test (categorical data) in GraphPad prism v9.4. Significance was set as P < 0.05.
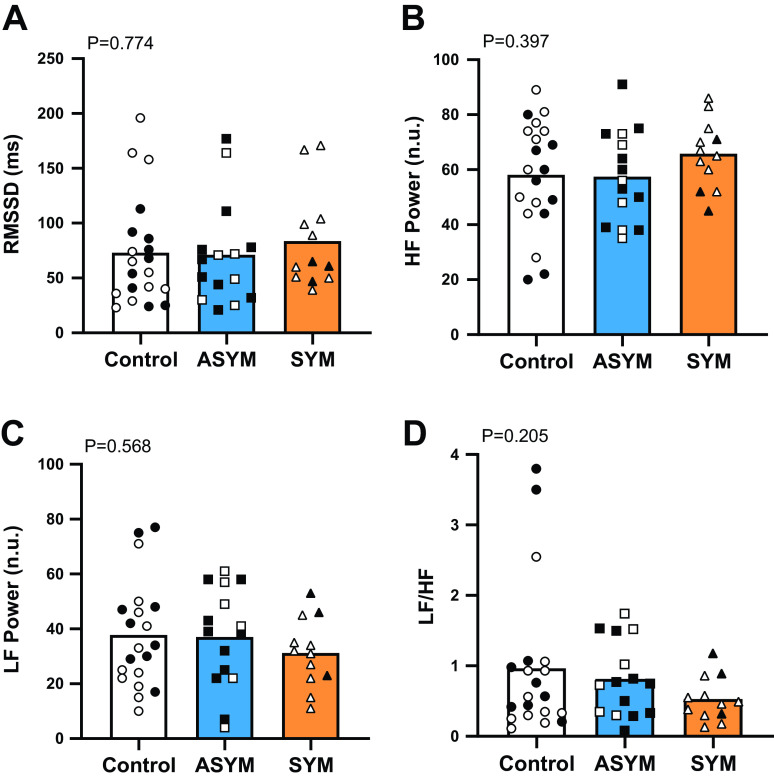
Resting heart rate and blood pressure were not different between the control, ASYM, and SYM groups (Table 1). Measures of HRV [RMSSD, LF power (n.u.), HF power (n.u.), and LF:HF ratio] are shown in Fig. 1 and were not different between groups. LF power measured in absolute units was also not different between control (2,139 ± 4,498 ms2), ASYM (1,359 ± 1,173 ms2), and SYM (1,493 ± 1,591 ms2; P = 0.549). Similarly, there were no differences in HF power in absolute units (control, 2,488 ± 3,305 ms2; ASYM, 2,621 ± 3,011 ms2; and SYM, 3,168 ± 2,960 ms2; P = 0.996). Cardiac BRS measures (overall gain, up gain, and down gain) are provided in Fig. 2 with no differences between groups. Cardiac baroreflex effectiveness index was also not different between control (62 ± 13%), ASYM (56 ± 16%), and SYM (61 ± 20%; P = 0.512).
Figure 1.
Group means and individual data for heart rate variability measures. A: root mean square of successive differences between normal heartbeats (RMSSD). B: high-frequency (HF) power. C: low-frequency (LF) power. D: LF-to-HF ratio. The control group (n = 20) is indicated by white bars and circles, the asymptomatic at the time of testing (ASYM; n = 15) are shown in blue bars and squares, and those who were still symptomatic at the time of testing (SYM; n = 12) are in orange bars and triangles. Male participants are indicated by filled symbols and females by open symbols. Comparisons between groups were made using ordinary one-way ANOVA, and significance was determined as P < 0.05.
Figure 2.
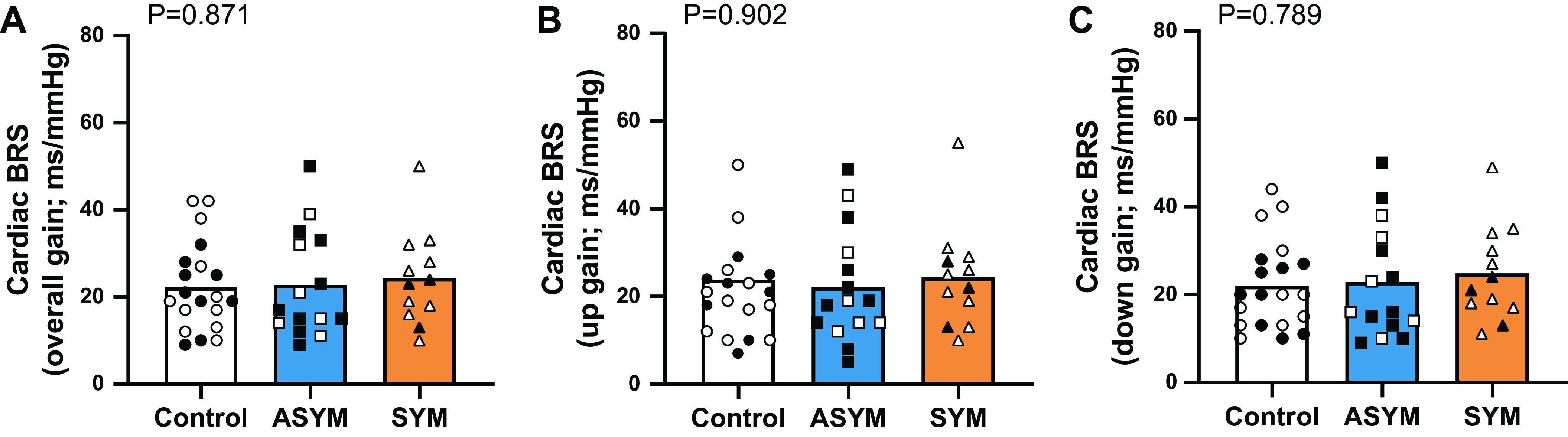
Group means and individual data for cardiac baroreflex sensitivity (BRS) measured as overall gain (A), up gain (B), and down gain (C). The control group (n = 20) is indicated by white bars and circles, the asymptomatic at time of testing (ASYM; n = 15) is shown in blue bars and squares, and those who were still symptomatic at the time of testing (SYM; n = 12) are in orange bars and triangles. Male participants are indicated by filled symbols and females by open symbols. Comparisons between groups were made using ordinary one-way ANOVA, and significance was determined as P < 0.05.
In terms of relationships, RMSSD was positively correlated with time since diagnosis (Fig. 3A), as was HF power (r = 0.385, P = 0.047). LF power trended toward a negative relationship with time since diagnosis (r = −0.361, P = 0.065), whereas there was a significant negative relationship between LF:HF and time since diagnosis (Fig. 3B). Lastly, there was a positive relationship between time since diagnosis and overall cardiac BRS gain (Fig. 3C), which was similar for up gain (r = 0.476, P = 0.012) and down gain (r = 0.471, P = 0.013).
Figure 3.

Correlations between time since diagnosis and the root mean square of successive differences between normal heartbeats (RMSSD; A), low-frequency (LF) to high-frequency power (HF) power ratio (B), cardiac baroreflex sensitivity (BRS) overall gain for all participants with COVID-19 (C). Participants who were asymptomatic at the time of testing (n = 15) are indicated by squares, and those who were still symptomatic (n = 12) are in triangles. Male participants are indicated by filled symbols and females by open symbols. Pearson’s correlation coefficient (r) was used to assess the relationship between time since diagnosis and outcome measures. Significance was determined as P < 0.05.
DISCUSSION
This study was the first to demonstrate that COVID-19 does not have a major impact on indices of cardiac autonomic function in young, otherwise healthy adults, regardless of symptomology. However, there were significant correlations with time since diagnosis indicating that transient effects of COVID-19 on HRV and cardiac BRS may be present closer to diagnosis. Together, these data indicate that cardiac autonomic function is minimally impacted and transient in young adults following COVID-19, an effect that appears unrelated to persistent symptoms.
In the present study, we report no difference in measures of HRV in young adults who had COVID-19 and had persistent symptoms compared with those without persistent symptoms and controls without a history of COVID-19. Our findings for vagally mediated control of heart rate contrast with the previous studies investigating HRV that reported either higher (6) or lower (7) RMSSD in young adults who also likely contracted the earlier variants of COVID-19. The reasons for the differences are unclear but may include a shorter time since diagnosis (i.e., within 8 wk) (6) and larger ranges for body mass and age (7). However, in agreement with the aforementioned studies, we also show no impact of COVID-19 on LF power or LF:HF in young adults (6, 7) suggesting that cardiac autonomic balance is unaltered with COVID-19. Measures of HRV in middle-aged or older adults who had COVID-19 have also been equivocal, with higher (16), reduced (17, 18), or no change (19, 20) being previously reported compared with controls who did not have COVID-19. These equivocal results may be due to differences in the age range between these studies and the potential existence of comorbidities in the participants. Importantly, our data indicate that altered HRV is not associated with persistent COVID-19-related symptoms in young, otherwise healthy adults. This is encouraging given the previously reported peripheral vascular dysfunction in those with persistent symptoms (9) and the high number of young adults who experienced persistent symptoms of COVID-19 (8). In line with our hypothesis, we found a relationship between indices of HRV and time since diagnosis, suggesting that there may be transient effects of COVID-19 on cardiac autonomic function. Nevertheless, our findings suggest that the impact of COVID-19 on vagal control of the heart and cardiac autonomic balance is minimal in young adults.
COVID-19 has also been reported to blunt cardiac BRS in middle-aged individuals (20). However, we were unable to recapitulate these findings in young adults, even when considering their symptomatology. Taken together with the findings from our previous work during the omicron wave (5), this suggests that cardiac autonomic function may be less susceptible to change following COVID-19 in young healthy adults. Middle-aged adults with persistent symptoms (e.g., fatigue) were observed to be more likely to experience orthostatic intolerance (3, 19), which would support the notion that symptomology may matter when it comes to autonomic dysfunction. Importantly, the participants in our study did not present with symptoms indicative of autonomic dysfunction (e.g., orthostatic intolerance), and thus our data did not align with our hypothesis, perhaps because they are young and otherwise healthy. Although the risk of future cardiovascular events may be increased in individuals who had COVID-19 (2), our findings demonstrate preserved cardiac autonomic function in young, otherwise healthy individuals following COVID-19 even if persistent symptoms are present. However, our finding of an association between cardiac BRS (and RMSSD) with time since diagnosis may suggest a temporal effect of COVID-19 on cardiac autonomic function that is more pronounced closer to diagnosis. This is similar to the data from middle-aged adults that showed cardiac BRS was lower <3 mo compared with >3 mo from diagnosis (20). Together, these findings align with previous data indicating a time-course improvement in blood pressure and arterial stiffness in young adults (21, 22) and suggest that similarly, any potential autonomic changes following COVID-19 are transient in young, otherwise healthy adults.
In summary, we showed no major effect of persistent COVID-19-related symptoms on measures of cardiac autonomic function (i.e., HRV and cardiac BRS) in young, otherwise healthy adults who had COVID-19. However, measures of HRV and cardiac BRS were correlated with time since diagnosis indicating a potential transient impact of COVID-19 that improves over time. Although elevated cardiovascular risk following COVID-19 is multifactorial, we would contend altered cardiac autonomic function does not contribute to this risk in young college-aged adults.
GRANTS
This work was supported by The University of Texas at Arlington College of Nursing and Health Innovation, National Institute of Diabetes and Digestive and Kidney Diseases Undergraduate Award R25DK07838 (to N.A.G.) and American Heart Association Predoctoral Fellowship Grants 827597 (to D.N.) and 20PRE34990010 (to B.Y.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.S., N.A.G., D.N., B.E.Y., and P.J.F. conceived and designed research; R.J.S., N.A.G., D.N., B.Y.S., A.N.W., A.-K.G., and B.E.Y. performed experiments; R.J.S., N.A.G. and D.N. analyzed data; R.J.S., N.A.G., D.N., B.E.Y., and P.J.F. interpreted results of experiments; R.J.S. and N.A.G. prepared figures; R.J.S. and N.A.G. drafted manuscript; R.J.S., N.A.G., D.N., B.Y.S., A.N.W., A.-K.G., B.E.Y., and P.J.F. edited and revised manuscript; R.J.S., N.A.G., D.N., B.Y.S., A.N.W., A.-K.G., B.E.Y., and P.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all participants for volunteering time for this research.
REFERENCES
- 1.Centers for Disease Control and Prevention. COVID Data Tracker (Online; last accessed, Sept 10, 2022). https://covid.cdc.gov/covid-data-tracker/#datatracker-home.
- 2. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 28: 583–590, 2022. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shouman K, Vanichkachorn G, Cheshire WP, Suarez MD, Shelly S, Lamotte GJ, Sandroni P, Benarroch EE, Berini SE, Cutsforth-Gregory JK, Coon EA, Mauermann ML, Low PA, Singer W. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res 31: 385–394, 2021. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res 69: 205–211, 2021. [Erratum in Immunol Res 2021]. doi: 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skow RJ, Nandadeva D, Grotle AK, Stephens BY, Wright AN, Fadel PJ. Impact of breakthrough COVID-19 cases during the omicron wave on vascular health and cardiac autonomic function in young adults. Am J Physiol Heart Circ Physiol 323: H59–H64, 2022. doi: 10.1152/ajpheart.00189.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stute NL, Stickford JL, Province VM, Augenreich MA, Ratchford SM, Stickford ASL. COVID-19 is getting on our nerves: sympathetic neural activity and haemodynamics in young adults recovering from SARS-CoV-2. J Physiol 599: 4269–4285, 2021. doi: 10.1113/JP281888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freire APCF, Lira FS, Morano AEVA, Pereira T, Coelho-E-Silva MJ, Caseiro A, Christofaro DGD, Marchioto Júnior O, Dorneles GP, Minuzzi LG, Pinho RA, Silva BSA. Role of body mass and physical activity in autonomic function modulation on post-COVID-19 condition: an observational subanalysis of fit-COVID study. Int J Environ Res Public Health 19: 2457, 2022. doi: 10.3390/ijerph19042457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, Chu HY. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open 4: e210830, 2021. [Erratum in JAMA Netw Open 4: e214572, 2021] doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nandadeva D, Young BE, Stephens BY, Grotle AK, Skow RJ, Middleton AJ, Haseltine FP, Fadel PJ. Blunted peripheral but not cerebral vasodilator function in young otherwise healthy adults with persistent symptoms following COVID-19. Am J Physiol Heart Circ Physiol 321: H479–H484, 2021. doi: 10.1152/ajpheart.00368.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hallal PC, Victora CG. Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Med Sci Sports Exerc 36: 556, 2004. doi: 10.1249/01.mss.0000117161.66394.07. [DOI] [PubMed] [Google Scholar]
- 11.Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93: 1043–1065, 1996. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 12. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health 5: 258, 2017. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young BE, Kaur J, Vranish JR, Stephens BY, Barbosa TC, Cloud JN, Wang J, Keller DM, Fadel PJ. Augmented resting beat-to-beat blood pressure variability in young, healthy, non-Hispanic black men. Exp Physiol 105: 1102–1110, 2020. doi: 10.1113/EP088535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Di Rienzo M, Parati G, Castiglioni P, Tordi R, Mancia G, Pedotti A. Baroreflex effectiveness index: an additional measure of baroreflex control of heart rate in daily life. Am J Physiol Regul Integr Comp Physiol 280: R744–751, 2001. doi: 10.1152/ajpregu.2001.280.3.R744. [DOI] [PubMed] [Google Scholar]
- 15. Adlan AM, Veldhuijzen van Zanten J, Lip GYH, Paton JFR, Kitas GD, Fisher JP. Cardiovascular autonomic regulation, inflammation and pain in rheumatoid arthritis. Auton Neurosci 208: 137–145, 2017. doi: 10.1016/j.autneu.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asarcikli LD, Hayiroglu M, Osken A, Keskin K, Kolak Z, Aksu T. Heart rate variability and cardiac autonomic functions in post-COVID period. J Interv Card Electrophysiol 63: 715–721, 2022. doi: 10.1007/s10840-022-01138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Acanfora D, Nolano M, Acanfora C, Colella C, Provitera V, Caporaso G, Rodolico GR, Bortone AS, Galasso G, Casucci G. Impaired vagal activity in long-COVID-19 patients. Viruses 14: 1035, 2022. doi: 10.3390/v14051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurtoğlu E, Afsin A, Aktaş İ, Aktürk E, Kutlusoy E, Çağaşar Ö. Altered cardiac autonomic function after recovery from COVID-19. Ann Noninvasive Electrocardiol 27: e12916, 2022. doi: 10.1111/anec.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Townsend L, Moloney D, Finucane C, McCarthy K, Bergin C, Bannan C, Kenny RA. Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLoS One 16: e0247280, 2021. doi: 10.1371/journal.pone.0247280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zanoli L, Gaudio A, Mikhailidis DP, Katsiki N, Castellino N, Lo Cicero L, Geraci G, Sessa C, Fiorito L, Marino F, Antonietta Di Rosolini M, Colaci M, Longo A, Montineri A, Malatino L, Castellino P; Methuselah Study Group. Vascular dysfunction of COVID-19 is partially reverted in the long-term. Circ Res 130: 1276–1285, 2022. doi: 10.1161/CIRCRESAHA.121.320460. [DOI] [PubMed] [Google Scholar]
- 21. Szeghy RE, Stute NL, Province VM, Augenreich MA, Stickford JL, Stickford ASL, Ratchford SM. Six-month longitudinal tracking of arterial stiffness and blood pressure in young adults following SARS-CoV-2 infection. J Appl Physiol (1985) 132: 1297–1309, 2022. doi: 10.1152/japplphysiol.00793.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nandadeva D, Skow RJ, Grotle AK, Stephens BY, Young BE, Fadel PJ. Impact of COVID-19 on ambulatory blood pressure in young adults: a cross-sectional analysis investigating time since diagnosis. J Appl Physiol (1985) 133: 183–190, 2022. doi: 10.1152/japplphysiol.00216.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ratchford SM, Stickford JL, Province VM, Stute N, Augenreich MA, Koontz LK, Bobo LK, Stickford ASL. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol 320: H404–H410, 2021. doi: 10.1152/ajpheart.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szeghy RE, Province VM, Stute NL, Augenreich MA, Koontz LK, Stickford JL, Stickford ASL, Ratchford SM. Carotid stiffness, intima-media thickness and aortic augmentation index among adults with SARS-CoV-2. Exp Physiol 107: 694–707, 2022. doi: 10.1113/EP089481. [DOI] [PMC free article] [PubMed] [Google Scholar]