Abstract
Background
Roxadustat is a newly listed oral hypoxia-inducible factor-proline enhancing enzyme inhibitor (HIF-PHI) in recent years. There have been some studies that have proved the efficacy of roxadustat on the treatment of renal anemia in patients with chronic kidney disease (CKD), but there are still different conclusions on its safety.
Methods
PubMed, Embase, Cochrane, and ClinicalTrials were searched for randomized controlled trials (RCTs) that assess efficacy and safety of roxadustat treatment for anemia in CKD patients. The Cochrane Literature Quality Evaluation Scale was used to evaluate the quality of included literature. We choose fixed-effects model or random effects model for data processing based on heterogeneity. It was considered statistically significant when p value <0.05.
Results
A total of 842 articles were retrieved, and 16 trials in the 15 articles were finally included. Roxadustat treatment significantly increased Hb levels. Iron (SMD 1.43, 95% CI 0.31 to 2.55), total iron-binding capacity (SMD 2.06, 95% CI 0.91 to 3.22), ferritin (WMD 21.33, 95% CI 3.04 to 39.62), transferrin saturation (SMD 4.17, 95% CI 3.90 to 4.45), and LDL-cholesterol (SMD -0.64, 95% CI -0.73 to -0.55) showed statistical significance in dialysis-dependent (DD) study. And hepcidin (SMD -1.56, 95% CI -2.63 to -0.50), transferrin (SMD 1.80, 95% CI 1.53 to 2.06), total iron-binding capacity (SMD 1.62, 95% CI 1.39 to 1.86), total cholesterol (SMD -0.88, 95% CI -1.68 to -0.09), ferritin (WMD -52.68, 95% CI -62.68 to -42.67), transferrin saturation (SMD -5.57, 95% CI -7.47 to -3.68), and LDL-cholesterol (SMD -0.85, 95% CI -1.37 to -0.34) showed statistical significance in not dialysis-dependent (NDD) study. In terms of safety, roxadustat treatment did not increase risk of total adverse events either in dialysis-dependent or not dialysis-dependent patients.
Conclusion
Roxadustat can effectively improve anemia in patients with chronic kidney disease. There was no significant difference in total adverse events compared with the control group.
1. Introduction
Renal anemia is one of the common complications of patients with chronic kidney disease (CKD). Nearly 90% of long-term dialysis patients have anemia. The lack of erythropoietin and the imbalance of iron metabolism are currently recognized causes of anemia in patients with chronic kidney disease. The use of erythropoiesis stimulators is one of the common methods of clinical treatment of renal anemia. However, under the influence of factors such as iron utilization disorder, chronic inflammation, malnutrition, and low patient compliance, fewer patients use exogenous EPO to treat renal anemia to achieve the goal of hemoglobin [1]. About 10-20% of patients respond poorly to EPO [1].
Roxadustat has been approved for the treatment of renal anemia in some countries as an oral hypoxia-inducible factor-proline hydroxylase inhibitor (HIF-PHI) newly marketed in recent years [2]. Research indicates that the kidneys of CKD patients still retain the ability to produce erythropoietin [3]. Hypoxia-inducible factor (HIF) is a cytokine that contains HIF-α (HIF-1α, HIF-2α, and HIF-3α) and HIF-β subunits. Under hypoxia, the proline hydroxylase is inactivated, and the concentration of HIF-α increases, which promotes the production of EPO by the interstitial cells around the renal tubules [4]. Roxadustat, as a proline hydroxylase inhibitor, can mimic the hypoxic environment, reduce the degradation of HIF, and increase the production of endogenous EPO [5]. A number of clinical studies have confirmed the effectiveness of roxadustat in correcting anemia. Many scholars believe that roxadustat has more adverse reactions in the treatment of renal anemia, mainly involving respiratory tract infections, hypertension, myocardial infarction, hyperkalemia, and gastrointestinal reactions [6]. In the study of Zheng et al., a higher incidence of adverse events (AEs) in the roxadustat group was significantly higher than that in the epoetin alfa group [7]. In recent years, a global phase 3 clinical trial showed that CKD 3-5 patients have a good tolerance to roxadustat [8]. A number of clinical trials have been carried out globally and have obtained new results this year. The results of meta-analyses on the safety of roxadustat are also inconsistent. Related meta-analysis was detailed in the analysis of the efficacy of roxadustat, and the occurrence of specific adverse events was rarely analyzed. We conducted a meta-analysis again including the latest high-quality RCTs to explore the efficacy and safety of roxadustat in the treatment of renal anemia, including the adverse events involved in each study.
2. Materials and Methods
2.1. Search Strategy
This meta-analysis was conducted to explore the efficacy and safety of roxadustat treatment for anemia in patients with chronic kidney disease (CKD). Our meta-analysis followed the Cochrane Handbook for Systematic Reviews of Interventions and Preferred Reporting for Systematic Review and Meta-Analysis (PRISMA). PubMed, Embase, Cochrane, and ClinicalTrials were searched for studies published through November 2021. We used (((renal dialysis) OR (chronic kidney disease) OR (end-stage kidney disease) OR (ESKD)) AND (roxadustat) AND (anemia)) as the search terms. Moreover, the cited references of included articles and systemic reviews were searched manually.
2.2. Selection Criteria
The literature screening was done independently by two authors, and the disagreements between the two authors were determined independently by the third reviewer. Studies that meet the following criteria were included: (1) studies as randomized controlled trials, (2) studies including dialysis patients or patients with stage 3-5 CKD who were not dependent on dialysis, (3) studies evaluating the efficacy and safety of roxadustat in treating anemia, (4) studies reporting the mean change from baseline in efficacy endpoints, and (5) studies reporting adverse events. Experiments where data were not available, nonhuman studies, case reports, systematic reviews, and meta-analysis were excluded. There are no restrictions on gender, race, or region.
2.3. Data Extraction
We extracted baseline characteristics such as first author, publication time, country, blinding method, patient and comparator, sample size, and duration from the included studies. Patient inclusion criteria, baseline data, baseline hemoglobin levels, and treatment options were also extracted. The main efficacy outcomes were mean changes from baseline in hemoglobin. Other outcomes included hepcidin, iron, transferrin, soluble transferrin receptor, total iron-binding capacity, total cholesterol, ferritin, transferrin saturation, low-density lipoprotein-cholesterol, triglycerides, and inflammatory markers. We intended to conduct a stratified analysis for hemoglobin levels in different C-reaction protein levels. Adverse events were extracted from the articles to assess the safety. When several articles were published on the same experiment, we selected the latest data.
2.4. Statistical Analysis
Statistical analysis was performed in Review Manager 5.3 software. The Cochrane Literature Quality Evaluation Scale was used to evaluate the quality of included literature. We examined heterogeneity by using the I2 statistics. If the I2 was >50%, the random effects model was adopted; otherwise, the fixed effects model was adopted. A sensitivity analysis was performed by removing each individual study when showing obvious heterogeneity. It was considered statistically significant when P value <0.05. Continuous variables were analyzed by the inverse variance method, and discontinuous variables were analyzed by the Mantel-Haenszel method. The publication bias was evaluated by funnel chart and Egger's test.
Data extraction and statistical analysis were completed by two authors independently, and differences were resolved by a third person.
3. Results
3.1. Literature Search
211 articles were retrieved from PubMed, 461 articles from Embase, 121 articles from the Cochrane, and 49 studies from ClinicalTrials. 335 duplicate documents were excluded. 570 irrelevant articles were excluded after screening titles and abstracts. A total of 89 articles were evaluated for the full text, and 15 articles were included in our meta-analysis finally. Figure 1 shows the flow chart of the included literature.
Figure 1.
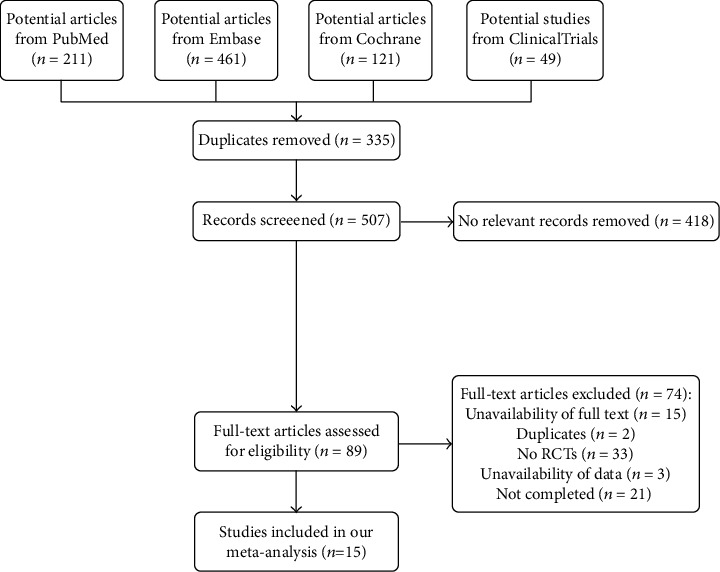
Flow diagram of the literature search and trial selection process.
3.2. Study Characteristics
The basic characteristics of 16 trials in the 15 articles were shown in Table 1. A total of 8 trials included patients with CKD stage 5 who were dialysis-dependent (DD) and 8 trials included patients who were not dialysis-dependent (NDD) with CKD stage 3–5 patients. The follow-up duration for patients was 6-104 weeks. Each study included 87-1043 patients. The patient baseline variables were similar in the roxadustat group and the control group. Patients had a baseline Hb < 12.0 g/dL. The results of literature quality evaluation were shown in Figure 2.
Table 1.
Baseline characteristics of studies included in the meta-analysis.
| Study | Country | Blinded | Patients | Comparator | Sample size | No. of experimental | No. of comparator | Duration (weeks) |
|---|---|---|---|---|---|---|---|---|
| Chen et al. [3] | China | Open-label | DD | Epoetin alfa | 304 | 204 | 100 | 27 |
| Hou et al. [9] | China | Open-label | PD | ESAs | 129 | 86 | 43 | 24 |
| Akizawa et al. [10] | Japan | Double-blind | HD | Darbepoetin | 303 | 151 | 152 | 24 |
| Csiky et al. [6] | Europe | Open-label | DD | ESAs | 836 | 415 | 421 | 104 |
| Chen et al. [11] | China | Double-blind/open-label | NDD/DD | Placebo/epoetin alfa | 91/96 | 61/74 | 30/22 | 8/6 |
| Provenzano et al. [12] | USA | Open-label | HD | Epoetin alfa | 144 | 108 | 36 | 19 |
| Provenzano et al. [13] | USA | Open-label | DD | Epoetin alfa | 1043 | 522 | 521 | 52 |
| Barratt et al. [14] | UK | Open-label | NDD | Darbepoetin alfa | 616 | 323 | 293 | 104 |
| Charytan et al. [15] | USA | Open-label | DD | Epoetin alfa | 741 | 370 | 371 | 52 |
| Akizawa et al. [16] | Japan | Open-label | NDD | Darbepoetin alfa | 332 | 201 | 131 | 52 |
| Chen et al. [17] | China | Double-blind | NDD | Placebo | 152 | 101 | 51 | 8 |
| Shutov et al. [18] | Russia | Double-blind | NDD | Placebo | 594 | 391 | 203 | 104 |
| Akizawa et al. [19] | Japan | Double-blind | NDD | Placebo | 107 | 80 | 27 | 6 |
| Besarab et al. [20] | USA | Single-blind | NDD | Placebo | 116 | 88 | 28 | 12 |
| Coyne et al. [8] | USA | Double-blind | NDD | Placebo | 922 | 616 | 306 | 52 |
DD: dialysis-dependent; NDD: not dialysis-dependent; PD: peritoneal dialysis; HD: hemodialysis.
Figure 2.
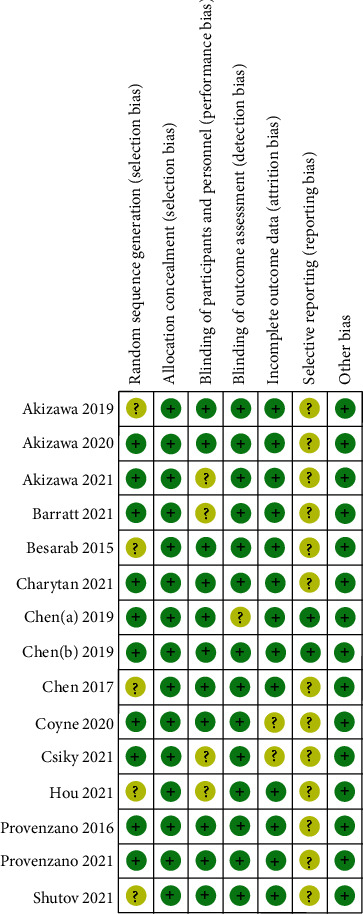
Risk of bias summary for each included study.
3.3. Efficacy Outcomes of Roxadustat in DD and NDD Study
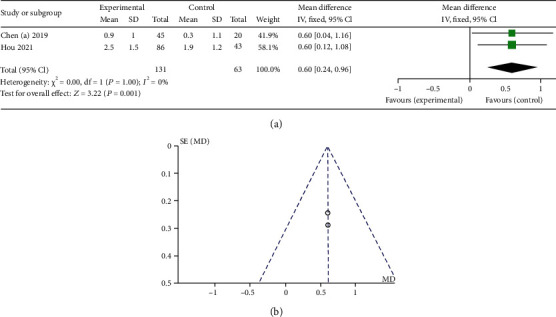
As shown in Figures 3 and 4, roxadustat treatment significantly increased Hb levels in NDD study (SMD 1.77, 95% CI 1.52 to 2.02, p < 0.00001) compared with placebo, and there was no statistical significance in DD study (SMD 0.21, 95% CI -0.10 to 0.52, p = 0.18) compared with ESAs. We conducted sensitivity analysis due to obvious heterogeneity. The results showed that there was statistical significance increased Hb levels in DD study after sensitivity analysis (SMD 0.36, 95% CI 0.15 to 0.58, p = 0.008). In the analysis that patients with a C-reactive protein (CRP) level above the upper limit of the normal range, roxadustat treatment had a greater Hb levels increased in DD study (WMD 0.60, 95% CI 0.24 to 0.96, p = 0.0001). Hb response was defined as an Hb rise not less than 1.0 g/dL from baseline. The forest plots of Hb response in DD study (RR 1.08, 95% CI 1.01 to 1.15, p = 0.02) and NDD study (RR 7.21, 95% CI 5.24 to 9.91, p < 0.00001) were shown in Figure 5.
Figure 3.
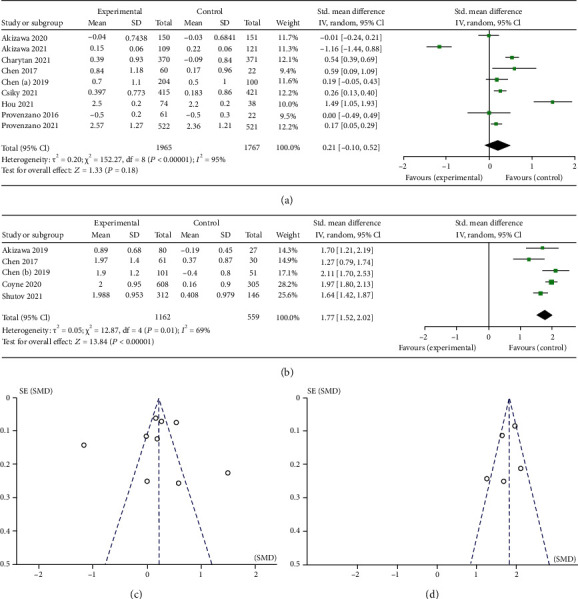
(a) Forest plot of mean change from baseline in Hb level in DD study. (b) Forest plot of mean change from baseline in Hb level in NDD study. (c) Funnel chart of mean change from baseline in Hb level in DD study. (d) Funnel chart of mean change from baseline in Hb level in NDD study.
Figure 4.
(a) Forest plot of mean change from baseline in Hb level in DD study in patient with a C-reactive protein level above the upper limit of the normal range. (b) Funnel chart of mean change from baseline in Hb level in DD study in patient with a C-reactive protein level above the upper limit of the normal range.
Figure 5.
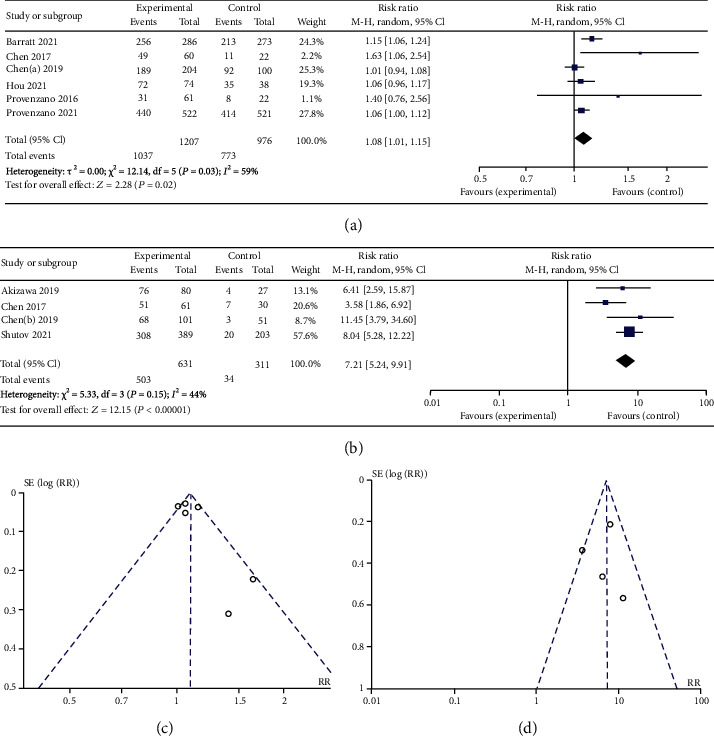
(a) Forest plot of Hb response in DD study. (b) Forest plot of Hb response in NDD study. (c) Funnel plot to assess publication bias in Hb response in DD study. (d) Funnel plot to assess publication bias in Hb response in NDD study.
As shown in Tables 2 and 3, iron (SMD 1.43, 95% CI 0.31 to 2.55, p = 0.01), total iron-binding capacity (SMD 2.06, 95% CI 0.91 to 3.22, p = 0.0005), ferritin (WMD 21.33, 95% CI 3.04 to 39.62, p = 0.02), transferrin saturation (SMD 4.17, 95% CI 3.90 to 4.45, p < 0.00001), and LDL-cholesterol (SMD -0.64, 95% CI -0.73 to -0.55, p < 0.00001) showed statistical significance in DD study. And hepcidin (SMD -1.56, 95% CI -2.63 to -0.50, p = 0.004), transferrin (SMD 1.80, 95% CI 1.53 to 2.06, p < 0.00001), total iron-binding capacity (SMD 1.62, 95% CI 1.39 to 1.86, p < 0.00001), total cholesterol (SMD -0.88, 95% CI -1.68 to -0.09, p = 0.03), ferritin (WMD -52.68, 95% CI -62.68 to -42.67, p < 0.00001), transferrin saturation (SMD -5.57, 95% CI -7.47 to -3.68, p < 0.00001), and LDL-cholesterol (SMD -0.85, 95% CI -1.37 to -0.34, p = 0.01) showed statistical significance in NDD study. The results showed that there was no change in the conclusion after sensitivity analysis.
Table 2.
Mean change from baseline in iron parameter and lipid levels in DD study.
| Parameter | p value for heterogeneity (p, I2) | SMD/WMD (95% CI) | p value |
|---|---|---|---|
| Hepcidin |
p = 0.05 I2 = 63% |
-0.12 (-0.39, 0.15) | p = 0.38 |
| Iron |
p < 0.00001 I2 = 99% |
1.43 (0.31, 2.55) | p = 0.01 |
| Transferrin |
p < 0.00001 I2 = 99% |
3.77 (0.04, 7.50) | p = 0.05 |
| Soluble transferrin receptor |
p = 0.009 I2 = 85% |
0.09 (-0.61, 0.79) | p = 0.80 |
| Total iron-binding capacity |
p < 0.00001 I2 = 99% |
2.06 (0.91, 3.22) | p = 0.0005 |
| Total cholesterol |
p < 0.00001 I2 = 99% |
-0.36 (-1.35, 0.62) | p = 0.47 |
| Ferritin |
p = 0.07 I2 = 53% |
21.33 (3.04, 39.62) | p = 0.02 |
| Transferrin saturation |
p = 0.46 I2 = 0% |
4.17 (3.90, 4.45) | p < 0.00001 |
| LDL-cholesterol |
p = 0.20 I2 = 35% |
-0.64 (-0.73, -0.55) | p < 0.00001 |
| Triglycerides |
p = 0.62 I2 = 0% |
-0.09 (-0.39, 0.21) | p = 0.56 |
LDL-cholesterol: low-density lipoprotein cholesterol.
Table 3.
Mean change from baseline in iron parameter and lipid levels in NDD study.
| Parameter | p value for heterogeneity (p, I2) | SMD/WMD (95% CI) | p value |
|---|---|---|---|
| Hepcidin |
p < 0.00001 I2 = 97% |
-1.56 (-2.63, -0.50) | p = 0.004 |
| Iron |
p = 0.50 I2 = 0% |
-0.06 (-0.29, 0.18) | p = 0.64 |
| Transferrin |
p = 0.25 I2 = 27% |
1.80 (1.53, 2.06) | p < 0.00001 |
| Total iron-binding capacity |
p = 0.28 I2 = 21% |
1.62 (1.39, 1.86) | p < 0.00001 |
| Total cholesterol |
p = 0.001 I2 = 90% |
-0.88 (-1.68, -0.09) | p = 0.03 |
| Ferritin |
p = 0.13 I2 = 47% |
-52.68 (-62.68, -42.67) | p < 0.00001 |
| Transferrin saturation |
p = 0.30 I2 = 18% |
-5.57 (-7.47, -3.68) | p < 0.00001 |
| LDL-cholesterol |
p = 0.03 I2 = 78% |
-0.85 (-1.37, -0.34) | p = 0.01 |
LDL-cholesterol: low-density lipoprotein cholesterol.
The analysis of IV iron use in DD study is shown in Figure 6. Compared with ESAs, roxadustat treatment reduced the use (RR 0.52, 95% CI 0.45 to 0.61, p = 0.04) and dose (SMD -30.97, 95% CI -36.59 to -25.35, p < 0.00001) of IV iron in patients.
Figure 6.
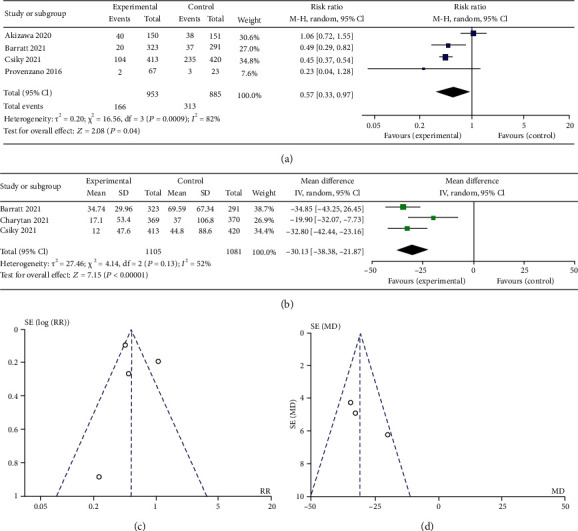
(a) Forest plot of no. of patients using IV iron in DD study. (b) Forest plot of the mean monthly dose IV iron in DD study. (c) Funnel plot of no. of patients using IV iron in DD study. (d) Funnel plot of the mean monthly dose IV iron in DD study.
3.4. Safety Outcomes of Roxadustat in DD and NDD Study
As shown in Figure 7 and Tables 4 and 5, there was no significant difference in total adverse events compared with the control group. Compared with ESAs, roxadustat treatment increased the risk of vomiting (RR 1.77, 95% CI 1.08 to 2.90, p = 0.02), hypotension (RR 1.45, 95% CI 1.08 to 1.96, p = 0.01), diarrhea (RR1.40, 95% CI 1.07 to 1.82, p = 0.01), and arteriovenous fistula thrombosis (RR 1.43, 95% CI 1.09 to 1.87, p = 0.009) in dialysis patients and reduced the risk of cardiac failure (RR 0.39, 95% CI 0.17 to 0.89, p = 0.03). Compared with placebo, roxadustat treatment increased the risk of hypertension (RR 1.45, 95% CI 1.12 to 1.87, p = 0.005), hyperkalemia (RR 1.41, 95% CI 1.08 to 1.85, p = 0.01), insomnia (RR 3.17, 95% CI 1.66 to 6.07, p = 0.0005), nausea (RR 1.79, 95% CI 1.26 to 2.55, p = 0.001), and peripheral edema (RR 1.38, 95% CI 1.02 to 1.87, p = 0.03) in NDD patients and reduced the risk of anemia (RR 0.18, 95% CI 0.11 to 0.31, p < 0.00001).
Figure 7.
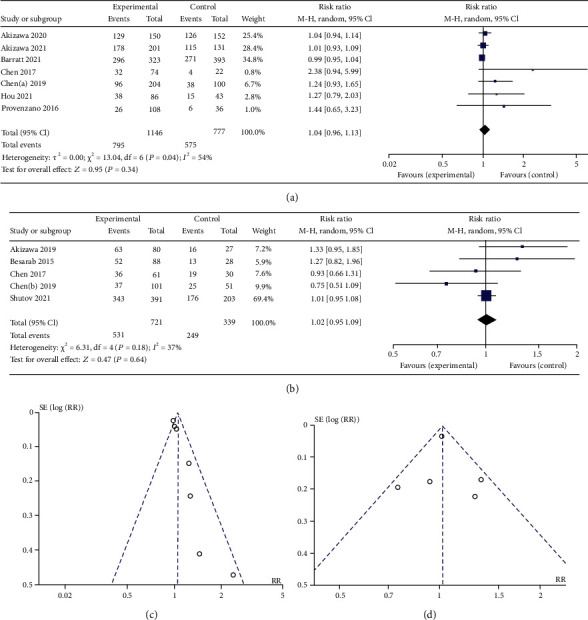
(a) Forest plot of the risk of the main adverse events in DD study. (b) Forest plot of the risk of the main adverse events in NDD study. (c) Funnel plot of the risk of the main adverse events in DD study. (d) Funnel plot of the risk of the main adverse events in NDD study.
Table 4.
TEAEs occurring in the treatment groups in DD study.
| Parameter | p value for heterogeneity (p, I2) | RR (95% CI) | p value |
|---|---|---|---|
| TEAEs | p = 0.04, I2 = 54% | 1.04 (0.96, 1.13) | p = 0.34 |
| Serious TEAEs | p = 0.68, I2 = 0% | 1.09 (0.97, 1.23) | p = 0.14 |
| Drug-related serious TEAEs | p = 0.63, I2 = 0% | 1.10 (0.96, 1.26) | p = 0.18 |
| Upper respiratory tract infection | p = 0.37, I2 = 7% | 1.07 (0.70, 1.64) | p = 0.76 |
| Urinary tract infection | p = 0.71, I2 = 0% | 1.76 (0.71, 4.40) | p = 0.22 |
| Pneumonia | p = 0.63, I2 = 0% | 1.03 (0.77, 1.37) | p = 0.86 |
| Hypertension | p = 0.71, I2 = 0% | 1.13 (0.93, 1.37) | p = 0.21 |
| Hypertensive crisis | p = 0.79, I2 = 0% | 0.79 (0.39, 1.60) | p = 0.52 |
| Hyperkalemia | p = 0.11, I2 = 44% | 1.03 (0.80, 1.33) | p = 0.83 |
| Headache | p = 0.43, I2 = 0% | 1.22 (0.92, 1.62) | p = 0.17 |
| Chest discomfort | p = 0.13, I2 = 56% | 3.31 (0.21, 52.28) | p = 0.39 |
| Vomiting | p = 0.44, I2 = 0% | 1.77 (1.08, 2.90) | p = 0.02 |
| Nausea | p = 0.02, I2 = 56% | 1.48 (0.83, 2.65) | p = 0.19 |
| Asthenia | p = 0.45, I2 = 0% | 2.30 (0.67, 7.87) | p = 0.19 |
| Alanine aminotransferase increase | p = 0.93, I2 = 0% | 1.48 (0.55, 3.98) | p = 0.43 |
| Dizziness | p = 0.65, I2 = 0% | 1.02 (0.65, 1.60) | p = 0.95 |
| Hypotension | p = 0.71, I2 = 0% | 1.45 (1.08, 1.96) | p = 0.01 |
| Muscle spasms | p = 0.004, I2 = 74% | 0.63 (0.28, 1.39) | p = 0.25 |
| Anemia | p = 0.26, I2 = 26% | 1.07 (0.63, 1.83) | p = 0.80 |
| Atrial fibrillation | p = 0.97, I2 = 0% | 0.81 (0.48, 1.37) | p = 0.43 |
| Diarrhea | p = 0.13, I2 = 47% | 1.40 (1.07, 1.82) | p = 0.01 |
| Constipation | p = 1.00, I2 = 0% | 1.51 (0.91, 2.50) | p = 0.11 |
| Pruritus | p = 0.94, I2 = 26% | 1.36 (0.80, 2.30) | p = 0.26 |
| Peritonitis | p = 0.58, I2 = 0% | 0.82 (0.40, 1.66) | p = 0.58 |
| Hyperparathyroidism secondary | p = 0.32, I2 = 0% | 1.09 (0.73, 1.64) | p = 0.66 |
| Injury, poisoning, and procedural complications | p = 0.75, I2 = 0% | 0.87 (0.68, 1.11) | p = 0.27 |
| Arteriovenous fistula thrombosis | p = 0.66, I2 = 0% | 1.43 (1.09, 1.87) | p = 0.009 |
| Coronary artery disease | p = 0.62, I2 = 0% | 0.22 (0.04, 1.35) | p = 0.10 |
| Acute myocardial infarction | p = 0.78, I2 = 0% | 0.59 (0.29, 1.21) | p = 0.15 |
| Cardiac failure | p = 0.32, I2 = 2% | 0.39 (0.17, 0.89) | p = 0.03 |
| Gastroenteritis | p = 0.72, I2 = 0% | 1.06 (0.26, 4.29) | p = 0.94 |
| Pancreatitis | p = 0.27, I2 = 19% | 4.99 (0.73, 34.09) | p = 0.10 |
| Cellulitis | p = 0.89, I2 = 0% | 0.82 (0.25, 2.75) | p = 0.75 |
| Sepsis | p = 0.47, I2 = 0% | 1.12 (0.60, 2.09) | p = 0.73 |
| Gangrene | p = 0.26, I2 = 27% | 1.34 (0.54, 3.29) | p = 0.53 |
TEAEs: treatment emerged adverse event.
Table 5.
TEAEs occurring in the treatment groups in NDD study.
| Parameter | p value for heterogeneity (p, I2) | RR (95% CI) | p value |
|---|---|---|---|
| TEAEs | p = 0.18, I2 = 37% | 1.02 (0.95, 1.09) | p = 0.64 |
| Serious TEAEs | p = 0.56, I2 = 0% | 1.09 (0.94, 1.25) | p = 0.26 |
| ESKD | p = 0.08, I2 = % | 1.38 (0.85, 2.25) | p = 0.19 |
| Upper respiratory tract infection | p = 0.76, I2 = 0% | 0.79 (0.58, 1.08) | p = 0.14 |
| Urinary tract infection | p = 0.44, I2 = 77% | 0.46 (0.05, 4.55) | p = 0.50 |
| Cough | p = 0.14, I2 = 54% | 0.61 (0.13, 2.98) | p = 0.54 |
| Pneumonia | p = 0.70, I2 = 0% | 1.14 (0.76, 1.71) | p = 0.52 |
| Viral upper respiratory tract infection | p = 0.14, I2 = 53% | 1.50 (0.87, 2.61) | p = 0.15 |
| Nasopharyngitis | p = 0.63, I2 = 0% | 1.27 (0.59, 2.75) | p = 0.54 |
| Hypertension | p = 0.72, I2 = 0% | 1.45 (1.12, 1.87) | p = 0.005 |
| Hyperkalemia | p = 0.94, I2 = 0% | 1.41 (1.08, 1.85) | p = 0.01 |
| Metabolic acidosis | p = 0.05, I2 = 74% | 1.77 (0.24, 13.04) | p = 0.58 |
| Insomnia | p = 0.17, I2 = 46% | 3.17 (1.66, 6.07) | p = 0.0005 |
| Gout | p = 0.18, I2 = 43% | 0.72 (0.43, 1.20) | p = 0.21 |
| Back pain | p = 0.04, I2 = 75% | 0.47 (0.03, 8.90) | p = 0.62 |
| Headache | p = 0.88, I2 = 0% | 1.16 (0.82, 1.66) | p = 0.40 |
| Vomiting | p = 0.69, I2 = 0% | 1.38 (0.85, 2.24) | p = 0.20 |
| Nausea | p = 0.38, I2 = 3% | 1.79 (1.26, 2.55) | p = 0.001 |
| Dizziness | p = 0.22, I2 = 32% | 0.77 (0.53, 1.11) | p = 0.16 |
| Muscle spasms | p = 0.12, I2 = 53% | 1.04 (0.20, 5.44) | p = 0.96 |
| Anemia | p = 0.52, I2 = 0% | 0.18 (0.11, 0.31) | p < 0.00001 |
| Diarrhea | p = 0.17, I2 = 38% | 1.38 (1.00, 1.92) | p = 0.05 |
| Peripheral edema | p = 0.71, I2 = 0% | 1.38 (1.02, 1.87) | p = 0.03 |
| Fever | p = 0.05, I2 = 73% | 1.02 (0.17, 6.12) | p = 0.98 |
| Pruritus | p = 0.09, I2 = 65% | 2.28 (0.65, 7.93) | p = 0.20 |
| Asthenia | p = 0.63, I2 = 0% | 1.48 (0.77, 2.85) | p = 0.24 |
| Gastrointestinal hemorrhage | p = 0.89, I2 = 0% | 0.82 (0.25, 2.75) | p = 0.75 |
TEAEs: treatment emerged adverse event.
4. Discussion
This meta-analysis included 6,518 patients from 16 trials. We evaluated the efficacy and safety of roxadustat in the treatment of renal anemia. The trials we included are all randomized controlled trials. In our results, roxadustat can effectively improve hemoglobin levels for both dialysis-dependent (DD) and not dialysis-dependent (NDD) patients. Compared with ESAs, roxadustat treatment increased the serum iron level, total iron-binding capacity, and ferritin and reduced the transferrin saturation in patients undergoing dialysis. Compared with placebo, roxadustat treatment reduced the hepcidin, ferritin, and transferrin saturation in NDD patients and increased the transferrin and total iron-binding capacity. In addition, roxadustat can reduce the use of intravenous iron. In the 12 included studies, intravenous iron supplementation was forbidden except for rescue treatment.
We have reached some different conclusions in our research. Compared with previous meta-analyses, we analyzed more adverse events in detail and obtained some new conclusions. Patient in the roxadustat group have an increased risk of vomiting, hypotension, diarrhea, and arteriovenous fistula thrombosis and have a lower risk of cardiac failure and reduced the use of IV iron compared with the ESA group. In NDD study, the risk of hypertension, hyperkalemia, insomnia, nausea, and peripheral edema may increase in the roxadustat group compared with placebo. In our meta-analysis, total adverse events were not statistically significant either in DD or NDD patients. This conclusion is different from the previous meta-analysis results. In the study of Zheng et al., a higher incidence of adverse events (AEs) in the roxadustat group was significantly higher than that in the epoetin alfa group [7].
In our meta-analysis, roxadustat can reduce the level of hepcidin in NDD patients, and it was not statistically significant in DD patients. This is a different result from the previous paper [7, 21]. The inconsistent conclusions may be attributable to short duration and low dose of roxadustat and the dialysis per se [12, 22]. According to Provenzano et al.'s study, significant changes of hepcidin and ferritin were noted in dialysis patients after 19-week treatment of roxadustat [22]. In addition to promoting the production of endogenous EPO, HIF-α can also promote the absorption of iron in the intestines and promote the transport of iron in the blood to the tissues [23]. The level of hepcidin is elevated in an inflammatory state, which is currently considered to be one of the reasons for the deficiency of iron utilization [23]. HIF-mediated hypoxia may inhibit the expression of hepatic hepcidin and increase iron utilization [23]. hs-CRP concentration is used as a marker of inflammation. In our results, patients with a C-reactive protein (CRP) level above the upper limit of the normal range, roxadustat treatment had a greater Hb levels increased in DD study. In animal model experiments, the inflammatory response of mice treated with roxadustat was also significantly weakened, which can be demonstrated by the decrease in the infiltration of macrophages and neutrophils and the downregulated expression of inflammatory cytokines [24]. Roxadustat has a positive effect in the treatment of chronic inflammation, and its mechanism is related to the redistribution of oxygen in the cell microenvironment [25]. In the study of Yin et al., the frequency of administration has different effects on inflammation [26]. Due to limited data, we are unable to perform more analysis on inflammation markers in our study. In our meta-analysis, roxadustat can reduce the level of LDL in patients. This may have a protective effect on atherosclerosis [27].
It is generally believed that ESAs will promote platelet function and production results [28]. In 3 studies with a total of 2489 patients, we got the result that roxadustat treatment has an increased risk of arteriovenous fistula thrombosis than ESA treatment. Hypoxia increases the concentration of HIF-1, thereby forming thrombi on atherosclerotic plaques through upregulation of prothrombotic factors [29]. This may be the mechanism that roxadustat treatment increases the risk of arteriovenous fistula thrombosis. A recent animal experiment proved that roxadustat has no effect on platelet production and function in healthy and 5/6 nephrectomized mice [30]. The mechanism is worthy of further exploration.
In our study, roxadustat increased the incidence of hypotension in DD patients and the incidence of hypertension in NDD patients. This is different from the conclusion of Wang et al. [21]. In the study of Yu et al., roxadustat can be used to treat hypertension associated with high renin-angiotensin system (RAS) activity [31]. The mechanism may be through stabilizing HIF-1α and then targeting eNOS, AGTR1, AGTR2, and oxidative stress [31]. This may increase the incidence of hypotension. HIF-1α and HIF-2α play an antagonistic effect in the long-term activation process, which may contribute to the progression of chronic heart failure, atherosclerosis, hypertension, vascular disease, and chronic kidney disease cardiac failure [32]. HIF-2α is the main stimulator of erythropoietin synthesis [33]. Roxadustat as an inhibition of isoform-selective prolyl hydroxylases can be achieved in selective activation of HIF-2α to ameliorate the development of cardiac failure [32]. Roxadustat increases the levels of HIF-1α and HIF-2α in CD4+ T cells, reduces their proliferation, and induces apoptosis [34]. Experimental data supports that roxadustat may increase infection by upregulating HIF-1α and affecting adaptive immune responses [34], but we did not get the result that roxadustat will increase the infection, either upper respiratory or urinary tract infection. This is the same conclusion as previous studies [21].
There are several limitations in our meta-analysis. (1) The random effects model is used when the heterogeneity is obvious. (2) We have not conducted a subgroup analysis of race, and we cannot rule out its influence. (3) Each study indicated a different initial dose, and we did not control the dose of roxadustat. The observation period of each study was different, which may have some influence on the efficacy and the occurrence of adverse events. The relevant information of inflammation markers is insufficient, and more attention should be paid in future work.
Data Availability
All data are available from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Zhou Y., Chen X. X., Zhang Y. F., Lou J. Z., Yuan H. B. Roxadustat for dialysis patients with erythropoietin hypo-responsiveness: a single-center, prospective investigation. Internal and Emergency Medicine . 2021;16(8):2193–2199. doi: 10.1007/s11739-021-02738-4. [DOI] [PubMed] [Google Scholar]
- 2.Sakashita M., Tanaka T., Nangaku M. Hypoxia-inducible factor-prolyl hydroxylase domain inhibitors to treat anemia in chronic kidney disease. Contributions to Nephrology . 2019;198:112–123. doi: 10.1159/000496531. [DOI] [PubMed] [Google Scholar]
- 3.Chen N., Hao C., Liu B. C., et al. Roxadustat treatment for anemia in patients undergoing long-term dialysis. New England Journal of Medicine . 2019;381(11):1011–1022. doi: 10.1056/NEJMoa1901713. [DOI] [PubMed] [Google Scholar]
- 4.Yap D. Y. H., McMahon L. P., Hao C. M., et al. Recommendations by the Asian Pacific society of nephrology (APSN) on the appropriate use of HIF-PH inhibitors. Nephrology . 2021;26(2):105–118. doi: 10.1111/nep.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanghani N. S., Haase V. H. Hypoxia-inducible factor activators in renal anemia: current clinical experience. Advances in Chronic Kidney Disease . 2019;26(4):253–266. doi: 10.1053/j.ackd.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csiky B., Schömig M., Esposito C., et al. Roxadustat for the maintenance treatment of anemia in patients with end-stage kidney disease on stable dialysis: a European phase 3, randomized, open-label, active-controlled study (PYRENEES) Advances in Therapy . 2021;38(10):5361–5380. doi: 10.1007/s12325-021-01904-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng Q., Yang H., Fu X., et al. The efficacy and safety of roxadustat for anemia in patients with chronic kidney disease: a meta-analysis. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association-European Renal Association . 2021;36(9):1603–1615. doi: 10.1093/ndt/gfaa110. [DOI] [PubMed] [Google Scholar]
- 8.Coyne D. W., Roger S. D., Shin S. K., et al. Roxadustat for CKD-related anemia in non-dialysis patients. Kidney International Reports . 2021;6(3):624–635. doi: 10.1016/j.ekir.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou Y. P., Mao X. Y., Wang C., et al. Roxadustat treatment for anemia in peritoneal dialysis patients: a randomized controlled trial. Journal of the Formosan Medical Association/Taiwan yi zhi . 2022;121(2):529–538. doi: 10.1016/j.jfma.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Akizawa T., Iwasaki M., Yamaguchi Y., Majikawa Y., Reusch M. Phase 3, randomized, double-blind, active-comparator (darbepoetin alfa) study of oral roxadustat in CKD patients with anemia on hemodialysis in Japan. Journal of the American Society of Nephrology: JASN . 2020;31(7):1628–1639. doi: 10.1681/ASN.2019060623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N., Qian J., Chen J., et al. Phase 2 studies of oral hypoxia-inducible factor prolyl hydroxylase inhibitor FG-4592 for treatment of anemia in China. Nephrology Dialysis Transplantation . 2017;32(8):1373–1386. doi: 10.1093/ndt/gfx011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Provenzano R., Besarab A., Wright S., et al. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. American Journal of Kidney Diseases . 2016;67(6):912–924. doi: 10.1053/j.ajkd.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Provenzano R., Shutov E., Eremeeva L., et al. Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrology, Dialysis, Transplantation . 2021;36(9):1717–1730. doi: 10.1093/ndt/gfab051. [DOI] [PubMed] [Google Scholar]
- 14.Barratt J., Andric B., Tataradze A., et al. Roxadustat for the treatment of anaemia in chronic kidney disease patients not on dialysis: a phase 3, randomized, open-label, active-controlled study (DOLOMITES) Nephrology, Dialysis, Transplantation . 2021;36(9):1616–1628. doi: 10.1093/ndt/gfab191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charytan C., Manllo-Karim R., Martin E. R., et al. A randomized trial of roxadustat in anemia of kidney failure: SIERRAS study. Kidney International Reports . 2021;6(7):1829–1839. doi: 10.1016/j.ekir.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akizawa T., Iwasaki M., Otsuka T., Yamaguchi Y., Reusch M. Phase 3 study of roxadustat to treat anemia in non-dialysis-dependant CKD. Kidney International Reports . 2021;6(7):1810–1828. doi: 10.1016/j.ekir.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N., Hao C., Peng X., et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. New England Journal of Medicine . 2019;381(11):1001–1010. doi: 10.1056/NEJMoa1813599. [DOI] [PubMed] [Google Scholar]
- 18.Shutov E., Sułowicz W., Esposito C., et al. Roxadustat for the treatment of anemia in chronic kidney disease patients not on dialysis: a phase 3, randomized, double-blind, placebo-controlled study (ALPS) Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association-European Renal Association . 2021;36(9):1629–1639. doi: 10.1093/ndt/gfab057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akizawa T., Iwasaki M., Otsuka T., Reusch M., Misumi T. Roxadustat treatment of chronic kidney disease-associated anemia in Japanese patients not on dialysis: a phase 2, randomized, double-blind, placebo-controlled trial. Placebo-Controlled Trial. Advances in Therapy . 2019;36(6):1438–1454. doi: 10.1007/s12325-019-00943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besarab A., Provenzano R., Hertel J., et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrology Dialysis Transplantation . 2015;30(10):1665–1673. doi: 10.1093/ndt/gfv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L., Yin H., Yang L., Zhang F., Wang S., Liao D. The efficacy and safety of roxadustat for anemia in patients with chronic kidney disease: a meta-analysis. Frontiers in Pharmacology . 2022;13, article 779694 doi: 10.3389/fphar.2022.779694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia L., Dong X., Yang J., Jia R., Zhang H. Effectiveness of hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat on renal anemia in non-dialysis-dependent chronic kidney disease: a systematic review and meta-analysis. Annals of Translational Medicine . 2019;7(23):p. 720. doi: 10.21037/atm.2019.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinozaki Y., Fukui K., Kobayashi H., Yoshiuchi H., Matsuo A., Matsushita M. JTZ-951 (enarodustat), a hypoxia-inducible factor prolyl hydroxylase inhibitor, improves iron utilization and anemia of inflammation: comparative study against recombinant erythropoietin in rat. European Journal of Pharmacology . 2021;898, article 173990 doi: 10.1016/j.ejphar.2021.173990. [DOI] [PubMed] [Google Scholar]
- 24.Miao A. F., Liang J. X., Yao L., Han J. L., Zhou L. J. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against renal ischemia/reperfusion injury by inhibiting inflammation. Renal Failure . 2021;43(1):803–810. doi: 10.1080/0886022X.2021.1915801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sulser P., Pickel C., Günter J., et al. HIF hydroxylase inhibitors decrease cellular oxygen consumption depending on their selectivity. FASEB Journal . 2020;34(2):2344–2358. doi: 10.1096/fj.201902240R. [DOI] [PubMed] [Google Scholar]
- 26.Yin D., Li Z., Ding Z. Y., Liu B. Effect of different frequency of FG-4592 on chronic kidney disease. Nephrology Dialysis Transplantation . 2021;36, article i328(Supplement 1) [Google Scholar]
- 27.Zhang X., Zhang Y., Wang P., et al. Adipocyte hypoxia-inducible factor 2α suppresses atherosclerosis by promoting adipose ceramide catabolism. Cell Metabolism . 2019;30(5):937–951.e5. doi: 10.1016/j.cmet.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Solomon S. D., Uno H., Lewis E. F., et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. The New England Journal of Medicine . 2010;363(12):1146–1155. doi: 10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]
- 29.Matsuura Y., Yamashita A., Iwakiri T., et al. Vascular wall hypoxia promotes arterial thrombus formation via augmentation of vascular thrombogenicity. Thrombosis and Haemostasis . 2015;114(7):158–172. doi: 10.1160/TH14-09-0794. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J., Xu Y., Xie J., Liu J., Zhang R., Yan X. Roxadustat does not affect platelet production, activation, and thrombosis formation. Arteriosclerosis, Thrombosis, and Vascular Biology . 2021;41(10):2523–2537. doi: 10.1161/ATVBAHA.121.316495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J., Wang S., Shi W., et al. Roxadustat prevents Ang II hypertension by targeting angiotensin receptors and eNOS. JCI. Insight . 2021;6(18) doi: 10.1172/jci.insight.133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Packer M. Mutual antagonism of hypoxia-inducible factor isoforms in cardiac, vascular, and renal disorders. JACC: Basic to Translational Science . 2020;5(9):961–968. doi: 10.1016/j.jacbts.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapitsinou P. P., Liu Q., Unger T. L., et al. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood . 2010;116(16):3039–3048. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eleftheriadis T., Pissas G., Liakopoulos V., Stefanidis I. On the increased event rate of urinary tract infection and pneumonia in CKD patients treated with roxadustat for anemia. Journal of the American Society of Nephrology . 2021;32(6):p. 1537. doi: 10.1681/ASN.2021020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the corresponding author.


