Background:
Meta-analysis was used to evaluate the efficacy of Fufang Biejia Ruangan Tablets in the treatment of chronic hepatitis B (CHB) liver fibrosis.
Methods:
Databases, including PubMed, China Knowledge Network (CNKI), China Biomedical Database (CBM), Wan Fang, VIP database, Embase, and Cochrane Library were searched. The time was searched up to May 2022. The participant intervention comparator outcomes of this study were as follows: P, patients with CHB liver fibrosis; I, Fufang Biejia Ruangan Tablets; C, pharmacological placebo; O, the efficacy rate, alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB), procollagen III protein (PIIIP), hyaluronic acid (HA), laminin (LN), collagen C type IV (IV-C), portal vein diameter, spleen thickness and HBV‐DNA negative conversion rate. The Cochrane Risk of Bias tool, Begg’s test and Egger’s test were used to evaluate the methodological quality of eligible studies. A randomized controlled trial of Fufang Biejia Ruangan Tablets was used to treat CHB liver fibrosis. Three reviewers independently selected trials, extracted data, cross-checked, and performed methodological quality assessments. Data analysis was completed by Review Manager 5.3.
Results:
Twenty-six studies with 2717 patients were included in the meta‐analysis. The meta-analysis showed that Fufang Biejia Ruangan Tablets was effective by increasing the efficacy. Fufang Biejia Ruangan Tablets was more efficient in improving ALT, AST, TBIL, ALB, PIIIP, HA, LN, IV-C, portal vein diameter, spleen thickness, and HBV‐DNA negative conversion rate with no serious adverse reactions.
Conclusion:
It was shown that Fufang Biejia Ruangan Tablets can effectively improve liver function and relieve liver fibrosis, but future research should focus on rigorously designed, multicenter, and large randomized controlled trials.
Keywords: chronic hepatitis B liver fibrosis, Fufang Biejia Ruangan Tablets, meta‐analysis, randomized controlled trials
1. Introduction
Hepatitis, liver cirrhosis, and liver cancer caused by chronic hepatitis B virus (HBV) infection are a trilogy that threatens human life and health, and brings severe disease burden.[1] There are about 250 million people infected by chronic hepatitis B (CHB) virus (HBV) in the world, of which about 600,000 die each year from HBV-induced cirrhosis and liver cancer. Among them, liver fibrosis is the pathological basis of all chronic liver diseases.[2] It is the necessary phase for the development of CHB as liver cirrhosis or even evolved into liver cancer. CHB liver fibrosis refers to the chronic hypersensitivity of CHB virus, excessive proliferation and abnormal deposition of extracellular matrix (ESC) components in the liver, resulting in pathological changes in structural and functional abnormalities of the liver, structurally manifesting as hepatic sinusoidal capillarization Fibrosis with intrahepatic lobules and portal areas.[3] Due to the reversibility of liver fibrosis, how to suppress or even reverse hepatic fibrosis has become a hot spot in the current study.
At present, the core treatment for liver fibrosis is antiviral therapy. Nucleoside drugs such as adefovir dipivoxil and entecavir can effectively inhibit HBV-DNA replication and relieve liver inflammation. However, due to the existence of a large base of existing HBV infection, nucleoside drugs can effectively inhibit the replication of HBV virus, thereby reducing liver inflammation and preventing the formation of fibrosis, but the effect of existing liver fibrosis is not ideal.[4] Therefore, the search for more effective anti-fibrosis treatment has always been the focus of current research.
Fufang Biejia Ruangan Tablets is a traditional Chinese medicine, which is developed based on the traditional Chinese medicine theory, used on anti-hepatic fibrosis. It is made by the Carapax Trionycis,Rhizoma Atractylodis, Radix Padoniae Rubra, Angelica sinensis, Radix Ginseng, Codonopsis pilosula, Astragalus membranaceus, Placenta Hominis, Ophiocordyceps sinensis, Radix Isatidis, Forsythia suspensa and other 11 Chinese herbs. Modern pharmacological studies have shown that the drug can effectively block hepatic fibrosis in the early stage, and at the same time significantly inhibit the proliferation of fat-storing cells, prevent excessive deposition of collagen in the blood vessel lumen, and reduce collagen synthesis.[5] For the Liver fibrosis that has been formed, Fufang Biejia Ruangan Tablets can play a certain role in dissolution and absorption, and can have a good inhibitory effect on the expression of liver fibrosis α2 (I) mRNA.[6] Due to the low quality of evidence-based medical research on the safety and efficacy of Fufang Biejia Ruangan Tablets for the treatment of CHB liver fibrosis, and the lack of a comprehensive meta-analysis, this study collected comprehensive randomized clinical trials to test the effectiveness and safety of Fufang Biejia Ruangan Tablets as adjuvant drugs for the treatment of CHB liver fibrosis and to provide evidence and references for clinicians to rationally use drugs.
2. Methods
The study has been registered on Prospective Systems Evaluation Registration Site (PROSPERO) with the registration number of CRD42018100007.
2.1. Inclusion criteria
The following criteria were included in this study:
Unlimited language;
Patients were included in accordance with a clear diagnostic criteria, and confirmed by the regular hospital for CHB liver fibrosis;
Age and gender of patients in this study were not limited;
All included studies were randomized controlled trials (RCTs) and quasi-RCTs;
The control group used conventional western medicine (entecavir, adefovir dipivoxil), and the experimental group used Fufang Biejia Ruangan Tablets on the basis of the control group.
2.2. Exclusion criteria
The exclusion criteria for this study were as follows:
Non-clinical trials such as review, animal experiments;
Repeat literature;
The experimental group or control group also combined other medicines during the treatment process;
Patients with history of drug allergy and severe liver and kidney dysfunction were excluded.
2.3. Research strategy and data extraction
By searching PubMed, China Knowledge Network (CNKI), China Biomedical Database (CBM), Wan Fang, VIP database, Embase, and Cochrane Library, with “Fufang Biejia Ruangan Tablets” “CHB Fibrosis” “CHB” “Liver Fibrosis” as search keywords. The search time is from the date of establishment of database to May 2022. The data extraction and quality assessment were independently completed by 3 researchers (Xiangbo Meng, Zhengqi Pan and Quansheng Feng) and resolved through discussion if there were objections. The information extracted by the 3 researchers included: author, time of publication, number of groups, interventions, quality of RCTs, duration of treatment, and outcome measures. The 3 researchers also searched the reference list and related reviews for more articles.
2.4. Statistical analysis
This meta-analysis was based on Cochrane RevMan 5.3 software. For the dichotomy, we calculated the odds ratio (OR). For the continuous variable, we calculated the mean difference (MD). The chi-square test and I2 test were used for consistency assessment. If P ≥ .1 and I2 ≤ 50%, heterogeneity is acceptable, and a fixed effect model was used for data analysis. If P < .1 and I2 > 50%, it indicated that there is significant heterogeneity, and the random effect model was used for data analysis. Finally, the study used the funnel plot, Begg’s and Egger’s test to explore whether the results of the study were affected by publication bias.
2.5. Retrieval study
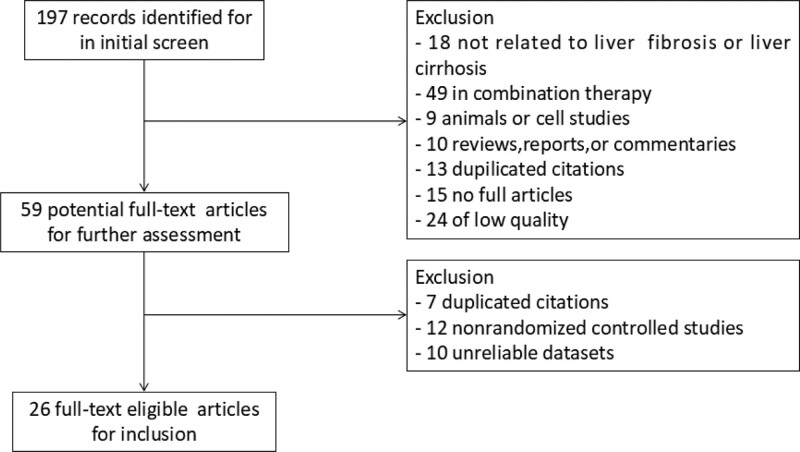
The 2 researchers initially searched for 197 related documents. After evaluating the studies, we selected inclusion criteria and excluded duplicate content, introduction of personal experience, reviews, non-RCTs, and animal experiments. There were 59 articles that met the inclusion criteria. After reading the full text, 26 clinical RCTs were included, as shown in Figure 1.
Figure 1.
Flow diagram of the literature selection for this study.
2.6. Inclusion study
This meta-analysis finally included 26 articles,[7–32] all being RCTS or quasi-RCTS. A total of 2717 patients participated in this study. All trials were parallel design trials conducted in China. All patients included in the literature were diagnosed with CHB fibrosis according to the definite diagnostic criteria. For the studies included in this study, all the control groups were used conventional treatments such as glutathione, polyphosphorylcholine, antiviral drugs (adefovir dipivoxil or entecavir), and other modern medicines. All the experimental groups were on the basis of the control group combined with Fufang Biejia Ruangan Tablets (Table 1).
Table 1.
Characteristics of included studies.
| Included study (yr) | Cases (E/C) | Intervening measure (E/C) | FFBJ dosage | Duration (mo) | Outcome measures |
|---|---|---|---|---|---|
| Wang 2015 | 68/68 | CT + FFBJ/CT | 2 g/d | 12 | ALT,ALB,TBIL,AST,ALP,GGT,PA,DPV,spleen thickness,efficacy rate |
| Wang 2014 | 112/108 | CT + FFBJ/CT | 2 g/d | 6 | HA,PIIIP,IV-C,LN |
| Li 2013 | 40/40 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C, LN,efficacy rate |
| Fan 2014 | 34/33 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C, LN,ALT,AST,TBIL |
| Fang 2013 | 50/50 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C, LN,ALT,AST,TBIL,efficacy rate |
| Qiu 2009 | 30/26 | CT + FFBJ/CT | 2 g/d | 6 | HBV-DNA,ALT,AST,TBIL |
| Meng 2009 | 72/70 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C, LN,HBV-DNA |
| Chai 2009 | 24/24 | CT + FFBJ/CT | 2 g/d | 12 | HA, PIIIP, IV-C, LN,SB |
| Wang 2010 | 49/49 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,HBV-DNA |
| Gong 2010 | 35/30 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,LN,DPV,spleen thickness |
| Lu 2010 | 42/40 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C, LN,GGT,HBV-DNA |
| Lv 2010 | 33/32 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C, LN,HBV-DNA,ALT |
| Hong 2011 | 62/48 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C, LN,ALT,AST,TBIL,HBV-DNA,SPV,DPV,spleen thickness,efficacy rate |
| Zhang 2016 | 71/63 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,LN |
| Zhang 2014 | 57/55 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,LN,DPV,spleen thickness |
| Zhang 2017 | 32/32 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,ALT,AST |
| Zhang 2015 | 100/100 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,LN |
| Wu 2017 | 45/47 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,LN,ALT,AST,TBIL,HBV-DNA |
| Wang 2017 | 48/48 | CT + FFBJ/CT | 2 g/d | 12 | TBIL,ALT,AST,ALB/GLB |
| Zhang 2012 | 43/43 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,efficacy rate |
| Xiao 2013 | 54/54 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,LN,ALT,AST |
| Wang 2011 | 46/50 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,LN,ALT,AST,HBV-DNA,ALB/GLB,efficacy rate |
| Sun 2017 | 38/38 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,LN,efficacy rate,HBV-DNA |
| Li 2017 | 108/108 | CT + FFBJ/CT | 2 g/d | 12 | ALT,AST,HBV-DNA,TBIL |
| Chen 2017 | 36/36 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,LN,efficacy rate |
| Shen 2010 | 46/50 | CT + FFBJ/CT | 2 g/d | 12 | HA,PIIIP,IV-C,LN,ALT,AST,HBV-DNA |
ALB = albumin, ALT = alanine transaminase, AST = aspartate aminotransferase, IV‐C = collagen C type IV, DPV = diameter of portal vein, E/C = experimental group/control group, FFBJ = Fufang Biejia Ruangan Tablets, HBV = hepatitis B virus, HA = hyaluronic acid, LN = laminin, PIIIP = procollagen III protein, TBIL = total bilirubin.
2.7. Quality of study
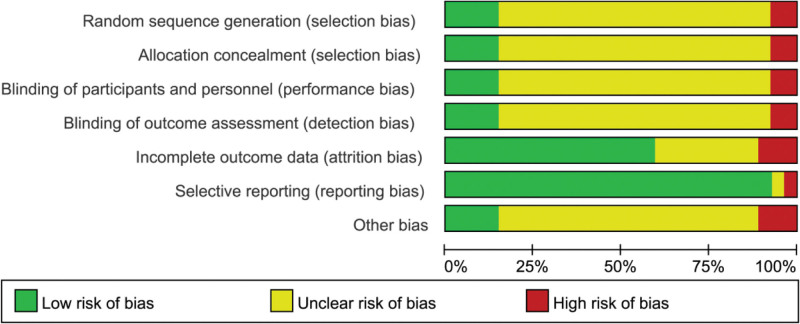
All 26 RCTs[7–32] included in this study referred to the use of randomization, of which 3 studies were randomized using a random number table method, 1 was grouped by admission order, and the remaining 22 studies did not detail specific randomization methods. All studies did not mention whether allocation concealment and blinding were used in these studies (Fig. 2).
Figure 2.
Methodological quality assessment of the risk of bias for each included study.
3. Results
3.1. Efficient
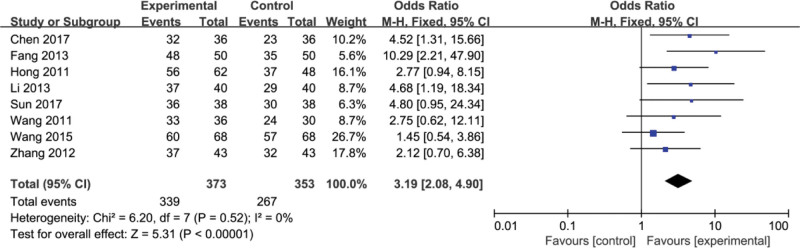
Eight articles included[8,9,11,19,20,22,28,30] in the literature reported the effectiveness. Heterogeneity tests showed that there was no more heterogeneity in these 8 studies (P = .52, I2 = 0%), and a fixed effect model was used for the combined analysis of the data. By comparing the results, there was a significant difference between the efficiency of experimental group and the efficiency of control group (OR = 3.19, 95%CI, [2.08, 4.90] P < .00001) (Fig. 3).
Figure 3.
The efficacy rate of Fufang Biejia Ruangan Tablets plus conventional treatment versus conventional treatment.
3.2. Hyaluronic acid (HA)
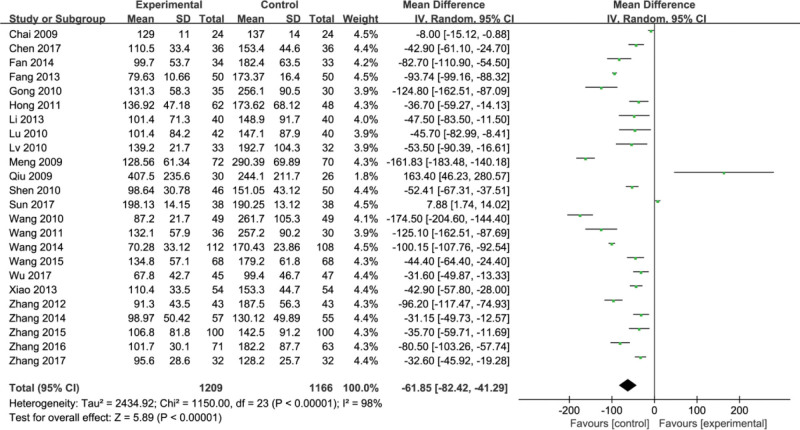
Twenty-four studies of RCTs[7–30] reported hyaluronic acid (HA). There was significant statistical heterogeneity among the studies (P ≤ .00001, I2 = 98%). A random effect model was used. Meta-analysis showed that compared with conventional therapy, the combination of conventional therapy and Fufang Biejia Ruangan Tablets can significantly improve the levels of HA index (MD = −61.85, 95% CI, [−82.42, −41.29] P < .00001) (Fig. 4).
Figure 4.
Forest plots of hyaluronic acid (HA) after treatment.
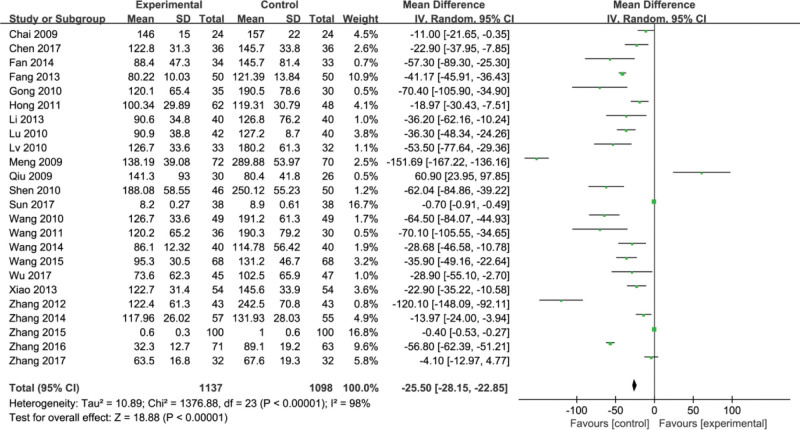
3.3. Procollagen III protein (PIIIP)
Twenty-four studies[7–30] reported procollagen III protein (PIIIP) with heterogeneity (P < .00001, I2 = 98%) and used a random effects model for meta-analysis. The results showed that compared with the control group, the treatment group could improve the PIIIP index (MD = −25.50, 95% CI, [−28.15, −22.85] P < .00001) (Fig. 5).
Figure 5.
Forest plots of procollagen III protein (PIIIP) after treatment.
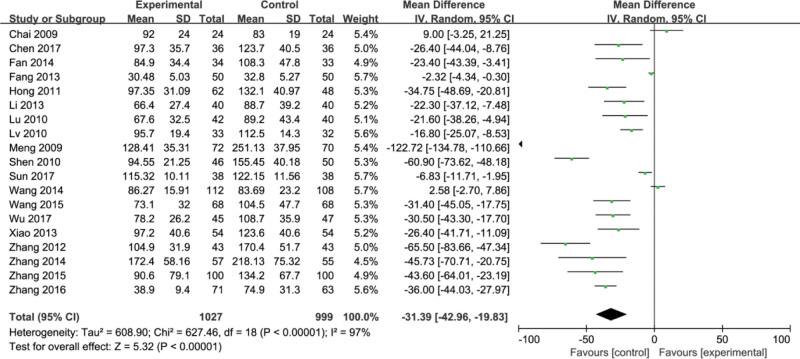
3.4. Collagen C type IV (IV-C)
Nineteen studies[7-11,13,14,17-24,26,27,29,30] reported collagen C type IV (IV-C). The 19 studies had heterogeneity between each other (P < .00001, I2 = 97%). Using the random effects model, meta-analysis showed that compared with the conventional group, the conventional group plus Fufang Biejia Ruangan Tablets can significantly improve IV-C index (MD = −31.39, 95%CI, [−42.96, −19.83] P < .00001) (Fig. 6).
Figure 6.
Forest plots of collagen C type IV (IV-C) after treatment.
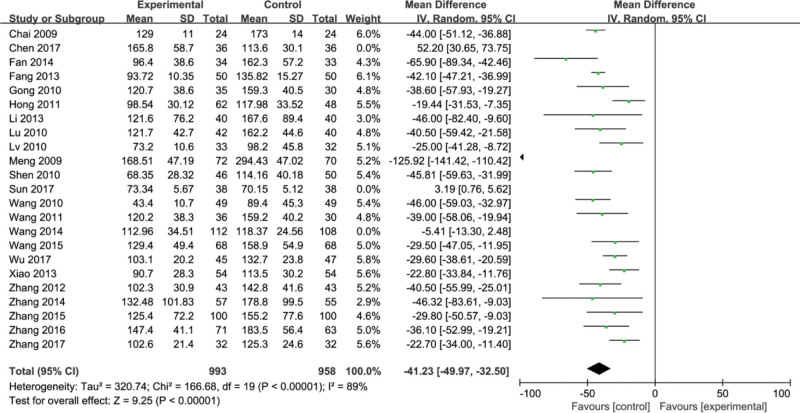
3.5. Laminin (LN)
Twenty-three studies[7–11,13–30] reported laminin (LN) of 1951 patients. Heterogeneity tests revealed heterogeneity in these 20 studies (P < .00001, I2 = 89%). Random effect models were used for data analysis. After comparison, it was found that the improvement effect of the experimental group on the LN index was significantly different from that of the control group (MD = −41.23, 95%CI, [−49.97, −32.50] P < .00001) (Fig. 7).
Figure 7.
Meta-analysis of the indices of liver fibrosis laminin (LN).
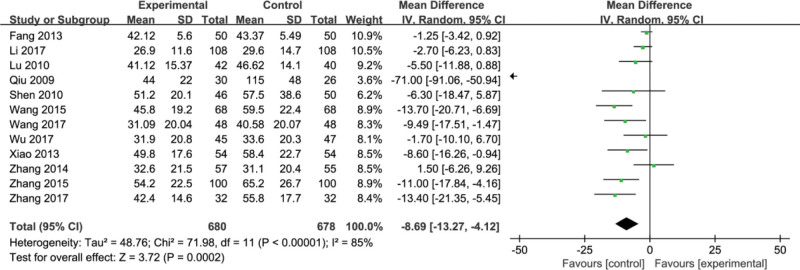
3.6. Alanine transaminase (ALT)
Twelve studies[8,11,12,17,21,24–27,29,31,32] reported alanine transaminase (ALT). Forest maps showed heterogeneity among the 12 studies (P < .00001, I2 = 85%). Random effect models were used to analyze the data. The results showed that there was a significant difference in the index of ALT between the conventional therapy and the combination of conventional therapy and Fufang Biejia Ruangan Tablets (MD = −8.69, 95% CI, [−13.27, −4.12] P < .00001) (Fig. 8).
Figure 8.
Meta-analysis of the indices of liver fibrosis alanine transaminase sALT).
3.7. Aspartate aminotransferase (AST)
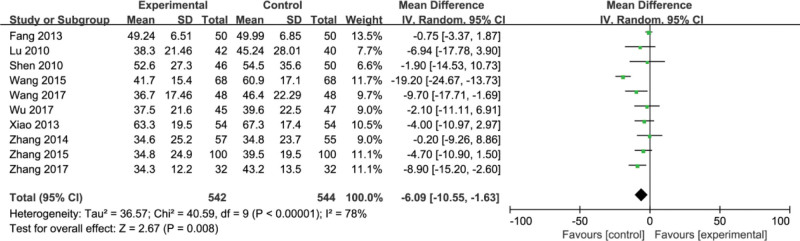
Ten studies[8,11,17,21,24–27,29,31] reported aspartate aminotransferase (AST). These 10 studies were heterogeneous (P < .00001, I2 = 78%). Random effects model was used for data combination analysis. The results showed that compared with the control group, the treatment group can significantly improve the AST index (MD = −6.09, 95%CI, [−10.55, −1.63] P = .0001) (Fig. 9).
Figure 9.
Forest plots of aspartate aminotransferase (AST) after treatment.
3.8. Albumin (ALB)
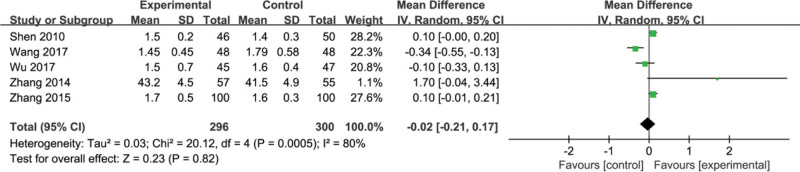
Five studies[21,24,26,27,31] reported albumin (ALB). Heterogeneity tests revealed heterogeneity among the 5 studies (P = .0005, I2 = 80%). Random effect models were used for data analysis. By comparison, we found that there is no significant difference between the experimental group and the control group in improving the ALB index (MD = −0.02, 95% CI, [−0.21, 0.17] P = .82) (Fig. 10).
Figure 10.
Forest plots of albumin (ALB) after treatment.
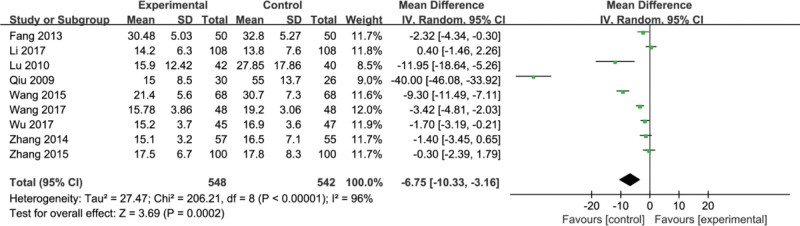
3.9. Total bilirubin (TBIL)
Five studies [21,24,26,27,31] reported ALB. Heterogeneity tests revealed heterogeneity among the 5 studies (P = .0005, I2 = 80%). Random effect models were used for data analysis. By comparison, we found that there is no significant difference between the experimental group and the control group in improving the ALB index (MD = −0.02, 95% CI, [−0.21, 0.17] P = .82) (Fig. 11).
Figure 11.
Forest plots of total bilirubin (TBIL) after treatment.
3.10. Spleen thickness
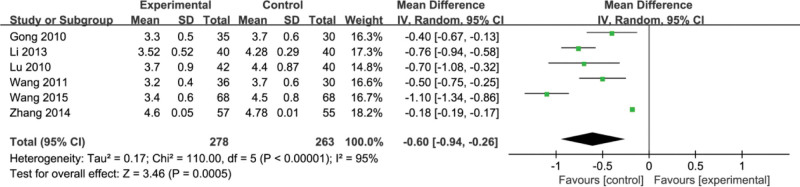
Six studies[8,9,16,17,24,28] reported spleen thickness. Heterogeneity tests revealed heterogeneity in the 6 studies (P < .00001, I2 = 95%). Random effect models were used for data analysis. By comparison, we found that the improvement effect of the experimental group on spleen thickness index had significant difference compared with the control group (MD = −0.60, 95% CI, [−0.94, −0.26] P = .0005) (Fig. 12).
Figure 12.
Improvement of spleen thickness.
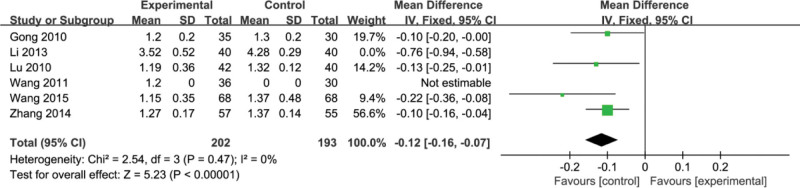
3.11. Portal vein diameter
Six studies[8,9,16,17,24,28] reported the internal diameter of the portal vein. Heterogeneity tests showed that there was no heterogeneity in these 4 studies (P = .47, I2 = 0%), and a fixed effect model was used for data analysis. By comparison, we found that the improvement effect of experimental group on the portal vein diameter index had a significant difference compared with the control group (MD = −0.12, 95% CI, [−0.16, −0.07] P < .0006) (Fig. 13).
Figure 13.
Forest plots of Portal vein diameter after treatment.
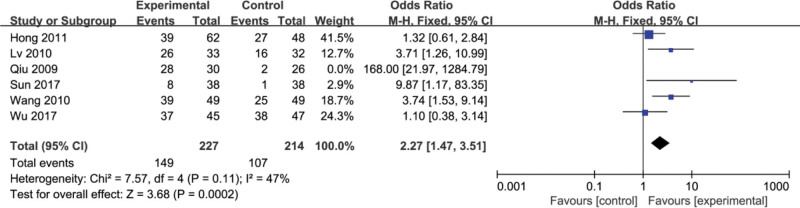
3.12. HBV-DNA negative conversion rate
Six studies[12,15,18,19,22,27] reported the negative conversion rate of HBV-DNA. The meta-analysis showed that there was heterogeneity among the 5 studies (P = .11, I2 = 47%), and a fixed effect model was used to analyze the data. The results showed that compared with conventional treatment, the use of conventional therapy combined with Fufang Biejia Ruangan Tablets can significantly improve the HA level (OR = 2.27, 95%CI, [1.47, 3.51] P = .0002) (Fig. 14).
Figure 14.
Meta-analysis of assessing the HBV‐DNA negative conversion rate. HBV = hepatitis B virus.
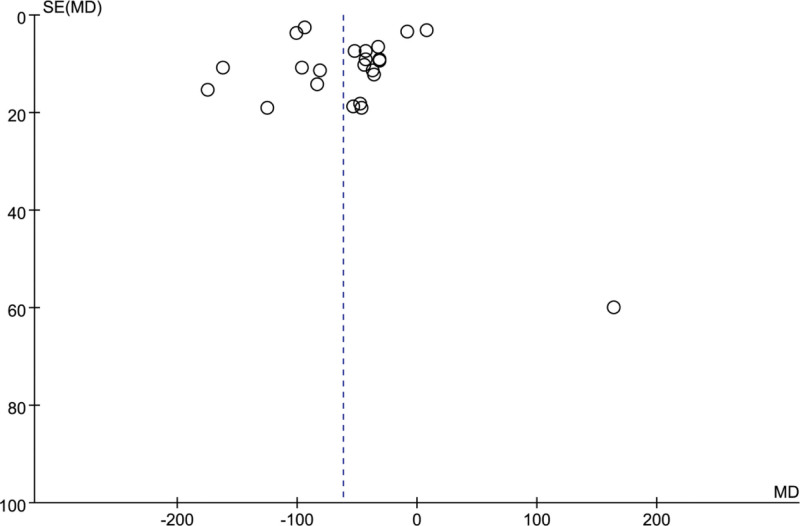
3.13. Publication bias
We used the funnel plot method to conduct a bias assessment of the 26 articles included in this study. The distribution of the inverted funnel maps is not completely symmetric, which suggests that there may be publication bias, or that the methodological quality of the trials included in this study is too low. Subsequently, in order to more clearly explore whether the results of this study are affected by the potential publication bias, we conducted the Begg’s and Egger’s test. As we have previously inferred, the precise statistical studies also proved that the results of this study are higher: Confidence (Begg: P = .472; Egger: P = .507) (Fig. 15).
Figure 15.
The funnel plot based on the data of hyaluronic acid (HA).
3.14. Adverse reactions
A total of 5 articles in this study reported adverse reactions.[9,10,18,23,26] The main adverse reactions were as follows: mild vertigo, nausea and fatigue. There were no serious adverse reactions. The above symptoms were untreated and cured on their own. Compared with the control group, there was no significant difference in the risk of adverse reaction events in the test group, indicating that Fufang Biejia Ruangan Tablets is safe as a supplementary drug for the treatment of CHB liver fibrosis.
4. Discussion
Liver fibrosis is the common pathological basis of various chronic liver diseases. The analysis of its pathogenesis is due to abnormal synthesis/degradation of extracellular matrix, which leads to abnormal deposition of connective tissue in the liver. The activation of hepatic stellate cells is a central link in the formation of hepatic fibrosis. Activation of HSC can be achieved through autocrine and paracrine pathways. The main cause of hepatic fibrosis is chronic inflammation of the liver tissue that caused by HBV infection.[33] Therefore, antiviral therapy is the primary method to prevent the formation of hepatic fibrosis. Science symptomatic clinical treatment has an important preventive effect on transformation of hepatic fibrosis to liver cirrhosis and liver cancer and can prolong the survival time of patients and improve their quality of life.
The World Health Organization has attested to the popularity and efficacy of indigenous herbal therapies, including CHM as a first line of treatment for some diseases including liver disorders.[34] In traditional Chinese medicine, some traditional Chinese medicines such as Salvia miltiorrhiza and Astragalus membranaceus are used in Danshen injection and Huangqi injection as drugs for treating liver fibrosis. However, patients with CHB treated with Astragalus injection may experience palpitation.[35] In addition, another study reported that Danshen injection may cause slight allergic reaction in a few patients.[36] As a traditional Chinese medicine, the Fufang Biejia Ruangan Tablets’ safety and effectiveness have been confirmed in animal experiments and clinical trials. Serum AST, ALT, ALB, and total bilirubin (TBIL) were the main indexes to evaluate liver function. Meta-analysis showed that Fufang Biejia Ruangan Tablets combined with antiviral drugs can significantly reduced serum AST, ALT, ALB, and TBIL levels compared with antiviral drugs alone, which means Fufang Biejia Ruangan Tablets combined with antiviral drugs can promote the recovery of liver function. HA, LN, VI-C and PC-III are the serum markers for liver fibrosis assessment. In this study, the levels of serum HA, LN, VI-C and PC-III were lower in the test group than in the control group. Therefore, the combined use of Chinese and Western medicines also shows a good anti-fibrotic effect. In addition, clinical studies have shown that Fufang Biejia Ruangan Tablets combined with antiviral drugs have a better improvement in the diameter of the portal vein and spleen thickness, effectively reduce the risk of variceal bleeding, and increase survival rate of patients with varicose veins that caused by CHB fibrosis.[37] In addition, through this study, it was found that the rate of HBV-DNA negative conversion after the treatment by using Fufang Biejia Ruangan Tablets combined with antiviral drugs was significantly increased. In conclusion, the total effective rate of Fufang Biejia Ruangan Tablets combined with antiviral drugs was significantly improved, and there was no significant difference in the incidence of adverse events after the combination was used, which also demonstrated its high safety.
Although the results of the meta-analysis showed that the use of Fufang Biejia Ruangan Tablets can improve the efficacy of CHB fibrosis, providing new ideas for clinical use of drugs, there are some objective factors and limitations in this study. In order to minimize the bias, we did not restrict the types of publications and languages and searched many common databases. Even so, all the studies were conducted in China, and the quality of most of the research methods was low. The sample size was small, and no specific random methods were described. All these factors affected the strength of this meta-analysis. Therefore, future research should focus on high-quality, large-sample, randomized, double-blind, controlled trials that provide more reliable evidence-based medical evidence for clinical treatment of liver fibrosis.
Author contributions
Data curation: Xiangbo Meng.
Formal analysis: Xiangbo Meng, Zhengqi Pan.
Funding acquisition: Quansheng Feng.
Investigation: Xiangbo Meng.
Methodology: Xiangbo Meng, Jiawei Zhao.
Project administration: Quansheng Feng.
Resources: Quansheng Feng.
Software: Xiangbo Meng, Zhengqi Pan.
Writing – original draft: Xiangbo Meng, Zhengqi Pan.
Writing – review & editing: Xiangbo Meng, Jiawei Zhao.
Abbreviations:
- ALB =
- albumin
- ALT =
- alanine transaminase
- AST =
- aspartate aminotransferase
- CHB =
- chronic hepatitis B
- IV-C =
- collagen C type IV
- HA =
- hyaluronic acid
- HBV =
- hepatitis B virus
- LN =
- laminin
- MD =
- mean difference
- OR =
- odds ratio
- PIIIP =
- procollagen III protein
- RCTs =
- randomized controlled trials
- TBIL =
- total bilirubin
The systematic review does not require ethical approval. This article will be published in peer-reviewed journals and presented at relevant conferences.
PROSPERO registration number: CRD42018100007.
An ethics statement is not applicable because this study is based exclusively on published literature.
XM, ZP, and JZ contributed equally to this work.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. The datasets generated during and/or analyzed during the current study are publicly available.
The current study was financially supported by Sichuan Provincial Department of Science and Technology (No. 2020YFS0301).
How to cite this article: Meng X, Pan Z, Zhao J, Feng Q. Efficacy and safety of Fufang Biejia Ruangan Tablets as an adjuvant treatment for chronic hepatitis B liver fibrosis: A systematic review and meta-analysis. Medicine 2022;101:46(e31664).
Contributor Information
Xiangbo Meng, Email: 2020kb004@stu.cdutcm.edu.cn.
Zhengqi Pan, Email: TcmPierre@stu.cdutcm.edu.cn.
Jiawei Zhao, Email: jarviechao123@stmail.hbtcm.edu.cn.
References
- [1].Maurizio S, Lydia G, Melchiorre C, et al. Non invasive tools for the diagnosis of liver cirrhosis. World J Gastroenterol. 2014;20:18131–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].The Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2016;22:18–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lange CM, Bojunga J, Hofmann WP, et al. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology. 2009;50:2001–6. [DOI] [PubMed] [Google Scholar]
- [5].Yang FR, Fang BW, Lou JS. Effects of Fufang Biejia Ruangan Pills on hepatic fibrosis in vivo and in vitro. World J Gastroenterol. 2013;19:5326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cai YJ, Dong JJ, Wang XD, et al. A diagnostic algorithm for assessment of liver fibrosis by liver stiffness measurement in patients with chronic hepatitis B. J Viral Hepat. 2017;17:34–6. [DOI] [PubMed] [Google Scholar]
- [7].Wang FM. Clinical observation on chronic hepatitis B liver fibrosis treated by compound Biejia Ruangan tablet combined with adefovir dipivoxil. Inner Mong Trad Chinese Med. 2014;36:39. [Google Scholar]
- [8].Wang XW. Adefovir dipivoxil combined with compound Biejia Ruangan tablets for the treatment of chronic hepatitis B complicated with hepatic fibrosis. Mod J Int Trad Chinese Western Med. 2015;24:2652–4. [Google Scholar]
- [9].Li YQ. Clinical observation of liver fibrosis treated with adefovir dip combined with compound Biejia Ruangan tablet. Global Chinese Med. 2013;S1:81–2. [Google Scholar]
- [10].Fan RQ, Wang C. Adefovir dip combined with compound Biejia Ruangan tablets for chronic hepatitis B liver fibrosis. J Yangtze Univ (Natural Science). 2014;06:17–21. [Google Scholar]
- [11].Fang ZY, Fang W. Adefovir dipivoxil combined with compound Biejia Ruangan tablets for the treatment of liver fibrosis in patients with chronic hepatitis B virus. Hebei Med Sci. 2013;09:1284–7. [Google Scholar]
- [12].Hong Q. Adefovir dipivoxil + compound Biejia Ruangan tablets in the treatment of hepatitis B liver fibrosis efficacy analysis. Shandong Med. 2009;05:78–9. [Google Scholar]
- [13].Meng SX. Effects of compound Biejia Ruangan tablets combined with adefovir dipivoxil on t lymphocyte subsets and liver fibrosis indexes in patients with chronic hepatitis B. China Sci Technol Trad Chinese Med. 2009;04:315. [Google Scholar]
- [14].Chai LX, Sun J, Ma QM. Compound Biejia Ruangan tablets combined with adefovir dipivoxil for treatment of chronic hepatitis B liver fibrosis in 24 cases. Chinese J Int Trad Western Med Dig. 2009;05:336–7. [Google Scholar]
- [15].Wang HR. Adefovir dip combined with compound Biejia Ruangan tablets for the treatment of chronic hepatitis B fibrosis. J Commun Med. 2010;03:27. [Google Scholar]
- [16].Gong YY. Clinical observation on chronic hepatitis B liver fibrosis treated with adefovir dip combined with compound Biejia Ruangan tablet. J Pract Liver Dis. 2010;02:126–8. [Google Scholar]
- [17].Lu N, Xu Y, Cheng WN. Clinical observation of hepatitis B liver fibrosis treated with adefovir dipter combined with compound Biejia Ruangan tablets. J Chinese Int Trad Chinese Med Soc Treat Liver Dis. 2010;04:209–11. [Google Scholar]
- [18].Lv F, Sun P, Lu SR. Observation of anti-fibrosis effect of compound Biejia Ruangan tablet on chronic hepatitis B. Clin J Trad Chinese Med. 2010;06:497–8. [Google Scholar]
- [19].Hong YQ, Chen ZJ, OuYang LJ. Adefovir combined with compound Biejia Ruangan tablets for the treatment of chronic hepatitis B liver fibrosis. Jilin Med J. 2011;07:1322–3. [Google Scholar]
- [20].Chen RJ. Observation of the effect of entecavir combined with compound Biejia Ruangan tablets in the treatment of chronic hepatitis B liver fibrosis. China Health Standard Manage. 2017;28:111–3. [Google Scholar]
- [21].Shen CR, Guo JC, Yu X, et al. Clinical observation of entecavir combined with compound Biejia Ruangan tablet in treating chronic hepatitis B liver fibrosis. J Zhejiang Univ Trad Chinese Med. 2010;03:370–1. [Google Scholar]
- [22].Sun YY, Zhu ZX. Clinical efficacy of entecavir combined with compound Biejia Ruangan tablets in treating chronic hepatitis B liver fibrosis. J Clin Rational Drug Appl. 2017;33:67–8. [Google Scholar]
- [23].Zhang XL. Clinical analysis of compound Biejia Ruangan tablets combined with entecavir in the treatment of chronic hepatitis B liver fibrosis. China Metallurg Ind Med J. 2016;06:680–1. [Google Scholar]
- [24].Zhang RF, Yao YJ, You ZL, et al. Clinical observation on chronic hepatitis B liver fibrosis treated by Fufang Biejia Ruangan tablets combined with entecavir. J Third Milit Med Univ. 2014;18:1961–3. [Google Scholar]
- [25].Zhang H, Hao XL. Clinical analysis of compound Biejia Ruangan Tablets combined with entecavir in patients with chronic hepatitis B liver fibrosis. Chinese For Med Care. 2017;01:119–21. [Google Scholar]
- [26].Zhang HT, Zhang XY, Me ZY, et al. Clinical observation on chronic hepatitis B liver fibrosis treated by compound Biejia Ruangan tablets combined with entecavir. China Coal Ind Med J. 2015;07:1120–2. [Google Scholar]
- [27].Wu ZG, Zhou GD, Chen YY, et al. Clinical study on compound Biejia Ruangan tablets combined with entecavir for chronic hepatitis B liver fibrosis. Drug Eval Res. 2017;03:351–5. [Google Scholar]
- [28].Wang T. Entecavir combined with compound Biejia Ruangan tablets for chronic hepatitis B liver fibrosis in 36 cases. Shaanxi J Trad Chinese Med. 2011;09:1115–6. [Google Scholar]
- [29].Xiao L, Yang YY, Xu L. Clinical efficacy of entecavir combined with compound Biejia Ruangan tablets in treating chronic hepatitis B liver fibrosis. China Pharm Sci. 2013;14:59–60. [Google Scholar]
- [30].Zhang ZG. The efficacy of entecavir plus compound Biejia Ruangan tablets in treating chronic hepatitis B liver fibrosis. Chinese Med Guide. 2012;36:456–8. [Google Scholar]
- [31].Wang YQ, Li XF. Effect of Fufang Biejia Ruangan Tablets combined with entecavir on liver function and quality of life in patients with chronic hepatitis B liver fibrosis. Henan Med Res. 2017;21:3903–4. [Google Scholar]
- [32].Li SS, Tan L, Jiang N. Clinical study on entecavir combined with compound Biejia Ruangan tablets for treatment of hepatic fibrosis in chronic hepatitis B. J Chin Int Trad Chin Med Liver Dis. 2017;01:28–30. [Google Scholar]
- [33].Chen SR, Chen XP, Lu JJ, et al. Potent natural products and herbal medicines for treating liver fibrosis. Chin Med. 2015;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Alex B, Yan Y, James A, et al. Anti-fibro-hepatocarcinogenic Chinese herbal medicines: a mechanistic overview. J Intercult Ethnopharmacol. 2016;5:278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhu CT, Cao H, Zhou XF, et al. Meta-analysis of the clinical value of Danshen injection and Huangqi injection in liver cirrhosis. Evid Based Complement Alternat Med. 2013;2013:842824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li S, Tan HY, Wang N, et al. Current status of herbal medicines in chronic liver disease therapy: the biological effects, molecular targets and future prospects. Int J Mol Sci . 2015;16:28705–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yang ZL, Yuan PJ. Clinical efficacy of combined use of traditional Chinese and western medicine in patients with Hepatitis B cirrhosis. J Guangxi Med Univ. 2021:330–2. [Google Scholar]