Abstract
With the increasing appreciation for sex as a biological variable and the inclusion of female mice in research, it is important to understand the influence of the estrous cycle on physiological function. Sex hormones are known to modulate vascular function, but the effects of the mouse estrous cycle phase on arterial stiffness, endothelial function, and arterial estrogen receptor expression remain unknown. In 23 female C57BL/6 mice (6 mo of age), we determined the estrous cycle stage via vaginal cytology and plasma hormone concentrations. Aortic stiffness, assessed by pulse wave velocity, was lower during the estrus phase compared with diestrus. In ex vivo assessment of isolated pressurized mesenteric and posterior cerebral arteries, the responses to acetylcholine, insulin, and sodium nitroprusside, as well as nitric oxide-mediated dilation, were not different between estrous cycle phases. In the aorta, expression of phosphorylated estrogen receptor-α was higher for mice in estrus compared with mice in proestrus. In the cerebral arteries, gene expression for estrogen receptor-β (Esr2) was lowest for mice in estrus compared with diestrus and proestrus. These results demonstrate that the estrus phase is associated with lower in vivo large artery stiffness in mice. In contrast, ex vivo resistance artery endothelial function is not different between estrous cycle phases. Estrogen receptor expression is modulated by the estrus cycle in an artery-dependent manner. These results suggest that the estrous cycle phase should be considered when measuring in vivo arterial stiffness in young female mice.
NEW & NOTEWORTHY To design rigorous vascular research studies using young female rodents, the influence of the estrous cycle on vascular function must be known. We found that in vivo aortic stiffness was lower during estrus compared with the diestrus phase in female mice. In contrast, ex vivo mesenteric and cerebral artery endothelial function did not differ between estrous cycle stages. These results suggest that the estrous cycle stage should be accounted for when measuring in vivo arterial stiffness.
Listen to this article’s corresponding podcast at https://ajpheart.podbean.com/e/vascular-function-during-the-estrous-cycle/.
Keywords: cerebral artery, estrogen receptors, nitric oxide, sex hormones, vascular function
INTRODUCTION
In the past, females were often excluded from research studies leading to a limited understanding of many physiological processes. In 2014, the National Institutes of Health announced a policy requiring both sexes to be represented in preclinical biomedical research for cells and animals unless otherwise justified (1), and many scientific journals are beginning to add this requirement (2). Proper design of rodent studies that include females necessitates an acknowledgment of the hormonal fluctuation throughout the estrous cycle and purposeful decisions about whether to control for these fluctuations. Therefore, it is crucial to elucidate the effects of the estrous cycle on physiological processes.
In women, the menstrual cycle phase impacts vascular function, in particular, stiffness of the large elastic arteries and endothelial function (3, 4). The large elastic arteries, specifically the aorta and carotid arteries, have a high elastin content contributing to their high compliance. Greater large artery stiffness corresponds with elevated systemic pulse pressure and increased risk for cardiovascular diseases and Alzheimer’s disease (5). At the same time, endothelial cell function is important for regulating vascular tone and can modulate the compliance of arteries (6). Endothelial cells produce nitric oxide (NO), a potent vasodilator, through the activation of endothelial nitric oxide synthase (eNOS). Impaired endothelial function, measured by endothelium-dependent vasodilation, is associated with cardiovascular and cerebrovascular diseases (7). Although numerous studies have examined changes to large artery stiffness and endothelial function during the menstrual cycle (3, 4, 8, 9), the effects of the estrous cycle phase on these vascular outcomes in mice have yet to be investigated.
The impact of the menstrual or estrous cycle on vascular function likely arises from modulation by sex hormones (10). In mice, the estrous cycle is on average 5 days in length and composed of four stages: proestrus, estrus, metestrus, and diestrus. Circulating estrogen in mice is highest in the proestrus and estrus phases and lowest in diestrus (4). Other sex hormones also vary during the estrous cycle, such as progesterone and testosterone. Estrogens bind to estrogen receptor α (ERα, Esr1 gene), estrogen receptor-β (ERβ, Esr2 gene), and estrogen G protein-coupled receptor (GPER, Gper1 gene) in vascular cells. Activation of ERα and GPER increases NO bioavailability, and deletion of Gper1 is associated with higher arterial stiffness in mice (11, 12). Sex hormones are highly specific and have a high affinity for their receptors, allowing the slightest differences in sex hormone concentrations to induce physiological changes (13). Thus, despite the short duration of the mouse estrous cycle and low concentrations of circulating sex hormones, it is possible for the changes in circulating sex hormone concentrations and subsequent downstream receptor-mediated signaling to modulate physiological function between estrous cycle phases.
Due to the importance of including young female rodents in research, it is pertinent to understand the effects of the estrous cycle on vascular physiology and related outcomes. Therefore, the purpose of this study was to determine the effect of the mouse estrous cycle phase on arterial stiffness, vascular endothelial function, arterial estrogen receptor expression, and cognitive function. We hypothesized that estrous cycle stages with high circulating estrogen, namely, proestrus and estrus compared with diestrus, would have lower aortic stiffness and higher cerebral and mesenteric artery endothelial function. We further hypothesized that arterial estrogen receptor expression and eNOS expression/activation would be highest during proestrus/estrus compared with diestrus. As vascular function has overarching effects on other organs, we sought to determine the effects of the estrous cycle on cognitive function and motor coordination. We hypothesized that proestrus/estrus would be associated with higher cognitive function and better motor coordination.
METHODS
Animals
Female C57BL/6 mice were purchased from Charles River and studied at 6 to 7 mo of age (n = 23). All mice were on a phytoestrogen-free diet (Envigo, Teklad Global Soy Protein-Free Extruded Rodent Diet, 2920X) with food and water provided ad libitum and housed in the animal care facility on a 12-h:12-h light/dark cycle at 24°C. Mice were euthanized by exsanguination under isoflurane immediately before vascular reactivity studies. In efforts to match the estrous cycles, female mice were induced into cycling by one cup of male bedding. All animal procedures conform with the Guide to the Care and Use of Laboratory Animals (8th ed., Revised 2011) and were approved by the Institutional Animal Care and Use Committee at the University of Oregon.
Estrous Cycle Tracking
The mouse estrous cycle was tracked via vaginal cytology at least 5 days before each test and at euthanasia. The vagina was flushed with a sterile transfer pipette containing sterile saline, and the sample was expelled into a 36-well plate and placed on a slide for staining. With the use of a rapid differential stain kit (Vet-One, Boise, ID), the samples were stained per kit instructions. Once the slide was dry, samples were observed with a LMi1 microscope (Lecia Microsystems, Wetzlar, Germany) by two investigators, and the cycle was determined. After analysis, two additional investigators confirmed the cycle stage, and any contradicting results were verified by the university’s veterinarian. Proestrus was identified by the presence of many nucleated epithelial cells, whereas estrus was identified by a predominance of cornified epithelial cells. Finally, diestrus was determined by the presence of polymorphonuclear leukocytes (14, 15) (see Supplemental Fig. S1). Metestrus was excluded from this study because of its limited duration within the estrous cycle.
Hormone Analysis
Plasma hormone concentrations for 17β-estradiol (E2), esterone (E1), progesterone, and testosterone were analyzed via liquid chromatography-mass spectrometry (LC-MS). Blood samples were collected via cardiac puncture with a heparin saline-coated syringe. The blood was centrifuged, then plasma was collected and frozen in liquid nitrogen. All plasma analyses were performed by the Endocrine Technologies Core at Oregon National Primate Research Center at Oregon Health & Science University. Estradiol and esterone detection was low, thus their quantification is less accurate due to concentrations below the standard limit for quantification. The ratio of E2 to E1 was calculated and reported (see Table 1).
Table 1.
Animal characteristics
| Variable | Proestrus | Estrus | Diestrus |
|---|---|---|---|
| n | 8 | 8 | 7 |
| Age, mo | 6.0 ± 0.3 | 5.9 ± 0.2 | 5.9 ± 0.2 |
| Body mass, mg | 23.9 ± 1.7 | 24.2 ± 1.7 | 24.4 ± 1.1 |
| Heart mass, mg | 0.11 ± 0.01 | 0.12 ± 0.02 | 0.12 ± 0.01 |
| Percent heart:body mass ratio | 0.47 ± 0.05 | 0.50 ± 0.07 | 0.51 ± 0.03 |
| Spleen mass, mg | 0.09 ± 0.02 | 0.11 ± 0.02 | 0.10 ± 0.01 |
| Percent spleen:body mass ratio | 0.41 ± 0.07 | 0.44 ± 0.06 | 0.42 ± 0.03 |
| WAT mass, mg | 0.76 ± 0.24 | 0.76 ± 0.36 | 0.77 ± 0.19 |
| Percent WAT:body mass ratio | 3.16 ± 0.88 | 3.08 ± 1.37 | 3.14 ± 0.67 |
| Gastroc mass, mg | 0.15 ± 0.02 | 0.18 ± 0.04 | 0.17 ± 0.03 |
| Percent gastroc:body mass ratio | 0.67 ± 0.11 | 0.75 ± 0.15 | 0.70 ± 0.13 |
| Uterus mass, mg | 0.10 ± 0.01 | 0.08 ± 0.01* | 0.08 ± 0.01* |
| Percent uterus:body mass ratio | 0.43 ± 0.04 | 0.33 ± 0.05* | 0.32 ± 0.03* |
| Esterone, ng/mL | 0.002 ± 0.001 | 0.001 ± 0.000* | 0.001 ± 0.001 |
| Estradiol, ng/mL | 0.008 ± 0.001 | 0.001 ± 0.000 | 0.002 ± 0.003 |
| E2:E1 ratio | 2.64 ± 1.74 | 0.49 ± 0.01 | 1.45 ± 0.76 |
| Progesterone, ng/mL | 2.32 ± 1.15 | 5.42 ± 2.80 | 4.40 ± 2.70 |
| Testosterone, ng/mL | 0.016 ± 0.012 | 0.024 ± 0.096 | 0.094 ± 0.050*† |
Values are means ± SD; n, number of animals. WAT, white adipose tissue; gastroc, gastrocnemius; E2, estradiol; E1, esterone. *P < 0.05 vs. proestrus †P < 0.05 vs. estrus.
Aortic Stiffness
Aortic stiffness was measured in vivo by pulse wave velocity (PWV). In a cohort of mice (n = 6), PWV was measured three times during a 5-day period, with 1 day between each measurement, with the goal to obtain measurements in each phase of the cycle. However, given the short duration of proestrus, we did not collect enough measurements during this stage to include in the analysis, and thus, data for only estrus and diestrus are presented for the repeated-measures comparison. For the remaining mice, PWV measures were collected only once. Mice were anesthetized using isoflurane (2% isoflurane in 100% oxygen) and laid supine on a temperature-controlled heating pad (35°C). Electrodes were placed on distal portions of limbs that recorded ECG, while two Doppler transducers were positioned on the aortic arch and abdominal aorta, and the distance between the two transducers was recorded. Using the Doppler signal processing workstation program (DSPW; Indus Industries, Webster, TX), we analyzed the time of a pulse to travel from the arch to the abdominal aorta, and the time was then divided by the distance between the transducers. Two researchers analyzed all the files independently, and the average was taken.
Vascular Reactivity
Endothelium-dependent vasodilation was assessed in ex vivo isolated pressurized mesenteric arteries and posterior cerebral arteries, as previously described in detail (16). Arteries were excised and cannulated onto glass micro pipettes in a myograph chamber (Danish MyoTechnology) filled with a physiological salt solution. All arteries were preconstricted with phenylephrine (1–6 μM to obtain 20%–40% preconstriction) and increases in luminal diameter in response to increasing concentrations of endothelium-dependent dilators acetylcholine (ACh: 1 × 10−9 to 1 × 10−4 M) and insulin (1 × 10−9 to 1 × 10−5 M) were determined. After ACh and insulin responses, arteries were incubated for 30 min with 0.1 mM of Nω-nitro-l-arginine methyl ester (l-NAME). ACh and insulin responses were repeated in the presence of l-NAME to determine the contribution of nitric oxide synthase to endothelium-dependent dilation. Endothelium-independent dilation was measured by the increases in lumen diameter to sodium nitroprusside (1 × 10−10 to 1 × 10−4 M).
Motor Coordination
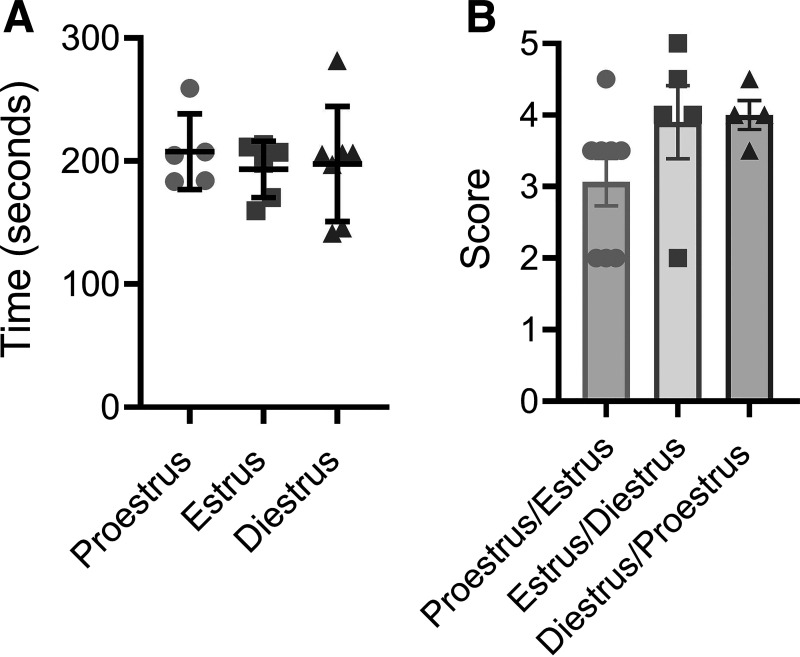
Motor coordination testing was performed using a rotarod (47650 Rota-Rod NG, Ugo Basile, Gemonio, Italy) over a 2-day period. The first day was a training session, and mice were required to stay on the rod rotating at 4 rpm for 90 s. The second day consisted of three trials, with a 10-min rest between trials, where the rod accelerated during each trial from 4 to 40 rpm with a cutoff time at 5 min. The time was recorded for when the mouse fell from the rod or rotated around the rod two consecutive times (17).
Nesting Behavior Test
Nest building tests instinctual behavior and was performed over a 12-h period. Mice were singly housed overnight in a cage with food, water, and a condensed cotton nestlet. In the morning, the researcher scored the nests and placed mice back in their communal cage. Nests were scored on a scale of 0–5, and the scoring criteria were as follows: 0, nestlet is untouched; 1, nestlet has been moved but no significant tearing; 2, significant tearing, but no formation of a nest; 3, significant tearing, indication of nest or nest is in the corner of the cage; 4, significant tearing, formation of a nest in the corner; and 5, significant tearing, formation of a nest in the corner and the nest walls are high, making a bowl-like nest (18). Because of mice changing cycle stages over the 12-h cycle period, the proestrus/estrus group consists of all proestrus and estrus mice. Mice in diestrus were placed in the diestrus/proestrus group. Mice that changed from estrus to diestrus or diestrus to proestrus overnight were placed in the estrus/diestrus and diestrus/proestrus groups, respectively.
Protein Expression
The thoracic aorta was excised and cleaned of fat and blood and frozen in liquid nitrogen. Samples were homogenized in total protein extracting reagent buffer, protease inhibitor cocktail and water then sonicated for 60 s twice at 50% power. Lysate protein concentration was assessed using a commercially available kit (Pierce BCA protein assay kit; Thermo Fisher, Waltham, MA). Precast Criterion TGX gels (4%–20%) were used for separation. Membranes were blocked with 5% goat serum in TBS-T. The transfer was verified with Ponceau red stain and then incubated in primary antibodies overnight in PBS containing 3% BSA. Primary antibody concentrations were as follows: Gapdh, 1:2,000; phosphorylated eNOS S1177, 1:1,000; total eNOS, 1:1,000; phosphorylated Akt S473, 1:1,000; total Akt, 1:1,000; phosphorylated Erα S118, 1:1,000; and Erα, 1:500. Following incubation, membranes were washed and incubated with a species-appropriate HRP-conjugated secondary antibody (1:1,000). Following washes, membranes were incubated with Pierce enhanced chemiluminescence Western blotting substrate (Pierce ECL, Thermo Fisher) and imaged with an Odyssey Fc (LI-COR, Lincoln, NE). ImageJ was used to determine protein band density, and relative protein expression was calculated as protein density normalized to Gapdh density. Values were normalized to the group in diestrus. See Supplemental Table S1 for the antibody list and validation and Supplemental Figs. S2–S6 for blot images with DNA ladder.
Gene Expression
In samples of mesenteric arteries and cerebral arteries (combined middle cerebral artery, posterior cerebral artery, and basilar artery), mRNA gene expression was quantified for Esr1, Esr2, and Gper1 through qPCR (see Supplement Table S2 for primer sequences). RNA from cerebral arteries and mesenteric arteries was isolated by a standard protocol using Qiazol and RNAeasy Micro Kits (Qiagen, Hilden, Germany) and then quantified using a NanoDrop 2000 (Thermo Scientific, Waltham, MA). Reverse transcription was performed to produce cDNA with a Qiagen Quantitect Reverse Transcription kit. The cDNA samples underwent real-time qPCR using ThermoFisher PowerUp Sybr Green and a Quantstudio 5 real-time PCR system (ThermoFisher Scientific, Waltham, MA). mRNA expression was calculated using the 2−ΔΔCT method. 18 s mRNA was used as a housekeeping gene control, and values were normalized to the group in diestrus.
Statistical Analysis
Statistical analyses were performed with IBM SPSS (v. 26, Armonk, NY) and GraphPad Prism 9.0. One-way analysis of variance (ANOVA) between proestrus, estrus, and diestrus groups was used unless otherwise noted. In cases of a significant F value, post hoc analyses were performed using a Tukey correction for preplanned comparisons. For the matched PWV measures, a paired t test was used to compare cycle phases. A repeated-measures ANOVA was used to determine group differences for dose responses. Pearson correlation analysis was used to assess bivariate relations of interest. Significance was set at P < 0.05, and values are represented as means ± SD. Outliers were identified as z-score > 2 and removed from the data set.
RESULTS
Animal Characteristics throughout the Estrous Cycle
There were no differences for body weight or any tissue weights, except for uterus weight, between the stages of the estrous cycle (all P > 0.05, Table 1). Uterus weight was higher in the proestrus phase compared with estrus (P = 0.0014) and diestrus (P = 0.007). Plasma testosterone concentration was higher for mice in diestrus compared with mice in proestrus and estrus (P = 0.0003, Table 1). Plasma esterone was elevated during the proestrus phase compared with estrus (P = 0.03, Table 1).
In Vivo Arterial Stiffness Is Dependent on Estrous Cycle Phase
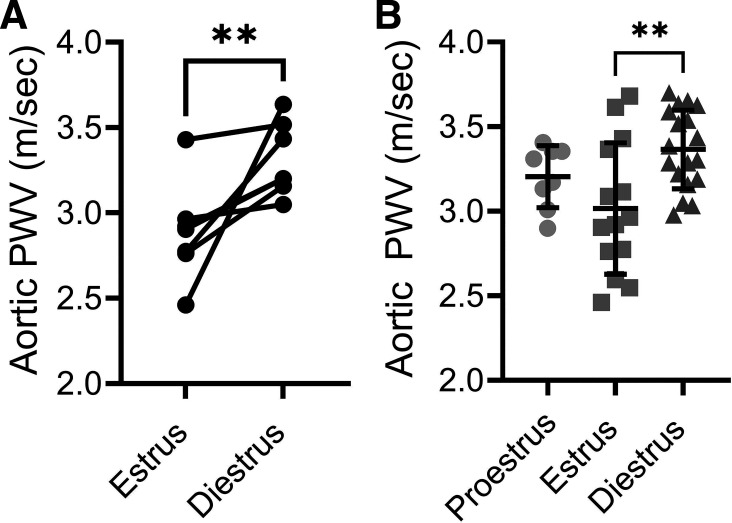
In a subset of mice that underwent repeated measures of arterial stiffness across the estrous cycle, aortic PWV increased from estrus to diestrus (P = 0.0046, Fig. 1A). In the full subject sample, PWV was lower for the mice in estrus when compared with mice in diestrus (P = 0.0048). Mice in proestrus did not differ from the other stages for aortic PWV (P = 0.32 vs. estrus, and P = 0.40 vs. diestrus, Fig. 1B). Heart rate did not differ between groups (P = 0.20, data not shown).
Figure 1.
Aortic stiffness is lower during estrus. A: repeated measures of aortic stiffness by pulse wave velocity (PWV) for mice in estrus and diestrus. n = 6/group. B: compiled aortic PWV for the entire cohort during proestrus, estrus, and diestrus. n = 8–18/group. One-way ANOVA revealed **P < 0.01. Data are means ± SD.
Endothelial Function Ex Vivo Is Independent of Estrous Cycle Phase
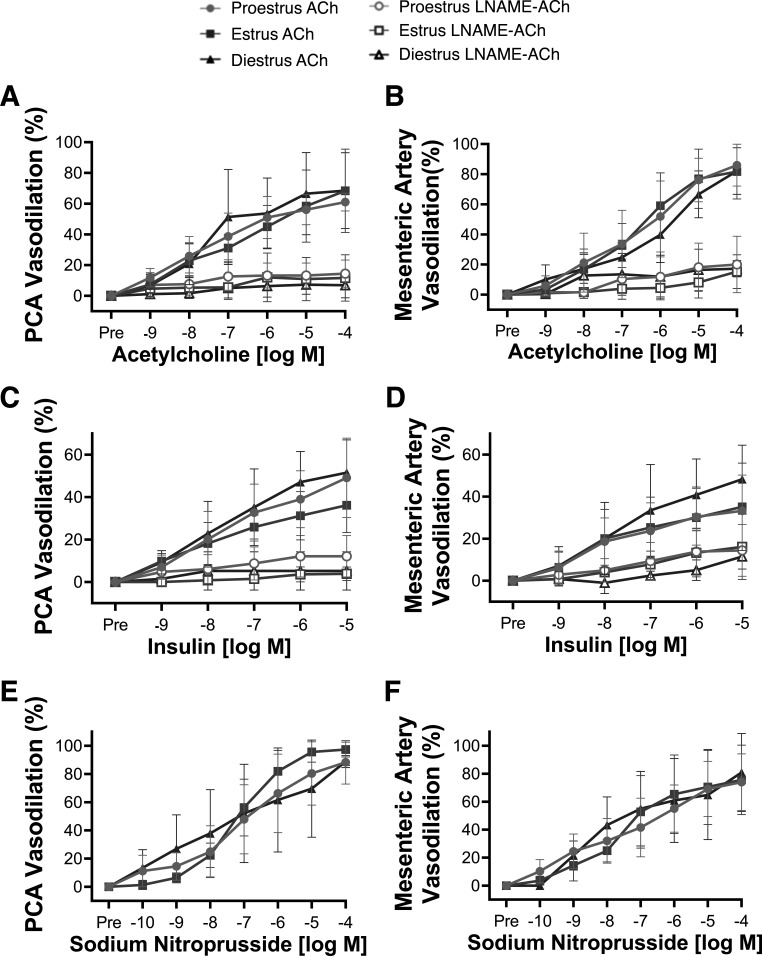
The posterior cerebral artery and mesenteric artery responses to ACh did not differ between estrous cycle phases (dose-response RM-ANOVA and maximal-responses ANOVA, all P > 0.05, Fig. 2, A and B). The maximal ACh responses in the cerebral and mesenteric arteries did not correlate with uterus weight (posterior cerebral artery, r = 0.04, P = 0.40; mesenteric artery, r = 0.21, P = 0.17), progesterone (cerebral artery, r = 0.37, P = 0.11; mesenteric artery, r = −0.14, P = 0.30), or testosterone (cerebral artery, r = 0.22, P = 0.24; mesenteric artery, r = 0.02, P = 0.47), further indicating no influence of the estrous cycle on endothelium-dependent vasodilation. Furthermore, the posterior cerebral artery and mesenteric artery responses to insulin were not different between estrous cycle phases (dose-response RM-ANOVA and maximal-responses ANOVA, all P > 0.05, Fig. 2, C and D). Likewise, the response to ACh and insulin in the presence of l-NAME was not different between estrous cycle phases for either artery (dose-response RM-ANOVA and maximal-responses ANOVA, all P > 0.05, Fig. 2, A–D). Endothelium-independent vasodilation in response to sodium nitroprusside was also not different between groups for either artery (dose-response RM-ANOVA and maximal-responses ANOVA, all P > 0.05, Fig. 2, E and F). Sensitivity (EC50) and preconstriction also did not differ between estrous cycle phases for any dose response (all P > 0.05, Supplemental Table S3). These data reveal that ex vivo endothelium-dependent and endothelium-independent vasodilatory responses in resistance arteries are independent of the estrous cycle phase.
Figure 2.
Ex vivo resistance artery endothelial function does not differ with estrous cycle phase. Endothelium-dependent dilation to acetylcholine (ACh) in the presence and absence of nitric oxide synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) in the posterior cerebral artery (PCA; A) and mesenteric artery (B) from mice in proestrus, estrus, or diestrus. Endothelium-dependent dilation to insulin in PCA (C) and mesenteric artery (D) and endothelium-independent dilation to sodium nitroprusside in PCA (E) and mesenteric artery (F) from mice in proestrus, estrus, or diestrus. Repeated-measure ANOVA showed no differences were found between groups. n = 7–9/group. Data are means ± SD.
Small Artery Gene Expression
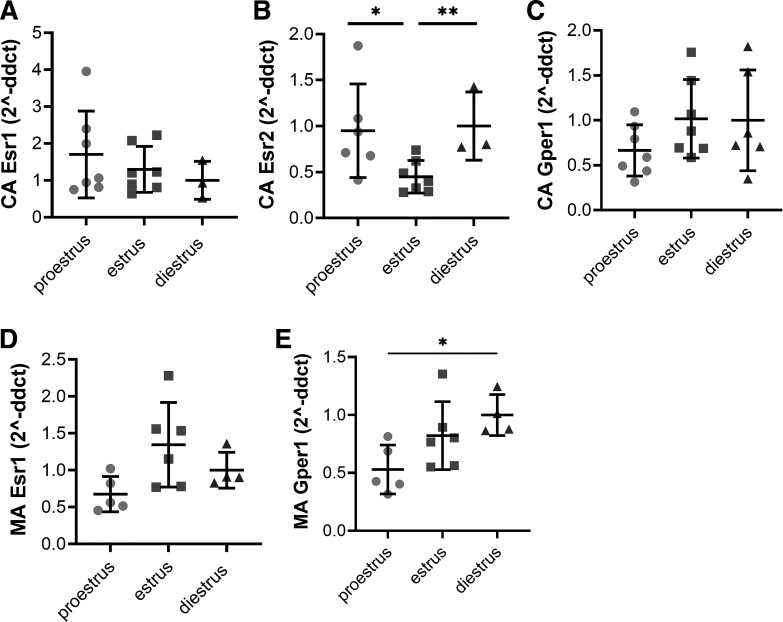
Gene expression did not differ between estrous cycle phases for Esr1 in either the cerebral or mesenteric arteries (all P > 0.05, Fig. 3, A and D). Cerebral artery Esr2 gene expression for mice in estrus was 57% lower than diestrus (P = 0.008) and 54% lower than proestrus (P = 0.04, Fig. 3B), whereas mesenteric artery Esr2 was not detectable. Gper1 expression in the mesenteric arteries during proestrus was lower than diestrus (P = 0.03, Fig. 3E), but Gper1 expression did not differ between phases in cerebral arteries (P > 0.05, Fig. 3C).
Figure 3.
Resistance artery estrogen receptor gene expression during the estrous cycle; lower Esr2 expression in cerebral arteries during estrus. Cerebral artery (CA) gene expression of Esr1 (estrogen receptor-α; A), Esr2 (estrogen receptor-β; B), and Gper1 (GPER; C) from mice in proestrus, estrus, or diestrus. Mesenteric artery (MA) gene expression of Esr1 (D) and Gper1 (E) from mice in proestrus, estrus, or diestrus. Esr2 was below the threshold for detection in the MA. n = 3–7/group. *P < 0.05; **P < 0.01. A one-way ANOVA with Tukey’s multiple comparison was used to test statistical significance. Data are means ± SD.
Large Artery Protein Expression
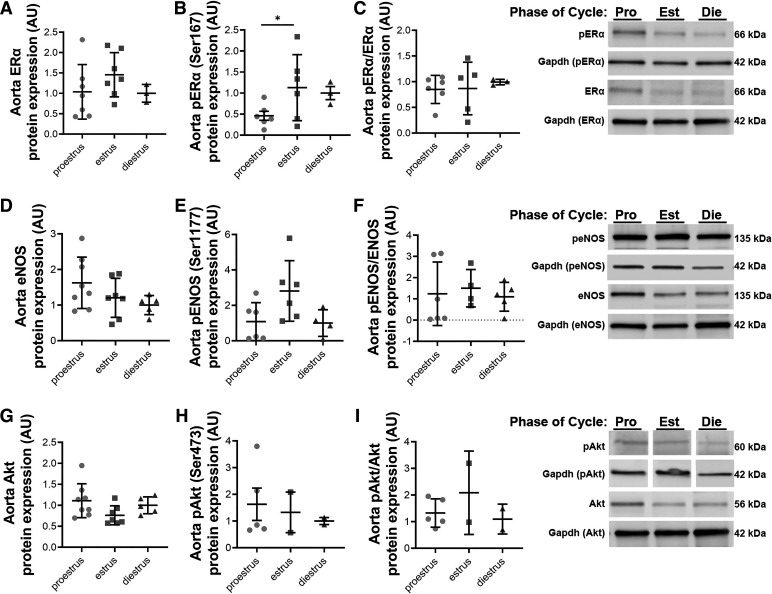
Aorta protein expression for ERα did not differ with estrous phase (P = 0.34, Fig. 4A), but p-Erα (Ser167) was elevated during the estrus phase compared with proestrus (P = 0.04, Fig. 4B). The ratio of p-Erα to total ERα was not different between phases (P = 0.85, Fig. 4C). Aorta protein expression of Akt, p-Akt (Ser 473), eNOS, p-eNOS (Ser1177), and their ratios did not differ between the stages of the estrous cycle (all P > 0.05, Fig. 4, D–I).
Figure 4.
Aorta protein expression during the estrous cycle; higher pERα expression in the aorta during estrus. Aorta protein expression for estrogen receptor-α (Erα; A), phosphorylated ERα at serine-167 (pERα; B), and pERα:ERα ratio (C), endothelial nitric oxide synthase (eNOS; D), phosphorylated eNOS at serine-1177 (peNOS; E), pENOS:eNOS ratio (F), Akt (G), and phosphorylated Akt at serine-473 (pAkt; H), and pAkt:Akt ratio (I) for mice in proestrus (P), estrus (E), and diestrus (D). Representative blot to the right. n = 2–8/group. *P < 0.05. pAkt and its Gapdh was taken from Supplemental Fig. 6. lanes 1 (P), 3 (D), and 5 (E). A one-way ANOVA with Tukey’s multiple comparison was used. Data are means ± SD.
Motor Coordination and Cognitive Function Is Independent of Estrous Cycle
Motor coordination, measured by the accelerating rotarod test, did not differ between estrous cycle stage (P = 0.80, Fig. 5A). Cognitive function, tested by nest building, also did not differ between estrous cycle stages (P = 0.34, Fig. 5B).
Figure 5.
Motor coordination and cognitive function did not differ with estrous cycle phase. A: amount of time on the rod during the accelerating rotarod test in mice during proestrus, estrus, and diestrus. B: nest building score for mice grouped by estrus cycle phase at the start of the test and phase at completion. n = 4–8/group. No differences were found between groups using a one-way ANOVA. Data are means ± SD.
DISCUSSION
The major finding of this investigation is that aortic stiffness measured in vivo is lower in the estrus phase of the estrous cycle compared with the diestrus phase. In contrast, we found no differences between estrous cycle phases for resistance artery endothelial function measured ex vivo in either cerebral or mesenteric arteries. Cerebral artery gene expression of Esr2 was lowest in the estrus phase, whereas gene expression of Gper1 was higher during the diestrus phase compared with proestrus. Esr1 was not different between estrous cycle phases in small resistance arteries. Our results also reveal that phosphorylated ERα was elevated in the aorta in the estrus phase, but eNOS and Akt expression did not appear to be affected by the estrous cycle with the aorta. Finally, we found no differences between estrous cycle phases in motor coordination or cognitive function. Taken together, these results suggest that the mouse estrous cycle impacts large elastic artery stiffness, but not small resistance artery endothelial function, and these effects are accompanied by differences in estrogen receptor gene expression and post-translational modifications.
Large Artery Stiffness: Modulation by Hormonal Fluctuations
Increased large artery stiffness is associated with cardiovascular diseases and poor overall health (5). With increasing age, large arteries become stiffer while circulating concentrations of sex steroid hormones decline progressively (19, 20), suggesting a potential relationship between large artery stiffness and lower levels of circulating sex hormones. Previous studies in human premenopausal females reveal that PWV (brachial-femoral or carotid-femoral) and aortic augmentation index were lower during the ovulatory or luteal phases (i.e., high estrogen phases) compared with during menses, suggesting that estrogen fluctuations during the menstrual cycle impact arterial stiffness (3, 8, 9). These results are similar to our findings of higher PWV in the diestrus phase, equivalent to the menses phase of a human. However, there are inconsistent findings in this regard in human studies, with some studies finding no effect of the menstrual cycle on arterial stiffness (21–25). Potential explanations for the varying results include inadequate tracking of the cycles, diets containing soy, or small sample sizes (26, 27). It is important to note that the mice in this study were on a phytoestrogen-free diet, whereas the typical research mouse diet contains phytoestrogen soy protein. Thus, the effects of estrous cycle on artery stiffness in mice fed a diet containing soy protein remain unknown. In summary, our results indicate that the mouse estrous cycle impacts large artery stiffness, and accounting for the estrous cycle in future studies will allow for further insights into how sex steroid hormones modulate the vasculature. More importantly, failure to account for the estrous cycle or phytoestrogen diet in studies of in vivo large artery stiffness may increase the variability of the data and mask potential differences.
Mechanisms of Modulation of Large Artery Stiffness
Due to the short duration of the estrous cycle, it is unlikely that structural changes to the aorta account for the differences in stiffness between estrous cycle phases. As such, the most likely mechanism for the effects of the estrous cycle on PWV is a change in arterial tone. Circulating estrogen affects arterial tone, primarily by inducing vasodilation through actions on receptors ERα and GPER1 (28). ERα expression is increased in human endothelial cells during the late follicular phase (29). In contrast, we did not find a difference in aortic ERα expression between mouse estrous cycle phases. This may be due to our measurement of the whole aorta in mice compared with the measurement of isolated endothelial cells in human subjects in the previous study. We did find that ERα phosphorylation at Serine-167 was highest in the aorta during the estrus phase. In the presence of estradiol, the estrogen receptors undergo a conformational change, enhancing the likelihood of being phosphorylated, and once phosphorylated the receptor is more likely to affect nuclear transcription (30). Thus, our findings suggest greater expression of pERα in the aorta during the estrus phase. However, the ratio of pERα/ERα was not different, suggesting that expression of ERα but not the activation changed with the estrus cycle. The non-genomic activity of ERα includes the phosphorylation of Akt, which in turn phosphorylates eNOS, leading to greater NO production that can occur within minutes after exposure to estrogen (31). In addition, ERα genomic signaling upregulates eNOS and superoxide dismutase expression (32). Gavin et al. (29) demonstrated that endothelial cell ERα expression is positively related to brachial artery endothelium-dependent dilation, as well as eNOS and p-eNOS (Ser1177) endothelial cell expression in healthy human females. eNOS expression is also higher in the brain during the proestrus phase compared with diestrus in rats (33), and NO may play a pivotal role in the regulation and timing of the estrous cycle (34, 35). Thus, our results indicate that aortic ERα phosphorylation is modulated by the estrous cycle, while previous studies suggest the menstrual cycle modulates ERα expression concomitant with changes to eNOS expression.
GPER1 also participates in nongenomic estrogen-related signaling and impacts large artery function (36). Aortas from middle aged and old female mice have lower expression of GPER protein when compared with aortas from young female mice, and the vasodilatory responses to GPER agonist G-1 is impaired in aged female mice (36). Furthermore, young female mice have a lower PWV compared with young male mice, and this sex difference is abolished by GPER deletion (37). GPER deletion also results in elevated pulse pressure, an indicator of greater large artery stiffness, in response to angiotensin II, but only in female mice (11). Unfortunately, we were unable to reliably measure GPER protein expression in this study. Thus, GPER has impacts on vascular tone and arterial stiffness; however, it remains unknown what role GPER protein expression plays in the aorta across the estrous cycle.
ERβ is also thought to impact vascular function but is also lowly expressed in the larger arteries (36), further suggesting the primary roles of ERα and GPER in large artery stiffness change during the estrous cycle. In general, ERβ is thought to negate the actions of Erα-mediated transcription (38) and have negative vascular consequences (39). Alterations in ER subtype expression with age and declining circulating estrogen concentration may mediate a shift in the ERα:ERβ ratio, toward more ERβ (39). This shift toward more ERβ may promote an adverse vascular phenotype and contributes to the development of hypertension and vascular injury, as previously found in the uterine arteries (40, 41). Given the low expression of ERβ in large arteries, we were not able to detect ERβ in our aorta samples. Thus, the relationship between estrogen receptors with aging and the estrous cycle warrants further investigation.
In addition to estrogen receptors and NO, changes to other vasodilators and hormones with the estrous cycle impact arterial tone. For example, cyclooxygenases have a role in the increased middle cerebral artery velocity during the late follicular phase of the menstrual cycle (42). Hydrogen peroxide-induced vasodilation may also change during the estrous cycle as estrogen increases superoxide dismutase expression resulting in increased conversion of superoxide to hydrogen peroxide (32). It is also important to note the fluctuation of other hormones throughout the estrous cycle and their impacts on arterial tone. The effects of progesterone on endothelial function remain controversial (43), but the majority of previous research shows progesterone can elicit negative effects on endothelial function (44, 45). Increased testosterone concentrations also correlate with endothelial dysfunction (43, 44, 46). Yet to be thoroughly investigated is the impact of luteinizing hormone and follicular stimulating hormone on endothelial function and arterial health. Thus, future studies are needed to determine the exact hormones and signaling involved in the changes to arterial stiffness during the estrous cycle.
Ex Vivo Small Artery Function and the Estrous Cycle
Despite the effects of the estrus stage on PWV, downstream small resistance artery endothelial function did not differ between estrous cycle phases in this study. These results could be due to the removal of the artery from the in vivo exposure to hormones when performing the ex vivo studies. Alternatively, estrogen potentially has a greater impact on conduit artery endothelial function compared with small resistance arteries (47, 48). Importantly for our study, the resistance artery results are consistent between two different endothelium-dependent vasodilators, ACh and insulin. In contrast, a meta-analysis of the impact of the menstrual cycle on vascular endothelial function found that conduit artery flow-mediated dilation was greatest during the late follicular phase. In contrast, this meta-analysis revealed no effect of the menstrual cycle on forearm or leg blood flow (47). A previous study also observed that the estrous cycle modulated the neurovascular response to angiotensin II, specifically angiotensin II-induced cerebral endothelial dysfunction and impaired neurovascular coupling was spared during proestrus and estrus phases compared with diestrus (49). One explanation for the conflicting results is that these previous studies were performed in vivo (i.e., with estrogen present) compared with our ex vivo studies. Thus, future studies could explore the acute versus chronic effects of the hormonal environment on vascular function throughout the estrous cycle.
Resistance Artery Estrogen Receptor Expression
Our data revealed that Esr2 expression was modulated by the estrous cycle in cerebral arteries, but Esr1 gene expression was independent of the estrous cycle in cerebral arteries and mesenteric arteries. As a lower ERα:ERβ ratio contributes to an adverse vascular phenotype, the lower Esr2 expression during estrus could serve as a vascular protective mechanism (40). Although our results for cerebral artery endothelium-dependent dilation do not have the same pattern as these Esr2 expression results, there are other potential cerebrovascular benefits of changes to estrogen receptor expressions, such as metabolic and antioxidant benefits (50–55). The ability to detect Esr2 gene expression in the cerebral vasculature, but not the mesenteric arteries, aligns with previous studies indicating relatively higher expression in the brain for Esr2 (56). The cerebral artery samples collected in this study could include other brain cells that surround these arteries, thus, it is unknown which specific cells are expressing Esr2 in these tissue samples. An implication of this finding is that treatments that activate estrogen receptors could have differential effects depending on which receptors are being highly expressed in that tissue.
We found that Gper1 expression was elevated during periods of low estrogen (i.e., the diestrus phase). It was previously shown in mesenteric arteries that females have better endothelium-dependent dilation in middle age compared with males, but these sex differences are absent in Gper1 knockout mice (57), suggesting that GPER plays a pivotal role in endothelial function in females. However, in our study, despite differences in Gper1 gene expression, no differences in endothelial function in mesenteric arteries were found between estrous cycle stages, thus dissociating Gper1 expression from endothelial function.
Estrous Cycle Stage, Cognitive Function, and Motor Coordination
In this study, estrous cycle stage had no effect on cognitive function measured by nest building, consistent with previous findings (58). Furthermore, motor coordination assessed by accelerating rotarod was not affected by the estrous cycle also in line with past literature (59, 60). It is important to note the difficulty in measuring nest building across the estrous cycle due to the short duration of the cycle phases and the likelihood of switching between phases during the 12-h test period. Overall, our data suggest that the estrous cycle does not need to be accounted for with motor or cognitive tasks.
Challenges of Measuring Steroid Hormones
The measurement of sex hormones and estrous cycle staging in rodents presents a number of challenges. The most commonly used technique to measure serum sex hormones in rodents is immunoassays, but these often overestimate steroid hormone concentrations because of the lack of specificity (61–68). As such, it has been recommended that all studies reporting steroid hormones as an endpoint use mass spectrometry-based assays to ensure specificity and reproducibility (69). The inconsistencies in the literature related to sex hormone concentrations during the mouse estrous cycle may arise from the varied accuracy of different measurement techniques (15, 70–76). Furthermore, serum sex hormone concentrations may vary widely within an estrous cycle stage (77) indicating that a single measurement for each stage is not sufficient for capturing the hormone status. Thus, caution should be used when measuring sex hormones by immunoassay or using sex hormones to stage the estrous cycle.
Limitations
Our study has a few limitations that should be noted. We did not measure passive stiffness or examine structural differences in the aorta. Thus, while we believe the short duration of the estrous cycle precludes structural changes to the aorta as the mechanisms for differences in stiffness, we do not have evidence from this study to support this hypothesis. Our samples for gene and protein expression are whole arteries, and thus, we cannot determine estrogen receptor abundance within specific cells, for example, endothelial cell versus smooth muscle cell expression. Another limitation of our study is the inability to measure protein expression in cerebral arteries due to limited tissue availability, and thus we cannot confirm that the differences seen in gene expression are indicative of protein expression. We fed the mice in this study a phytoestrogen-free diet to limit confounding effects. However, standard rodent chows often contain phytoestrogen soy that may modulate the influence of the estrous cycle on the vasculature. Finally, our findings may be specific to the C57BL/6 strain and we encourage further investigation with other rodent strains.
Conclusions
These results suggest that the estrous cycle should be accounted for when measuring in vivo large artery stiffness in young female mice. However, given that estrous cycle stage did not affect ex vivo measures of endothelial function in small arteries, it appears that accounting for estrous cycle in these outcomes is not needed. Changes to estrogen receptor expression or activation may contribute to these vascular outcomes. We specifically find that during the estrous cycle, Erβ gene expression fluctuates in cerebral arteries and p-Erα protein expression is modulated in the aorta. In summary, it is important to consider sex as a biological variable and accounting for hormonal fluctuation during in vivo measurements of vascular function will likely improve the quality of results. Acknowledging the effect of the estrous cycle on vascular function may explain the variability found within female rodents and further the understanding of the effects of sex hormones on vascular function.
SUPPLEMENTAL DATA
Supplemental Tables S1 and S3 and Supplemental Figs. S1–S6: https://doi.org/10.6084/m9.figshare.20324889.v2.
GRANTS
This work was supported by National Institutes of Health (NIH) Grant R01 AG064016 and the John L Luvaas Family Fund. The Endocrine Technologies Core is supported, in part by NIH Grant P51 OD011092 for operation of the Oregon National Primate Research Center and NIH Grant S10OD026701.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.N.K., B.R.B., and A.E.W. conceived and designed research; M.N.K., B.R.B., A.E.C., A.K., H.J.D., G.D.H., and A.E.W. performed experiments; M.N.K., B.R.B., A.E.C., A.K., H.J.D., G.D.H., and A.E.W. analyzed data; M.N.K., B.R.B., and A.E.W. interpreted results of experiments; M.N.K. prepared figures; M.N.K., B.R.B., and A.E.W. drafted manuscript; M.N.K., B.R.B., A.E.C., A.K., H.J.D., G.D.H., and A.E.W. edited and revised manuscript; M.N.K., B.R.B., A.E.C., A.K., H.J.D., G.D.H., and A.E.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Audrey Cannon, Sky Ferguson, and Dr. Kathy Snell for assistance with vaginal cytology and Dr. David Erikson (Endocrine Technology Core at Oregon Health and Science University) for assistance and advice with steroid hormone measurements.
REFERENCES
- 1. Clayton JA, Collins FS. NIH to balance sex in cell and animal studies. Nature 509: 282–283, 2014. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lindsey ML, LeBlanc AJ, Ripplinger CM, Carter JR, Kirk JA, Hansell Keehan K, Brunt KR, Kleinbongard P, Kassiri Z. Reinforcing rigor and reproducibility expectations for use of sex and gender in cardiovascular research. Am J Physiol Heart Circ Physiol 321: H819–H824, 2021. doi: 10.1152/ajpheart.00418.2021. [DOI] [PubMed] [Google Scholar]
- 3. Robb AO, Mills NL, Din JN, Smith IBJ, Paterson F, Newby DE, Denison FC. Influence of the menstrual cycle, pregnancy, and preeclampsia on arterial stiffness. Hypertension 53: 952–958, 2009. doi: 10.1161/HYPERTENSIONAHA.109.130898. [DOI] [PubMed] [Google Scholar]
- 4. Hong K, Choi Y. Role of estrogen and RAS signaling in repeated implantation failure. BMB Rep 51: 225–229, 2018. doi: 10.5483/BMBRep.2018.51.5.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease. J Am Coll Cardiol 74: 1237–1263, 2019. doi: 10.1016/j.jacc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sandoo A, van Zanten J, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J 4: 302–312, 2010. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 1: 183–198, 2005. [PMC free article] [PubMed] [Google Scholar]
- 8. Stamatelopoulos KS, Georgiopoulos G, Papaioannou T, Lambrinoudaki I, Kouzoupis A, Vlachopoulos C, Georgiou SP, Manios E, Alevizaki M, Papamichael CM, Sfikakis PP. Can premenstrual syndrome affect arterial stiffness or blood pressure? Atherosclerosis 224: 170–176, 2012. doi: 10.1016/j.atherosclerosis.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 9. Madhura M, Sandhya T. Effect of different phases of menstrual cycle on reflection index, stiffness index and pulse wave velocity in healthy subjects. J Clin Diagn Res 8: BC01–BC04, 2014. doi: 10.7860/JCDR/2014/7385.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Natoli AK, Medley TL, Ahimastos AA, Drew BG, Thearle DJ, Dilley RJ, Kingwell BA. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension 46: 1129–1134, 2005. doi: 10.1161/01.HYP.0000187016.06549.96. [DOI] [PubMed] [Google Scholar]
- 11. Ogola BO, Clark GL, Abshire CM, Harris NR, Gentry KL, Gunda SS, Kilanowski-Doroh I, Wong TJ, Visniauskas B, Lawrence DJ, Zimmerman MA, Bayer CL, Groban L, Miller KS, Lindsey SH. Sex and the G protein–coupled estrogen receptor impact vascular stiffness. Hypertension 78: e1–e14, 2021. doi: 10.1161/HYPERTENSIONAHA.120.16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pastore MB, Talwar S, Conley MR, Magness RR. Identification of differential ER-alpha versus ER-beta mediated activation of eNOS in ovine uterine artery endothelial cells. Biol Reprod 94: 139, 2016. doi: 10.1095/biolreprod.115.137554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim W, Mayer B, Pawson T. Cell Signaling Principles and Mechanisms (1st ed.). New York: Garland Science, 2014. [Google Scholar]
- 14. Salinero AE, Robison LS, Gannon OJ, Riccio D, Mansour F, Abi‐Ghanem C, Zuloaga KL. Sex-specific effects of high-fat diet on cognitive impairment in a mouse model of VCID. FASEB J 34: 15108–15122, 2020. doi: 10.1096/fj.202000085R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cora MC, Kooistra L, Travlos G. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol 43: 776–793, 2015. doi: 10.1177/0192623315570339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cole JA, Kehmeier MN, Bedell BR, Krishna Kumaran S, Henson GD, Walker AE. Sex differences in the relation between frailty and endothelial dysfunction in old mice. J Gerontol A Biol Sci Med Sci 77: 416–423, 2022. doi: 10.1093/gerona/glab317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deacon RM. Measuring motor coordination in mice. J Vis Exp 29: e2609, 2013. doi: 10.3791/2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deacon R. Assessing burrowing, nest construction, and hoarding in mice. J Vis Exp 59: e2607, 2012. doi: 10.3791/2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rossi P, Francès Y, Kingwell BA, Ahimastos AA. Gender differences in artery wall biomechanical properties throughout life. J Hypertens 29: 1023–1033, 2011. doi: 10.1097/HJH.0b013e328344da5e. [DOI] [PubMed] [Google Scholar]
- 20. Hougaku H, Fleg JL, Najjar SS, Lakatta EG, Harman SM, Blackman MR, Metter EJ. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Physiol 290: E234–E242, 2006. doi: 10.1152/ajpendo.00059.2005. [DOI] [PubMed] [Google Scholar]
- 21. Williams MRI, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Physiol 86: 5389–5395, 2001. doi: 10.1210/jcem.86.11.8013. [DOI] [PubMed] [Google Scholar]
- 22. Hayashi K, Miyachi M, Seno N, Takahashi K, Yamazaki K, Sugawara J, Yokoi T, Onodera S, Mesaki N. Variations in carotid arterial compliance during the menstrual cycle in young women. Exp Physiol 91: 465–472, 2006. doi: 10.1113/expphysiol.2005.032011. [DOI] [PubMed] [Google Scholar]
- 23. Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 235: 111–118, 2010. doi: 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spaczyński RZ, Mitkowska A, Florczak M, Banaszewska B, Krauze T, Wykrętowicz A, Guzik P, Pawelczyk L. Decreased large-artery stiffness in midluteal phase of the menstrual cycle in healthy women of reproductive age. Ginekol Pol 85: 771–777, 2014. [PubMed] [Google Scholar]
- 25. Ounis-Skali N, Mitchell GF, Solomon CG, Solomon SD, Seely EW. Changes in central arterial pressure waveforms during the normal menstrual cycle. J Investig Med 54: 321–326, 2006. doi: 10.2310/6650.2006.05055. [DOI] [PubMed] [Google Scholar]
- 26. Ajayi AF, Akhigbe RE. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract 6: 5, 2020. doi: 10.1186/s40738-020-00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Man B, Cui C, Zhang X, Sugiyama D, Barinas-Mitchell E, Sekikawa A. The effect of soy isoflavones on arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr 60: 603–614, 2021. doi: 10.1007/s00394-020-02300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev 60: 210–241, 2008. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor α is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab 94: 3513–3520, 2009. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lannigan DA. Estrogen receptor phosphorylation. Steroids 68: 1–9, 2003. doi: 10.1016/S0039-128X(02)00110-1. [DOI] [PubMed] [Google Scholar]
- 31. Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol 116: 135–170, 2019. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Z, Gou Y, Zhang H, Zuo H, Zhang H, Liu Z, Yao D. Estradiol improves cardiovascular function through up-regulation of SOD2 on vascular wall. Redox Biol 3: 88–99, 2014. doi: 10.1016/j.redox.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knauf C, Ferreira SP, Hamdane M, Mailliot C, Prevot V, Beauvillain J-C, Croix D. Variation of endothelial nitric oxide synthase synthesis in the median eminence during the rat estrous cycle: an additional argument for the implication of vascular blood vessel in the control of GnRH release. Endocrinology 142: 4288–4294, 2001. doi: 10.1210/endo.142.10.8443. [DOI] [PubMed] [Google Scholar]
- 34. Zhang X, Lin HY, Liu GY, Wang HM, Li QL, Zhu C. Expressions and regulation of endothelial and inducible nitric oxide synthases in mouse uterus during the estrous cycle and early pregnancy. Front Biosci 10: 3172–3182, 2005. doi: 10.2741/1773. [DOI] [PubMed] [Google Scholar]
- 35. Drazen DL, Klein SL, Burnett AL, Wallach EE, Crone JK, Huang PL, Nelson RJ. Reproductive function in female mice lacking the gene for endothelial nitric oxide synthase. Nitric Oxide 3: 366–374, 1999. doi: 10.1006/niox.1999.0251. [DOI] [PubMed] [Google Scholar]
- 36. Gurrala R, Kilanowski-Doroh IM, Hutson DD, Ogola BO, Zimmerman MA, Katakam PVG, Satou R, Mostany R, Lindsey SH. Alterations in the estrogen receptor profile of cardiovascular tissues during aging. GeroScience 43: 433–442, 2021. doi: 10.1007/s11357-021-00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ogola BO, Zimmerman MA, Sure VN, Gentry KM, Duong JL, Clark GL, Miller KS, Katakam PVG, Lindsey SH. G Protein-coupled estrogen receptor protects from angiotensin II-induced increases in pulse pressure and oxidative stress. Front Endocrinol (Lausanne) 10: 586, 2019. doi: 10.3389/fendo.2019.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hall JM, McDonnell DP. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates erα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140: 5566–5578, 1999. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 39. Maggi A, Cignarella A, Brusadelli A, Bolego C, Pinna C, Puglisi L. Diabetes undermines estrogen control of inducible nitric oxide synthase function in rat aortic smooth muscle cells through overexpression of estrogen receptor-β. Circulation 108: 211–217, 2003. doi: 10.1161/01.CIR.0000079311.39939.94. [DOI] [PubMed] [Google Scholar]
- 40. Novensà L, Novella S, Medina P, Segarra G, Castillo N, Heras M, Hermenegildo C, Dantas AP. Aging negatively affects estrogens-mediated effects on nitric oxide bioavailability by shifting ERα/ERβ balance in female mice. PLoS One 6: e25335, 2011. doi: 10.1371/journal.pone.0025335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Connelly PJ, Casey H, Montezano AC, Touyz RM, Delles C. Sex steroids receptors, hypertension, and vascular ageing. J Hum Hypertens 36: 120–125, 2022. doi: 10.1038/s41371-021-00576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peltonen GL, Harrell JW, Aleckson BP, LaPlante KM, Crain MK, Schrage WG. Cerebral blood flow regulation in women across menstrual phase: differential contribution of cyclooxygenase to basal, hypoxic, and hypercapnic vascular tone. Am J Physiol Regul Integr Comp Physiol 311: R222–R231, 2016. doi: 10.1152/ajpregu.00106.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab 24: 805–813, 2004. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- 44. Barbagallo M, Dominguez LJ, Licata G, Shan J, Bing L, Karpinski E, Pang PKT, Resnick LM. Vascular effects of progesterone. Hypertension 37: 142–147, 2001. doi: 10.1161/01.HYP.37.1.142. [DOI] [PubMed] [Google Scholar]
- 45. Faulkner JL, Kennard S, Huby A-C, Antonova G, Lu Q, Jaffe IZ, Patel VS, Fulton DJR, Belin de Chantemèle EJ. Progesterone predisposes females to obesity-associated leptin-mediated endothelial dysfunction via upregulating endothelial MR (mineralocorticoid receptor) expression. Hypertension 74: 678–686, 2019. doi: 10.1161/HYPERTENSIONAHA.119.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vlachopoulos C, Ioakeimidis N, Miner M, Aggelis A, Pietri P, Terentes-Printzios D, Tsekoura D, Stefanadis C. Testosterone deficiency: a determinant of aortic stiffness in men. Atherosclerosis 233: 278–283, 2014. doi: 10.1016/j.atherosclerosis.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 47. Williams JS, Dunford EC, MacDonald MJ. Impact of the menstrual cycle on peripheral vascular function in premenopausal women: systematic review and meta-analysis. Am J Physiol Heart Circ Physiol 319: H1327–H1337, 2020. doi: 10.1152/ajpheart.00341.2020. [DOI] [PubMed] [Google Scholar]
- 48. Higashi Y, Sanada M, Sasaki S, Nakagawa K, Goto C, Matsuura H, Ohama K, Chayama K, Oshima T. Effect of estrogen replacement therapy on endothelial function in peripheral resistance arteries in normotensive and hypertensive postmenopausal women. Hypertension 37: 651–657, 2001. doi: 10.1161/01.hyp.37.2.651. [DOI] [PubMed] [Google Scholar]
- 49. Capone C, Anrather J, Milner TA, Iadecola C. Estrous cycle–dependent neurovascular dysfunction induced by angiotensin ii in the mouse neocortex. Hypertension 54: 302–307, 2009. doi: 10.1161/HYPERTENSIONAHA.109.133249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gupte AA, Pownall HJ, Hamilton DJ. Estrogen: an emerging regulator of insulin action and mitochondrial function. J Diabetes Res 2015: 916585, 2015. doi: 10.1155/2015/916585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol 22: 609–622, 2008. doi: 10.1210/me.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lopez Sanchez MIG, Shearwood A-MJ, Chia T, Davies SMK, Rackham O, Filipovska A. Estrogen-mediated regulation of mitochondrial gene expression. Mol Endocrinol 29: 14–27, 2015. doi: 10.1210/me.2014-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin C-T, Buddo KA, Fix AM, Smith CA, Gilliam LA, Karvinen S, Lowe DA, Spangenburg EE, Zeczycki TN, Shaikh SR, Neufer PD. 17β-Estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab 27: 167–179.e7, 2018. doi: 10.1016/j.cmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arias-Loza P-A, Muehlfelder M, Pelzer T. Estrogen and estrogen receptors in cardiovascular oxidative stress. Pflugers Arch 465: 739–746, 2013. doi: 10.1007/s00424-013-1247-7. [DOI] [PubMed] [Google Scholar]
- 55. Bellanti F, Matteo M, Rollo T, Rosario FD, Greco P, Vendemiale G, Serviddio G. Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy. Redox Biol 1: 340–346, 2013. doi: 10.1016/j.redox.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maioli S, Leander K, Nilsson P, Nalvarte I. Estrogen receptors and the aging brain. Essays Biochem 65: 913–925, 2021. doi: 10.1042/EBC20200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ogola BO, Abshire CM, Visniauskas B, Kiley JX, Horton AC, Clark-Patterson GL, Kilanowski-Doroh I, Diaz Z, Bicego AN, McNally AB, Zimmerman MA, Groban L, Trask AJ, Miller KS, Lindsey SH. Sex differences in vascular aging and impact of GPER deletion. Am J Physiol Heart Circ Physiol 323: H336–H349, 2022. doi: 10.1152/ajpheart.00238.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Deacon RM. Assessing nest building in mice. Nat Protoc 1: 1117–1119, 2006. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 59. Meziane H, Ouagazzal A-M, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav 6: 192–200, 2007. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 60. Chari T, Griswold S, Andrews NA, Fagiolini M. The stage of the estrus cycle is critical for interpretation of female mouse social interaction behavior. Front Behav Neurosci 14: 113, 2020. doi: 10.3389/fnbeh.2020.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stanczyk FZ, Clarke NJ. Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol 121: 491–495, 2010. doi: 10.1016/j.jsbmb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 62. Stanczyk FZ, Jurow J, Hsing AW. Limitations of direct immunoassays for measuring circulating estradiol levels in postmenopausal women and men in epidemiologic studies. Cancer Epidemiol Biomarkers Prev 19: 903–906, 2010. doi: 10.1158/1055-9965.EPI-10-0081. [DOI] [PubMed] [Google Scholar]
- 63. Schiøler V, Thode J. Six direct radioimmunoassays of estradiol evaluated. Clin Chem 34: 949–952, 1988. [PubMed] [Google Scholar]
- 64. Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev 16: 1713–1719, 2007. doi: 10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- 65. Lee JS, Ettinger B, Stanczyk FZ, Vittinghoff E, Hanes V, Cauley JA, Chandler W, Settlage J, Beattie MS, Folkerd E, Dowsett M, Grady D, Cummings SR. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab 91: 3791–3797, 2006. doi: 10.1210/jc.2005-2378. [DOI] [PubMed] [Google Scholar]
- 66. Cook NJ, Read GF. Oestradiol measurement in women on oral hormone replacement therapy: the validity of commercial test kits. Br J Biomed Sci 52: 97–101, 1995. [PubMed] [Google Scholar]
- 67. Dowsett M, Folkerd E. Deficits in plasma oestradiol measurement in studies and management of breast cancer. Breast Cancer Res 7: 1–4, 2005. doi: 10.1186/bcr960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, Ekins S. Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol 14: 33, 2014. doi: 10.1186/1472-6890-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Handelsman DJ, Wartofsky L. Requirement for mass spectrometry sex steroid assays in the journal of clinical endocrinology and metabolism. J Clin Endocrinol Metab 98: 3971–3973, 2013. doi: 10.1210/jc.2013-3375. [DOI] [PubMed] [Google Scholar]
- 70. Kubota K, Cui W, Dhakal P, Wolfe MW, Rumi MAK, Vivian JL, Roby KF, Soares MJ. Rethinking progesterone regulation of female reproductive cyclicity. Proc Natl Acad Sci USA 113: 4212–4217, 2016. doi: 10.1073/pnas.1601825113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wood GA, Fata JE, Watson KLM, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction 133: 1035–1044, 2007. doi: 10.1530/REP-06-0302. [DOI] [PubMed] [Google Scholar]
- 72. Visser JA, Durlinger ALL, Peters IJJ, van den Heuvel ER, Rose UM, Kramer P, de Jong FH, Themmen APN. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Müllerian hormone null mice. Endocrinology 148: 2301–2308, 2007. doi: 10.1210/en.2006-1265. [DOI] [PubMed] [Google Scholar]
- 73. Asai S, Ohta R, Shirota M, Sato M, Watanabe G, Taya K. Reproductive endocrinology in Hatano high- and low-avoidance rats during the estrous cycle. Endocrine 18: 161–166, 2002. doi: 10.1385/ENDO:18:2:161. [DOI] [PubMed] [Google Scholar]
- 74. Inoue S. Neural basis for estrous cycle-dependent control of female behaviors. Neurosci Res 176: 1–8, 2022. doi: 10.1016/j.neures.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 75. Zenclussen ML, Casalis PA, Jensen F, Woidacki K, Zenclussen AC. Hormonal fluctuations during the estrous cycle modulate heme oxygenase-1 expression in the uterus. Front Endocrinol (Lausanne) 5: 32, 2014. doi: 10.3389/fendo.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McQuillan HJ, Han SY, Cheong I, Herbison AE. GnRH pulse generator activity across the estrous cycle of female mice. Endocrinology 160: 1480–1491, 2019. doi: 10.1210/en.2019-00193. [DOI] [PubMed] [Google Scholar]
- 77. Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96: 219–226, 1975. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables S1 and S3 and Supplemental Figs. S1–S6: https://doi.org/10.6084/m9.figshare.20324889.v2.