Keywords: cachexia, inflammation, lung cancer, muscle wasting, ribosomal RNA
Abstract
Preclinical models have been instrumental to elucidate the mechanisms underlying muscle wasting in lung cancer (LC). We investigated anabolic deficits and the expression of proinflammatory effectors of muscle wasting in the LP07 and Lewis lung carcinoma (LLC) tumor models. Tumor growth resulted in significant weakness in LP07 but not in LLC mice despite similar reductions in gastrocnemius muscle mass in both models. The LP07 tumors caused a reduction in ribosomal (r)RNA and a decrease in rRNA gene (rDNA) transcription elongation, whereas no changes in ribosomal capacity were evident in LLC tumor-bearing mice. Expression of RNA Polymerase I (Pol I) elongation-associated subunits Polr2f, PAF53, and Znrd1 mRNAs was significantly elevated in the LP07 model, whereas Pol I elongation-related factors FACT and Spt4/5 mRNAs were elevated in the LLC mice. Reductions in RPS6 and 4E-BP1 phosphorylation were similar in both models but were independent of mTOR phosphorylation in LP07 mice. Muscle inflammation was also tumor-specific, IL-6 and TNF-α mRNA increased with LLC tumors, and upregulation of NLRP3 mRNA was independent of tumor type. In summary, although both models caused muscle wasting, only the LP07 model displayed muscle weakness with reductions in ribosomal capacity. Intracellular signaling diverged at the mTOR level with similar reductions in RPS6 and 4E-BP1 phosphorylation regardless of tumor type. The increase in proinflammatory factors was more pronounced in the LLC model. Our results demonstrate novel divergent anabolic deficits and expression of proinflammatory effectors of muscle wasting in the LP07 and LLC preclinical models of lung cancer.
NEW & NOTEWORTHY We provide novel data demonstrating significant divergence in anabolic deficits and the expression of proinflammatory effectors of muscle wasting consequent to different lung-derived tumors.
INTRODUCTION
Lung cancer (LC) is one the most prevalent cachexia-inducing malignancies in the United States, with a greater risk for inpatient death than all other cancers combined (1, 2). Current treatment options for LC-associated cachexia have shown limited effectiveness at preventing muscle wasting, in part, due to the limited understanding of the mechanisms underlying this condition. The use of preclinical models of LC has facilitated the identification of several molecular mechanisms promoting muscle wasting, yet their use continues to pose unique challenges. Differences in host genetic background, immune status, tumor origin, administration route, differential onset of tumor growth, and tumor burden can introduce confounding variables that limit the elucidation of the mechanism driving muscle wasting in LC (3–10). In addition to the scarcity of human clinical data on LC-induced muscle wasting, these limitations may hinder progress toward the development of therapeutic interventions to prevent or reverse muscle wasting in cancer cachexia (11).
Much of the current knowledge about the mechanisms promoting muscle wasting in LC was generated using the Lewis lung carcinoma (LLC) model (5, 6, 8, 12–14). Following LLC tumor development, muscle wasting is associated with impaired muscle function (5, 6, 8, 9, 14), deficits in protein synthesis and elevated proteolysis (5, 6, 14, 15), increased systemic and local inflammation (5, 9), and mitochondrial dysfunction (8, 14). More recently, the LP07 lung adenocarcinoma (LP07) model revealed myopathogenic similarities with the LLC model (3, 4, 10). However, despite both tumor types originating from the lung and producing muscle wasting, whether they involve similar anabolic deficits remains unknown. This is an important problem as muscle wasting in cancer is associated with a reduction in ribosomal capacity (16–19). Anabolic impairments play a critical role in muscle wasting because muscle mass is largely determined by alterations in protein synthesis rates, which in turn depends on the ribosomal capacity of the muscle (20, 21). In addition to a lower ribosomal production, alterations in intracellular signaling involved in translational control may also promote muscle wasting in LC (4, 5, 13). Whether these anabolic mechanisms are similarly affected in skeletal muscle of LP07 and LLC tumor models remains to be investigated.
Congruent with the alterations in protein metabolism, an increase in systemic proinflammatory cytokines is often associated with tumor burden (16) and has been consistently demonstrated in preclinical models (5–7, 17) and late-stage patients with LC with muscle wasting (22–25). Based on their effects on whole body metabolism, systemic cytokines can be classified as pro- (e.g., IL-1b, IL6, and TNF) or anticachectic (e.g., IL-4, IL-10, and IL-13) (26) and can play a role in local cytokine production to promote muscle wasting (27–29). A potential mediator of in situ cytokine production is the nucleotide-binding oligomerization domain-like receptor family and pyrin domain containing 3 (NLRP3) inflammasome. In response to inflammatory insults, NLRP3 mediates IL-1β and IL-18 production (30) to control local tissue inflammation (31). Currently, there are no data available about the involvement of the NLRP3 inflammasomes in cancer-induced muscle wasting. Because of the potential inflammatory responses to different tumor types and the role of NLRP3 in modulating local muscle low-grade chronic inflammation during the progression of muscle wasting, we asked whether the different models of LC could elicit the coordinated expression of NLRP3 and downstream cytokines IL-1β and IL-18.
The goal of this study was to examine whether muscle wasting in the LP07 and LLC preclinical models of LC involved similar anabolic deficits and the local expression of proinflammatory factors. We hypothesized that muscle wasting following LP07 or LLC tumor implantation will be associated with a reduction in ribosomal capacity as a consequence of lower rDNA transcription together with alterations in mTOR and downstream signaling involved in translational control. In addition, we sought to determine whether the expression of local proinflammatory effectors of muscle wasting was similar regardless of tumor type. Our findings revealed that despite the LP07 and LLC tumor cells originating from the lung and producing similar levels of muscle wasting, the LP07 model resulted in a larger reduction in force production. Anabolic deficits involved a specific decline in rDNA transcription elongation in the LP07 model. A reduction in mTOR phosphorylation was only evident in the LLC model, but RPS6 and 4E-BP1 phosphorylation was negatively affected regardless of the tumor type. Muscle expression of the proinflammatory cytokines IL-6 and TNF-α was higher in LLC mice, and although NLRP3 expression was similar in LP07 and LLC models, IL-1β and IL-18 were not induced in either model. Overall, our findings show that despite producing a similar muscle-wasting phenotype, these preclinical models of LC induce muscle wasting via divergent anabolic deficits, and neuromuscular and proinflammatory mechanisms.
METHODS
Animals
All animal experiments were approved by both the animal care and use committees of the Universitat Pompeu Fabra and Pennsylvania State University for the LP07 and LLC models respectively, in accordance with the ethical standards set in the 1964 Declaration of Helsinki. The LP07 model was generated as previously described (4). Briefly, 4 × 105 LP07 tumor cells (RRID: CVCL_W868) resuspended in 200-μL MEM were subcutaneously inoculated under anesthesia into the right flank of 8-wk-old randomly selected female BALB/c mice and 200-μL MEM alone was injected in control mice. The LLC model was generated as recently described (15) by injecting of 5 × 105 LLC cells (RRID: CVCL_4358, CRL-1642, ATCC, Manassas, VA) in 100-μL sterile PBS subcutaneously into the right flank of 8-wk-old randomly selected female C57BL6/129 mice. Control mice were injected with 100 μL of sterile PBS. Both LP07 and LLC models were monitored for tumor growth over a 28- to 30-day period, and on euthanasia, the gastrocnemius muscles were excised, frozen, and stored at −80°C for subsequent analysis. Age-matched BALB/c or C57BL6/129 mice were used as controls for the LP07 and LLC models, respectively.
Determination of Muscle Function
Forelimb force production was determined via the grip strength test before tumor cell inoculation and before euthanasia as previously described (3, 32). Briefly, mice were allowed to grasp a wire mesh attached to a force transducer (Bioseb, Vitrolles, France) while held by the base of the tail. Three pulls were recorded with 5 s of rest between trials and absolute grip strength was calculated as the average of the peak forces from the three trials.
RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
Total RNA was extracted using Direct-zol RNA MiniPrep kit (Cat. No. R2051, Zymo Research, Irvin, CA) following the manufacturer’s instructions as previously described (16). Briefly, following extraction, RNA was quantified spectrophotometrically using a CLARIOstar Microplate Reader with a LVis Plate (BMG Labtech, Ortenberg, Germany) and cDNA synthesized through reverse transcription PCR using the SuperScript VILO cDNA synthesis kit (Cat. No. 11756500, Zymo Research, Irvin, CA). The cDNA products were then used to quantify the respective mRNA and rRNA quantities via qRT-PCT (BioRad CX384) utilizing GoTaq qPCR Master (Cat. No. A6002, Promega, Madison, WI). The full list of qRT-PCR primers is found in Table 1. All analyzed transcripts via qPCR were normalized to GAPDH by the comparative Ct (ΔΔCt) method.
Table 1.
Primers used in this study
| Target | Forward | Reverse |
|---|---|---|
| 45S pre-rRNA (ETS) | CCAAGTGTTCATGCCACGTG | CGAGCGACTGCCACAAAAA |
| 45S pre-rRNA (ITS) | CCGGCTTGCCCGATTT | GCCAGCAGGAACGAAACG |
| UBF | CGCGCAGCATACAAAGAATACA | GTTTGGGCCTCGGAGCTT |
| SSRP1 | CAGAGACATTGGAGTTCAACGA | GCCCGTCTTGCTGTTCTTAAAG |
| Spt16 | TGGGACCTCGCGTGATTCTC | GCCACGTCTGTAAGGCAGTTG |
| Spt4 | AGTGGCAGCGAGTCAGTAAC | AGTGGCAGCGAGTCAGTAAC |
| Spt5 | CTGGAGAAAGAAGAGATTGAAGCC | TCAGACCCTCCATACACCGT |
| Twtstnb | CTGAGCCTGGGCAGACGTTA | CAGGCTTAGGGATAGAGGCGT |
| PAF-53 | CTCGGTTCTCCTGCCCTCC | AGAAAGAGGTACACGCCGCC |
| Znrd1 | TCACACCAGACAGATGCGCT | AAAGCATGGTAGCCGGAGGG |
| Polr2f | TCGACGGCGACGACTTTGAT | GGCCCGCTCATACTTGGTCA |
| IL-6 | AGCCAGAGTCCTTCAGAGAGA | GGAGAGCATTGGAAATTGGGG |
| TNF-α | GTCCCCAAAGGGATGAGAAGT | TTTGCTACGACGTGGGCTAC |
| NLRP3 | AGAGTGGATGGGTTTGCTGG | CGTGTAGCGACTGTTGAGGT |
| IL-1β | TGCCAAAAGGAAGATGATGCT | ACCCTCCCCACCTAACTTTG |
| IL-18 | GCACCTTCTTTTCCTTCATCTTTG | GTTGTTCATCTCGGAGCCTGT |
| Myostatin | TGGCCATGATCTTGCTGTAA | CCTTGACTTCTAAAAAGGGATTCA |
| GAPDH | ACTGAGCAAGAGAGGCCCTA | TATGGGGGTCTGGGATGGAA |
Western Blot Analysis
Samples were homogenized and extracted in RIPA lysis buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% Triton X-100, 0.1% SDS) supplemented with Pierce Protease and Phosphatase Inhibitor Mini Tablets (Cat. No. A32959, Thermo Fisher Scientific, Waltham, MA). The extracted proteins (10 µg) were separated by SDS-page on polyacrylamide gels, transferred to PVDF membranes as previously described (16), and subjected immunoblotting using the following antibodies: 4E-BP1 [1:2,000, Cell Signaling Technology (CST); Cat. No. 9644, RRID: AB_2097841]; Phospho-S6 Ribosomal Protein (S235/236; 1:2,000, CST; Cat. No. 2211, RRID: AB_331679), S6 Ribosomal Protein (1:2,000, CST; Cat. No. 2217, RRID: AB_331355), mTOR (1:2,000, CST; Cat. No. 2983, RRID: AB_2105622), Phospho-mTOR (Ser2448; 1:1,000, CST; Cat. No. 2971, RRID: AB_330970). All blots were verified for equal loading using whole membrane staining with 0.2% India ink in PBS (Alfa Aesar; Cat. No. J61007AP, Thermo Fisher Scientific, Waltham, MA).
Statistical Analysis
All data are reported as means ± standard deviation (SD) or mean standard error (SE) where appropriate and represented as either fold-change or as percentage change from control. To reduce variability, potential outliers were identified by a ROUT test with the maximum false discovery rate set to 1%. A two-tail unpaired t test was used when comparing the model tumor weights and tumor-to-BW ratios. All other comparisons were analyzed by a two-way ANOVA (model × tumor). A planned Tukey’s multiple-comparison test was performed if there was a significant interaction or main effect. P values ≤ 0.05 were considered significant. Correlations between the changes in muscle mass and rRNA, elongation subunits, pol I subunits, translational signaling factors, and cytokine mRNA expression were calculated using Pearson’s product-moment correlation coefficients where appropriate. All statistical analyses were performed and graphically represented using GraphPad Prism 8 (GraphPad Prism, RRID: SCR_002798, San Diego, CA).
RESULTS
Skeletal Muscle Wasting Is Similar in LP07 and LLC Models of Cachexia
Changes in tumor-free body weight relative to initial body weight revealed a significant decrease in the LLC tumor group only (P = 0.008; Fig. 1A). In addition, analyses of both raw tumor weight (P = 0.001; Fig. 1B) and the percentage of the tumor weight relative to whole body weight (P = 0.023; Fig. 1C) were significantly higher in the LLC model when compared with the LP07. Relative gastrocnemius muscle mass was significantly lower in both the LP07 (P = 0.026) and LLC (P < 0.002) tumor mice (Fig. 1D). Gastrocnemius wet muscle weight was also significantly decreased in both the LP07 (P = 0.031) and the LLC (P < 0.001) models. However, despite similar reductions in relative muscle weight, the LP07 tumor model experienced a decline in forelimb grip strength (P < 0.001) which was not apparent in the LLC model (P = 0.645; Fig. 1F).
Figure 1.

Skeletal muscle wasting is similar in LP07 and LLC models of cachexia. A: change in body weight (BW) was calculated as the percentage difference between initial and tumor-free end endpoint BW in grams. B: tumor weights are expressed in grams. C: the ratio of tumor weights was divided by the end BW and expressed in percentages. D: end gastrocnemius wet weight in milligrams was normalized as a percentage relative to their respective control. E: end gastrocnemius wet weight in milligrams. F: change in forelimb grip strength was calculated as the percent difference between initial and endpoint force in Newtons. Data are expressed as means ± SD. A two-way ANOVA followed by a Tukey’s multiple-comparisons test was performed for the change in tumor-free BW, relative muscle weight, and grip strength; and an unpaired two-tail t test was used for tumor weight and ratio of tumor to total end BW. Significant difference from LP07 control: **P < 0.01; from LLC control: ##P < 0.01, ###P < 0.001; between tumor groups: †P < 0.05, ††P < 0.01, †††P < 0.001. gastroc, gastrocnemius; LLC, Lewis lung carcinoma.
LP07 Tumors Reduced rRNA Levels and rDNA Transcription Elongation
Losses in total rRNA content occurred in the LP07 tumor mice (P = 0.008), whereas no observable differences were found in the LLC model (P = 0.916; Fig. 2A). When compared with their LLC counterparts, we found that total rRNA content was ∼60% lower in the tumor-bearing LP07 mice (P = 0.002), and strongly correlated with losses in muscle mass (LP07: r = 0.708, P = 0.022; Fig. 2B). Although no alterations were found in 45S pre-rRNA 5' external transcribed spacer (ETS) transcription, a reduction in the 45S pre-RNA internal transcribed spacer 1 (ITS) was observed in LP07 mice when compared with controls (P = 0.007) and no detectable differences in either ETS or ITS transcripts in the LLC model (Fig. 2C).
Figure 2.
Tumor-bearing LP07 mice exhibited significantly lower ribosomal (r)RNA and 45S pre-rRNA internal transcribed spacer (ITS) compared with controls and LLC tumor mice. A: quantification of total rRNA. B: Pearson’s product-moment correlations between rRNA and gastrocnemius muscle mass in LP07 and Lewis lung carcinoma (LLC) inoculated mice. C: expression of 45S pre-rRNA 5’ external transcribed spacer (ETS) and ITS. Data are expressed as fold-change from controls ± SE. A two-way ANOVA followed by a Tukey’s multiple-comparisons test was performed for all direct comparisons. Correlations were calculated using Pearson’s product-moment correlation. Significant difference from LP07 control: *P < 0.05; between tumor groups: †P < 0.05.
RNA Pol I Elongation-Related Subunits Are Upregulated in LP07 Tumor-Bearing Mice
To further investigate possible explanations for the loss of rRNA and ITS transcripts observed in the LP07 model, we next analyzed the expression of pol I subunits associated with 45S pre-rRNA elongation. Examination of pol I subunit mRNA showed significant increases in Polr2f in LP07 tumor mice compared with both control (P = 0.006) and LLC tumor-bearing mice (P = 0.001) and significantly which negatively correlated to muscle mass loss (r = −0.785, P = 0.007). In addition, increased PAF53 (P = 0.022) and Znrd1 (P = 0.004) mRNA transcripts were uncovered in the LP07 model only (Fig. 3A) with significant correlations to muscle mass (PAF53: r = −0.720, P = 0.019; Znrd1: r = −0.865, P = 0.001; Fig. 3B).
Figure 3.
RNA polymerase I (RNA pol I) subunit mRNA expression was increased in LP07 model. A: expression of RNA pol I elongation associated subunit mRNAs. B: correlations between RNA pol I elongation associated subunit mRNAs and gastrocnemius muscle mass. Data are expressed as mean fold-change from controls ± SE. A two-way ANOVA followed by a Tukey’s multiple-comparisons test was performed for all direct comparisons. Correlations were calculated using Pearson’s product-moment correlation. Significant differences from LP07 controls: *P < 0.05, **P < 0.01; between tumor groups: ††P < 0.01.
RNA Pol I-Associated Elongation Factors Are Significantly Elevated in the LLC Tumor-Bearing Mice
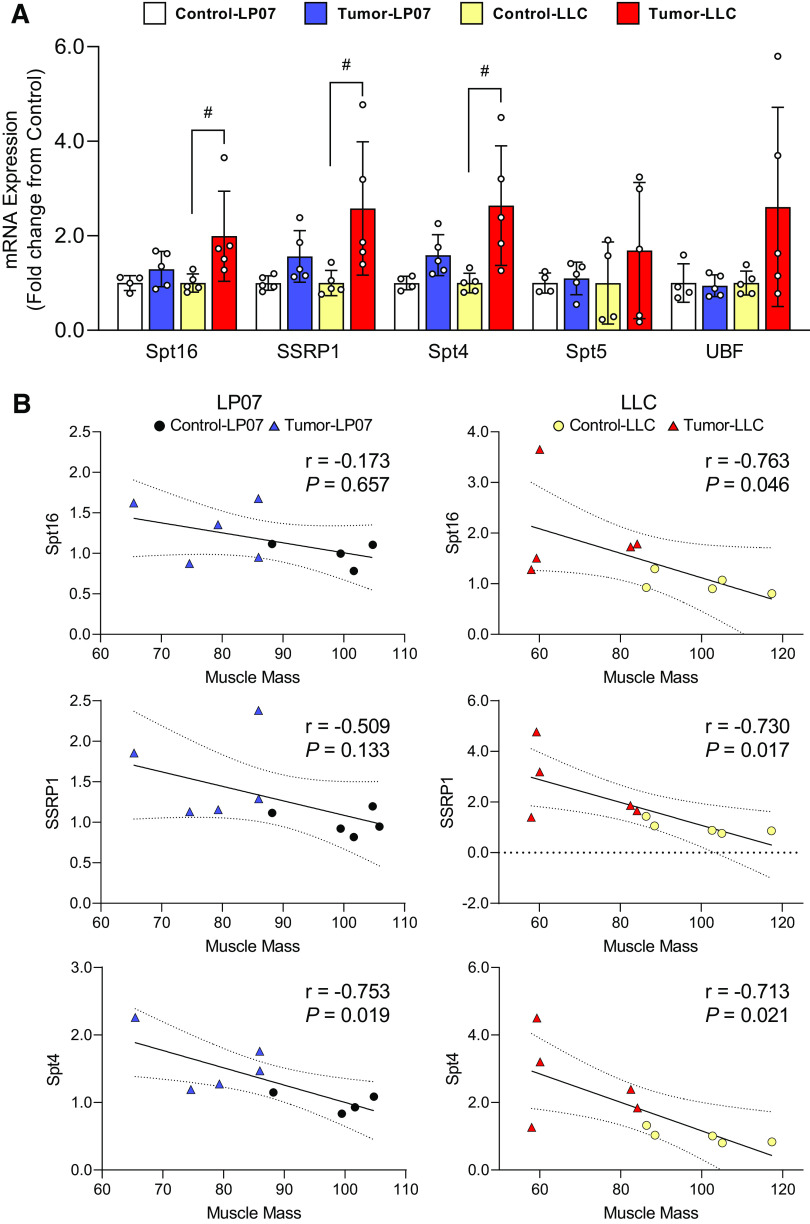
Due to the disruption in rDNA transcription in 45S pre-rRNA elongation, we next examined several RNA pol I-associated elongation factors. Of the five factors evaluated, only SSRP1, Spt4, and Spt16 mRNA transcripts were significantly elevated in LLC tumor-bearing mice when compared with controls (Spt16: P = 0.049; SSRP1: P = 0.024; Spt4: P = 0.010), whereas no changes were observed in the LP07 (Fig. 4A). Further analyses revealed strong correlations between Spt16 (r = −0.763, P = 0.046), SSRP1 (r = −0.730, P = 0.017), and Spt4 (r = −0.713, P = 0.021) and the losses in muscle mass in the LLC model (Fig. 4B).
Figure 4.
An increase in RNA polymerase I (RNA pol I) elongation factors was found in the Lewis Lung Carcinoma (LLC) model for Spt16, SSRP1, and Spt4 mRNA transcripts. A: expression of Spt16, SSRP1, Spt4, Spt5, and UBF mRNA. B: Pearson’s product-moment correlations between RNA pol I elongation factor mRNA and gastrocnemius muscle mass in LP07 and LLC inoculated mice. Data are expressed as mean fold-change from controls ± SE. A two-way ANOVA followed by a Tukey’s multiple-comparisons test was performed for all direct comparison. Correlations were calculated using Pearson’s product-moment correlation. Significant differences from LLC control: #P < 0.05.
Phosphorylation Status of Translational Control Factors Differs between LP07 and LLC Models
We then investigated the anabolic deficits in the LP07 and LLC models by examining changes in translational control factor signaling. Western blots were used to compare the phosphorylation status of mTOR(S2448) relative to total mTOR, the γ band of 4E-BP1 relative to total 4E-BP1 as a surrogate for phosphorylation, and the phosphorylation status of RPS6(S235/236) relative to total RPS6. Here, we show a significant reduction in mTOR phosphorylation in the tumor-bearing LLC mice when compared with their controls (P = 0.042) and the LP07 tumor-bearing group (P = 0.036; Fig. 5A) that strongly correlated to losses in muscle mass (r = 0.929, P < 0.001; Fig. 5C). Downstream of mTORC1, we uncovered similar decreases in RPS6 phosphorylation (LP07: P = 0.020; LLC: P = 0.003) and 4E-BP1 phosphorylation (LP07: P < 0.001; LLC: P < 0.001; Fig. 5A) with significant correlations to lower muscle mass in both models (RPS6: r = 0.827, P = 0.011; 4E-BP1: r = 0.792, P = 0.019; Fig. 5C).
Figure 5.

A: quantification of mTOR, RPS6, and 4E-BP1 Western blots. B: representative Western blots for mTOR, RPS6, and 4E-BP1. The 4E-BP1 γ subunit was normalized to total 4E-BP1 and phosphorylated ribosomal protein S6 at S235/236 (p-RPS6(S235/236)) was normalized to total RPS6 to assess protein phosphorylation status. C: correlations between mTOR, RPS6, and 4E-BP1 Western blots and gastrocnemius muscle mass. Data are expressed as mean fold-change from controls ± SD. A two-way ANOVA followed by a Tukey’s multiple-comparisons test was performed for all direct comparisons. Correlations were calculated using Pearson’s product-moment correlation. Significant differences from LP07 controls: *P < 0.05, ***P < 0.001; from LLC controls: ###P < 0.001. LLC, Lewis lung carcinoma.
IL-6 and TNF-α mRNA Are Significantly Elevated in LLC Mice, Whereas NLRP3 mRNA Is Induced Regardless of Tumor Type
To assess specific proinflammatory cytokine profiles in these models, we examined the mRNA levels of selected effectors of muscle wasting. In the LLC tumor mice, muscle IL-6 and TNF-α mRNA levels were significantly elevated when compared with both their respective control groups (IL-6: P < 0.001; TNF-α: P < 0.001) and the LP07 group (IL-6: P < 0.001; TNF-α: P = 0.028; Fig. 6A), and negatively correlated with reductions in muscle mass (IL-6: r = −0.787, P = 0.007; TNF-α: r = −0.562, P = 0.091; Fig. 6B). Expression of the NLRP3 inflammasome, IL-1β, and IL-18 did not differ between models. However, NLRP3 mRNA levels were elevated in the tumor-bearing mice of both models (LP07: P < 0.001; LLC: P = 0.004). The expression of myostatin remained unaltered in either tumor model at the time points studied (Fig. 6A).
Figure 6.
Proinflammatory cytokine mRNA expression was only elevated in the skeletal muscle of cachectic LLC mice. A: expression of proinflammatory cytokines (IL-6, TNF-α, NLRP3, IL-1β, and IL-18) and myostatin mRNA. B: correlations between proinflammatory cytokine mRNA levels and gastrocnemius muscle mass. Data are expressed as mean fold-change from controls ± SE. A two-way ANOVA followed by a Tukey’s multiple-comparisons test was performed for all direct comparisons. Correlations were calculated using Pearson’s product-moment correlation. Significant differences from LP07 controls: *P < 0.05, ***P < 0.001; from LLC controls: ###P < 0.001. LLC, Lewis lung carcinoma.
DISCUSSION
We investigated key mechanisms underlying anabolic deficits and the expression of proinflammatory cytokines using two established preclinical models of LC with muscle wasting. Clinically, cancer cachexia is defined by a loss of body weight, weakness, and wasting of skeletal muscle and adipose tissue that is not reversible by nutritional interventions (33–35). The emergence of new preclinical models of cancer cachexia has raised awareness about potential differences that may challenge their translational relevance for the understanding of muscle wasting in humans (11, 36). This motivated us to determine if tumors originating from the same tissue involved similar mechanisms of muscle wasting or whether they entail tumor-specific mechanisms. LP07 tumors have been previously shown to cause a significant reduction in body (∼8%), gastrocnemius (∼25%), and diaphragm (∼25%) mass (3), whereas LLC tumor-bearing mice also experienced reductions in lean body mass (∼10%), tibialis anterior (∼25%), extensor digitorum longus (∼15%), soleus (∼25%), and gastrocnemius (∼20%) mass (15). Contrary to our hypothesis, we report findings demonstrating divergent anabolic deficits and local expression of proinflammatory cytokines in these established preclinical models of LC-associated muscle wasting.
Impaired force production often accompanies muscle wasting and is a hallmark of cancer cachexia (33, 35). We found that although both tumor types caused similar levels of muscle wasting, the LP07 tumors resulted in a severe reduction in grip strength whereas the LLC tumor-bearing mice remained unaffected. This suggests that either the LP07 tumor impacts the neuromuscular system in a tumor-specific manner, or that the Balb/c mouse strain is more susceptible to neuromuscular impairments following an oncogenic challenge. Recent findings from two independent laboratories using the C26, MC38, or HCT116 tumors as models of colorectal cancer reported that muscle wasting was associated with disruptions of neuromuscular junctions (37, 38). The underlying cause of the force deficits was a defect in presynaptic axon terminal morphology, which did not appear to show any tumor selectivity in the CD2F1 mouse strain (38). Thus, although it seems likely that force deficits in the LP07 may be a consequence of defective neuromuscular remodeling, it remains to be determined whether the LLC tumors can impact the neuromuscular junction in a similar manner at a later time point, or whether the C57BL6/129 mice are resistant to tumor-induced neuromuscular junction alterations.
Reduced skeletal muscle anabolism has been consistently reported in preclinical models of cancer (16–19) and is largely determined by a diminished ribosomal capacity (15). Here, we report that muscle rRNA levels were significantly reduced in LP07 but not in the LLC tumor-bearing mice. The reduction in rRNA was consistent with our recent findings in the ES-2 ovarian cancer model (16), but in the LP07 model, the deficit in rRNA production was a consequence of decreased rDNA elongation as reflected by a lower (∼40%) ITS signal. Because disruptions in Pol I elongation can impair rRNA processing and hence rRNA levels (39), we examined the expression of Pol I subunits and associated elongation factors to find out if the deficit in Pol I elongation was a consequence of a reduced expression of these factors. The Pol I holoenzyme is composed of 13 subunits, of which Polr2f, PAF53, Znrd1, and Twistnb promote efficient transcription elongation (40, 41). For example, mutations in rpa49 (yeast ortholog of PAF53) result in defective Pol I elongation (40), and ablation of the core Pol I subunit ABC23 (yeast ortholog of Polr2f) leads to the loss of rpa43 (Twistnb), reduces rpa14 stability, and consequently impairs rRNA elongation (41). Contrary to our assumption, we found that the expression of Pol I subunits involved in transcription elongation was higher in LP07 tumor-bearing mice. This intriguing finding resembles the expression pattern of Pol I subunits previously reported in the ES-2 model with reduced rDNA transcription, where the expression of Pol I factors was elevated in response to lower rRNA production (16). In the LP07 model, the specific response involved the upregulation of selected Pol I elongation subunits, likely to counter the reduction in rDNA transcription elongation. Another contrasting finding was the elevated expression of Pol I-associated elongation factors in the LLC model. Due to the specific reduction in ITS levels, we focused on two complexes involved in Pol I elongation. The Facilitates Chromatin Transcription (FACT) complex is a heterotrimeric protein complex composed of the Spt16 and SSRP1 subunits (42) and is responsible for maintaining the euchromatic state to facilitate transcription elongation (43). The Spt4/5 complex interacts with Pol I to promote processivity along the rDNA repeat (44). Although we did not find any differences in the LP07, the expression level of Spt16, SSRP1, and Spt5 mRNA was significantly elevated in the LLC tumor-bearing mice. Clearly, although the LP07 tumor impaired transcription elongation, the LLC model appears to maintain rRNA levels likely via the upregulation of elongation-specific factors. These observations suggest that model-specific regulatory mechanisms exist at the level of rDNA transcription elongation that either impair (LP07) or maintain (LLC) rRNA production. This is in contrast with our previous findings in the ES-2 ovarian cancer model where deficits in rDNA transcription occurred at the initiation level (16).
In addition to the discrepant alterations in ribosomal capacity, the LP07 and LLC models also produced dissimilar changes in muscle intracellular signaling. A reduction in mTOR phosphorylation was only apparent in LLC mice; however, a significant reduction in RPS6 and 4E-BP1 phosphorylation was detectable in both models. Although our results are consistent with previous findings in cachectic LLC mice (5, 6, 13), a recent clinical investigation of late-stage nonsmall cell lung cancer (NSCLC) patients reported reductions in p70S6k and 4E-BP1 phosphorylation independent of changes in mTOR phosphorylation (22). Thus, it appears that the phosphorylation patterns induced by the LP07 model resemble those described in muscle from patients with LC. The dissociation in mTOR phosphorylation from its downstream targets is intriguing because mTOR is recognized as the putative upstream modulator of RPS6(S235/236) and 4E-BP1. A possible explanation for the discrepancy between mTOR phosphorylation and downstream targets is the input from Ras/Raf/ERK signaling, which may be operant in parallel to mTOR (6, 45). For example, pharmacological inhibition of ERK with the MEK inhibitor U0126 can reduce RPS6(S235/236) phosphorylation to a greater extent than rapamycin (45), and the ERK inhibitor LY-294002 can significantly reduce 4E-BP1 phosphorylation. Thus, it is possible that ERK signaling may operate along mTOR to modulate RPS6 and 4E-BP1 phosphorylation (46), which suggest that dysregulation of ERK signaling also contributes to the reduction in RPS6 and 4E-BP1 phosphorylation in LC independent of mTOR. Whereas both LP07 and LLC tumor-bearing mice experienced a reduction in RPS6 and 4E-BP1 phosphorylation, a clear detrimental signaling effect exists at the level of mTOR phosphorylation in the LLC tumor-bearing mice.
Changes in systemic proinflammatory cytokines have been consistently associated with muscle wasting in cancer (26, 34). Specifically in patients with late-stage LC, elevated TNF-α and IL-6 levels are concurrent with the progression of cachexia (22, 23) and can aggravate the proinflammatory milieu by stimulating in situ cytokine production (27, 52). Increased systemic inflammation has been well-documented in both LP07 and LLC models (4, 5, 9); however, it is unclear if local skeletal muscle cytokine expression is conserved in these models (27, 47). We found that LLC tumors induced a significant increase in muscle IL-6 and TNF-α mRNA, but the LP07 tumor model did not significantly alter the expression of these cytokines. NLRP3, considered to be important for local cytokine production via the inflammasome (30), was similar in both models, but the proinflammatory cytokines IL-1β and IL-18 were not induced in either model. This was intriguing and contrary to our hypothesis that NLRP3, IL-1β, and IL-18 are transcriptionally coregulated. One possible explanation is that the elevation in NLRP3 mRNA may serve as a priming mechanism for IL-1β and IL-18 processing, therefore once these cytokines are produced, NLRP3 facilitates their maturation into active cytokines. Previous studies have shown that sepsis-induced muscle wasting stimulates local NLRP3-dependent IL-1β production (31). However, because the expression of these cytokines is transient and most severe following acute insults (31, 48), the lack of changes in IL-1β or IL-18 mRNA suggests that these cytokines may be induced during the acute phase of the wasting process rather than at this later stage. This interpretation is consistent with recent findings demonstrating downregulation of proinflammatory cytokines during the late stages (4 wk) of muscle wasting in LLC tumor-bearing mice (49). We also examined if the LP07 and LLC tumors caused any changes in myostatin, a potent negative regulator of muscle growth (50). Myostatin mRNA expression was not induced by either tumor, which is in line with clinical findings showing that myostatin mRNA was unaltered in muscle biopsies from patients with late-stage NSCLC (22, 23). Together with the lack of changes in IL-1β and IL-18 expression, our results suggest that myostatin may play a more prominent role in the early stages of the wasting process (51). Altogether, the cytokine findings suggest that although IL-6 and TNF-α appear to contribute to muscle wasting in a tumor-specific manner (i.e., elevated in LLC), the specific responses of other proinflammatory and growth-suppressing factors may be conserved but at earlier time points. The fact that NLRP3 expression was similar in both models suggests that inflammasome-mediated local cytokine production may be a general response to tumors. Clearly, although the LP07 and LLC preclinical models of LC exhibit divergent proinflammatory phenotypes, more research is warranted to establish their temporal involvement in these two models of LC-induced muscle wasting.
In summary, although both the LP07 and LLC preclinical models of LC resulted in significant muscle wasting, clear differences in anabolic deficits, neuromuscular function, intracellular signaling, and proinflammatory cytokine profiles demonstrate tumor-specific mechanisms of action that promote muscle wasting and loss of function. A limitation of this study is that we were not able to define the role of tumor burden because we utilized two tumor types in different genetic backgrounds. However, it is important to highlight that the LLC developed into larger tumors compared with the LP07 with approximately twofold higher tumor weight to body mass ratio. Yet, muscle wasting was relatively similar in the two models studied. Further investigations designed to address the role of tumor burden will help clarify how different tumor types induce anabolic deficits and the expression of proinflammatory effectors in preclinical models of muscle wasting.
GRANTS
This study was supported by grants from the National Institutes of Health Grants AR073385 and AR078430 (to G.A.N.), and by the Instituto de Salud Carlos-III, contract Grant Nos., CIBERES, FIS 18/00075 (FEDER), Spanish Ministry of Science and Innovation (to E.B.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Esther Barreiro is an editor of Journal of Applied Physiology and was not involved and did not have access to information regarding the peer-review process or final disposition of this article. An alternate editor oversaw the peer-review and decision-making process for this article.
AUTHOR CONTRIBUTIONS
D.W., E.B., and G.A.N. conceived and designed research; D.J.B., M.G., B.H., and H.-G.K. performed experiments; D.J.B., M.G., B.H., and H.-G.K. analyzed data; D.J.B., H.-G.K., and G.A.N. interpreted results of experiments; D.J.B. and H.-G.K. prepared figures; D.J.B., H.-G.K., and G.A.N. drafted manuscript; D.J.B., B.H., H.-G.K., D.W., E.B., and G.A.N. edited and revised manuscript; D.J.B., M.G., B.H., H.-G.K., D.W., E.B., and G.A.N. approved final version of manuscript.
REFERENCES
- 1. Arthur ST, Van Doren BA, Roy D, Noone JM, Zacherle E, Blanchette CM. Cachexia among US cancer patients. J Med Econ 19: 874–880, 2016. doi: 10.1080/13696998.2016.1181640. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 69: 7–34, 2019. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3. Chacon-Cabrera A, Fermoselle C, Urtreger AJ, Mateu-Jimenez M, Diament MJ, de Kier Joffé EDB, Sandri M, Barreiro E. Pharmacological strategies in lung cancer-induced cachexia: effects on muscle proteolysis, autophagy, structure, and weakness. J Cell Physiol 229: 1660–1672, 2014. doi: 10.1002/jcp.24611. [DOI] [PubMed] [Google Scholar]
- 4. Chacon-Cabrera A, Mateu-Jimenez M, Langohr K, Fermoselle C, García-Arumí E, Andreu AL, Yelamos J, Barreiro E. Role of PARP activity in lung cancer-induced cachexia: Effects on muscle oxidative stress, proteolysis, anabolic markers, and phenotype. J Cell Physiol 232: 3744–3761, 2017. doi: 10.1002/jcp.25851. [DOI] [PubMed] [Google Scholar]
- 5. Puppa MJ, Gao S, Narsale AA, Carson JA. Skeletal muscle glycoprotein 130’s role in Lewis lung carcinoma-induced cachexia. FASEB J 28: 998–1009, 2014. doi: 10.1096/fj.13-240580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown JL, Lee DE, Rosa-Caldwell ME, Brown LA, Perry RA, Haynie WS, Huseman K, Sataranatarajan K, Van Remmen H, Washington TA, Wiggs MP, Greene NP. Protein imbalance in the development of skeletal muscle wasting in tumour-bearing mice. J Cachexia Sarcopenia Muscle 9: 987–1002, 2018. [Erratum in J Cachexia Sarcopenia Muscle 10: 712, 2019]. doi: 10.1002/jcsm.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang G, Liu Z, Ding H, Miao H, Garcia JM, Li YP. Toll-like receptor 4 mediates Lewis lung carcinoma-induced muscle wasting via coordinate activation of protein degradation pathways. Sci Rep 7: 2273, 2017. doi: 10.1038/s41598-017-02347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown JL, Rosa-Caldwell ME, Lee DE, Blackwell TA, Brown LA, Perry RA, Haynie WS, Hardee JP, Carson JA, Wiggs MP, Washington TA, Greene NP. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J Cachexia Sarcopenia Muscle 8: 926–938, 2017. doi: 10.1002/jcsm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohe Y, Podack ER, Olsen KJ, Miyahara Y, Miura K, Saito H, Koishihara Y, Ohsugi Y, Ohira T, Nishio K, Saijo N. Interleukin-6 cDNA transfected Lewis lung carcinoma cells show unaltered net tumour growth rate but cause weight loss and shorten survival in syngeneic mice. Br J Cancer 67: 939–944, 1993. doi: 10.1038/bjc.1993.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chacon-Cabrera A, Fermoselle C, Salmela I, Yelamos J, Barreiro E. MicroRNA expression and protein acetylation pattern in respiratory and limb muscles of Parp-1-/- and Parp-2-/- mice with lung cancer cachexia. Biochim Biophys Acta 1850: 2530–2543, 2015. doi: 10.1016/j.bbagen.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 11. Ballarò R, Costelli P, Penna F. Animal models for cancer cachexia. Curr Opin Support Palliat Care 10: 281–287, 2016. doi: 10.1097/SPC.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 12. Rosa-Caldwell ME, Fix DK, Washington TA, Greene NP. Muscle alterations in the development and progression of cancer-induced muscle atrophy: a review. J Appl Physiol (1985) 128: 25–41, 2020. doi: 10.1152/japplphysiol.00622.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bohnert KR, Gallot YS, Sato S, Xiong G, Hindi SM, Kumar A. Inhibition of ER stress and unfolding protein response pathways causes skeletal muscle wasting during cancer cachexia. FASEB J 30: 3053–3068, 2016. doi: 10.1096/fj.201600250RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim S, Deaver JW, Rosa-Caldwell ME, Haynie WS, Morena da Silva F, Cabrera AR, Schrems ER, Saling LW, Jansen LT, Dunlap KR, Wiggs MP, Washington TA, Greene NP. Development of metabolic and contractile alterations in development of cancer cachexia in female tumor-bearing mice. J Appl Physiol (1985) 132: 58–72, 2022. doi: 10.1152/japplphysiol.00660.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hain BA, Xu H, VanCleave AM, Gordon BS, Kimball SR, Waning DL. REDD1 deletion attenuates cancer cachexia in mice. J Appl Physiol (1985) 131: 1718–1730, 2021. doi: 10.1152/japplphysiol.00536.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim H-G, Huot J, Pin F, Guo B, Bonetto A, Nader G. Reduced rDNA transcription diminishes skeletal muscle ribosomal capacity and protein synthesis in cancer cachexia. FASEB J 35: e21335, 2021. doi: 10.1096/fj.202002257R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Emery PW, Edwards RH, Rennie MJ, Souhami RL, Halliday D. Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br Med J (Clin Res Ed) 289: 584–586, 1984. doi: 10.1136/bmj.289.6445.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baracos VE, DeVivo C, Hoyle DH, Goldberg AL. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol Endocrinol Physiol 268: E996–E1006, 1995. doi: 10.1152/ajpendo.1995.268.5.E996. [DOI] [PubMed] [Google Scholar]
- 19. Goodlad GAJ, Clark CM. Response of skeletal muscle RNA polymerases I and II to tumour growth. Biochim Biophys Acta 950: 296–302, 1988. doi: 10.1016/0167-4781(88)90125-x. [DOI] [PubMed] [Google Scholar]
- 20. Millward DJ, Garlick PJ, Nnanyelugo DO, Waterlow JC. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem J 156: 185–188, 1976. doi: 10.1042/bj1560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Millward DJ, Garlick PJ, James WPT, Nnanyelugo DO, Ryatt JS. Relationship between protein synthesis and RNA content in skeletal muscle [15]. Nature 241: 204–205, 1973. doi: 10.1038/241204a0. [DOI] [PubMed] [Google Scholar]
- 22. Murton AJ, Maddocks M, Stephens FB, Marimuthu K, England R, Wilcock A. Consequences of late-stage non–small-cell lung cancer cachexia on muscle metabolic processes. Clin Lung Cancer 18: e1–e11, 2017. doi: 10.1016/j.cllc.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 23. Op Den Kamp CM, Langen RC, Snepvangers FJ, De Theije CC, Schellekens JM, Laugs F, Dingemans AMC, Schols AM. Nuclear transcription factor κB activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am J Clin Nutr 98: 738–748, 2013. doi: 10.3945/ajcn.113.058388. [DOI] [PubMed] [Google Scholar]
- 24. Paval DR, Patton R, McDonald J, Skipworth R, Gallagher I, Laird B; Caledonian Cachexia Collaborative. A systematic review examining the relationship between cytokines and cachexia in incurable cancer. J Cachexia Sarcopenia Muscle 13: 824–838, 2022. doi: 10.1002/jcsm.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pelosi M, De Rossi M, Barberi L, Musarò A. IL-6 impairs myogenic differentiation by downmodulation of p90RSK/eEF2 and mTOR/p70S6K axes, without affecting AKT activity. Biomed Res Int 2014: 206026, 2014. doi: 10.1155/2014/206026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Argilés JM, López‐Soriano FJ. The role of cytokines in cancer cachexia. Med Res Rev 19: 223–248, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 27. Webster JM, Kempen LJAP, Hardy RS, Langen RCJ. Inflammation and skeletal muscle wasting during cachexia. Front Physiol 11: 597675, 2020. doi: 10.3389/fphys.2020.597675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Podbregar M, Lainscak M, Prelovsek O, Mars T. Cytokine response of cultured skeletal muscle cells stimulated with proinflammatory factors depends on differentiation stage. Sci World J 2013: 617170, 2013. doi: 10.1155/2013/617170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luo G, Hershko DD, Robb BW, Wray CJ, Hasselgren P-O. IL-1β stimulates IL-6 production in cultured skeletal muscle cells through activation of MAP kinase signaling pathway and NF-κB. Am J Physiol Regul Integr Comp Physiol 284: R1249–R1254, 2003. doi: 10.1152/ajpregu.00490.2002. [DOI] [PubMed] [Google Scholar]
- 30. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 10: 417–426, 2002. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 31. Huang N, Kny M, Riediger F, Busch K, Schmidt S, Luft FC, Slevogt H, Fielitz J. Deletion of Nlrp3 protects from inflammation-induced skeletal muscle atrophy. Intensive Care Med Exp 5: 3, 2017. doi: 10.1186/s40635-016-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonetto A, Andersson DC, Waning DL. Assessment of muscle mass and strength in mice. Bonekey Rep 4: 732, 2015. doi: 10.1038/bonekey.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489–495, 2011. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 34. Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 14: 754–762, 2014. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 35. Vanhoutte G, Van De Wiel M, Wouters K, Sels M, Bartolomeeussen L, De Keersmaecker S, Verschueren C, De Vroey V, De Wilde A, Smits E, Cheung KJ, De Clerck L, Aerts P, Baert D, Vandoninck C, Kindt S, Schelfhaut S, Vankerkhoven M, Troch A, Ceulemans L, Vandenbergh H, Leys S, Rondou T, Dewitte E, Maes K, Pauwels P, De Winter B, Van Gaal L, Ysebaert D, Peeters M. Cachexia in cancer: what is in the definition? BMJ Open Gastroenterol 3: e000097, 2016. doi: 10.1136/bmjgast-2016-000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Penna F, Busquets S, Argilés JM. Experimental cancer cachexia: Evolving strategies for getting closer to the human scenario. Semin Cell Dev Biol 54: 20–27, 2016. doi: 10.1016/j.semcdb.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 37. Sartori R, Hagg A, Zampieri S, Armani A, Winbanks CE, Viana LR, Haidar M, Watt KI, Qian H, Pezzini C. Perturbed BMP signaling and denervation promote muscle wasting in cancer cachexia. Sci Transl Med 13: eaay9592, 2021. doi: 10.1126/scitranslmed.aay9592. [DOI] [PubMed] [Google Scholar]
- 38. Huot JR, Pin F, Bonetto A. Muscle weakness caused by cancer and chemotherapy is associated with loss of motor unit connectivity. Am J Cancer Res 11: 2990–3001, 2021. [Erratum in Am J Cancer Res 12: 1435, 2022]. [PMC free article] [PubMed] [Google Scholar]
- 39. Schneider DA, Michel A, Sikes ML, Vu L, Dodd JA, Salgia S, Osheim YN, Beyer AL, Nomura M. Transcription elongation by RNA polymerase I is linked to efficient rRNA processing and ribosome assembly. Mol Cell 26: 217–229, 2007. doi: 10.1016/j.molcel.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Albert B, Léger-Silvestre I, Normand C, Ostermaier MK, Pérez-Fernández J, Panov KI, Zomerdijk JCBM, Schultz P, Gadal O. RNA polymerase I-specific subunits promote polymerase clustering to enhance the rRNA gene transcription cycle. J Cell Biol 192: 277–293, 2011. doi: 10.1083/jcb.201006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lanzendörfer M, Smid A, Klinger C, Schultz P, Sentenac A, Carles C, Riva M. A shared subunit belongs to the eukaryotic core RNA polymerase. Genes Dev 11: 1037–1047, 1997. doi: 10.1101/gad.11.8.1037. [DOI] [PubMed] [Google Scholar]
- 42. Orphanides G, LeRoy G, Chang C-H, Luse DS, Reinberg D. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell 92: 105–116, 1998. doi: 10.1016/s0092-8674(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 43. Birch JL, Tan BCM, Panov KI, Panova TB, Andersen JS, Owen-Hughes TA, Russell J, Lee SC, Zomerdijk JCBM. FACT facilitates chromatin transcription by RNA polymerases I and III. EMBO J 28: 854–865, 2009. doi: 10.1038/emboj.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schneider DA, French SL, Osheim YN, Bailey AO, Vu L, Dodd J, Yates JR, Beyer AL, Nomura M. RNA polymerase II elongation factors Spt4p and Spt5p play roles in transcription elongation by RNA polymerase I and rRNA processing. Proc Natl Acad Sci USA 103: 12707–12712, 2006. doi: 10.1073/pnas.0605686103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem 282: 14056–14064, 2007. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Le Plénier S, Goron A, Sotiropoulo A, Archambault E, Guihenneuc C, Walrand S, Salles J, Jourdan M, Neveux N, Cynober L, Moinard C. Citrulline directly modulates muscle protein synthesis via the PI3K/MAPK/4E-BP1 pathway in a malnourished state: evidence from in vivo, ex vivo, and in vitro studies. Am J Physiol Endocrinol Physiol 312: E27–E36, 2017. doi: 10.1152/ajpendo.00203.2016. [DOI] [PubMed] [Google Scholar]
- 47. Argilés JM, Stemmler B, López-Soriano FJ, Busquets S. Inter-tissue communication in cancer cachexia. Nat Rev Endocrinol 15: 9–20, 2018. doi: 10.1038/s41574-018-0123-0. [DOI] [PubMed] [Google Scholar]
- 48. Voss JG, Shagal AG, Tsuji JM, MacDonald JW, Bammler TK, Farin FM, St Pierre Schneider B. Time course of inflammatory gene expression following crush injury in murine skeletal muscle. Nurs Res 66: 63–74, 2017. doi: 10.1097/NNR.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 49. Blackwell TA, Cervenka I, Khatri B, Brown JL, Rosa-Caldwell ME, Lee DE, Perry RA Jr, Brown LA, Haynie WS, Wiggs MP, Bottje WG, Washington TA, Kong BC, Ruas JL, Greene NP. Transcriptomic analysis of the development of skeletal muscle atrophy in cancer-cachexia in tumor-bearing mice. Physiol Genomics 50: 1071–1082, 2018. doi: 10.1152/physiolgenomics.00061.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang DT, Yang YJ, Huang RH, Zhang ZH, Lin X. Myostatin activates the ubiquitin-proteasome and autophagy-lysosome systems contributing to muscle wasting in chronic kidney disease. Oxid Med Cell Longev 2015: 684965, 2015. doi: 10.1155/2015/684965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Busquets S, Toledo M, Orpí M, Massa D, Porta M, Capdevila E, Padilla N, Frailis V, López-Soriano FJ, Han HQ, Argilés JM. Myostatin blockage using actRIIB antagonism in mice bearing the Lewis lung carcinoma results in the improvement of muscle wasting and physical performance. J Cachexia Sarcopenia Muscle 3: 37–43, 2012. doi: 10.1007/s13539-011-0049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gao S, Durstine JL, Koh HJ, Carver WE, Frizzell N, Carson JA. Acute myotube protein synthesis regulation by IL-6-related cytokines. Am J Physiol Cell Physiol 313: C487–C500, 2017. doi: 10.1152/ajpcell.00112.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]