Background:
Isotonic crystalloids are the preferred solution for the initial clinical management of patients with multiple trauma, among which lactated Ringer’s solution and normal saline are the most widely used, but both have clinical limitations. Bicarbonated Ringer’s solution (BRS), which provides physiological levels of bicarbonate ions and electrolyte ions, can be used to supplement missing extracellular fluid and correct metabolic acidosis.
Methods:
A prospective, randomized controlled study enrolled 63 patients with traumatic hepatic rupture and hemorrhagic shock. They were randomly assigned to the Bicarbonated group (n = 33) or the Control group (n = 30), which received restrictive fluid resuscitation with sodium bicarbonate Ringer’s solution or sodium lactate Ringer’s solution, respectively. The levels of interleukin (IL)-6, tumor necrosis factor (TNF)-α, arterial blood lactic acid and potential of hydrogen (pH) were measured prior to, 1, 3, 24, and 72 hours following resuscitation. The primary outcomes were patient survival, shock-related complications, and comparison of the inflammatory factors.
Results:
The incidence of complications in the Bicarbonated group was significantly lower than in the Control group (15.15% vs 40.0%; P < .05). The intensive care unit length of stay and mechanical ventilation time in the Bicarbonated group were significantly shorter than in the Control group (all P < .01). The levels of IL-6 and TNF-α in the Bicarbonated group were significantly lower 1 hour following resuscitation than prior to resuscitation (P < .01), whereas these levels in the Control group were increased following 1h of resuscitation as compared with before resuscitation (P < .01). Following resuscitation, the levels of IL-6, TNF-α and lactate in the Bicarbonated group were significantly lower than in the Control group (P < .01). Moreover, in the Bicarbonated group, the lactic acid level decreased and the pH value increased significantly following resuscitation, whereas there was no difference in lactic acid levels and pH value between pre- and 1 hour post-resuscitation in the Control group (P > .05).
Conclusion:
The shock-related complications were dramatically reduced from using BRS in these patients. Additionally, the BRS was found to better inhibit the expression of inflammatory factors in their peripheral blood and could correct acidosis.
Keywords: cytokines, early resuscitation, shock, sodium bicarbonate Ringer’s solution, traumatic hepatic rupture
1. Introduction
Trauma from traffic accidents, mechanical accidents, and fall injuries has now become the principal cause of death among people under 40 years of age.[1,2] Nearly 50% of the deaths have been attributed to hemorrhagic post-traumatic shock.[3] The liver has been the most likely ruptured, which can induce massive hemorrhagic shock[4] resulting in death within 2 hours.[5,6] Therefore, timely antishock treatment is the key to saving lives.
The sine qua non of severe hypovolemic shock is insufficient tissue perfusion, leading to cellular hypoxia, with resultant microcirculatory dysfunction, causing a shift to anaerobic metabolism and increased lactate production that upsets the acid-base balance resulting in metabolic acidosis.[7,8] Timely resuscitation of body fluids and restoration of circulating blood volume are the main clinical antishock treatments. In the last 25 years, restrictive fluid resuscitation has been clinically accepted, showing a significantly better efficacy than large volume fluid resuscitation.[9,10] However, the choice of resuscitation fluids remains controversial. Normal saline and sodium lactate Ringer’s solution have until recently been the most commonly used crystalloids for resuscitation in clinical practice.[11,12] But both have limitations. Normal saline has a high chloride ion content, which can cause hyperchloremic metabolic acidosis and aggravate the disorders of water and electrolyte balance during rapid replenishment.[13] Although sodium lactated Ringer’s solution (LRS) avoided the hyperchloremia, it reportedly had the potential of aggravating the shock-induced lactic acidosis.[14] The lactate in LRS and the acetate in Sodium acetate Ringer’s liquid require metabolism in the liver,[15,16] and in the setting of shock and a traumatized liver, may only exacerbate the problem.[17] Sodium bicarbonate Ringer’s solution is a new formulation of crystalloid solution composed of sodium, magnesium, potassium, and calcium ions.[18] Sodium bicarbonate Ringer’s solution has been demonstrated to have a beneficial clinical effect in supplementing circulating blood volume and improving metabolic acidosis.[19,20]
In the early stage of shock, the body’s inflammatory system is also stimulated, and many inflammatory factors are released into the blood, which can potentially evolve into the systemic inflammatory response syndrome.[21] In view of this, this paper adopts Bicarbonated Ringer’s solution to treat traumatic hepatic rupture with hemorrhagic shock.
2. Materials and methods
2.1. Patients
The study included 63 patients with traumatic hepatic rupture and hypovolemic shock who were admitted to our hospital from June 2020 to June 2022. According to the random number table method, they were divided into the Bicarbonate group (resuscitated with Bicarbonated Ringer’s solution) and the Control group (resuscitated with LRS), 33 vs 30 cases, respectively. The Bicarbonate group included 20 males and 13 females, aged 19 to 67 years; the Control group included 16 males and 14 females, aged 18 to 68 years (Table 1). The clinical characteristics of the 2 groups were comparable. This study was approved by the Ethics Committee of Lu’an Hospital of Anhui Medical University (batch number: 2020LL017), and the patient’s family members were informed of the relevant information, and informed consent was obtained for all participants.
Table 1.
The clinical data of the 2 groups.
| Item | Bicarbonate group n = 33 (%) | Control group n = 30 (%) | t/χ2 | P |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 20 (60.6) | 16 (53.3) | 0.339 | .56 |
| Female | 13 (39.4) | 14 (46.7) | ||
| Age (yr) | 38.48 ± 12.6 | 36.8 ± 11.2 | 0.558 | .579 |
| Injury severity score (points) | 21.18 ± 3.47 | 21.80 ± 4.07 | 0.650 | .518 |
| Time from injury to resuscitation (hr) | 2.52 ± 0.55 | 2.72 ± 0.67 | 1.307 | .196 |
| Cause of injury, n (%) | ||||
| Traffic accident | 16 (48.5) | 14 (46.7) | 0.292 | .962 |
| Fall | 8 (24.2) | 7 (23.3) | ||
| Crush | 6 (18.2) | 5 (16.7) | ||
| Others | 3 (9.1) | 4 (13.3) | ||
| Degree of shock, n (%) | ||||
| Mild | 9 (27.3) | 10 (33.3) | 0.897 | .639 |
| Moderate | 16 (48.5) | 11 (36.7) | ||
| Severe | 8 (24.2) | 9 (30) | ||
| Routine blood test | ||||
| Red blood cell (×1012/L) | 3.18 ± 0.46 | 3.40 ± 0.49 | 1.885 | .064 |
| Hemoglobin (g/L) | 101.76 ± 8.78 | 102.37 ± 8.52 | 0.279 | .781 |
| Hematocrit | 0.30 ± 0.05 | 0.30 ± 0.04 | 0.022 | .982 |
| Electrolyte | ||||
| Na (mmol/L) | 136.48 ± 5.88 | 136.73 ± 4.94 | 0.181 | .857 |
| K (mmol/L) | 4.04 ± 0.49 | 3.98 ± 0.48 | 0.540 | .591 |
| CL (mmol/L) | 102.27 ± 3.98 | 100.87 ± 5.21 | 1.195 | .237 |
| The osmolarity (mmol/L) | 347.24 ± 31.08 | 346.10 ± 31.33 | 0.145 | .885 |
| Systolic pressure (mm Hg) | 66.48 ± 9.43 | 68.10 ± 8.94 | 0.696 | .489 |
| Diastolic pressure (mm Hg) | 41.58 ± 3.91 | 39.93 ± 4.34 | 1.580 | .119 |
| Mean arterial pressure (mm Hg) | 49.82 ± 4.98 | 49.37 ± 4.72 | 0.369 | .714 |
| Heart rate (bmp) | 107.67 ± 9.74 | 110.20 ± 12.54 | 0.900 | .372 |
| Crystalloid infused in 24 hours (L) | 1.45 ± 0.27 | 1.54 ± 0.25 | 1.429 | .158 |
| Total red blood cell in 24 hours (units) | 3.44 ± 0.99 | 3.43 ± 1.08 | 0.023 | .982 |
| Plasma in 24 hours (mL) | 552.88 ± 83.67 | 547.50 ± 86.19 | 0.251 | .802 |
| INR | 1.36 ± 0.10 | 1.35 ± 0.08 | 0.122 | .903 |
| pH | 7.24 ± 0.20 | 7.25 ± 0.21 | 0.209 | .835 |
| Length of hospitalization (d) | 14.33 ± 5.41 | 14.93 ± 6.30 | 0.407 | .686 |
| Intensive care unit length of stay (d) | 4.52 ± 0.97 | 5.82 ± 1.73 | 3.615 | .001 |
| Mechanical ventilation time (hr) | 54.52 ± 10.65 | 63.57 ± 13.63 | 2.950 | .004 |
INR = international normalized ratio, pH = potential of hydrogen.
Inclusion criteria: hock index ≥ 1; mean arterial pressure at admission < 60 mm Hg; and age ≥ 18 years old. Exclusion criteria: hemorrhage from abdominal organs or mesentery in addition to the liver confirmed at operation; patients who received blood transfusion or surgery within 1 hour of resuscitation; and moderate to severe traumatic brain injury with associated persistent hypertension.
2.2. Assessment of shock degree
Shock index (SI), calculated by heart rate/systolic blood pressure (BP), was used to assess the degree of shock in patients.[22] 1.0 ≤ SI < 1.5 indicated mild shock; 1.5 ≤ SI < 2.0 indicated moderate shock; SI ≥ 2.0 indicated severe shock.
2.3. Treatment
All patients were immediately resuscitated upon admission by maintaining the airway, undertaking oxygen supplementation and tracheal intubation and mechanical ventilation as necessary, establishing large venous access and invasive BP monitoring through arterial catheterization, indwelling urinary catheter, correcting acid-base imbalance, and providing analgesia and sedation as indicated. Restricted fluid resuscitation was conducted within 1 h using lactated Ringer’s solution (Anhui Shuanghe Pharmaceutical Co., Ltd, Wuhu, China) in the Control group, and sodium bicarbonate Ringer’s solution (Jiangsu Hengrui Pharmaceutical Co., Ltd, Lianyungang, China) in the Bicarbonate group. Fluid infusion was administered with a target mean arterial pressure of 60 to 70 mm Hg within half an hour (permissive hypotension) and maintained for the subsequent 30 minutes by adjusting the amount and the speed of fluid infusion.[23] Then the infusion rate was reduced, and the ratio of crystalloids, plasma and packed red blood cell was approximately 1:1:1. Both groups underwent targeted surgical treatments according to their sites and types of injuries. These included 24 with liver rupture that underwent suture repair, 11 had left external lobectomy, 9 had right posterior lobe resection, 6 had gauze packing hemostasis, and 13 had hepatic resection.
2.4. Outcome measures
Admission vital signs and peripheral venous blood were obtained prior to resuscitation, supplemented by the demographic data of the 2 patient groups. Repeat venous blood was obtained 1 hour after resuscitation, and peripheral serum interleukin (IL)-6 and tumor necrosis factor (TNF)-α were analyzed by ELISA, and blood gas analysis was performed to determine lactic acid levels at the same time.
The primary outcomes of patient hospital survival (survival rate = number of surviving patients/total number of patients × 100%), and shock-related complications (number of patients with complications/total number of patients × 100%). The patients were observed until death or hospital discharge. The primary outcome was 28-day survival. Additionally, both intragroup and intergroup comparisons of inflammatory factors and lactate levels prior to and following fluid resuscitation were analyzed. Total amount of crystalloid infused in 24 hours; total red blood cell, plasma; international normalized ratio; blood potential of hydrogen (pH); length of hospitalization, Intensive care unit length of stay, mechanical ventilation time also were recorded.
2.5. Statistical methods
SPSS 19.0 software (SPSS, Inc., Chicago, IL) was used for statistical analyses. Count data were expressed as number of cases (n, %), and processed by χ2 test (test level: 2 sided α = 0.05). The measurement data in this study conformed to normal distribution and were expressed as mean ± standard deviation ( ± SD). Independent sample t test was used for the comparison between groups, and paired t test was used for comparison before and after resuscitation within the same group, 2-way ANOVA was used for comparison each time within the 2 groups (test level: 2-sided α = 0.05). P < .05 indicates a statistically significant difference.
3. Results
3.1. Baseline data
The clinical data of the 2 groups are shown in Table 1. Both the Bicarbonated group and the Control group showed a majority of male patients (64.0% and 76.0%, respectively) and the principal cause of injury was traffic accidents (48.5% and 46.7%, respectively). There were no significant differences between the 2 groups regarding gender, age, injury severity score, routine blood tests, electrolytes, osmolarity, BP, time from injury to resuscitation, cause of injury, or degree of shock (all P > .05); the 2 groups were comparable. The comparison of the components between the 2 solutions is shown in Table 2.
Table 2.
Comparing the components of these 2 solution.
| Components | Bicarbonated Ringer’s solution | Lactated Ringer’s solution |
|---|---|---|
| Na+ (mmol/L) | 130 | 131 |
| K+ (mmol/L) | 4 | 4 |
| Mg2+ (mmol/L) | 1 | – |
| Ca2+ (mmol/L) | 1.5 | 1.4 |
| Cl− (mmol/L) | 109 | 110 |
| Buffer system | HCO3− | lactate |
| pH | 7.3 | 6.5 |
3.2. The levels of IL-6 and TNF-α prior to and following resuscitation
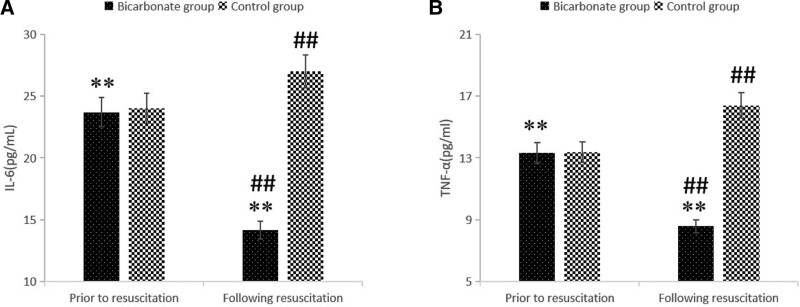
Prior to resuscitation, there was no significant difference in the levels of IL-6 and TNF-α between the 2 groups (all P > .01). However, following resuscitation, the Control group showed a significant increase in IL-6 and TNF-α as compared to levels prior to resuscitation (all P < .01); in contradistinction, the Bicarbonate group demonstrated significantly decreased IL-6 and TNF-α levels, comparing post- to pre-resuscitation (P < .01). Commensurately, the Bicarbonated group had lower levels of IL-6 and TNF-α than the Control group following resuscitation (all P < .01; Fig. 1, Table 3).
Figure 1.
The levels of IL-6 and TNF-α prior to and following resuscitation. (A) IL-6; (B) TNF-α. The Bicarbonate group showed lower IL-6 and TNF-α than the Control group following resuscitation. Compared with prior to resuscitation within the group, **P < 01; compared with Control group, ##P < .01. IL-6 = interleukin-6, TNF-α = tumor necrosis factor α.
Table 3.
Paired design t test comparing levels of cellular inflammatory factors before and after resuscitation between the 2 groups.
| Group | IL-6 (pg/mL) | t | P | TNF-α (pg/mL) | t | P | ||
|---|---|---|---|---|---|---|---|---|
| Prior to resuscitation | Following resuscitation | Prior to resuscitation | Following resuscitation | |||||
| Bicarbonate group (n = 33) | 23.96 ± 1.59 | 14.15 ± 1.41 | 27.10 | <.01 | 13.33 ± 1.33 | 8.57 ± 0.91 | 17.48 | <.01 |
| Control group (n = 30) | 24.00 ± 1.73 | 27.00 ± 1.18 | 14.61 | <.01 | 13.39 ± 1.28 | 16.40 ± 1.35 | 10.94 | <.01 |
3.3. Levels of lactic acid and ph prior to and following resuscitation
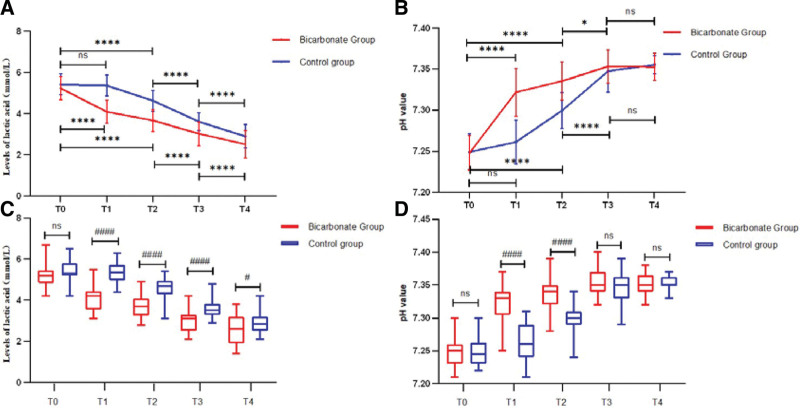
Prior to resuscitation, there was no significant difference in the blood lactic acid level and pH value between the 2 groups (P > .05). Following resuscitation, however, at each time, the blood lactic acid level in the Bicarbonated group was significantly reduced compared to the pre-resuscitation level (P < .0001) (Fig. 2A). The Bicarbonated group also showed a lower blood lactic acid level than the Control group at 1, 3, 24, and 72 hours following resuscitation. At the same time, the pH value in the Bicarbonated group was significantly raised compared to the pre-resuscitation level at each time (P < .0001) (Fig. 2B). At 3 hours after resuscitation, the levels of lactate decreased and pH value increased in the control group, so in early resuscitation, sodium bicarbonate ringer liquid can more quickly correct acidosis and reduce lactic acid levels. The t test indicated that the level of lactate in the sodium Bicarbonate group decreased more significantly at each time following resuscitation (Fig. 2C) than the control group, and pH (Fig. 2D) increased more significantly than the control group at 1 and 3 hours following resuscitation (P < .0001).
Figure 2.
Levels of lactic acid (A) and pH (B) prior to and following resuscitation by 2-way ANOVA. The levels of lactic acid (C) and pH (D) between the 2 group compared by t test. T0: prior to resuscitation, T1: 1 hr following resuscitation, T2: 3 hr following resuscitation, T3: 24 hr following resuscitation, T4: 72 hr following resuscitation. The Bicarbonate group showed lower lactic acid and higher pH value compared with prior to resuscitation at each time point, however, whether it is lactate or pH, there was no differenceat between the 1 hr following resuscitation and prior to resuscitation. Compared with prior to resuscitation within the group, ****P < .0001, *P < .05; compared with Control group, ####P < .0001, #P < .05. pH = potential of hydrogen.
3.4. Success rate of resuscitation and incidence of complications
No difference was found in the success rate of resuscitation between the 2 groups (84.8% vs 76.7%; P > .05). In striking contrast, the overall incidence of complications in the Bicarbonate group was significantly lower than in the Control group (15.15% vs 40.0%; P < .05; Table 4).
Table 4.
Success rate of resuscitation and incidence of complications.
| Item | Bicarbonate group (n = 33) | Control group (n = 30) | χ2 | P |
|---|---|---|---|---|
| Result of resuscitation, n (%) | ||||
| Survival | 28 (84.8) | 23 (76.7) | 0.682 | .409 |
| Death | 5 (15.2) | 7 (23.3) | ||
| Complications, n (%) | ||||
| DIC | 1 (3.03) | 2 (6.67) | ||
| ARDS | 3 (9.09) | 5 (16.67) | ||
| MODS | 1 (3.03) | 5 (16.67) | ||
| Total | 5 (15.15) | 12 (40.00) | 4.925 | .026 |
ARDS = adult respiratory distress syndrome, DIC = disseminated intravascular coagulation, MODS = multiple organ dysfunction syndrome.
4. Discussion
Patients in shock require timely fluid resuscitation to restore the effective circulating intravascular volume, increase BP, and ensure blood perfusion and oxygenation of tissues and organs.[23] The currently accepted method of restrictive fluid resuscitation reduces the excessive dilution of blood and profound extravascular tissue edema that accompanied the previous traditional aggressive fluid resuscitation which could also aggravate coagulation dysfunction.[24] Nevertheless, further refinement of restrictive fluid resuscitation could potentially improve patient outcomes in hypovolemic shock.
In the present study, the outcomes of using sodium bicarbonate Ringer’s solution and sodium lactate Ringer’s solution were compared in the early restricted fluid resuscitation of patients who had suffered traumatic hepatic rupture and hypovolemic shock. The sudden drop in blood volume, leading to hypoperfusion of tissues and organs results in anaerobic metabolism, elevated lactate levels, and an inflammatory response. IL-6 and TNF-α are at the center of the inflammatory cascade, which can intensify the deleterious consequences of shock. TNF-α can affect the physiological function of endothelial cells, changing the permeability of capillaries, potentially resulting in ischemia and thrombosis.[25,26] The marked reduction of renal perfusion can be heralded by an elevated IL-6 level as a predictor of acute kidney injury in shock patients.[27] Sodium lactate Ringer’s solution was shown to cause granulocyte respiration bursts, releasing a large amount of oxygen free radicals and other inflammatory response mediators to aggravate systemic inflammation.[28] The inflammation response can increase capillary permeability and plasma extravasation as well as decrease the blood volume, which can aggravate the hemorheology. An important result of the present study showed that IL-6 and TNF-α levels in patients who were resuscitated with sodium bicarbonate Ringer’s solution were significantly lower than those in the sodium lactate Ringer’s solution group, Furthermore, both inflammatory factors, already elevated in response to the serious hemorrhagic shock at the time of admission, were reduced just 1 hour following resuscitation with the bicarbonate Ringer’s solution. On the contrary, these factors showed an upward trend when lactate Ringer’s solution was used for resuscitation. Clearly, these results would indicate that sodium bicarbonate Ringer’s solution inhibited the expression of the inflammatory factors, undoubtedly blunting the overall inflammatory response. The specific molecular mechanism and signaling pathways involved are not yet clear and deserve further study.
In further support of bicarbonate Ringer’s solution, it is impressive to recognize that the metabolic rate of sodium bicarbonate is 6 times greater than lactic acid, which would reduce the metabolic burden to the liver.[29] Concordant with the present study, bicarbonate Ringer’s solution has been effective in the early resuscitation of patients with severe multiple injuries and traumatic shock, improving coagulation function and lactic acid metabolism, thereby reducing the risk of related complications and improving patient outcome.[30] This may be related to the HCO3− buffer system that can neutralize acidity without increasing lactic acid. An animal experiment in Japan also confirmed that sodium bicarbonate Ringer’s solution could improve metabolic acidosis in dogs with hemorrhagic shock, and its effect was significantly better than sodium lactate Ringer’s solution. Not surprisingly, sodium bicarbonate Ringer’s solution has been identified as an ideal crystalloid solution for the treatment of metabolic acidosis and maintenance of electrolyte balance.[19,31]
The Bicarbonated group did not achieve a survival advantage compared to the Control group. However, the overall incidence of complications in the Bicarbonated group was significantly lower than the Control group (15.15% vs 40.00%), the Intensive care unit length of stay and Mechanical ventilation time in the Bicarbonated group were significantly shorter than in the Control group (all P < .01), suggesting that sodium bicarbonate Ringer’s solution may be a better choice for early fluid resuscitation in patients with traumatic hepatic rupture with hemorrhagic shock.
There are limitations in this study. The sample size in this study was small which may have allowed statistical bias. The specific mechanism of resuscitation with sodium bicarbonate Ringer’s solution that accounted for the improvement of the inflammatory factors was not investigated. We plan to conduct a larger sample size study to further clarify the specific mechanism of sodium bicarbonate Ringer’s solution in patients with severe multiple injuries and traumatic shock.
Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Author contributions
All the authors contributed to the conception and design of the study. Material preparation, data collection, and analysis were performed by Sheng-Jin Han, Cui Yang, and Zheng-Wu Zhou. The first draft of the manuscript was written by Sheng-Jin Han and all authors commented on the previous versions of the manuscript. All authors have read and approved the final manuscript.
Conceptualization: Peng Gu.
Data curation: Sheng-Jin Han, Cui Yang, Peng Gu.
Formal analysis: Sheng-Jin Han, Zheng-Wu Zhou, Peng Gu.
Funding acquisition: Sheng-Jin Han, Zheng-Wu Zhou.
Investigation: Sheng-Jin Han, Cui Yang.
Methodology: Sheng-Jin Han.
Project administration: Sheng-Jin Han, Kun-Peng Wei.
Resources: Sheng-Jin Han, Zheng-Wu Zhou, Zeng-Fei Chu.
Software: Sheng-Jin Han, Zeng-Fei Chu.
Supervision: Jian-Zhong Ma.
Validation: Zeng-Fei Chu.
Writing – original draft: Sheng-Jin Han, Peng Gu.
Writing – review & editing: Sheng-Jin Han, Zheng-Wu Zhou.
Abbreviations:
- BP =
- blood pressure
- BRS =
- Bicarbonated Ringer’s solution
- IL-6 =
- interleukin-6
- LRS =
- Lactated Ringer’s solution
- pH =
- potential hydrogen
- SI =
- shock index
- TNF-α =
- tumor necrosis factor-α
This study was funded by the Clinical Research Special Grant of the China Primary Health Research Foundation (YLGX-JZ-2020011).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of Lu’an Hospital of Anhui Medical University (batch number: 2020LL017), and the patient’s family members were informed of the relevant information, and informed consent was obtained for all participants.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Han S-J, Zhou Z-W, Yang C, Wei K-P, Ma J-Z, Chu Z-F, Gu P. Hemorrhagic, hypovolemic shock resuscitated with Ringer’s solution using bicarbonate versus lactate: A CONSORT-randomized controlled study comparing patient outcomes and blood inflammatory factors. Medicine 2022;101:46(e31671).
Contributor Information
Sheng-Jin Han, Email: hanshengjin381@163.com.
Cui Yang, Email: 297490156@qq.com.
Kun-Peng Wei, Email: 13956142921@163.com.
Jian-Zhong Ma, Email: 13865750912@163.com.
Zeng-Fei Chu, Email: 13956132173@163.com.
Peng Gu, Email: 15955543663@163.com.
References
- [1].Harrois A, Soyer B, Gauss T, et al. Traumabase® Group. Prevalence and risk factors for acute kidney injury among trauma patients: a multicenter cohort study. Crit Care. 2018;22:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kuza CM, Hirji SA, Englum BR, et al. Pancreatic injuries in abdominal trauma in US adults: analysis of the national trauma data bank on management, outcomes, and predictors of mortality. Scand J Surg. 2020;109:193–204. [DOI] [PubMed] [Google Scholar]
- [3].Wang IJ, Bae BK, Park SW, et al. Prehospital modified shock index for prediction of massive transfusion and mortality in trauma patients. Am J Emerg Med. 2020;38:187–90. [DOI] [PubMed] [Google Scholar]
- [4].Unalp Haluk R, Yilmaz Y, Durak E, et al. Rupture of liver hydatid cysts into the peritoneal cavity. A challenge in endemic regions. Saudi Med J. 2010;31:37–42. [PubMed] [Google Scholar]
- [5].Hamada SR, Pirracchio R, Beauchesne J, et al. Effect of fibrinogen concentrate administration on early mortality in traumatic hemorrhagic shock: a propensity score analysis. J Trauma Acute Care Surg. 2020;88:661670. [DOI] [PubMed] [Google Scholar]
- [6].Jehan F, Con J, McIntyre M, et al. Pre-hospital shock index correlates with transfusion, resource utilization and mortality; the role of patient first vitals. Am J Surg. 2019;218:1169–74. [DOI] [PubMed] [Google Scholar]
- [7].Smith AA, Ochoa JE, Wong S, et al. Prehospital tourniquet use in penetrating extremity trauma: decreased blood transfusions and limb complications. J Trauma Acute Care Surg. 2019;86:43–51. [DOI] [PubMed] [Google Scholar]
- [8].Sperry JL, Guyette FX, Brown JB, et al. PAMPer Study Group. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–26. [DOI] [PubMed] [Google Scholar]
- [9].Corl KA, Prodromou M, Merchant RC, et al. The restrictive IV fluid trial in severe sepsis and septic shock (RIFTS): a randomized pilot study. Crit Care Med. 2019;47:951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gazmuri RJ, Whitehouse K, Whittinghill K, et al. Early and sustained vasopressin infusion augments the hemodynamic efficacy of restrictive fluid resuscitation and improves survival in a liver laceration model of hemorrhagic shock. J Trauma Acute Care Surg. 2017;82:317–27. [DOI] [PubMed] [Google Scholar]
- [11].Arnemann PH, Hessler M, Kampmeier T, et al. Resuscitation with hydroxyethyl starch maintains hemodynamic coherence in ovine hemorrhagic shock. Anesthesiology. 2020;132:131–9. [DOI] [PubMed] [Google Scholar]
- [12].Gao X, Tao Q, Zhou X, et al. Lactated Ringer’ solution may be superior to saline-based 6% hydroxyethyl starch 130/0.4 for early resuscitation within 12 hours from hemorrhagic shock. J Invest Surg. 2019;32:515–22. [DOI] [PubMed] [Google Scholar]
- [13].Huang K, Hu Y, Wu Y, et al. Hyperchloremia is associated with poorer outcome in critically ill stroke patients. Front Neurol. 2018;9:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lima R, Villela N, Castiglione R, et al. Dissociation between macroand microvascular parameters in the early phase of hemorrhagic shock. Microvasc Res. 2019;126:103909. [DOI] [PubMed] [Google Scholar]
- [15].Smyth DH. The rate and site of acetate metabolism in the body. J Physiol. 1947;105:299–315. [PubMed] [Google Scholar]
- [16].Adeva-Andany M, López-Ojén M, Funcasta-Calderón R, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion. 2014;17:76–100. [DOI] [PubMed] [Google Scholar]
- [17].Marko P, Gabrielli A, Caruso LJ. Too much lactate or too little liver? J Clin Anesth. 2004;16:389–95. [DOI] [PubMed] [Google Scholar]
- [18].Pakfetrat M, Malekmakan L, Salmanpour Z, et al. Comparison of normal saline, Ringer’s lactate, and sodium bicarbonate for prevention of contrast-induced nephropathy in patients with coronary angiography: a randomized double-blind clinical trial. Indian J Nephrol. 2019;29:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Satoh K, Ohtawa M, Katoh M, et al. Pharmacological study of BRS, a new bicarbonated Ringer’s solution, in haemorrhagic shock dogs. Eur J Anaesthesiol. 2005;22:703–11. [DOI] [PubMed] [Google Scholar]
- [20].Kim HJ, Son YK, An WS. Effect of sodium bicarbonate administration on mortality in patients with lactic acidosis: a retrospective analysis. PLoS One. 2013;8:e65283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Caserta S, Mengozzi M, Kern F, et al. Severity of systemic inflammatory response syndrome affects the blood levels of circulating inflammatory-relevant MicroRNAs. Front Immunol. 2018;8:1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].El Ayadi AM, Nathan HL, Seed PT, et al. Vital sign prediction of adverse maternal outcomes in women with hypovolemic shock: the role of shock index. PLoS One. 2016;11:e0148729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maegele M, Fröhlich M, Caspers M, et al. Volume replacement during trauma resuscitation: a brief synopsis of current guidelines and recommendations. Eur J Trauma Emerg Surg. 2017;43:439–43. [DOI] [PubMed] [Google Scholar]
- [24].Taylor SP, Karvetski CH, Templin MA, et al. Initial fluid resuscitation following adjusted body weight dosing is associated with improved mortality in obese patients with suspected septic shock. J Crit Care. 2018;43:7–12. [DOI] [PubMed] [Google Scholar]
- [25].Ferguson KL, Taheri P, Rodriguez J, et al. Tumor necrosis factor activity increases in the early response to trauma. Acad Emerg Med. 1997;4:1035–40. [DOI] [PubMed] [Google Scholar]
- [26].Yang Z, Zhang XR, Zhao Q, et al. Knockdown of TNF-α alleviates acute lung injury in rats with intestinal ischemia and reperfusion injury by upregulating IL-10 expression. Int J Mol Med. 2018;42:926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chawla LS, Seneff MG, Nelson DR, et al. Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J Am Soc Nephrol. 2007;2:22–30. [DOI] [PubMed] [Google Scholar]
- [28].Post EH, Donadello K, Taccone FS, et al. The harmful effects of hypertonic sodium lactate administration in hyperdynamic septic shock. Shock. 2016;46:663–71. [DOI] [PubMed] [Google Scholar]
- [29].Wang Y, Guo W, Gao D, et al. Effects of plasmalyte a, lactated Ringer’s, and normal saline on acid-base status and intestine injury in the initial treatment of hemorrhagic shock. Am J Emerg Med. 2017;35:317–21. [DOI] [PubMed] [Google Scholar]
- [30].Ma J, Han S, Liu X, et al. Sodium bicarbonated Ringer’s solution effectively improves coagulation function and lactic acid metabolism in patients with severe multiple injuries and traumatic shock. Am J Transl Res. 2021;13:5043–50. [PMC free article] [PubMed] [Google Scholar]
- [31].Ergin B, Kapucu A, Guerci P, et al. The role of bicarbonate precursors in balanced fluids during haemorrhagic shock with and without compromised liver function. Br J Anaesth. 2016;117:521–8. [DOI] [PubMed] [Google Scholar]