Keywords: apnea, breathing variability, obesity, sleep-disordered breathing, sleep fragmentation
Abstract
Obesity is associated with sleep-disordered breathing (SDB) and unrefreshing sleep. Residual daytime sleepiness and sleep impairments often persist after SDB treatment in patients with obesity, which suggests an independent effect of obesity on breathing and sleep. However, examining the relationship between sleep architecture and SDB in patients with obesity is complex and can be confounded by multiple factors. The main goal of this study was to examine the relationship between obesity-related changes in sleep architecture and SDB. Sleep recordings were performed in 15 lean C57BL/6J and 17 diet-induced obesity (DIO) mice of the same genetic background. Arousals from sleep and apneas were manually scored. Respiratory arousals were classified as events associated with ≥30% drops in minute ventilation (VE) from baseline. We applied Poincaré analysis of VE during sleep to estimate breathing variability. Obesity augmented the frequency of arousals by 45% and this increase was independent of apneas. Respiratory arousals comprised only 15% of the arousals in both groups of mice. Breathing variability during non-rapid-eye-movment (NREM) sleep was significantly higher in DIO mice, but it was not associated with arousal frequency. Our results suggest that obesity induces sleep fragmentation independently of SDB severity.
NEW & NOTEWORTHY Our diet-induced obesity (DIO) model reproduces sleep features of human obesity, including sleep fragmentation, increased apnea frequency, and larger breathing variability. DIO induces sleep fragmentation independently of apnea severity. Sleep fragmentation in DIO mice is mainly attributed to non-respiratory arousals. Increased breathing variability during sleep did not account for the higher arousal frequency in DIO. Our results provide a rationale to examine sleep in patients with obesity even when they are adequately treated for sleep-disordered breathing.
INTRODUCTION
Obesity is a major public health problem affecting 35% of US adults (1). Obesity leads to sleep fragmentation and unrefreshing sleep (2, 3). In patients with obesity, a major source of sleep fragmentation is sleep-disordered breathing (SDB) (4, 5). SDB in obesity can manifest as obstructive sleep apnea and obesity hypoventilation syndrome. Continuous positive airway pressure (CPAP) is an efficacious treatment for SDB in patients with obesity. Nevertheless, residual alterations in sleep architecture or daytime sleepiness persist in many adequately treated patients (6–9). These findings may suggest that obesity has a detrimental effect on sleep independent of SDB.
Examining the relationship between sleep architecture and SDB in obesity is complex. Sleep in individuals with obesity is influenced by genetic background, comorbid conditions, socio-economic background, and environmental factors. In addition, while apneas are well delineated, the definition of hypopneas has not been clearly established. In human studies, criteria for hypopneas differ by the inclusion of arousals and can be confounded by the effects of obesity on oxygenation (10, 11), which can yield significantly different estimations of SDB severity (12, 13). Thus, objective characterization of ventilation across the entire sleep, such as breathing variability, may complement standard approaches to measuring SDB in obesity.
We leveraged our group’s animal model of diet-induced obesity (DIO) to compare sleep and breathing in mice of similar genetic backgrounds with and without obesity. We hypothesized that obesity impairs sleep independently of SDB. We examined the relationship between obesity-related changes in sleep architecture and SDB using three approaches. First, we analyzed the relationships between arousals and apneas. Second, we examined whether sleep fragmentation was attributable to post-apnea arousals. Third, we applied an objective measurement of breathing variability to determine whether SDB increases sleep fragmentation in DIO.
METHODS
Animals
In total, 17 male DIO mice on the C57BL/6J background and 15 male lean C57BL/6J mice were used. All mice were purchased from Jackson Laboratory (Stocks Nos. 380050 and 000664, Bar Harbor, MA). DIO mice were purchased with 12 wk of age and fed with high-fat diet (5.4 kcal/g, 58.4% of kcal from fat, TD 03584, Teklad) until they reached 20 wk of age. Age-matched lean C57BL/6J mice (19.9 ± 0.7 wk-old) were fed with a chow diet (3.0 kcal/g, 4.4% fat, 13% kcal from fat). All animals received water and food ad libitum. Mice were housed at 22°C in a 12 h-light/dark cycle (09:00 AM–09:00 PM lights on and 09:00 PM–09:00 AM lights off). The study was approved by the Johns Hopkins University Animal Care and Use Committee (ACUC) under protocol number MO18M211 and complied with the American Physiological Society Guidelines for Animal Studies and ARRIVE guidelines.
Surgical Procedures
All mice were implanted with EEG and EMG electrodes for sleep studies as previously reported (14–18). All surgical procedures were performed under sterile and aseptic conditions. Mice were anesthetized with 1%–2% isoflurane administered through a facemask. Body temperature was kept at ∼37°C using a heating pad. Mice were placed in a stereotaxic frame and a longitudinal midline incision was performed on their skull. Surgical sites were washed with betadyne scrub solution. The underlying fascia was gently removed and an EEG/EMG headmount (Pinnacle Technology, Lawrence, KS) was glued above the bregma. Two pairs of silver electrodes were screwed to the headmount with silver conductive epoxy in frontal and parietal regions bilaterally. Insulated EMG leads were placed over the nuchal muscles. The incisions were sutured with 6-0 silk suture and the area around the headmount was closed with dental acrylic. Buprenorphine 0.05 mg/kg was administered subcutaneously immediately after the surgery and for at least 3 days after the surgery or until no signs of distress or pain were observed. All animals recovered for at least 1 wk before sleep studies.
Sleep Studies
Sleep studies were conducted in a modified whole body plethysmography (WBP) chamber (Buxco, Wilmington, NC) as previously done by our group (14–18). Mice were acclimated to the WBP chambers for at least 1 day before the sleep studies. Recordings were performed under a constant temperature of ∼29°C and ∼90% humidity for 6 h (from 10 AM to 4 PM). A continuous bias flow was maintained inside of the WBP chamber using positive and negative pressure sources in series with mass flow controllers (Alicat Scientific, Tucson, AZ) and high-resistance elements. Slow leaks were used to maintain the pressure inside the WBP chamber at the atmospheric level. Signals were processed at 1,000 Hz (sampling frequency per channel) and recorded in LabChart 7 Pro (version 7.2). The WBP tidal volume was calculated from the WBP pressure signal using the Drorbaugh and Fenn equation (19).
Sleep-wake states were manually scored based on 10-s epochs of EEG and EMG activity. Epochs with low amplitude in the high-frequency band (∼10–20 Hz) in the EEG and high levels of EMG activity were classified as wakefulness. Epochs with high EEG amplitude in the low-frequency band (∼2–5 Hz) and low EMG tonus were classified as NREM sleep. Epochs with low-amplitude and mixed frequencies in the EEG (∼5–10 Hz) and muscle atonia were scored as REM sleep. Sleep efficiency was calculated as total sleep time divided by recording time after sleep onset (14–18).
Ventilation Assessment
Minute ventilation (VE) was quantified throughout all sleep epochs. VE was calculated by the rectified moving average of the flow and divided by 2 to account for the inclusion of both inspiration and expiration (Fig. 1A). VE values were averaged every 0.5 s to generate individual data points. The 0.5 s duration was arbitrarily chosen because it exceeded one breath, but was shorter than two breaths duration in both groups in all stages of sleep, allowing to analyze the relationships between respiratory variability and apneas defined as two missing breaths. Analyses were performed in MATLAB (MathWorks, Natick, MA).
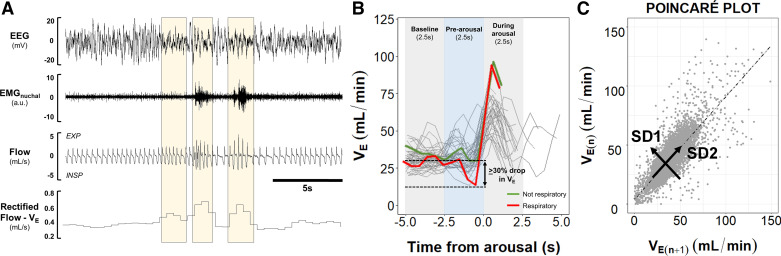
Figure 1.
Schematic representation of data extraction and analyses. A: representative trace of NREM sleep showing that minute ventilation (VE) was calculated by the rectified moving average of the flow. Shaded areas indicate increases in the rectified VE associated with arousals and EMG activity. VE was averaged every 0.5 s throughout all sleep epochs. B: schematic representation of arousal phases (baseline, prearousal, and during arousal) and classification of arousals. Each arousal phase had a 2.5 s duration. Respiratory arousals were defined as events related to ≥30% drop in VE during prearousal phase compared with the baseline (red line) (reprinted from Kim et al. (20) with permission of John Wiley and Sons). C: representative Poincaré plot that was used to analyze breathing variability during sleep. Current VE data point [VE(n+1)] was plotted against the preceding value [VE(n)]. Standard deviations perpendicular (SD1) and parallel (SD2) to the line of identity were calculated to estimate the short-term and long-term breathing variability, respectively.
Apneas Scoring
Sleep apneas were manually scored as previously described (18, 20). Apneas were defined as ≥90% drops in the flow channel during sleep for at least two breath cycles or ≥0.7 s regardless of the presence of arousals. The apnea index was calculated by dividing the number of apneas by the total sleep time (in hours).
Arousals Scoring and Analysis
Brief arousals are transient waking events occurring within sleep, characterized by their short duration (<16 s), cortical activation, and a burst in EMG activity (21, 22). We manually scored brief arousals interrupting specifically NREM sleep by visual inspection of the EEG and EMG traces and fast Fourier Transform (FFT) spectrums of one EEG derivation using SleepSign for Animals analysis software (Kissei Comtec Co., Japan). Respiratory tracings were removed from the recordings for the arousal scoring. Sleep studies with any kind of EMG artifact were considered not reliable for the arousals scoring. In total, arousals were scored in 12 DIO mice and 9 lean C57BL/6J mice. All studies were scored by a single investigator, who was blinded to the study groups (C.A.).
We examined whether sleep fragmentation in each mouse strain was attributed to respiratory alterations, we defined respiratory arousals as arousals that were preceded by apneas or at least a 30% reduction from baseline in VE (Fig. 1B). A 30% reduction in ventilation was arbitrarily chosen by analogy with the definition for hypopneas in human studies (12, 13, 23). We examined the range of changes in ventilation before arousals at multiple time points preceding each arousal. On average, mice exhibited a 2.5-s interval between the peak of VE at arousal (time from arousal 0 s) and the return of VE to baseline values (time from arousal 2.5 s) (Fig. 1B). Based on this interval, we defined three arousal phases with 2.5 s duration/each: 1) Baseline (5.0–2.5 s preceding the arousal), 2) pre-arousal (2.5 s preceding the arousal), and 3) during arousal (0.0–2.5 s after the arousal). We plotted line graphs of VE before and immediately after each arousal (Fig. 1B). We calculated the nadir of VE during the pre-arousal phase and divided it by the averaged VE at baseline for each arousal. Arousals with pre-arousal/baseline ratios ≤0.7 (≥30% drop in VE) were classified as respiratory arousals, while ratios of >0.7 were classified as not respiratory. The frequency of respiratory arousals was quantified and the indexes of respiratory and non-respiratory arousals were calculated by dividing the number of each arousal type by the total sleep time.
Breathing Variability Analysis
Poincaré plots were used to estimate breathing variability. Poincaré analysis is a geometrical method to assess information regarding short-term cycling and overall variability of periodic physiologic phenomena, such as electrocardiac patterns (e.g., heart rate variability) and breathing (24–29). This method examines the correlation between a pair of consecutive data points over some period of time. In our study, Poincaré analyses were performed by plotting each 0.5 s VE data point (VE(n+1)) against the preceding VE point [VE(n)], generating scatterplots for each mouse (Fig. 1C). In the Poincaré plots, visual analyses of the distribution of the data points (shape of the plot) can provide a qualitative estimation of variability, in which a greater spread of data points represents a larger variability of the physiological parameter. Quantitatively, the variability can be estimated by calculating the standard deviation of the distance from the line of identity (short-term variability, SD1) or along the line of identity (long-term variability, SD2) (24–28). Here, higher short-term and long-term breathing variabilities during sleep were represented by increases in SD1 and SD2 values, respectively (Fig. 1C). To examine whether changes in VE during sleep were driven by fluctuations in tidal volume (VT) or respiratory rate (RR), we performed additional Poincaré analyses. We sampled breaths across NREM periods as previously described (15–18). Briefly, we selected at least 20 s of breaths during quiet NREM sleep every 30 min of recording. Each breath was further analyzed to determine VT and RR. Poincaré plots of VT and RR were generated by plotting one breath [VT(n+1) or RR(n+1)] against the preceding breath [VT(n) or RR(n)].
To examine the relationship between breathing variability and sleep fragmentation or apnea severity, we identified the VE data points that were related to arousals or apneas, respectively. We defined VE related to arousals or apneas as VE data points occurring within 0.5 s of an arousal or an apnea. We chose this interval because the timing of arousals and apneas was recorded with a markedly higher resolution than our VE sampling interval of 0.5 s in the Poincaré analyses, and the start or end of an apnea or hypopnea invariably occurred within 0.5 s of a data point. In addition, using a 0.5-s window allowed us to account for the vast majority of acute VE transitions from the end of an apnea/arousal to back to normal breathing. VE data points related to arousals or apneas were then excluded from the Poincaré plots and SD1/2 were recalculated.
Statistical Analysis
Normality and homogeneity of variance were tested using the Shapiro–Wilk test and F test, respectively. Normally distributed variables were analyzed by independent t test. Non-normally distributed data were analyzed by Mann–Whitney U test. The frequency of respiratory arousals was compared between groups using χ2 test. Linear regression models were used to analyze the predictors of sleep fragmentation. SD1, SD2, and apnea index were used as independent variables and were mean-centered to reduce collinearity. Effects of apneas or arousals exclusions in the Poincaré plots were examined by calculating the statistical power of the analyses (power 1-β), using the software G*Power 3.1.9.4 (30). Statistical analyses were performed using R version 4.2.1. Descriptive statistics were obtained from the summary tables of each statistical analysis and mentioned in the text as means ± standard error. Graphically, data were plotted using boxplots [median ± 1.5*interquartile range (IQR)] showing individual mice. P < 0.05 was considered statistically significant.
RESULTS
Obesity Induces Sleep Fragmentation Independently of Apnea Severity
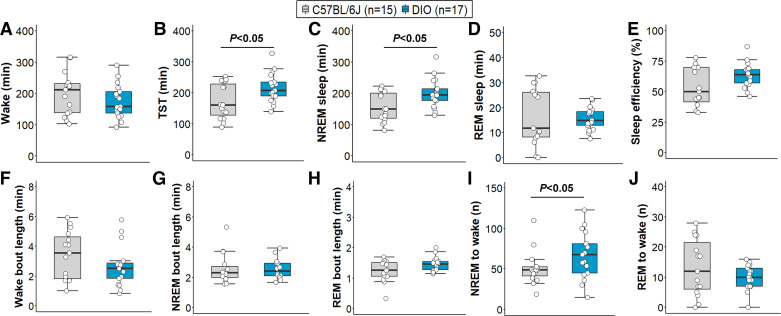
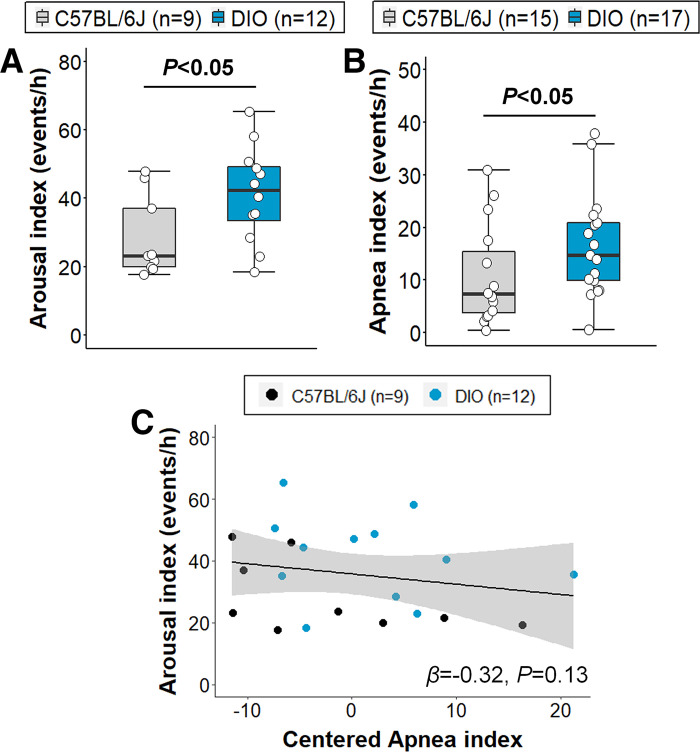
All DIO mice developed obesity under high-fat diet, indicated by significantly higher body weight compared with lean C57BL/6J mice on the day of the sleep recording (47.1 ± 0.7 vs. 30.9 ± 0.6 g, P < 0.001). The degree of body weight increase among DIO mice was also consistent. Sleep architecture in DIO and lean mice is presented in Fig. 2. Wake time was similar between groups (Fig. 2A). Total sleep time was significantly increased in DIO mice (P < 0.05, Fig. 2B), which was driven by an increased amount of NREM sleep, but not REM sleep (Fig. 2, C and D). Sleep efficiency and sleep-wake bout length were not significantly affected by obesity (Fig. 2, E–H). DIO mice had an increased number of NREM sleep-to-wake transitions (P < 0.05; Fig. 2I). Arousal index was 45% higher in DIO compared to lean mice (41.3 ± 4.0 vs. 28.5 ± 4.0 events/h, P < 0.05, Fig. 3A). Obesity also induced SDB indicated by a higher apnea index in DIO mice compared with lean C57BL/6J (16.5 ± 2.4 vs. 10.7 ± 2.5 events/h, P < 0.05, Fig. 3B). To examine whether increased sleep apnea severity accounts for more frequent arousals from sleep in obesity, we fit linear regression models and found that a higher apnea index was not associated with increased sleep fragmentation, regardless of obesity (Fig. 3C). Although our sample sizes were small, the downward slope of the relationship between arousal and apnea indices suggests that a significant positive association would not be found even if sample sizes were markedly increased.
Figure 2.
Sleep architecture in lean C57BL/6J and diet-induced obesity (DIO) mice. A: wake duration was similar between groups. Obese mice showed increased total sleep time (TST; B) and NREM sleep (C) duration, but not REM sleep (D). No effects of obesity were observed on sleep efficiency and the length of sleep-wake bouts (E–H). Frequency of transitions to wakefulness was higher in obese mice during NREM sleep (I), but not in REM sleep (J). Median ± 1.5 × IQR are shown.
Figure 3.
Obesity induced sleep fragmentation, which was not mainly attributed to apnea severity. Diet-induced obesity (DIO) mice showed augmented frequency of arousals (A) and apnea index (B) compared with lean mice (reprinted from Kim et al. (20) with permission of John Wiley and Sons). However, apnea severity (C) did not predict sleep fragmentation in the linear regression model. Apneas were scored in all mice. Arousals were scored in 9 lean C57BL/6J and 12 DIO mice. Sleep studies with any artifacts in electromyogram were considered not reliable and excluded from arousal scoring. Median ± 1.5 × IQR are shown. Independent variables were mean-centered in the linear regression model. β, β coefficient.
Sleep Fragmentation in Obesity Is Mainly Attributed to Nonrespiratory Arousals
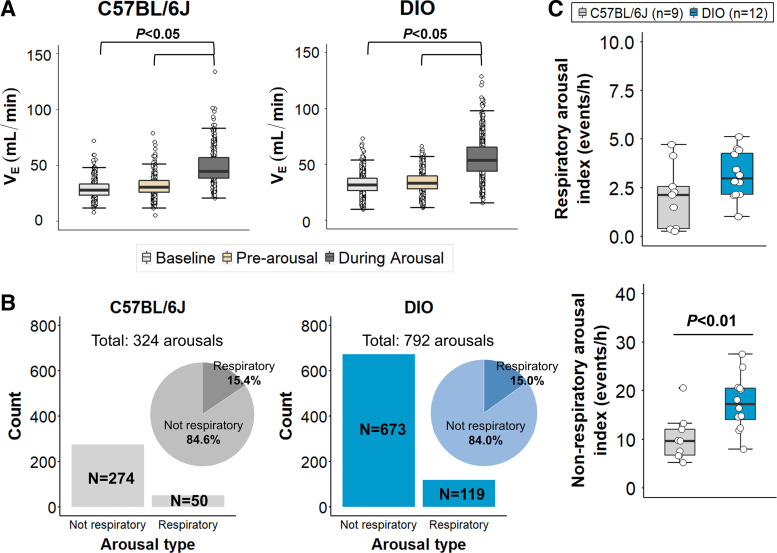
To further explore the relationship between SDB and sleep fragmentation in obesity, we analyzed ventilation at baseline (>2.5 s before arousals) immediately preceding (within 2.5 s of arousals) and during arousals in both groups (Fig. 1B; see methods for more details). In total, we analyzed 1,116 arousals. As expected, VE during arousal was significantly higher than at baseline and pre-arousal phase in both groups (Fig. 4A, P < 0.05). However, ventilation during the period preceding arousals did not differ from baseline in DIO and lean mice, suggesting that most of the arousals were not preceded by apneas or hypopneas. To characterize the etiology of arousals, we defined respiratory-related arousals. As shown in Fig. 4B, DIO mice had an increased number of respiratory and nonrespiratory arousals compared with lean mice, suggesting that obesity might nonspecifically augment arousals from sleep. Indeed, the proportion of respiratory arousals was similar between groups, representing only 15% of total arousals, and the respiratory arousal index was not significantly different between lean and obese mice (Fig. 4C). On the other hand, nonrespiratory arousal index was significantly higher in DIO mice compared with lean C57BL/6J (Fig. 4C, P < 0.01). Thus, these findings suggest that sleep fragmentation associated with obesity is mainly attributed to spontaneous arousals and not to respiratory arousals.
Figure 4.
Sleep fragmentation in obesity is mainly attributed to nonrespiratory arousals. A: during arousal, minute ventilation (VE) was significantly increased, but ventilation immediately preceding the arousal (prearousal phase) was similar to baseline in both groups. B: total number of arousals was significantly higher in diet-induced obesity (DIO) mice compared with lean mice (reprinted from Kim et al. (20) with permission of John Wiley and Sons). However, the proportion of respiratory arousals was similar between groups (∼15%). C: respiratory arousal index did not differ between obese and lean mice. Nonrespiratory arousal index was significantly higher in obese mice. Median ± 1.5 × IQR are shown.
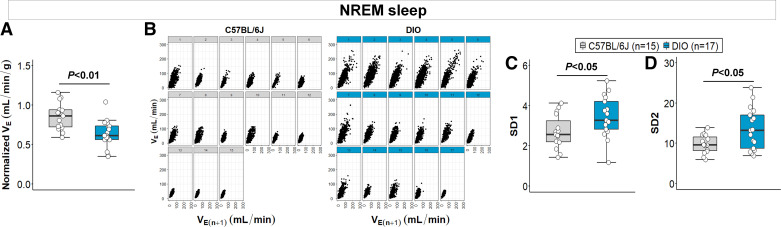
Obesity Induces Hypoventilation and Increases Breathing Variability during Sleep, but These Effects Are Independent of Sleep Fragmentation
In the preceding analyses, we used generally accepted definitions of apneas and hypopneas to measure SDB. The apnea index is a measure of breathing instability during sleep. Nevertheless, apnea frequency does not directly reflect ventilation and, based on our findings, does not predict sleep impairments induced by obesity (Fig. 3C). Thus, to further analyze the association between obesity-induced SDB and sleep fragmentation, we quantified the rectified VE across the entire sleep recording (Fig. 1A) and estimated the breathing variability during sleep. Obesity was associated with hypoventilation during NREM sleep indicated by decreased weight-normalized VE in DIO mice compared with lean mice (0.64 ± 0.04 vs. 0.86 ± 0.04 mL/min/g, P < 0.01, Fig. 5A). We applied Poincaré analyses to examine the effects of obesity on breathing variability using VE (Fig. 5B). A larger variability in the distribution of VE data points was observed in the Poincaré plots of DIO mice during NREM sleep (Fig. 5B). SD1 and SD2 values were significantly higher in obese mice compared with lean animals in NREM sleep (P < 0.05, power 1-β: 0.97, Fig. 5, C and D), suggesting an increased short-term and long-term breathing variability in obesity, respectively. Considering that VE was calculated from the rectified moving average of the flow, we were not able to quantify VT and RR. To examine whether the increased VE variability in obesity was driven by changes in VT or RR, we performed additional Poincaré analyses using sampled breaths across NREM periods. As shown in the Supplemental Fig. S1 (all Supplemental material is available at https://doi.org/10.6084/m9.figshare.20235588.v3), obesity was associated with increased short-term and long-term breathing variability during sleep in the VT-based analyses, but not in RR. In REM sleep, DIO mice also showed a decreased VE compared with lean mice (0.67 ± 0.05 vs. 0.85 ± 0.04 mL/min/g, P < 0.05), but no significant differences in the breathing variability (see Supplemental Fig. S2).
Figure 5.
Obesity is associated with decreased ventilation and augmented breathing variability during NREM sleep. A: normalized minute ventilation (VE) was significantly lower in diet-induced obesity (DIO) mice. B: individual Poincaré plots showed a larger dispersion of VE data points parallel and perpendicular to the line of identity in diet-induced obesity (DIO) mice compared with lean mice. DIO mice showed an augmented short-term and long-term breathing variability during NREM sleep, indicated by higher SD1 (C) and SD2 (D) values, respectively. Median ± 1.5 × IQR are shown (Reprinted from Kim et al. (20) with permission of John Wiley and Sons).
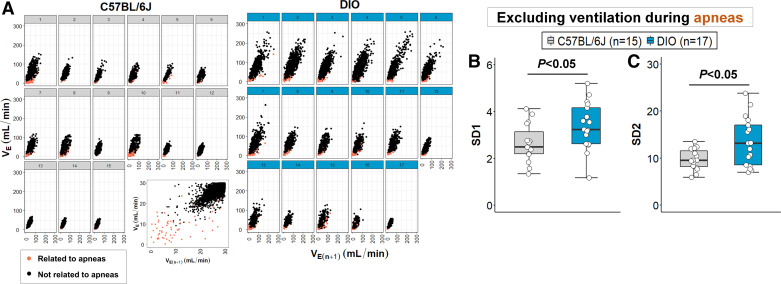
Interestingly, the frequency of apneas did not significantly affect breathing variability in our model. VE data points within 0.5 s of apnea were marked as related to a respiratory event (Fig. 6A) and then excluded from the Poincaré analysis. In total, apnea-related VE points accounted for only 0.25% and 0.32% of all VE data points during NREM sleep in lean and obese mice, respectively. After removing apnea-related VE, SD1 and SD2 values remained significantly higher in DIO mice compared with lean mice (P < 0.05, Fig. 6, B and C). The removal of VE data points related to apneas did not significantly affect the analysis power (power 1 − β: 0.98). In parallel, we performed autocorrelation analyses of ventilation for each mouse and did not find an increase in regular periodic breathing between lean and DIO mice. These findings suggest that obesity in our DIO model is associated with a higher sleep apnea frequency, but the obesity-related breathing variability during NREM sleep cannot be attributed only to recurrent apneas or other forms of periodic breathing.
Figure 6.
Obesity-related breathing variability during NREM sleep is not entirely attributed to sleep apneas. A: minute ventilation (VE) data points related to apneas were identified in the Poincaré plots (orange dots) and were mainly distributed in the bottom left quadrant of the scatterplots in both groups (VE values closer to zero). Apnea-related VE points were excluded from the analysis and SD1 (B) and SD2 (C) remained increased in diet-induced obesity (DIO) mice. Median ± 1.5 × IQR are shown (Reprinted from Kim et al. (20) with permission of John Wiley and Sons).
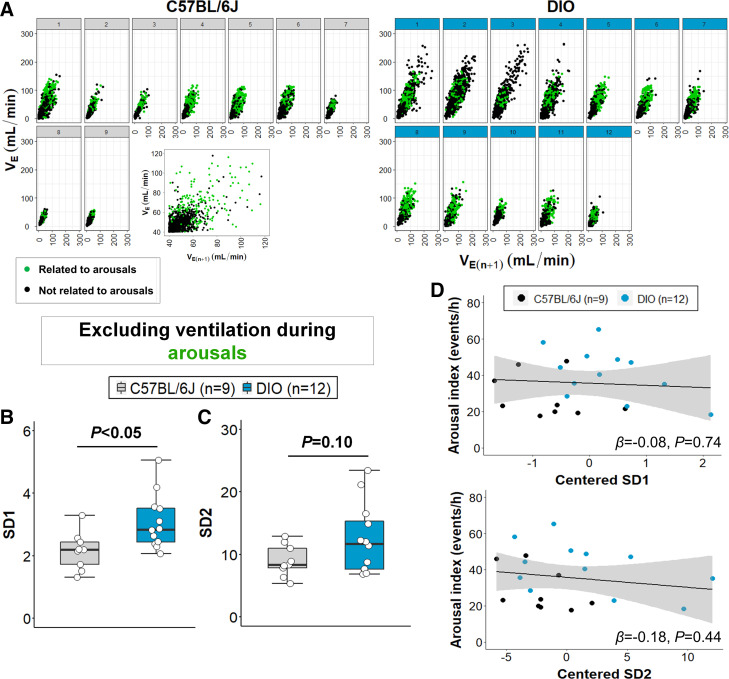
The relationship between breathing variability and sleep fragmentation was assessed by identifying VE data points that were related to arousals (within 0.5 s of an arousal) in the Poincaré plots. As shown in Fig. 7A, most of the arousals were associated with higher VE points in the plots (upper right quadrant) in most mice. Even after the removal of the arousal-related VE, our Poincaré analysis had a statistical power of >85%. The exclusion of arousal-related VE points did not significantly affect SD1 values (Fig. 7B), suggesting that arousals from sleep do not entirely explain the elevated short-term breathing variability in obese mice. SD2 was no longer significantly higher in DIO mice after excluding the points related to arousals (Fig. 7C), which may suggest that sleep fragmentation accounts for changes in long-term breathing variability. However, linear regression models showed that both SD1 and SD2 values do not significantly predict differences in arousal frequency (Fig. 7D). Thus, increased sleep fragmentation is not related to the increased respiratory variability in obesity.
Figure 7.
Sleep fragmentation does not entirely account for the increased breathing variability during NREM sleep in obesity. A: minute ventilation (VE) data points related to arousals were identified in the Poincaré plots (green dots) and were mainly distributed in the upper right quadrant of the scatterplots in both groups (higher VE values). Arousal-related VE points were excluded from the analysis and SD1 (B) remained increased in diet-induced obesity (DIO) mice. C: SD2 was no longer significant, but linear regression models (D) showed no significant association between arousal index and SD1/2 values, suggesting that short-term and long-term breathing variability in obesity was not attributed to sleep fragmentation. Median ± 1.5 × IQR are shown. Independent variables were mean-centered in the linear regression model. β, β coefficient (Reprinted from Kim et al. (20) with permission of John Wiley and Sons).
DISCUSSION
In this study of genetically similar mice, we analyzed the effects of obesity on sleep and breathing. Our study has three major novel findings. First, obese mice had increased sleep fragmentation and SDB severity, but no significant association between arousal index and apnea frequency was observed. Second, sleep fragmentation in DIO mice was mainly attributed to non-respiratory arousals. Third, although obesity augmented respiratory variability during sleep, this increase did not account for the higher arousal frequency in DIO.
To the best of our knowledge, this is the first study to analyze the independent effects of obesity on sleep architecture and SDB in DIO mice. Although population-based studies have shown that obesity is associated with increased sleep fragmentation (2), SDB remains a significant confounder. In fact, sleep disruptions frequently persist in patients with obesity in whom SDB is fully treated (6–9). To address some limitations of human studies, we performed several different analyses. We analyzed the relationship between the arousal from sleep and respiratory indices and found no association between arousal and apnea frequencies over the course of a sleep period. However, the apnea index does not fully characterize SDB. The Poincaré analysis is a simple way to quantify respiratory variability across the entire night without sampling techniques, adding an objective quantification to respiratory analysis during sleep. We found an increased breathing variability during NREM sleep in obese mice, which could not be attributed to apnea frequency. The increased Poincaré indices in obesity may reflect variations in the output of respiratory motoneurons or slow respiratory oscillations due to mechanical impairments of obesity. Here, we showed that the increased VE variability during sleep in obesity was driven by an augmented VT instability. Several types of respiratory neurons predominantly regulate VT rather than RR during sleep (31, 32). An adequate measure of respiratory variability may have a significant predictive value (33). However, the clinical implications of our Poincaré analysis are not entirely clear. We found that short-term and long-term breathing variability did not predict sleep fragmentation. Thus, our findings suggest that sleep fragmentation is not entirely related to breathing during sleep in obesity.
Next, we directly examined the role of breathing in arousals. In humans, multiple conditions are associated with non-respiratory arousals, including periodic limb movements, pain, and environment. The effects of obesity on nonrespiratory arousals are not known and may be underestimated because of difficulties with scoring arousals. We performed a rigorous arousal scoring in which the scorer was blinded to the experimental groups, as well as the ventilation channels to remove bias (34). All arousals lead to transient increases in VE (35). We defined a respiratory arousal by a decrease ≥30% in VE immediately before the event. We observed that only 15% of arousals were related to reductions in ventilation in both lean and obese mice. Thus, our study provides rationale to carefully examine sleep in patients with obesity to determine if residual daytime sleepiness, despite treatment of SDB, arises from the effects of obesity on sleep fragmentation.
The main respiratory-related trigger of arousals is CO2 elevation. In our study, however, the increased sleep fragmentation in obesity was mainly attributed to spontaneous arousals, which were not temporally related to reductions in ventilation. These findings suggest that increased sensitivity to hypercapnia was not the major contributor to arousals in DIO mice. In this sense, the underlying mechanisms by which obesity induces arousals independently of SDB are more likely related to the imbalance of sleep-wake neurotransmission systems. Cortical arousals are regulated by a complex and not yet fully understood system. Orexin neurons in the lateral hypothalamus play an important role in promoting arousals and stabilizing a waking state (36, 37). Central administration of orexin increases wakefulness in rodents (38, 39), whereas the deficiency in orexinergic signaling is related to the pathophysiology of narcolepsy (40, 41), a central disorder of hypersomnolence. Leptin, an adipocyte-produced hormone, stimulates ventilation (15, 42) and inhibits orexin neurons in a dose-dependent manner (43). Leptin resistance is a key trait of human obesity, characterized by poor leptin signaling in the central nervous system despite the high circulating levels of leptin (44–46). Reduced leptin signaling could lead to an enhanced orexin neuron firing in the lateral hypothalamus, promoting a higher frequency of cortical arousals in obesity. Activation of orexin neurons in the lateral hypothalamus also stimulates breathing during sleep (47). The effects of orexin neurons on ventilation may be offset by reductions in leptin signaling. Nevertheless, a combination of increased arousals and stimulation of breathing may still result in respiratory instability. It is therefore conceivable that leptin resistance of obesity may lead to respiratory instability via the orexin pathway without significantly altering overall ventilation. Thus, mechanisms of dissociative effects of obesity on sleep architecture and breathing during sleep are unknown, but leptin resistance is a likely candidate for both pathways.
In our study, sleep fragmentation was associated with increased sleep duration in obese mice. Other investigators have shown similar findings, suggesting that high-fat feeding increases the amount of NREM sleep, but not REM sleep (48, 49). This body of evidence from animal models reproduces in part some features of human obesity. Obese patients without sleep apnea are sleepier than nonobese individuals and excessive daytime sleepiness is associated with a higher sleep efficiency at night (50). In this sense, obesity may directly lead to increased sleep pressure, which predisposes to a reduced ability to maintain wakefulness during the daytime. Our findings of increased sleep fragmentation in DIO suggest an alternative pathogenic pathway in sleep prolongation and hypersomnolence that reflects microsleep architecture alterations independent of SDB severity.
Our study has several limitations. We performed an observational study to assess the effects of obesity on sleep fragmentation and SDB, which did not allow us to explore the potential mechanisms of this association. However, our findings provide new insights into the pathogenesis of SDB in obesity, suggesting independent pathways in obesity-induced sleep fragmentation and SDB. Poincaré analysis has not been validated to assess the severity of SDB. Nevertheless, respiratory instability during sleep is observed in patients with increased loads on the respiratory system and disturbed sleep (51). The sole purpose of using this analysis in our study was to examine relationships between arousals from sleep and breathing instability comprehensively. The impact of the Poincaré-based measurements of respiratory variability on cardiovascular, cognitive, and metabolic outcomes also remains unknown. We quantified VE based on the rectified moving average of the flow signal, which does not allow us to distinguish the effects of RR and VT. However, we showed here that the increased VE variability during sleep in obese mice was driven by changes in VT using a simple sampling technique. We defined VE points related to arousals or apneas as data points within 0.5 s of these events. Although this window allowed us to account for the majority of ventilatory transitions from an apnea or an arousal, we did not test other window durations. Longer window durations could provide a broader notion of the contribution of apneas/arousals to breathing variability during sleep. We did not analyze the relationships between sleep fragmentation and SDB during REM sleep. In our experience, DIO mice have very short REM episodes, accounting for only 5%–8% of total sleep time (16). We analyzed only male mice. It was exceedingly difficult to induce severe obesity in a large number of female mice. Sex differences in DIO have been previously reported, showing that female mice are more resistant to weight gain and are protected against DIO-induced metabolic dysfunctions (52, 53). Our analysis was limited to the 6-h period during the light phase. We were not able to perform 24-h sleep recordings since the mice were food and water deprived in the WBP chamber. We were not able to distinguish apneas as central or obstructive. In our experience, although DIO mice have significant inspiratory flow limitation indicative of impaired upper airway patency, complete obstruction does not occur and apneas are almost exclusively central (16). We did not assess arterial blood gases, which may impact the analysis of the dissociative effects of obesity on breathing and sleep. However, our group has previously described DIO mice as a model of obesity hypoventilation, characterized by lower pH and higher partial pressure of arterial CO2 during wakefulness compared with lean mice (16). In addition, our analysis of respiratory arousals partially accounted for discrete periods of hypoventilation, defined based on drops in VE. Finally, it is possible that our study was underpowered to detect a small effect of respiratory-related arousals in obesity. Nevertheless, we had a 90% power to detect a standardized effect of 0.5 or greater, suggesting that it is unlikely that SDB-related arousals accounted for a large portion of obesity-related sleep fragmentation.
Conclusions
In male mice, obesity induces sleep fragmentation independently of SDB severity. Our DIO model reproduces sleep features of SDB in human obesity, manifested by robust sleep fragmentation, increased apnea frequency, and larger breathing variability during sleep. The underlying mechanisms by which obesity augments cortical arousals independently of respiratory events should be further examined.
SUPPLEMENTAL DATA
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.20235588.v3.
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this article will be made available upon reasonable request.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant R01 HL135483 and NHLBI 2R01 HL133100-05, American Academy of Sleep Medicine Foundation 238-BS-20, and American Thoracic Society Unrestricted Award (to L.V.P.), National Institutes of Health (NIH) R01 HL128970, R01 HL133100, and R01 HL13892 (to V.Y.P.), NIH R01 NS112266 (to A.L.), Johns Hopkins Blaustein Pain Research Grant (to C.A. and A.L.), and American Heart Association Postdoctoral Fellowship Award 828142 (to L.J.K.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.J.K., V.Y.P., and L.V.P. conceived and designed research; L.J.K., H.P., A.L., and L.V.P. performed experiments; L.J.K., C.A., H.P., A.L., and L.V.P. analyzed data; L.J.K., C.A., H.P., A.L., V.Y.P., and L.V.P. interpreted results of experiments; L.J.K. prepared figures; L.J.K., V.Y.P., and L.V.P. drafted manuscript; L.J.K., C.A., H.P., A.L., V.Y.P., and L.V.P. edited and revised manuscript; L.J.K., C.A., H.P., A.L., V.Y.P., and L.V.P. approved final version of manuscript.
ACKNOWLEDGMENTS
Graphical abstract image created with BioRender and published with permission.
REFERENCES
- 1. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 303: 235–241, 2010. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2. Zhao B, Sun S, He X, Yang J, Ma X, Yan B. Sleep fragmentation and the risk of obesity: the Sleep Heart Health Study. Obesity (Silver Spring) 29: 1387–1393, 2021. doi: 10.1002/oby.23193. [DOI] [PubMed] [Google Scholar]
- 3. Koolhaas CM, Kocevska D, Te Lindert BHW, Erler NS, Franco OH, Luik AI, Tiemeier H. Objectively measured sleep and body mass index: a prospective bidirectional study in middle-aged and older adults. Sleep Med 57: 43–50, 2019. doi: 10.1016/j.sleep.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 4. de Sousa AGP, Cercato C, Mancini MC, Halpern A. Obesity and obstructive sleep apnea-hypopnea syndrome. Obes Rev 9: 340–354, 2008. doi: 10.1111/j.1467-789X.2008.00478.x. [DOI] [PubMed] [Google Scholar]
- 5. Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest 137: 711–719, 2010. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kingshott RN, Vennelle M, Hoy CJ, Engleman HM, Deary IJ, Douglas NJ. Predictors of improvements in daytime function outcomes with CPAP therapy. Am J Respir Crit Care Med 161: 866–871, 2000. doi: 10.1164/ajrccm.161.3.9905053. [DOI] [PubMed] [Google Scholar]
- 7. Kribbs NB, Pack AI, Kline LR, Getsy JE, Schuett JS, Henry JN, Maislin G, Dinges DF. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis 147: 1162–1168, 1993. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 8. Sforza E, Krieger J. Daytime sleepiness after long-term continuous positive airway pressure (CPAP) treatment in obstructive sleep apnea syndrome. J Neurol Sci 110: 21–26, 1992. doi: 10.1016/0022-510x(92)90004-5. [DOI] [PubMed] [Google Scholar]
- 9. Pack AI, Black JE, Schwartz JRL, Matheson JK. Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med 164: 1675–1681, 2001. doi: 10.1164/ajrccm.164.9.2103032. [DOI] [PubMed] [Google Scholar]
- 10. Kapur VK, Wilsdon AG, Au D, Avdalovic M, Enright P, Fan VS, Hansel NN, Heckbert SR, Jiang R, Krishnan JA, Mukamal K, Yende S, Barr RG. Obesity is associated with a lower resting oxygen saturation in the ambulatory elderly: results from the cardiovascular health study. Respir Care 58: 831–837, 2013. doi: 10.4187/respcare.02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Littleton SW, Tulaimat A. The effects of obesity on lung volumes and oxygenation. Respir Med 124: 15–20, 2017. doi: 10.1016/j.rmed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 12. Ho V, Crainiceanu CM, Punjabi NM, Redline S, Gottlieb DJ. Calibration model for apnea-hypopnea indices: impact of alternative criteria for hypopneas. Sleep 38: 1887–1892, 2015. doi: 10.5665/sleep.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manser RL, Rochford P, Pierce RJ, Byrnes GB, Campbell DA. Impact of different criteria for defining hypopneas in the apnea-hypopnea index. Chest 120: 909–914, 2001. doi: 10.1378/chest.120.3.909. [DOI] [PubMed] [Google Scholar]
- 14. Hernandez AB, Kirkness JP, Smith PL, Schneider H, Polotsky M, Richardson RA, Hernandez WC, Schwartz AR. Novel whole body plethysmography system for the continuous characterization of sleep and breathing in a mouse. J Appl Physiol (1985) 112: 671–680, 2012. doi: 10.1152/japplphysiol.00818.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pho H, Hernandez AB, Arias RS, Leitner EB, Van Kooten S, Kirkness JP, Schneider H, Smith PL, Polotsky VY, Schwartz AR. The effect of leptin replacement on sleep-disordered breathing in the leptin-deficient ob/ob mouse. J Appl Physiol (1985) 120: 78–86, 2016. doi: 10.1152/japplphysiol.00494.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleury Curado T, Pho H, Berger S, Caballero-Eraso C, Shin M-K, Sennes LU, Pham L, Schwartz AR, Polotsky VY. Sleep-disordered breathing in C57BL/6J mice with diet-induced obesity. Sleep 41: zsy089, 2018. doi: 10.1093/sleep/zsy089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pho H, Berger S, Freire C, Kim LJ, Shin M-K, Streeter SR, Hosamane N, Cabassa ME, Anokye-Danso F, Dergacheva O, Amorim MR, Fleury-Curado T, Jun JC, Schwartz AR, Ahima RS, Mendelowitz D, Polotsky VY. Leptin receptor expression in the dorsomedial hypothalamus stimulates breathing during NREM sleep in db/db mice. Sleep 44: zsab046, 2021. doi: 10.1093/sleep/zsab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim LJ, Shin M-K, Pho H, Otvos L, Tufik S, Andersen ML, Pham LV, Polotsky VY. Leptin receptor blockade attenuates hypertension, but does not affect ventilatory response to hypoxia in a model of polygenic obesity. Front Physiol 12: 688375, 2021. doi: 10.3389/fphys.2021.688375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955. doi: 10.1542/peds.16.1.81. [DOI] [PubMed] [Google Scholar]
- 20. Kim LJ, Alexandre C, Pho H, Latremoliere A, Polotsky VY, Pham LV. Obesity-induced breathing variability during sleep is independent of apneas and sleep fragmentation. FASEB J 36, 2022. doi: 10.1096/fasebj.2022.36.S1.R2594. [DOI] [Google Scholar]
- 21. Freire C, Pho H, Kim LJ, Wang X, Dyavanapalli J, Streeter SR, Fleury-Curado T, Sennes LU, Mendelowitz D, Polotsky VY. Intranasal leptin prevents opioid induced sleep disordered breathing in obese mice. Am J Respir Cell Mol Biol 63: 502–509, 2020. doi: 10.1165/rcmb.2020-0117OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol Regul Integr Comp Physiol 261: R198–R208, 1991. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 23. Tobler I, Gaus SE, Deboer T, Achermann P, Fischer M, Rülicke T, Moser M, Oesch B, McBride PA, Manson JC. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature 380: 639–642, 1996. doi: 10.1038/380639a0. [DOI] [PubMed] [Google Scholar]
- 24. Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, Troester MT, Vaughn BV. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med 13: 665–666, 2017. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raetz SL, Richard CA, Garfinkel A, Harper RM. Dynamic characteristics of cardiac R-R intervals during sleep and waking states. Sleep 14: 526–533, 1991. doi: 10.1093/sleep/14.6.526. [DOI] [PubMed] [Google Scholar]
- 26. Fechtner L, El Ali M, Sattar A, Moore M, Strohl KP. Fentanyl effects on breath generation in C57BL/6J and A/J mouse strains. Respir Physiol Neurobiol 215: 20–29, 2015. doi: 10.1016/j.resp.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peng Y-J, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 2α (HIF-2α) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci USA 108: 3065–3070, 2011. [Erratum in Proc Natl Acad Sci USA 119: e2210923119, 2022]. doi: 10.1073/pnas.1100064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hsu C-H, Tsai M-Y, Huang G-S, Lin T-C, Chen K-P, Ho S-T, Shyu L-Y, Li C-Y. Poincaré plot indexes of heart rate variability detect dynamic autonomic modulation during general anesthesia induction. Acta Anaesthesiol Taiwan 50: 12–18, 2012. doi: 10.1016/j.aat.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 29. Welch R, Kolbe J, Lardenoye M, Ellyett K. Novel application of Poincaré analysis to detect and quantify exercise oscillatory ventilation. Physiol Meas 42: 04NT01, 2021. doi: 10.1088/1361-6579/abf05d. [DOI] [PubMed] [Google Scholar]
- 30. Angel C, Glovak ZT, Alami W, Mihalko S, Price J, Jiang Y, Baghdoyan HA, Lydic R. Buprenorphine depresses respiratory variability in obese mice with altered leptin signaling. Anesthesiology 128: 984–991, 2018. doi: 10.1097/ALN.0000000000002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41: 1149–1160, 2009. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 32. Gutierrez G, Williams J, Alrehaili GA, McLean A, Pirouz R, Amdur R, Jain V, Ahari J, Bawa A, Kimbro S. Respiratory rate variability in sleeping adults without obstructive sleep apnea. Physiol Rep 4: e12949, 2016. doi: 10.14814/phy2.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas RJ. Arousals in sleep-disordered breathing: patterns and implications. Sleep 26: 1042–1047, 2003. doi: 10.1093/sleep/26.8.1042. [DOI] [PubMed] [Google Scholar]
- 34. Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K. I, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38: 701–713, 2003. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 35. Williams RH, Burdakov D. Hypothalamic orexins/hypocretins as regulators of breathing. Expert Rev Mol Med 10: e28, 2008. doi: 10.1017/S1462399408000823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med 158: 1333–1337, 1998. doi: 10.1001/archinte.158.12.1333. [DOI] [PubMed] [Google Scholar]
- 37. McNicholas WT, Hansson D, Schiza S, Grote L. Sleep in chronic respiratory disease: COPD and hypoventilation disorders. Eur Respir Rev 28: 190064, 2019. doi: 10.1183/16000617.0064-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron 87: 946–961, 2015. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burke PG, Kanbar R, Basting TM, Hodges WM, Viar KE, Stornetta RL, Guyenet PG. State-dependent control of breathing by the retrotrapezoid nucleus. J Physiol 593: 2909–2926, 2015. doi: 10.1113/JP270053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol (1985) 116: 302–313, 2014. doi: 10.1152/japplphysiol.00649.2013. [DOI] [PubMed] [Google Scholar]
- 41. Jordan AS, Eckert DJ, Wellman A, Trinder JA, Malhotra A, White DP. Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am J Respir Crit Care Med 184: 1183–1191, 2011. doi: 10.1164/rccm.201106-0975OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 437: 1257–1263, 2005. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 43. Alexandre C, Andermann ML, Scammell TE. Control of arousal by the orexin neurons. Curr Opin Neurobiol 23: 752–759, 2013. doi: 10.1016/j.conb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci USA 101: 4649–4654, 2004. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DNC, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA 96: 10911–10916, 1999. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98: 365–376, 1999. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 47. Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 14: 318–328, 2015. doi: 10.1016/S1474-4422(14)70218-2. [DOI] [PubMed] [Google Scholar]
- 48. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295, 1996. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 49. Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 296: R493–R500, 2009. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jenkins JB, Omori T, Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased in mice with obesity induced by high-fat food. Physiol Behav 87: 255–262, 2006. doi: 10.1016/j.physbeh.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 51. Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased by weight gain and decreased by weight loss in mice. Sleep 31: 627–633, 2008. doi: 10.1093/sleep/31.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pettersson US, Waldén TB, Carlsson P-O, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One 7: e46057, 2012. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Casimiro I, Stull ND, Tersey SA, Mirmira RG. Phenotypic sexual dimorphism in response to dietary fat manipulation in C57BL/6J mice. J Diabetes Complications 35: 107795, 2021. doi: 10.1016/j.jdiacomp.2020.107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figs. S1 and S2: https://doi.org/10.6084/m9.figshare.20235588.v3.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available upon reasonable request.