Abstract
Adjuvant endocrine therapy (AET) is known to reduce the risk of hormone receptor-positive (HR+) breast cancer (BC) recurrence and mortality rates, but its impact on cardiovascular disease (CVD) events is unclear. The primary objective of this study was to analyze the association of HR status with CVD mortality in patients with stage I to III BC. A retrospective study of patients with stage I to III BC was conducted using the 2004 to 2016 Surveillance, Epidemiology, and End Results (SEER) database, and patients were grouped according to their HR status. Propensity score matching (PSM) was used to adjust for heterogeneity between the groups. The cumulative incidence rate of CVD mortality was evaluated via a cumulative incidence curve. Univariate and multivariate Fine and Gray’s competing risk regression models were used to identify risk factors associated with CVD mortality. In total, 399,209 patients with BC were included in this study, and 329,958 patients (82.65%) were HR-positive. The cumulative incidence of CVD death was 8.28% in stage I to III BC patients. In the constituent ratio analysis, primary BC was the leading cause of death (45.29%, N = 31,465), followed by heart disease (16.07%, N = 11,166). Compared to the second year following BC diagnosis, the risk of CVD-specific death gradually increased. After PSM, 65,952 pairs of patients were matched, which led to the equilibrium of all variables between the HR-negative cohort and HR+ cohort. Multivariate analysis indicated that HR status was not significantly associated with the risk of CVD mortality, with a hazard ratio of 1.01 (P = .895). This study highlights the importance of understanding the associations between risk factors and CVD for BC patients. HR status was not associated with the risk of CVD mortality in this study.
Keywords: breast cancer, cardiovascular disease mortality, hormone receptor status, SEER database
1. Introduction
Cardiovascular disease (CVD) and malignant tumors are the leading causes of mortality worldwide.[1] Among 28 cancer types, 38% of the cancer patients die of malignant neoplasms, and 11% die of CVD.[2,3] Advances in cancer therapies have led to an increasing number of survivors, and these patients now live long enough to encounter other entities, especially CVD, which will terminate life expectancy.[2–4]
Of the almost 17 million cancer survivors in the US in 2019, over 3.8 million are breast cancer (BC) survivors.[5] With recent advancements in BC detection and treatment, the population of BC survivors is continuously growing.[2,6,7] Although the population of BC survivors is substantial, the importance of cardiology care for this patient population is underestimated. BC survivors are at high risk of CVD due to shared cardiometabolic risk factors (including obesity, diabetes, hypertension, and dyslipidemia), and the risk is exacerbated by BC treatment.[2,8–10] The success of BC treatment is increasingly tempered by cardiotoxicity due to several therapeutic approaches, including cytotoxic chemotherapy (anthracyclines), novel molecular targeted therapies, radiation therapy and possible cardiometabolic toxicity of endocrine therapy.[11–14] Therefore, cardiology care of BC survivors has become particularly important. For BC survivors, the most efficient strategy for the primary prevention and management of CVD is likely achieved through identifying and characterizing risk factors associated with CVD. Understanding any associations between risk factors and CVD is critical to inform the prevention and management of CVD events in BC patients.
Patients with hormone receptor-positive (HR+) BC commonly receive adjuvant endocrine therapy (AET), such as tamoxifen and aromatase inhibitors (AIs), to reduce the risk of postoperative recurrence.[15] These drugs can instigate cardiovascular events, including venous thromboembolism, myocardial infarction, ischemic heart disease and arrhythmia.[14,16–18] However, studies comparing BC patients treated with tamoxifen or AI to those who are HR-negative (HR−) regarding the risk of CVD have shown contradictory results. Previous studies have even suggested a possible cardioprotective effect of tamoxifen.[14,16,19] However, an increased risk of stroke and venous thromboembolism caused by tamoxifen has also been found.[18,20,21] In a more recent meta-analysis including 26 studies, tamoxifen treatment was not associated with an increased risk of stroke.[17] Although some studies have claimed significant CVD risk effects related to AI exposure, the outcomes have been divergent and even contradictory across studies.[16,17,19,21–23]
The Surveillance, Epidemiology, and End Results (SEER) database has one of the largest cohorts of BC patients with long-term follow-up and detailed CVD-specific mortality. AET is recommended for all patients with HR + BC.[15] Currently, there are no long-term follow-ups or large datasets available with the association of HR status with CVD-specific mortality in patients with stage I to III BC.
To understand CVD mortality in the general population of BC patients and to derive more knowledge regarding the association of HR status with CVD mortality in the real-world setting, we conducted this retrospective study, including patients stratified by HR status, using the SEER database.
2. Patients and methods
2.1. Data sources
This retrospective study was approved by the Institutional Ethics Committee of the People’s Hospital of Ganzhou. The SEER database (SEER 18 Registries research plus Data, Nov 2020 Sub) was accessed using the SEER program (www.seer.cancer.gov), which represents approximately 28% of the United States population. Data were extracted using SEER*Stat software (version 8.3.9).
2.2. Study population and definitions
We downloaded the data of BC patients registered from 2004 through 2016. The major inclusion criteria are listed as follows: female patients older than 18 years of age; pathological diagnosis of primary BC (pathological types were selected as infiltrating ductal cancer [codes: 8500 and 8521], lobular carcinoma [codes: 8520], infiltrating duct and lobular carcinoma [codes: 8522], and other BC); diagnosis of stage I/II/III BC according to the AJCC staging system sixth edition; 1 primary malignant tumor only (C50.x [Breast codes range were C50.0–C50.6, C50.8–C50.9]); and previous primary surgery performed (SEER surgery codes range were 20–24 [Breast conserving surgery], 30/40–49/75/80 [Mastectomy], and 50–59/60–69/70–74 [Radical mastectomy]). Key exclusion criteria were insufficient information required by this study and death within 1 month after BC diagnosis. According to estrogen receptor (ER) and progesterone receptor (PR) status, patients were divided into HR− (ER−/PR−) and HR+ (ER+/PR+, ER+/PR−, or ER−/PR+) cohorts.
The following variables were extracted from the SEER database: age at diagnosis, race, laterality, pathological type, pathologic grade, surgical procedure, stage, T stage, N stage, tumor size, radiotherapy, chemotherapy, ER status, PR status, months of survival, vital status and cause of death.
Follow-up duration was defined as the time from the date of BC diagnosis to the date of last follow-up, death or the end of follow-up (December 31, 2018). CVD-specific death was defined as death from CVD, including heart diseases; hypertension without heart disease; cerebrovascular diseases; atherosclerosis; aortic aneurysm/dissection; or other diseases of arteries, arterioles, and capillaries.[2]
2.3. Statistical analysis
Data are presented as the numbers (%) or medians (range) as appropriate. Propensity score matching (PSM) was performed at 1:1 with a caliper value of 0.001 to match HR− and HR+ cohorts. A standardized mean difference (SMD) of less than 0.1 represents a negligible difference in covariates between groups before and after matching.[24,25] The cumulative incidence rate of CVD mortality was evaluated via a cumulative incidence curve. Univariate and multivariate Fine and Gray’s competing risk regression models were used to identify risk factors associated with CVD mortality.[26] Variables with P ≤ .10 in univariate analysis were selected for multivariate analysis. Differences were considered statistically significant at p values < 0.05. Data were analyzed using R (www.R-project.org) and Empower Stats software (https://www.empowerstats.net/cn/).
3. Results
3.1. Patient characteristics
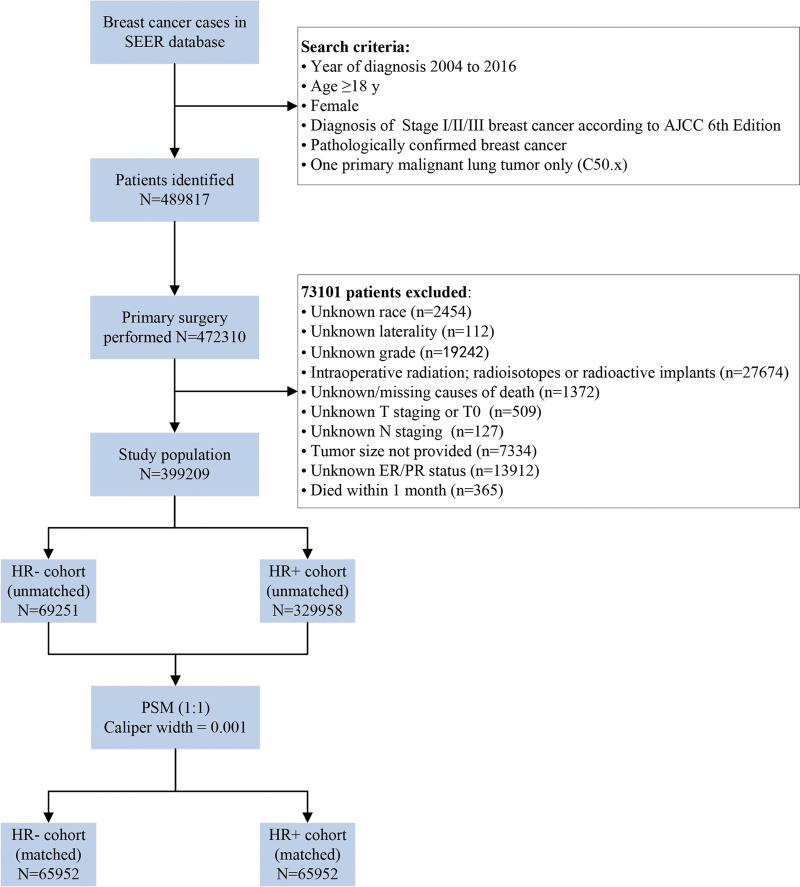
A total of 399,209 patients entered the study. The flow chart of the selection of this study and the reasons for exclusion are presented in Figure 1. Among them, 329,958 patients (82.65%) were HR-positive. The proportions of patients stratified by HR status differed by age, race, histologic type, grade, surgical procedure, chemotherapy, stage, T stage, N stage and tumor size (Table 1). No significant differences were found in laterality, radiation or follow-up time between groups. After PSM, 65,952 pairs of patients were matched, which led to the equilibrium of all variables between the HR-negative cohort and HR + cohort, as shown in Table 1.
Figure 1.
Flow chart of the screened patients. AJCC = American joint committee on cancer, ER = estrogen receptor, HR+/− = hormone receptor-positive/negative, LN = lymph nodes, PR = progesterone receptor, SEER = surveillance, epidemiology, and end results.
Table 1.
The baseline clinical characteristics of enrolled patients with stage I/II/III breast cancer before and after PSM
| Clinical parameters | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| HR− cohort (N = 69,251) | HR + cohort (N = 329,958) | SMD* | HR− cohort (N = 65,952) | HR+ cohort (N = 65,952) | SMD | |
| Age, yrs (range) | 56 (18–100) | 60 (18–100) | 0.230 | 56 (18–100) | 56 (18–100) | 0.005 |
| Race | 0.260 | 0.034 | ||||
| Black | 50846 (73.42%) | 268775 (81.46%) | 49410 (74.92%) | 49164 (74.55%) | ||
| White | 12274 (17.72%) | 29533 (8.95%) | 10500 (15.92%) | 10126 (15.35%) | ||
| Others | 6131 (8.85%) | 31650 (9.59%) | 6042 (9.16%) | 6662 (10.10%) | ||
| Laterality | 0.023 | 0.014 | ||||
| Left | 35804 (51.70%) | 166843 (50.56%) | 34001 (51.55%) | 34449 (52.23%) | ||
| Right | 33447 (48.30%) | 163115 (49.44%) | 31951 (48.45%) | 31503 (47.77%) | ||
| Histologic type | 0.498 | 0.028 | ||||
| Infiltrating ductal cancer | 60229 (86.97%) | 244140 (73.99%) | 57863 (87.74%) | 57644 (87.40%) | ||
| Infiltrating lobular cancer | 678 (0.98%) | 31150 (9.44%) | 678 (1.03%) | 825 (1.25%) | ||
| Mixed ductal and lobular | 1070 (1.55%) | 23810 (7.22%) | 1070 (1.62%) | 1222 (1.85%) | ||
| Other types | 7274 (10.50%) | 30858 (9.35%) | 6341 (9.61%) | 6261 (9.49%) | ||
| Grade | 1.372 | 0.017 | ||||
| Well differentiated | 1438 (2.08%) | 88245 (26.74%) | 1438 (2.18%) | 1322 (2.00%) | ||
| Moderately differentiated | 12601 (18.20%) | 160376 (48.60%) | 12601 (19.11%) | 12376 (18.77%) | ||
| Poorly differentiated | 54191 (78.25%) | 80149 (24.29%) | 51034 (77.38%) | 51425 (77.97%) | ||
| Undifferentiated; anaplastic | 1021 (1.47%) | 1188 (0.36%) | 879 (1.33%) | 829 (1.26%) | ||
| Surgical procedure | 0.236 | 0.024 | ||||
| BCS | 34593 (49.95%) | 197576 (59.88%) | 33367 (50.59%) | 32564 (49.38%) | ||
| Mastectomy | 16056 (23.19%) | 73585 (22.30%) | 15292 (23.19%) | 15673 (23.76%) | ||
| Radical mastectomy | 18602 (26.86%) | 58797 (17.82%) | 17293 (26.22%) | 17715 (26.86%) | ||
| Radiation | 0.086 | 0.022 | ||||
| No | 32604 (47.08%) | 141276 (42.82%) | 30459 (46.18%) | 31188 (47.29%) | ||
| Yes | 36647 (52.92%) | 188682 (57.18%) | 35493 (53.82%) | 34764 (52.71%) | ||
| Chemotherapy | 0.788 | 0.009 | ||||
| No | 18080 (26.11%) | 206277 (62.52%) | 18065 (27.39%) | 17804 (27.00%) | ||
| Yes | 51171 (73.89%) | 123681 (37.48%) | 47887 (72.61%) | 48148 (73.00%) | ||
| Stage | 0.358 | 0.021 | ||||
| I | 24481 (35.35%) | 173192 (52.49%) | 23537 (35.69%) | 22947 (34.79%) | ||
| II | 32475 (46.89%) | 120491 (36.52%) | 30710 (46.56%) | 30923 (46.89%) | ||
| III | 12295 (17.75%) | 36275 (10.99%) | 11705 (17.75%) | 12082 (18.32%) | ||
| T stage | 0.403 | 0.006 | ||||
| T1 | 31281 (45.17%) | 212483 (64.40%) | 30295 (45.93%) | 30122 (45.67%) | ||
| T2 | 29323 (42.34%) | 95755 (29.02%) | 27868 (42.25%) | 27982 (42.43%) | ||
| T3 | 5613 (8.11%) | 15881 (4.81%) | 5108 (7.75%) | 5129 (7.78%) | ||
| T4 | 3034 (4.38%) | 5839 (1.77%) | 2681 (4.07%) | 2719 (4.12%) | ||
| N stage | 0.163 | 0.039 | ||||
| N0 | 43600 (62.96%) | 227656 (69.00%) | 40876 (61.98%) | 39634 (60.10%) | ||
| N1 | 17108 (24.70%) | 75412 (22.86%) | 16784 (25.45%) | 17667 (26.79%) | ||
| N2 | 5074 (7.33%) | 18012 (5.46%) | 5000 (7.58%) | 5188 (7.87%) | ||
| N3 | 3469 (5.01%) | 8878 (2.69%) | 3292 (4.99%) | 3463 (5.25%) | ||
| Tumor size, cm | 0.401 | 0.010 | ||||
| <=2 | 31624 (45.67%) | 213235 (64.62%) | 27414 (41.57%) | 27108 (41.10%) | ||
| >2, <=5 | 30499 (44.04%) | 98543 (29.87%) | 32151 (48.75%) | 32440 (49.19%) | ||
| >5 | 7128 (10.29%) | 18180 (5.51%) | 6387 (9.68%) | 6404 (9.71%) | ||
| Median FU, mo (range) | 74 (1–179) | 77 (1–179) | 0.031 | 74 (1–179) | 76 (1–179) | 0.026 |
Abbreviations: BCS = breast conserving surgery, FU = follow-up time, HR+/− = hormone receptor-positive/negative, PSM = propensity score-matching, SMD = standardized mean difference.
SMDs of 0.1 or more represent meaningful differences in covariates between groups before and after matching. Data are shown as the medians (range) or n (%).
3.2. CVD-related death in stage I to III BC before PSM
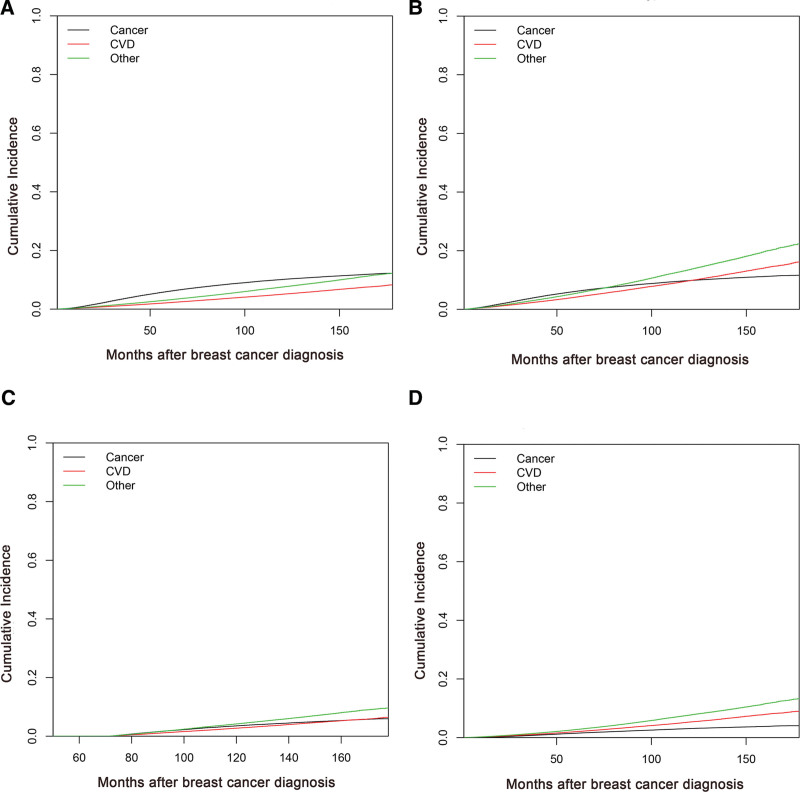
The cumulative incidence curve resulting from all causes of death in the study population is shown in Figure 2A. Other noncancer and non-CVD causes were the leading causes of death, with cumulative rates of 12.26%, closely followed by BC (12.21%). The cumulative incidence of CVD-related death (8.28%) was lower than that arising from BC or other reasons. The cumulative mortality resulting from CVD for women ≥ 60 years old, with a follow-up time greater than 6 years and with stage I BC, was higher than that associated with BC (Fig. 2B–D). The cumulative incidence curve for all causes of death in select population groups is shown in Supplemental Digital Content (Fig. S1, http://links.lww.com/MD/H971). Cancer was still the leading cause of death for women < 60 years of age, follow-up time within 6 years and with stage II/III BC (Supplemental Digital Content Fig. S1, http://links.lww.com/MD/H971).
Figure 2.
Cumulative incidence curves of all causes of death for women: (A) in the study population, (B) of ≥ 60 yrs, (C) of follow-up time greater than 6 yrs, and (D) with stage I BC. BC = breast cancer.
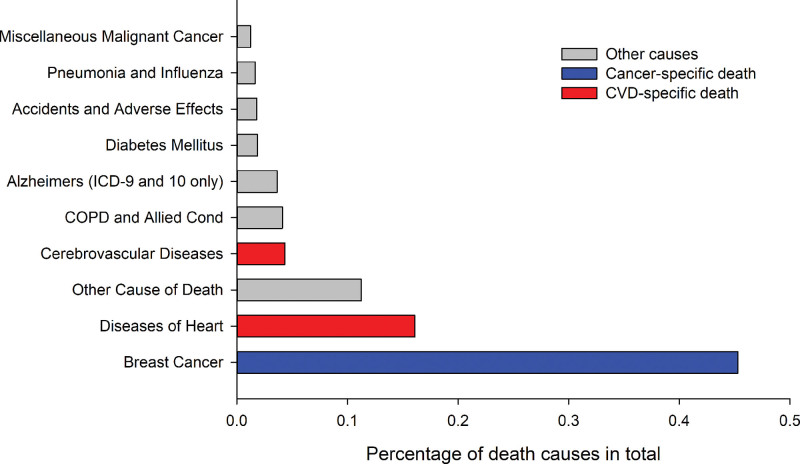
A total of 69,473 patients (17.40%) died from primary stage I to III BC, CVD, or other causes in the period of 2004 to 2016. Among all of these cancer survivors (329,736 cases), 217,422 (65.94%) survived more than 5 years beyond their cancer diagnosis, and 87,149 (26.43%) survivors were considered 10-year survivors. A total of 15,391 patients (3.86%) died of CVDs. Among them, 2284 (3.30%) and 13,107 (3.97%) patients succumbed to CVD in the HR− and HR + groups, respectively. In the constituent ratio analysis, Figure 3 summarizes the top ten causes of death in stage I to III BC patients. BC was the leading cause of death for our cohort before PSM (45.29%, N = 31,465), followed by heart disease (16.07%, N = 11,166). Notably, the plurality (92.12%) of all CVD-related mortality in stage I to III BC was caused by heart diseases (72.55%) and cerebrovascular diseases (19.57%).
Figure 3.
Top 10 causes of death among patients with stage I to III breast cancer. COPD = chronic obstructive pulmonary disease.
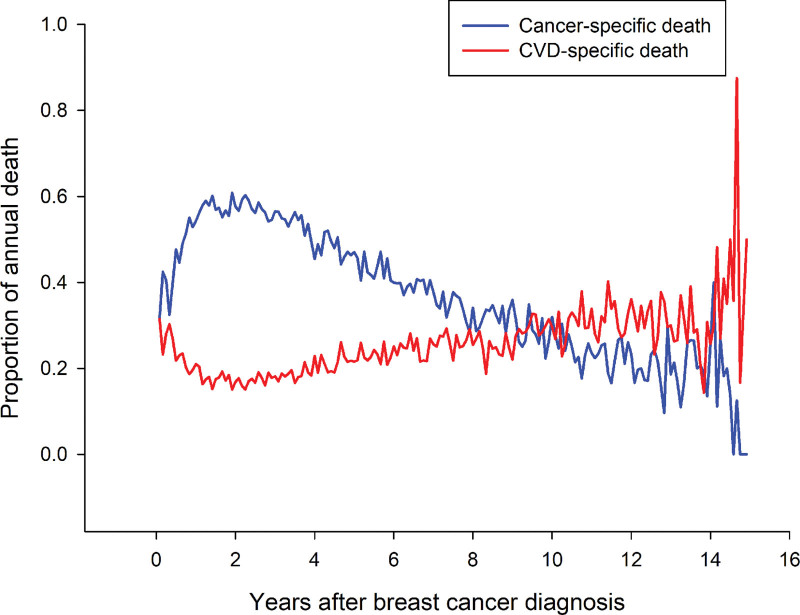
We analyzed the constituent ratio of cancer-related mortality and CVD-related mortality and observed its alterations after the cancer diagnosis, including trends of increased CVD-related mortality and decreased cancer-related mortality over time (Fig. 4). Compared to the second year following BC diagnosis, the risk of CVD-specific death gradually increased (Fig. 4).
Figure 4.
Trends of CVD- and cancer-specific death in patients with stage I to III breast cancer during the follow-up time after diagnosis. CVD = cardiovascular disease.
3.3. Risk factors for CVD-related death in stage I to III BC after PSM
To reduce the risk of bias, PSM was performed with a 1:1 matching protocol to balance important patient characteristics between HR-negative and HR-positive cohorts. Univariate analysis revealed that CVD-related death was significantly associated with age, race, laterality, histologic type, grade, radiation, chemotherapy, stage, N stage, and HR status (Table 2). The multivariate competing risk regression model revealed that age, race, laterality, histologic type, grade, radiation, chemotherapy, stage, and N stage were independent risk factors for CVD-specific mortality (Table 2). We analyzed the impact of HR status on CVD-related death, and the multivariate analyses showed no significant association between HR status and the risk of CVD-related death (HR = 1.01, 95% CI 0.93–1.08, P = .895). The results of univariate and multivariate analyses for risk factors for CVD-specific death in patients with BC before PSM are shown in Supplemental Digital Content (Table S1, http://links.lww.com/MD/H972). This result was consistent with our previous multivariate analysis, and HR status was not associated with the risk of CVD-related death (multivariate HR = 0.95, 95% CI 0.89–1.01, P = .079).
Table 2.
Univariate and multivariate analyses of cardiovascular disease-related mortality using a Fine-Gray hazard model
| Variable name | Univariate analysis (N = 131,904) | Multivariate analysis (N = 131904) | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI for HR | P value | HR | 95% CI for HR | P value | |||
| Age, yrs (>50 vs =<50) | 11.28 | 9.69 | 13.14 | <.0001 | 12.37 | 10.21 | 14.98 | <.0001 |
| Race | ||||||||
| White | Reference | Reference | ||||||
| Black | 1.09 | 1.00 | 1.19 | .052 | 0.98 | 0.88 | 1.08 | .634 |
| Others | 0.56 | 0.47 | 0.66 | <.0001 | 0.57 | 0.48 | 0.68 | <.0001 |
| Laterality (Right vs Left) | 0.92 | 0.86 | 0.98 | .005 | 0.97 | 0.90 | 1.04 | .371 |
| Histologic type | ||||||||
| Infiltrating ductal cancer | Reference | Reference | ||||||
| Infiltrating lobular cancer | 1.48 | 1.16 | 1.90 | .002 | 0.93 | 0.70 | 1.23 | .622 |
| Mixed ductal and lobular | 1.02 | 0.80 | 1.30 | .863 | 0.88 | 0.67 | 1.16 | .364 |
| Other types | 1.48 | 1.35 | 1.62 | <.0001 | 1.16 | 1.05 | 1.28 | .004 |
| Grade | ||||||||
| Well differentiated | Reference | Reference | ||||||
| Moderately differentiated | 0.82 | 0.68 | 0.98 | .029 | 1.12 | 0.92 | 1.36 | .261 |
| Poorly differentiated | 0.58 | 0.49 | 0.70 | <.0001 | 1.02 | 0.85 | 1.23 | .829 |
| Undifferentiated; anaplastic | 0.92 | 0.69 | 1.22 | .567 | 1.44 | 1.06 | 1.96 | .021 |
| Surgical procedure | ||||||||
| (Mastectomy/Radical mastectomy vs BCS) | 1.03 | 0.97 | 1.09 | .383 | — | — | — | — |
| Radiation (Yes vs No) | 0.51 | 0.48 | 0.55 | <.0001 | 0.69 | 0.64 | 0.74 | <.0001 |
| Chemotherapy (Yes vs No) | 0.19 | 0.18 | 0.21 | <.0001 | 0.26 | 0.24 | 0.28 | <.0001 |
| Stage (II/III vs I) | 0.90 | 0.84 | 0.96 | .001 | 1.46 | 1.34 | 1.59 | <.0001 |
| T stage (T3/4 vs T1/2) | 1.03 | 0.94 | 1.13 | .490 | — | — | — | — |
| N stage (N + vs N0) | 0.73 | 0.69 | 0.78 | <.0001 | 0.85 | 0.78 | 0.93 | .000 |
| Tumor size, cm | ||||||||
| <=2 | Reference | — | ||||||
| >2, <=5 | 1.05 | 0.99 | 1.12 | .114 | — | — | — | — |
| >5 | 0.92 | 0.82 | 1.02 | .122 | — | — | — | — |
| HR status (HR + vs HR−) | 0.93 | 0.87 | 0.99 | .022 | 1.01 | 0.93 | 1.08 | .895 |
P < .05 was considered significant. Model adjusted for multivariate analysis: Age, Race, Laterality, Histologic type, Grade, Radiation, Chemotherapy, Stage, N stage and HR status.
Abbreviations: BCS = breast conserving surgery, HR+− = hormone receptor-positive/negative.
4. Discussion
BC, a hormone-dependent tumor, generally includes 4 molecular subtypes based on ER, PR and Her-2 status.[27] In addition to operation and chemotherapy, endocrine therapy, including tamoxifen and AI, is one of the standard treatments for HR + BC.[15,21] AET is well established to reduce the risk of HR-positive BC recurrence and mortality rates[28–31] and is recommended for 5 to 10 years following primary treatment as part of standard care.[32,33] Knowledge of any associations between risk factors and CVD is an important prerequisite for preventing and managing CVD events in BC patients. However, given the reported contradictory results, the association of AET with CVD in patients with BC remains unclear.[14,16,17,19,21–23] In this retrospective study, our results indicated that CVD-specific mortality (cumulative incidence 8.28%) remains a challenge in BC survivors, especially in postoperative patients. The cumulative mortality resulting from CVD in select population groups was higher than that associated with BC. Moreover, in the constituent ratio analysis, BC was the leading cause of death (45.29%, N = 31,465), followed by heart disease (16.07%, N = 11,166). We also observed that, compared to the second year following BC diagnosis, patients had a continually elevated risk of CVD mortality. In line with previous studies,[2,4,8] our results highlighted a need to study risk factors of CVD mortality and the necessity of cardio-oncology in patients prior to, during, and following treatment.
Although there is a lack of direct endocrine treatment information from the SEER database, the association between endocrine therapy and HR status has been firmly established.[10,28–31] Previous studies have reported that specific estimates of AET adherence range from 75% to over 90% in patients with HR-positive BC.[34,35] A SEER-based study showed that of the 743 patients eligible for endocrine therapy, 80 (10.8%) never initiated therapy, 112 (15.1%) started therapy but discontinued prematurely.[36] The factors associated with initiation included race/ethnicity, worry about recurrence and sufficient information receipt about these agents. Factors associated with persistence included younger age and concurrent medication use. It may therefore be concluded that Enhanced patient education about potential side effects and the effectiveness of AET in improving outcomes may improve initiation and persistence rates and optimize BC survival.[36] We identified several risk factors for CVD mortality in patients with stage I to III BC, including age, race, laterality, histologic type, grade, radiation, chemotherapy, stage, and N stage. Similar risk factors (age, ethnicity, laterality, histology type, chemotherapy and radiotherapy) for CVD mortality were identified in another study in patients with lung cancer.[37]
Moreover, our study provided no support for a potential link between HR status and CVD mortality (HR = 1.01, 95% CI 0.93–1.08, P = .895). This result is consistent with findings from a study of patients in a community-based population, suggesting that rates of myocardial infarction and stroke for patients on AIs or tamoxifen did not differ significantly from BC patients not receiving endocrine therapy.[23] Another study also showed that comparisons with those receiving no endocrine therapy showed no higher cardiovascular outcomes risk for either drug class.[21] However, meta-analyses of randomized controlled trials investigating the side effects of extended adjuvant AI compared with those not receiving it have shown conflicting results, with an increased risk of cardiovascular events with extended AIs in 1 study[16] but no difference in 2 other studies.[19,38] Additional studies are needed to better assess and manage cardiovascular risk factors for BC patients.
Our study was methodologically different than previous studies using no endocrine therapy as a comparator. First, the definition of outcomes differed from that used in our study investigating CVD death as an event, whereas the other studies used several CVD outcomes (including venous thromboembolism, myocardial infarction, ischemic heart disease and arrhythmia) as events. Furthermore, the lack of adherence remains a problem, and not all patients with HR-positive BC adhere to AET, which should be considered when interpreting our results. Finally, the dataset of our study was relatively large, with 399,209 participants. Another strength of the present study is the long follow-up time, with an average of more than 70 months.
Despite the insights into the association of HR status with CVD mortality in patients with stage I to III BC, there are a few limitations of this study. First, this study was a retrospective study based on the SEER database, and there were large differences in baseline clinical characteristics between the 2 groups. PSM was utilized and resulted in well-matched groups. Second, the SEER database lacks information regarding cancer therapy (such as immune checkpoint inhibitors and anti-HER-2 treatment), preexisting cardiovascular risk factors and CVDs, which might influence CVD mortality. HR-positive BC patients at risk of CVD are more likely not to receive AET in clinical, which will underestimate the results of this study. Finally, information about endocrine treatment was not available in the SEER database. Therefore, we paid attention to the association of HR status with CVD mortality. Either way, this finding provides additional indirect evidence that cardiovascular death may not increase significantly in patients treated with AET compared to patients not receiving AET.
In summary, this study shows the cumulative incidence of CVD-specific mortality and the constituent ratio of all causes of death and identifies some risk factors for CVD-specific mortality based on SEER data in stage I to III BC patients. Notably, HR status was not associated with the risk of CVD-specific mortality in our study. This study highlights the importance of understanding the associations between risk factors and CVD for BC patients. Further studies involving more detailed information on risk factors are needed.
Acknowledgments
We acknowledge the SEER program for providing these data.
Author contributions
Investigation: Jing He.
Resources: Zhihua Lai
Software: Xiaohong Liao.
Supervision: Yuanping Chen, Chao Liu, Chen Wang.
Writing – original draft: Luxia Wang, Zhihua Lai.
Writing – review and editing: Jing He.
Supplementary Material
Abbreviations:
- AET =
- adjuvant endocrine therapy
- AIs =
- aromatase inhibitors
- BC =
- breast cancer
- CVD =
- cardiovascular disease
- ER =
- estrogen receptor
- HR− =
- HR-negative
- HR+ =
- hormone receptor-positive
- PR =
- progesterone receptor
- PSM =
- propensity score matching
- SEER =
- surveillance, epidemiology, and end results
- SMD =
- standardized mean difference
ZL and LW contributed equally to this work.
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Lai Z, Wang L, Liao X, Chen Y, Liu C, Wang C, He J. Association of hormone receptor status with cardiovascular disease mortality in 399,209 patients with stage I to III breast cancer: A population-based study. Medicine 2022;101:46(e31911).
Contributor Information
Zhihua Lai, Email: 283770460@qq.com.
Luxia Wang, Email: goestbaby@163.com.
Xiaohong Liao, Email: 919524781@qq.com.
Yuanping Chen, Email: 382505721@qq.com.
Chao Liu, Email: 282067371@qq.com.
Chen Wang, Email: goestbaby@163.com.
References
- [1].GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England). 2015;385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Herrmann J. From trends to transformation: where cardio-oncology is to make a difference. Eur Heart J. 2019;40:3898–900. [DOI] [PubMed] [Google Scholar]
- [4].Patnaik JL, Byers T, DiGuiseppi C, et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ramirez AG, Muñoz E, Long Parma D, et al. Quality of life outcomes from a randomized controlled trial of patient navigation in Latina breast cancer survivors. Cancer Med. 2020;9:7837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Alonso-Molero J, Molina AJ, Jiménez-Moleón JJ, et al. Cohort profile: the MCC-Spain follow-up on colorectal, breast and prostate cancers: study design and initial results. BMJ Open. 2019;9:e031904e031904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alonso-Molero J, Dierssen-Sotos T, Gomez-Acebo I, et al. Quality of life in a cohort of 1078 women diagnosed with breast cancer in Spain: 7-year follow-up results in the MCC-spain study. Int J Environ Res Public Health. 2020;17:8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Armenian SH, Xu L, Ky B, et al. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol. 2016;34:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kwan ML, Cheng RK, Iribarren C, et al. Risk of cardiometabolic risk factors in women with and without a history of breast cancer: the pathways heart study. J Clin Oncol. 2022;40:1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Christensen RAG, Kirkham AA. Time-restricted eating: a novel and simple dietary intervention for primary and secondary prevention of breast cancer and cardiovascular disease. Nutrients. 2021;13:3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McLaughlin M, Florida-James G, Ross M. Breast cancer chemotherapy vascular toxicity: a review of mediating mechanisms and exercise as a potential therapeutic. Vasc Biol. 2021;3:R106–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harris EER. Breast radiation and the heart: cardiac toxicity and cardiac avoidance. Clin Breast Cancer. 2021;21:492–6. [DOI] [PubMed] [Google Scholar]
- [13].Gonciar D, Mocan L, Zlibut A, et al. Cardiotoxicity in HER2-positive breast cancer patients. Heart Fail Rev. 2021;26:919–35. [DOI] [PubMed] [Google Scholar]
- [14].Sund M, Garcia-Argibay M, Garmo H, et al. Aromatase inhibitors use and risk for cardiovascular disease in breast cancer patients: a population-based cohort study. Breast (Edinburgh, Scotland). 2021;59:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gradishar WJ, Moran MS, Abraham J, et al. NCCN Guidelines® insights: breast cancer, version 4.2021. J Natl Compr Canc Net. 2021;19:484–93. [DOI] [PubMed] [Google Scholar]
- [16].Goldvaser H, Barnes TA, Šeruga B, et al. Toxicity of extended adjuvant therapy with aromatase inhibitors in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2018;110. [DOI] [PubMed] [Google Scholar]
- [17].Matthews A, Stanway S, Farmer RE, et al. Long term adjuvant endocrine therapy and risk of cardiovascular disease in female breast cancer survivors: systematic review. BMJ (Clinical research ed). 2018;363:k3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yu Q, Xu Y, Yu E, et al. Risk of cardiovascular disease in breast cancer patients receiving aromatase inhibitors vs. tamoxifen: a systematic review and meta-analysis. J Clin Pharm Ther. 2022;47:575–87. [DOI] [PubMed] [Google Scholar]
- [19].Qian X, Li Z, Ruan G, et al. Efficacy and toxicity of extended aromatase inhibitors after adjuvant aromatase inhibitors-containing therapy for hormone-receptor-positive breast cancer: a literature-based meta-analysis of randomized trials. Breast Cancer Res Treat. 2020;179:275–85. [DOI] [PubMed] [Google Scholar]
- [20].Bushnell CD, Goldstein LB. Risk of ischemic stroke with tamoxifen treatment for breast cancer: a meta-analysis. Neurology. 2004;63:1230–3. [DOI] [PubMed] [Google Scholar]
- [21].Matthews AA, Peacock Hinton S, Stanway S, et al. Endocrine therapy use and cardiovascular risk in postmenopausal breast cancer survivors. Heart (British Cardiac Society). 2021;107:1327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jacobse JN, Schaapveld M, Boekel NB, et al. Risk of heart failure after systemic treatment for early breast cancer: results of a cohort study. Breast Cancer Res Treat. 2021;185:205–14. [DOI] [PubMed] [Google Scholar]
- [23].Ligibel JA, James O’Malley A, Fisher M, et al. Risk of myocardial infarction, stroke, and fracture in a cohort of community-based breast cancer patients. Breast Cancer Res Treat. 2012;131:589–97. [DOI] [PubMed] [Google Scholar]
- [24].Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pincus D, Jenkinson R, Paterson M, et al. Association between surgical approach and major surgical complications in patients undergoing total hip arthroplasty. Jama. 2020;323:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Amer Statist Assoc. 1999;94:496–509. [Google Scholar]
- [27].Li Y, Kong X, Xuan L, et al. Prolactin and endocrine therapy resistance in breast cancer: the next potential hope for breast cancer treatment. J Cell Mol Med. 2021;25:10327–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–92. [DOI] [PubMed] [Google Scholar]
- [29].Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–802. [DOI] [PubMed] [Google Scholar]
- [30].Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. [DOI] [PubMed] [Google Scholar]
- [31].Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet (London, England). 2011;378:771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37:423–38. [DOI] [PubMed] [Google Scholar]
- [33].Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Toivonen KI, Williamson TM, Carlson LE, et al. Potentially modifiable factors associated with adherence to adjuvant endocrine therapy among breast cancer survivors: a systematic review. Cancers 2020;13:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moon Z, Moss-Morris R, Hunter MS, et al. Barriers and facilitators of adjuvant hormone therapy adherence and persistence in women with breast cancer: a systematic review. Patient Prefer Adherence. 2017;11:305–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Friese CR, Pini TM, Li Y, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sun JY, Zhang ZY, Qu Q, et al. Cardiovascular disease-specific mortality in 270,618 patients with non-small cell lung cancer. Int J Cardiol. 2021;330:186–93. [DOI] [PubMed] [Google Scholar]
- [38].Xu L, Zhang Z, Xiang Q, et al. Extended adjuvant therapy with aromatase inhibitors for early breast cancer: a meta-analysis of randomized controlled trials. Clin Breast Cancer. 2019;19:e578–88. [DOI] [PubMed] [Google Scholar]