Abstract
The exuberant immunoinflammatory response that is associated with Pseudomonas aeruginosa infection is the major source of the morbidity and mortality in cystic fibrosis (CF) patients. Previous studies have established that an exoproduct of P. aeruginosa (exoenzyme S) is a mitogen for human T lymphocytes and activates a larger percentage of T cells than most superantigens, which may contribute to the immunoinflammatory response. An animal model would facilitate studies of the pathophysiologic consequences of this activation. As a first step toward developing an animal model, the murine lymphocyte response to exoenzyme S was examined. When stimulated with exoenzyme S, splenocytes isolated from naive mice entered S phase and proliferated. The optimum response occurred after 2 to 3 days in culture, at 4 × 105 cells per well and 5.0 μg of exoenzyme S per ml. The response was not due to lipopolysaccharide, since Rhodobacter sphaeroides lipid A antagonist did not block the response. Other preparations of exoenzyme S stimulated lymphocyte proliferation, since the response to recombinant exoenzyme S (rHisExo S) cloned from strain 388 was similar to the response to exoenzyme S from strain DG1. There was evidence that genetic variability influenced the response, since A/J, CBA/J, and C57BL/6 mice were high responders and BALB/cJ mice were low responders following stimulation with exoenzyme S. Both splenic T and B lymphocytes entered the cell cycle in response to exoenzyme S. Thus, murine lymphocytes, like human lymphocytes, respond to P. aeruginosa exoenzyme S, which supports the development of a murine model that may facilitate our understanding of the role that exoenzyme S plays in the pathogenesis of P. aeruginosa infections in CF patients.
Cystic fibrosis (CF) is the most common lethal inherited disorder found in the Caucasian population (2). In CF, a chronic respiratory infection causes pulmonary pathology (6) that is the major source of morbidity and mortality (7). Pseudomonas aeruginosa, the most common respiratory pathogen found in CF patients (9), has been hypothesized to play a major role in eliciting damage to the pulmonary tract. Moreover, the host response to P. aeruginosa is a complex immunoinflammatory interaction that confines this aggressive pathogen to the lung but results in tremendous damage to the airways and parenchyma of the lung. Such exuberant immunoinflammatory responses are often the result of neutrophil influx followed by its attendant oxidative and enzymatic release. However, under some circumstances, T lymphocytes can trigger a vigorous immunoinflammatory response when they are responding to microbial mitogens and superantigens (13, 18, 19). We have been studying the ability of a P. aeruginosa exoproduct to activate T cells and potentially contribute to the pathogenesis of CF. Previous studies have established that exoenzyme S stimulates T and B lymphocytes from a large percentage of adults to proliferate (20). Further, exoenzyme S is a novel mitogen for T lymphocytes and activates a larger percentage of T lymphocytes than many superantigens (4). To further study the role of exoenzyme S in the immunoinflammatory response that might contribute to the respiratory pathology seen in CF, the development of an animal model would be of great benefit.
Animal studies have shown that experimental infection with P. aeruginosa results in damage to the lung that is similar to that seen in CF patients (22, 33) and that exoenzyme S, a secreted P. aeruginosa exoproduct, contributes significantly to this pathology (24, 37). Exoenzyme S is an ADP-ribosylating enzyme produced by P. aeruginosa in both a secreted form and membrane-bound form (12). There are a number of different preparations of exoenzyme S, and although there are differences in some of their properties, the 50-kDa exoenzyme S from P. aeruginosa DG1, the 49-kDa recombinant form expressed in PA103 with the pUCP exoS expression vector inserted (16), and the 52-kDa recombinant form (rHisExo S) expressed in Escherichia coli BL21(DE3) (14) all activate human T cells (5). The present studies were performed to determine whether exoenzyme S stimulates murine lymphocyte proliferation.
The mouse is an obvious candidate for the development of an animal model, as the genetics are well understood for inbred strains, immunologic reagents are available, and murine models of CF exist (28, 30). To investigate the ability of murine lymphocytes to proliferate in response to exoenzyme S, splenocytes were stimulated with exoenzyme S under various conditions, and the uptake of [3H]thymidine ([3H]TdR) and lymphocyte cell counts were determined. As exoenzyme S is a purified bacterial exoproduct, the contribution of lipopolysaccharide (LPS) contamination to proliferation was investigated. Lymphocytes were stimulated with exoenzyme S, and the ability of Rhodobacter sphaeroides lipid A antagonist to block the response was assessed. To determine whether different preparations of exoenzyme S were capable of stimulating lymphocytes, the response of purified exoenzyme S from P. aeruginosa DG1 was compared to that of recombinant exoenzyme S cloned from P. aeruginosa 388 and expressed in E. coli BL21(DE3). To determine whether genetic variability between different inbred strains of mice influenced the response, lymphocyte proliferations of A/J, BALB/cJ, C57BL/6J, CBA/J, DBA/2J, C3H/HeJ, and C3H/OuJ mice were compared. Finally, to determine the cell population that proliferates in response to exoenzyme S, cell cycle analysis of T and B cells was performed by cell surface labeling and propidium iodide staining with flow cytometry.
MATERIALS AND METHODS
Mice.
Eight- to 10-week-old male A/J, BALB/cJ, C3H/HeJ, C3H/OuJ, C57BL/6J, CBA/J, and DBA/2J mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Mice used in these experiments were not previously sensitized with P. aeruginosa or P. aeruginosa exoenzyme S. Mice were maintained in a pathogen-free unit and were supplied with food and water ad libitum.
Preparation of P. aeruginosa exoenzyme S.
exoenzyme S was prepared from P. aeruginosa as previously described (36). Briefly, P. aeruginosa DG1 was grown for 36 h at 32°C in S medium. The protein in the culture filtrate was precipitated with ammonium sulfate, dissolved in Tris buffer, and separated by DEAE-Sephacel (Pharmacia, Uppsala, Sweden) column chromatography. The protein was reprecipitated with acetone and then dissolved in Tris buffer and separated by G-100 (Pharmacia) gel filtration column chromatography. Protein-containing eluted fractions were stored at −70°C until they were used. Exoenzyme S purified by this method does not contain ADP-ribosylation activity and migrates as a single, homogeneous band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Endotoxin levels were measured by using a Limulus amoebocyte lysate kit (Associates of Cape Cod, Woods Hole, Mass.) and were found to be less than 0.4 ng of LPS per ml.
Preparation of recombinant exoenzyme S.
rHisExo S was purified from E. coli BL21(DE3) with an inserted pETrHisExoS expression vector (a kind gift from Joseph Barbieri) as previously described (14). Briefly, an overnight culture of E. coli BL21(DE3) carrying the pETrHisExoS vector was diluted 1/30 in L broth containing 100 μg of ampicillin (Aldrich, Milwaukee, Wis.) per ml and shaken at 250 rpm at 30°C. After 2 h, IPTG (isopropyl-β-d-thiogalactopyranoside) (Vector Biosystems, Toronto, Ontario, Canada) was added at a final concentration of 0.5 mM, and the culture was shaken for another 2 h. Phenylmethylsulfonyl fluoride (Sigma, St. Louis, Mo.) was then added to the culture at a final concentration of 1 mM, and the mixture was immediately centrifuged at 6,000 rpm for 8 min in a previously refrigerated GS3 rotor (Beckman). The pellet was resuspended in binding buffer containing a cocktail of protease inhibitors, and cells were broken in a French press as previously described (14). Cellular extracts were centrifuged at 16,000 rpm for 16 min in a previously refrigerated SS34 rotor (Beckman) and then passed through a 0.22-μm-pore-size filter (Millipore, Bedford, Mass.). The filtrate was then subjected to Ni2+ affinity chromatography (2-ml column; Qiagen, Santa Clarita, Calif.). After loading, the column was washed with 20 ml of binding buffer and then with 20 ml of binding buffer containing 50 mM imidazole (Sigma). rHisExo S was eluted in binding buffer containing 0.5 M imidazole, and 2-ml fractions were collected and analyzed for protein content by measurement of the A280.
Splenocyte isolation and proliferation assays.
Splenocytes were obtained by passing freshly isolated spleens through a 60-mesh stainless steel sieve. Erythrocytes were removed by incubating the splenocytes in lysing buffer (0.15 M ammonium chloride, 0.01 M potassium hydrogen carbonate, 0.001 M EDTA) for 5 min at 4°C. The cells were then washed three times in Hanks balanced salt solution by centrifugation (800 × g) for 10 min at 4°C and suspended in culture medium containing RPMI 1640, 5% fetal calf serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 U of penicillin per ml, and 100 μg of streptomycin per ml (all from Gibco BRL, Burlington, Ontario, Canada) and 5 × 10−3 M 2-mercaptoethanol (Sigma). Cells were counted and viability was assessed by trypan blue exclusion.
Between 1 × 104 and 8 × 105 cells per well were incubated at 37°C in 5% CO2 in the presence or absence of stimuli for 8 days in 96-well flat-bottom plastic tissue culture plates (Nunclon; Nunc, Roskilde, Denmark). Stimuli used were exoenzyme S (0.005 to 10 μg/ml), P. aeruginosa serotype Habs 10 LPS (1 μg/ml) (Sigma), Staphylococcus enterotoxin B (1 μg/ml) (Toxin Technologies, Sarasota, Fla.), or concanavalin A (5 μg/ml) (Sigma). For some experiments R. sphaeroides lipid A antagonist (1 to 10 μg/ml) (Sigma) was combined with exoenzyme S or LPS in culture. Sixteen hours before the end of incubation, 1 μCi of [3H]TdR (ICN, Montreal, Quebec, Canada) was added to each well. Cells were harvested onto glass fiber filters, and the number of counts per minute was determined in a liquid scintillation counter. Data are presented as the stimulation index (SI), which is calculated by dividing the stimulated counts per minute by the unstimulated counts per minute.
The number of cells that were present in culture following stimulation was monitored by preincubating the cells in medium (as described above) at 2 × 106 cells per well in 24-well flat-bottom plastic tissue culture plates (Nunclon) for 3 days. The cells were then aspirated from the plates, counted by trypan blue exclusion, and plated for 0 to 6 days in 96-well flat-bottom plates at 2 × 105 cells per well in the presence of exoenzyme S, concanavalin A, or medium alone. After 0 to 6 days, the cells were removed and lymphocytes were counted by using trypan blue. This was done in quadruplicate for each group on each day.
Cell cycle analysis of splenocyte subsets stimulated with P. aeruginosa exoenzyme S.
To determine which splenocyte subset(s) was proliferating in response to stimulation with P. aeruginosa exoenzyme S, propidium iodide incorporation on gated B lymphocytes, or T lymphocytes was assessed. Briefly, unstimulated or stimulated cells were labeled with fluorescein isothiocyanate-conjugated anti-B220 (B cells) (Pharmingen, Mississauga, Ontario, Canada) or anti-Thy 1.2 (T cells) (Sigma). Cells were treated with 75% ethanol at −20°C for 1 h, followed by addition of RNase (1 mg/ml) (Sigma) and propidium iodide (5 μg/ml) (Sigma). Analysis for DNA content was performed by flow cytometric analysis (FACScan; Becton Dickinson) with CellQuest software (Becton Dickinson).
Statistics.
Data are given as the mean SI ± standard error of the mean (SEM). Statistical analysis was performed by using the Fisher least significant difference, when allowed by the F value (analysis of variance; Statview 512+; BrainPower Inc., Calabasas, Calif.). For these tests, a P value of < 0.05 was considered significant.
RESULTS
Murine lymphocytes respond to stimulation with P. aeruginosa exoenzyme S.
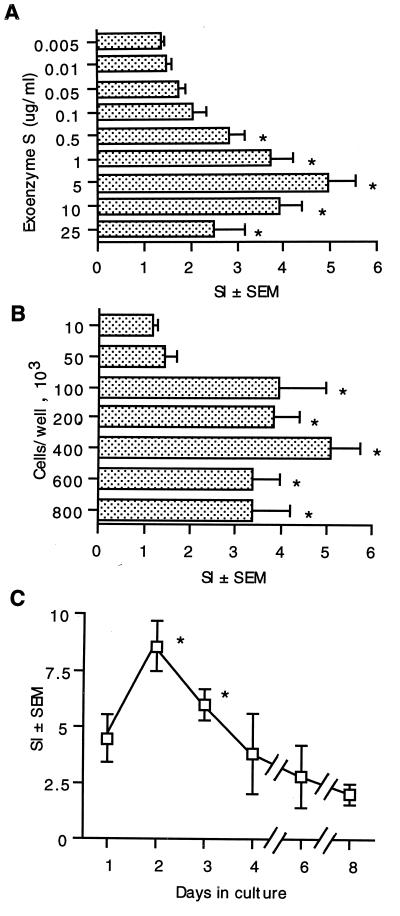
Experiments were performed to determine whether murine lymphocytes respond to stimulation with exoenzyme S and to determine the optimal conditions for lymphocyte proliferation. The response was determined by measuring the uptake of [3H]TdR into splenocytes. The response to 0.005 μg of exoenzyme S per ml was undetectable (SI = 1.41 ± 0.13). The response increased in a dose-dependent fashion, with an optimal concentration of 5 μg/ml (SI = 4.71 ± 0.96) (Fig. 1A). There was no further increase in [3H]TdR incorporation at higher concentrations of exoenzyme S.
FIG. 1.
Conditions required for murine splenocyte proliferation in response to stimulation with exoenzyme S. (A) Splenocytes from C3H/HeJ mice (4 × 105/well) were cultured with 0.005 to 25 μg of exoenzyme S per ml for 3 days. *, P < 0.05 compared to splenocytes stimulated with 0.005 μg of exoenzyme S per ml. [3H]TdR incorporation in unstimulated splenocytes = 2.7 (± 0.4) × 103 cpm; in response to 1 μg of Staphylococcus enterotoxin B per ml, SI = 9.1 ± 1.0. (B) Splenocytes (1 × 104 to 8 × 105/well) from C3H/HeJ mice were cultured with 1 μg of exoenzyme S per ml for 3 days. *, P < 0.05 compared to 10 × 103 splenocytes per well. (C) Splenocytes from C3H/HeJ mice (4 × 105/well) were cultured with 1 μg of exoenzyme S per ml for 1 to 8 days. *, P < 0.05 compared to splenocytes stimulated for 8 days. Values represent the mean SI ± SEM from eight different experiments.
The effect of varying the number of splenocytes was also examined. The response of 104 cells was undetectable (SI = 1.16 ± 0.10). Responses were detected when greater than 1 × 105 cells were present (SI = 3.93 ± 1.05), and the maximum response occurred at 4 × 105 cells (SI = 5.12 ± 1.03), although the difference in the magnitude of [3H]TdR incorporation between 1 × 105 and 8 × 105 cells was modest (Fig. 1B).
The time course of the response of murine lymphocytes to exoenzyme S was also assessed. Cells were stimulated with exoenzyme S, and the [3H]TdR incorporation was measured at days 1, 2, 3, 4, 6, and 8. The response was greatest on day 2, was similar on day 3, and declined on the following days (Fig. 1C).
Murine lymphocytes proliferate in response to stimulation with exoenzyme S.
[3H]TdR incorporation is a measure of DNA synthesis and is often used to indicate lymphocyte proliferation. To ensure that lymphocyte proliferation followed [3H]TdR incorporation, the number of lymphocytes was determined after stimulation. Stimulation of murine splenocytes with exoenzyme S resulted in a significant increase in the number of lymphocytes per well on days 4 and 6 (Table 1). The increase in the number of lymphocytes followed the peak [3H]TdR incorporation by 2 to 3 days and was similar to the increase in cell number in response to the positive control (concanavalin A).
TABLE 1.
Murine splenocytes proliferate in response to stimulation with exoenzyme Sa
| Stimulant | Cells/well (105) on day:
|
|||
|---|---|---|---|---|
| 0 | 2 | 4 | 6 | |
| None | 2.4 ± 0.1 | 2.1 ± 0.2 | 2.2 ± 0.1 | 2.3 ± 0.1 |
| Exoenzyme S | 2.4 ± 0.1 | 2.3 ± 0.1 | 2.9 ± 0.2b | 2.7 ± 0.03b |
| Concanavalin A | 2.6 ± 0.1 | 2.1 ± 0.1 | 2.8 ± 0.1b | 2.8 ± 0.1b |
Cells were stimulated with either exoenzyme S (5 μg/ml) or concanavalin A (5 μg/ml) or left unstimulated. The number of cells per well was determined by trypan blue exclusion. The experiment was repeated twice with similar results. Values are means ± SEMs.
P < 0.05, as calculated by analysis of variance, compared with results for unstimulated cells on the corresponding day.
The proliferative response to exoenzyme S is not due to LPS contamination.
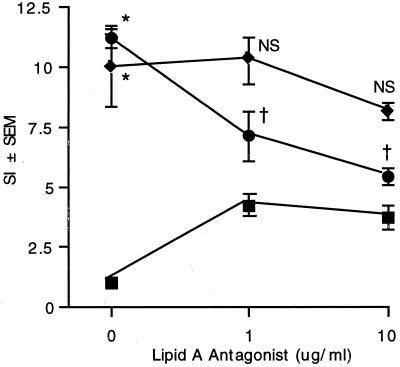
Since exoenzyme S was obtained from gram-negative bacterial culture supernatants, we considered the possibility that splenocyte proliferation was due to contaminating LPS. To determine if the proliferation in response to exoenzyme S was due to contaminating LPS, the effect of LPS was blocked with a lipid A antagonist from R. sphaeroides. R. sphaeroides lipid A did not block the proliferative response to exoenzyme S at either 1 or 10 μg/ml. However, it did reduce the LPS-induced proliferation in a dose-dependent fashion, and at 10 μg/ml proliferation was reduced nearly to the value when lipid A alone was present in culture (Fig. 2). The increase in lymphocyte proliferation with lipid A alone reflects its partial agonist qualities (27). Further, there was no lymphocyte proliferation in response to 0.5 ng of Habs 10 LPS per ml, which was similar to the concentration of contaminating LPS in the preparation of exoenzyme S (SI = 1.2 ± 0.2). Additionally, the proliferation of splenocytes from non-LPS-responsive C3H/HeJ mice was similar to that of splenocytes from genetically related but LPS-responsive C3H/OuJ mice (data not shown). These findings suggest that LPS is not responsible for splenocyte proliferation in response to exoenzyme S.
FIG. 2.
The proliferative response of splenocytes to exoenzyme S is not due to contaminating LPS. Splenocytes (4 × 105/well) from LPS-responsive DBA/2J mice were stimulated with exoenzyme S (⧫) (1 μg/ml) or P. aeruginosa LPS (●) (1 μg/ml) or left unstimulated (■) in the presence of various concentrations of R. sphaeroides lipid A antagonist. Values represent the mean SI of [3H]TdR incorporation ± SEM from five experiments. *, P < 0.05 compared to unstimulated splenocytes. NS, not significant compared to exoenzyme S without lipid A antagonist. †, P < 0.05 compared to P. aeruginosa LPS without lipid A antagonist. [3H]TdR incorporation in unstimulated splenocytes = 3.1 (± 0.5) × 103 cpm.
Different preparations of exoenzyme S.
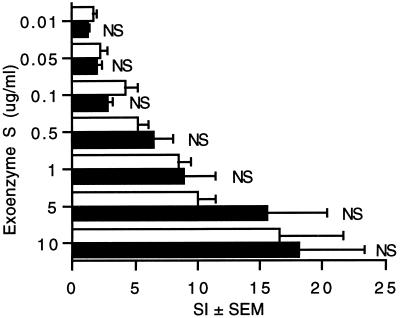
The experiments described above had been performed with exoenzyme S derived from P. aeruginosa DG1. To ensure that exoenzyme S from other strains of P. aeruginosa were also capable of stimulating splenocyte proliferation, the response to exoenzyme S from strain DG1 was compared to that of recombinant exoenzyme S that was cloned from strain 388 and expressed in E. coli BL21(DE3). The responses to the two preparations were similar across a broad range of concentrations (Fig. 3). This suggests that the molecular features that are responsible for lymphocyte proliferation are shared by the two preparations of exoenzyme S.
FIG. 3.
Different preparations of exoenzyme S stimulate splenocytes to proliferate. Splenocytes (4 × 105/well) from DBA/2J mice were cultured for 3 days in the presence of exoenzyme S purified from P. aeruginosa DG1 (□) or rHisExo S expressed in E. coli BL21(DE3) (■). Values represent the mean SI of [3H]TdR incorporation ± SEM from five experiments. NS, not significant compared to the corresponding concentration of exoenzyme S purified from P. aeruginosa DG1.
Different strains of mice vary in their proliferative responses to exoenzyme S.
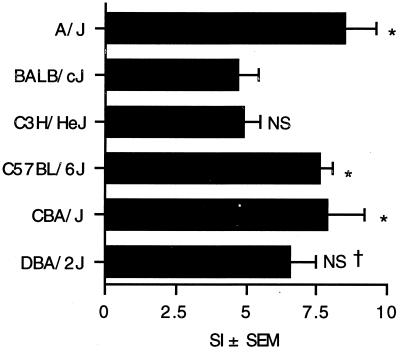
The availability of inbred strains of mice allow the comparison of potential genetic differences in the response to a stimulus. In an attempt to identify genetic differences that might influence the response to P. aeruginosa exoenzyme S, the responses of six widely genetically divergent inbred strains of mice were examined. Splenocytes from A/J, BALB/cJ, C3H/HeJ, C57BL/6J, CBA/J, and DBA/2J mice were stimulated with exoenzyme S, and [3H]TdR incorporation was assessed. There was a statistical difference between the highest responders, i.e., the A/J, C57BL/6J, and CBA/J mice, and the lowest responders, i.e., the BALB/cJ and C3H/HeJ mice (Fig. 4). This suggests that one or more of the genes that account for the diversity between these strains of mice influence the magnitude of the response to exoenzyme S.
FIG. 4.
Splenocyte proliferation in different strains of inbred mice in response to exoenzyme S. Splenocytes (4 × 105/well) from A/J, BALB/cJ, C3H/HeJ, C57BL/6J, CBA/J, or DBA/2J mice were cultured for 3 days in medium alone or with exoenzyme S (5 μg/ml). The bars represent the mean SI of [3H]TdR incorporation ± SEM from four mice. *, P < 0.05 compared to BALB/cJ and C3H/HeJ mice. NS, not significant compared to BALB/cJ mice. †, not significant compared to A/J, C57BL/6J, or CBA/J mice.
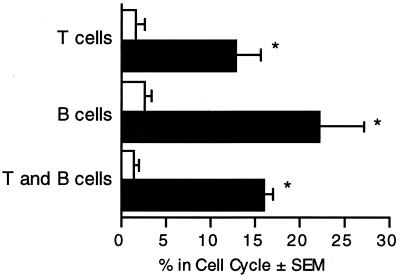
B cells and T cells enter the cell cycle upon stimulation with exoenzyme S.
The murine spleen is composed of several types of lymphocytes, with the most common being B lymphocytes (55%) and T lymphocytes (35%) (21). To identify the subset that was proliferating in response to exoenzyme S, splenocytes from DBA/2J mice were labeled with fluorescein-conjugated antibodies against T- or B-cell-specific markers and stained for DNA content with propidium iodide. Analysis of the DNA content on ungated splenocytes demonstrated that they entered the S or G2/M phase of the cell cycle. Moreover, B lymphocytes and T lymphocytes entered the cell cycle in response to stimulation with exoenzyme S (Fig. 5). The experiment was repeated with C3H/HeJ mice, with similar results (data not shown).
FIG. 5.
B cells and T cells enter the cell cycle in response to stimulation by P. aeruginosa exoenzyme S. Splenocytes (4 × 105/well) were cultured with medium alone (open bars) or 5 μg of exoenzyme S per ml (solid bars) for 3 days. Cells were labeled with either anti-Thy 1.(T cells), anti-B220 (B cells), or no antibody (T and B cells) and analyzed for DNA content by propidium iodide labeling with flow cytometry. The percentage of cells in the cell cycle was determined by CellQuest analysis. The bars represent the mean percentage of cells in the cycle ± SEM from five different experiments. *, P < 0.05 compared to the corresponding unstimulated group.
DISCUSSION
We have made four observations. (i) Murine splenocytes proliferated in response to stimulation with exoenzyme S, and optimal [3H]TdR incorporation occurred after incubation for 2 to 3 days at 2 × 105 to 4 × 105 cells per well in the presence of 5 μg of exoenzyme S per ml. (ii) This response was not due to contaminating LPS, and different preparations of exoenzyme S were capable of stimulating proliferation. (iii) Under these conditions, splenocytes from different strains of mice, including A/J, CBA/J, and C57BL/6J mice, were high responders, while BALB/cJ and C3H/HeJ mice were low responders. (iv) Both T and B cells were found to enter the cell cycle upon stimulation with exoenzyme S.
This study was undertaken to determine the feasibility of developing a murine model to study the in vivo pulmonary immune response to exoenzyme S. Previous studies have demonstrated that exoenzyme S is a major contributor to the virulence of P. aeruginosa in mice. Strains of P. aeruginosa that produce exoenzyme S cause more lung damage in a rat model (24, 34, 35, 37) and have greater dissemination from burn wounds to blood and other tissues in a mouse model (23). However, these studies did not determine the mechanism of tissue injury. It is possible that exoenzyme S is directly toxic to the epithelium, as suggested by studies of epithelial injury by exoenzyme S (8, 15). Additionally, the present studies also support the possibility that exoenzyme S-induced activation of T cells may contribute to the pathology of chronic Pseudomonas infections, as suggested by our previous work.
When murine splenocytes responded to the purified P. aeruginosa exoenzyme S, we considered the possibility that the response could actually be a result of contaminating LPS. Murine lymphocytes are highly responsive to LPS compared to human lymphocytes, and therefore experiments were performed to determine whether the lymphocyte proliferation was due to contaminating LPS. There are a number of pieces of evidence indicating that the response was not due to contaminating LPS. C3H/HeJ mice are unresponsive to LPS (25), but splenocytes from these mice proliferated in response to exoenzyme S. The concentration of P. aeruginosa LPS that was present in the preparation of exoenzyme S did not induce significant splenocyte proliferation in DBA/2 mice. The response of non-LPS-responsive C3H/HeJ splenocytes was similar to the response of LPS-responsive C3H/OuJ splenocytes. Finally, R. sphaeroides lipid A, which antagonizes the response to LPS, was unable to block the response to exoenzyme S despite its ability to block the response to LPS. These results indicate that the response to exoenzyme S was not due to contaminating LPS.
Studies of the time course of lymphocyte proliferation demonstrated that the peak of [3H]TdR incorporation occurred after 2 to 3 days in culture. The kinetics of this response are typical for mitogenic lectins such as concanavalin A and phytohemagglutinin, which usually peak on day 2 to 4, in contrast to recall antigens, which frequently peak after 7 days of culture. The increase in the number of lymphocytes peaked 2 days later, which is also typical of lymphocyte proliferation assays in vitro and is presumably because [3H]TdR incorporation indicates that cells have entered S phase, which is followed by an increase in the number of cells. The proliferative response in mice occurs earlier and is of greater magnitude than the response in humans. This suggests that the response in mice is even more vigorous than the response in humans, which may assist in studies attempting to model the human disease.
There has been debate about the similarities and differences of particular preparations of exoenzyme S. There are differences in the biochemistry of exoenzyme S preparations from strain 388 and strain DG1 and some differences in the biological activities elicited by these different preparations (for a review, see reference 9a). Despite these differences, we have determined that human T cells are activated in response to exoenzyme S purified from P. aeruginosa DG1 by the method of Woods and Que (36) or in response to recombinant exoenzyme S purified by the method of Kulich et al. (16) from strain PA103 (rExo S) or by the method of Knight et al. (14) from E. coli BL21(DE3) as a histidine-tagged fusion protein (rHisExo S) (5). The present studies extend these observations and demonstrate that the properties responsible for murine splenocyte proliferation are shared by exoenzyme S from strain DG1 and recombinant exoenzyme S expressed in E. coli BL21(DE3) (rHisExo S).
The genetics of the mouse are well understood and have been studied in great detail. Murine models of infectious disease have allowed us to study genetic traits that confer susceptibility or resistance to many pathogens, including Leishmania, Plasmodium, Trichinella, Mycobacterium, and Pseudomonas (1, 3, 17, 22, 26, 32). One strain of mice may be resistant to one infection but susceptible to another. For example, C57BL/6 mice are resistant to Leishmania and susceptible to Mycobacterium, while the converse is true for BALB/c mice (1, 11, 29). These inbred strains of mice have allowed us to map the phenotype to host defense genes. We had previously observed considerable variability in the magnitude of the human T-cell proliferation in response to exoenzyme S, which raised the possibility that genetic traits influenced the magnitude of the response. Previous studies utilizing P. aeruginosa-impregnated agar beads had demonstrated that BALB/c mice were resistant to infection, while A/J, C57BL/6, and DBA/2 mice were susceptible (10, 31). This correlates with our observation that A/J and C57BL/6 mice are high responders, DBA/2 mice are intermediate responders, and BALB/c mice are low responders, and it supports our hypothesis that lymphocyte proliferation in response to exoenzyme S causes a mitogenic lymphocyte response that impairs the host defense to P. aeruginosa. Further studies will be required to determine whether the same genes are responsible for the magnitude of the lymphocyte response and the impaired clearance of P. aeruginosa (22).
Our previous observations have demonstrated that human T cells and B cells proliferate in response to P. aeruginosa exoenzyme S and that the B-cell response is T-cell dependent (20). The present experiments demonstrate that both murine T cells and B cells proliferate. Our previous observations with humans had demonstrated that the T-cell response was somewhat greater than the B-cell response, while the converse was true in the murine experiments. There are a number of potential explanations for this. The murine spleen contains a larger fraction of B lymphocytes (approximately 55%) than T lymphocytes (35%) (21), while the converse is true for human peripheral blood. Additionally, although experiments with the murine and human systems both utilized measures of entry into S phase, the assays were different, which may alter the magnitude of the response.
The present studies demonstrate that murine lymphocytes proliferate in response to P. aeruginosa exoenzyme S and support the development of a murine model to study the lymphocyte response to exoenzyme S. Similar to the response of humans, a vigorous response occurs in naive mice, suggesting that exoenzyme S is a mitogen for mice, as it is for humans (4). The response in mice is optimal at concentrations of exoenzyme S similar to those for humans, and the number of lymphocytes that are required for an optimal response is similar. However, investigations in a murine model must take into account two differences between the responses of mice and humans. First, the response in mice is even more vigorous than the response in humans, in that it occurs 4 days earlier. Second, while T cells contribute substantially to the response, the response of B cells in mice is even more vigorous than it is in humans. Provided that these differences are taken into account, the murine model will be a valuable addition to our armamentarium to study the pathogenesis of P. aeruginosa infections.
ACKNOWLEDGMENTS
This work was supported by grants from the Alberta Lung Association and the Canadian Cystic Fibrosis Foundation. C.H.M. is a Scholar of the Alberta Heritage Foundation for Medical Research.
We thank Laurie Robertson for assistance with flow cytometry.
REFERENCES
- 1.Bach M, Hoffenbach A. Strain-dependent protective effect of adult thymectomy on murine infection by Mycobacterium lepraemurium. Clin Exp Immunol. 1987;68:521–527. [PMC free article] [PubMed] [Google Scholar]
- 2.Boat T F, Welsh M J, Beaudet A L. Cystic fibrosis. In: Scriver C R, Beadet A L, Sly W S, Valle D, editors. The metabolic basis of inherited disease. New York, N.Y: McGraw-Hill; 1989. pp. 2649–2680. [Google Scholar]
- 3.Boom W H, Liebster L, Abbas A K, Titus R G. Patterns of cytokine secretion in murine leishmaniasis: correlation with disease progression or resolution. Infect Immun. 1990;58:3863–3870. doi: 10.1128/iai.58.12.3863-3870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruno T F, Syme R M, Buser D E, Woods D E, Mody C H. Pseudomonas aeruginosa exoenzyme S is a mitogen, but not a superantigen for adult T lymphocytes. Infect Immun. 1998;66:3072–3079. doi: 10.1128/iai.66.7.3072-3079.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno T F, Woods D E, Mody C H. Recombinant Pseudomonas exoenzyme S and exoenzyme S from Pseudomonas aeruginosa strain DG1 stimulate T lymphocytes to proliferate. Can J Microbiol. 1999;45:1–5. .. [PubMed] [Google Scholar]
- 6.Chartrand S A, Marks M I. Pulmonary infections in cystic fibrosis: pathogenesis and therapy. In: Pennington J E, editor. Respiratory infections: diagnosis and management. New York, N.Y: Raven Press; 1983. pp. 201–214. [Google Scholar]
- 7.Fiel S B. Clinical management of pulmonary disease in cystic fibrosis. Lancet. 1993;341:1070–1074. doi: 10.1016/0140-6736(93)92423-q. [DOI] [PubMed] [Google Scholar]
- 8.Frithz-Lindsten E, Du Y, Rosqvist R, Forsberg A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via a type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol Microbiol. 1997;25:1125–1139. doi: 10.1046/j.1365-2958.1997.5411905.x. [DOI] [PubMed] [Google Scholar]
- 9.Gilligan P H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991;4:35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Goranson J, Frank D W. Genetic analysis of exoenzyme S by Pseudomonas aeruginosa. FEMS Microbiol Lett. 1996;135:149–155. doi: 10.1111/j.1574-6968.1996.tb07981.x. [DOI] [PubMed] [Google Scholar]
- 10.Gosselin D, DeSanctis J, Boule M, Skamene E, Matouk C, Radzioch D. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995;63:3272–3278. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinzel F P, Sadick M D, Holaday B J, Coffman R L, Locksley R M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iglewski B H, Sadoff J C, Bjorn M J, Maxwell E S. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc Natl Acad Sci USA. 1978;75:3211–3215. doi: 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jupin C, Anderson S, Damais C, Alouf J E, Parant M. Toxic shock syndrome toxin 1 as an inducer of human tumor necrosis factors and g-interferon. J Exp Med. 1988;167:752–761. doi: 10.1084/jem.167.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight D A, Finck-Babancon V, Kulich S M, Barbieri J T. Functional domains of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1995;63:3182–3186. doi: 10.1128/iai.63.8.3182-3186.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kudoh I, Wiener-Kronish J P, Hashimoto S, Pittet J, Frank D W. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar epithelial injury. Am J Physiol. 1994;267:L551–L556. doi: 10.1152/ajplung.1994.267.5.L551. [DOI] [PubMed] [Google Scholar]
- 16.Kulich S, Frank D W, Barbieri J T. Expression of recombinant exoenzyme S of Pseudomonas aeruginosa. Infect Immun. 1995;63:1–8. doi: 10.1128/iai.63.1.1-8.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Locksley R M, Heinzel F P, Sadick M D, Holaday B J, Gardner K D. Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann Inst Pasteur/Immunol. 1987;138:744–749. doi: 10.1016/s0769-2625(87)80030-2. [DOI] [PubMed] [Google Scholar]
- 18.Marrack P, Blackman M, Kushnir E, Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990;171:455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer P H, Wagner H. T cell mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mody C H, Buser D E, Syme R M, Woods D E. Pseudomonas aeruginosa exoenzyme S induces proliferation of human T lymphocytes. Infect Immun. 1995;63:1800–1805. doi: 10.1128/iai.63.5.1800-1805.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore J. A simplified method for the coordinate examination of apoptosis and the surface phenotype of murine lymphocytes. J Immunol Methods. 1995;188:219–228. doi: 10.1016/0022-1759(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 22.Morissette C, Skamene E, Gervais F. Endobronchial inflammation following Pseudomonas aeruginosa infection in resistant and susceptible strains of mice. Infect Immun. 1995;63:1718–1724. doi: 10.1128/iai.63.5.1718-1724.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicas T I, Bradley J, Lochner J E, Iglewski B H. The role of exoenzyme S in infections with Pseudomonas aeruginosa. J Infect Dis. 1985;152:716–721. doi: 10.1093/infdis/152.4.716. [DOI] [PubMed] [Google Scholar]
- 24.Nicas T I, Frank D W, Stenzel P, Lile J D, Iglewski B H. Role of exoenzyme S in chronic Pseudomonas aeruginosa lung infections. Eur J Clin Microbiol. 1985;4:175–179. doi: 10.1007/BF02013593. [DOI] [PubMed] [Google Scholar]
- 25.Pier G B, Markham R B, Eardley D. Correlation of the biologic responses of C3H/HeJ mice to endotoxin with the chemical and structural properties of lipopolysaccharides from Pseudomonas aeruginosa and Escherichia coli. J Immunol. 1981;127:184–191. [PubMed] [Google Scholar]
- 26.Pond L, Wassom D L, Hayes C E. Influence of resistant and susceptible genotype, IL-1, and lymphoid organ on Trichinella spiralis-induced cytokine secretion. J Immunol. 1992;149:957–965. [PubMed] [Google Scholar]
- 27.Rose J R, Christ W J, Bristol J R, Kawata T, Rossignol D P. Agonistic and antagonistic activities of bacterially derived Rhodobacter sphaeroides lipid A: comparison with activities of synthetic material of the proposed structure and analogs. Infect Immun. 1995;63:833–839. doi: 10.1128/iai.63.3.833-839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozmahel R, Wilschanski M, Matin A, Plyte S, Oliver M, Auerbach W, Moore A, Forstner J, Durie P, Nadeau J, Bear C, Tsui L C. Modulation of disease severity in cystic fibrosis transmembrane conductance regulator deficient mice by a secondary genetic factor. Nat Genet. 1996;12:280–287. doi: 10.1038/ng0396-280. [DOI] [PubMed] [Google Scholar]
- 29.Sadick M D, Locksley R M, Tubbs C, Raff H V. Murine cutaneous leishmaniasis: resistance correlates with the capacity to generate interferon-gamma in response to Leishmania antigens in vitro. J Immunol. 1986;136:655–661. [PubMed] [Google Scholar]
- 30.Snouwaert J N, Brigman K K, Latour A M, Malouf N N, Boucher R C, Smithies O, Koller B H. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson M M, Kondratieva T K, Apt A S, Tam M F, Skamene E. In vitro and in vivo T cell responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clin Exp Immunol. 1995;99:98–105. doi: 10.1111/j.1365-2249.1995.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor-Robinson A W, Phillips R S, Severn A, Moncada S, Liew F Y. The role of TH1 and TH2 cells in rodent malaria infection. Science. 1993;260:1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 33.Wiener-Kronish J P, Sakuma T, Kudoh I, Pittet J F, Frank D, Dobbs L, Vasil M L, Matthay M A. Alveolar epithelial injury and pleural empyema in acute Pseudomonas aeruginosa pneumonia in anesthetized rabbits. J Appl Physiol. 1993;75:1661–1669. doi: 10.1152/jappl.1993.75.4.1661. [DOI] [PubMed] [Google Scholar]
- 34.Woods D E, Hwang W S, Shahrabadi M S, Que J U. Alteration of pulmonary structure by Pseudomonas aeruginosa exoenzyme S. J Med Microbiol. 1988;26:133–141. doi: 10.1099/00222615-26-2-133. [DOI] [PubMed] [Google Scholar]
- 35.Woods D E, Lam J S, Paranchych W, Speert D P, Campbell M, Godfrey A J. Correlation of Pseudomonas aeruginosa virulence factors from clinical and environmental isolates with pathogenicity in the neutropenic mouse. Can J Microbiol. 1997;43:541–551. doi: 10.1139/m97-077. [DOI] [PubMed] [Google Scholar]
- 36.Woods D E, Que J U. Purification of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1987;55:579–586. doi: 10.1128/iai.55.3.579-586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods D E, Sokol P A. Use of transposon mutants to assess the role of exoenzyme S in chronic pulmonary disease due to Pseudomonas aeruginosa. Eur J Clin Microbiol. 1985;4:163–169. doi: 10.1007/BF02013591. [DOI] [PubMed] [Google Scholar]