Abstract
Immune checkpoint inhibitors (ICIs) have changed the status of tumor immunotherapy. ICIs-related adverse events (irAEs) have the high incidence and are difficult to predict and prevent. Researches have suggested that changes of cytokines were associated with irAEs. This study focused on the changes of interleukin-6 (IL-6) and interferon-γ in patients before and after irAEs and trying to find the biomarkers of irAEs. Collect basic data of patients who were treated with ICIs in China-Japan Friendship Hospital from January 2017 to August 2021 and had irAEs. Make statistics on IL-6 and INF-γ in the blood before and after irAEs. A total of 10 patients were enrolled, including 7 males and 3 females. According to statistical analysis, the IL-6 concentration level after irAEs was significantly higher than before, and the difference was statistically significant (P = .023); the interferon-γ concentration level was not changed significantly from before, the difference was not statistically significant (P = .853). The elevation of IL-6 was associated with the occurrence of adverse reactions in ICIs.
Keywords: biomarks, changes, IFN-γ, IL-6, IrAEs
1. Introduction
Since it was approved for the treatment of advanced melanoma in 2011, Immune checkpoint inhibitors (ICIs) have been gradually applied to more and more tumors, changing the status quo of tumor immunotherapy.[1] At present, it has become the first-line recommended regimen for various malignant tumors such as melanoma and lung cancer.[2] And of course, patients benefit a lot from these ICIs.[3] However, the application of such drugs will disturb the immune balance of the body to a certain extent, and even lead to the occurrence of ICIs-related adverse events (irAEs).[4] If irAEs cannot be detected and dealt with in time, it will not only affect the progress and efficacy of immunotherapy for cancer patients, but also cause serious consequences, and even lead to the death of patients in severe cases. However, due to the complex and diverse symptoms of irAEs and the highly unpredictable timing of occurrence, irAEs are difficult to predict and prevent. It is precise because of the existence of irAEs that it has become an important reason for restricting clinicians to choose ICIs therapy.[5] Therefore, it is of great significance to discover the change rules of certain indicators before and after the occurrence of irAEs from the existing clinical examination and test data, and use them to assist in predicting or preventing the occurrence of adverse reactions. Changes in cytokines are caused by dysregulation of the immune system in vivo at the time of irAEs. However, changes in external performances may occur later than changes in serological factors in vivo. Therefore, changes in serologically relevant factors can be monitored in advance and used to predict and prevent irAEs.
In recent years, studies have found that certain cytokines such as interleukin-6 (IL-6), interferon-γ (IFN-γ) will have obvious changes before and after the occurrence of irAEs,[4,6] which may have some predictive significance for the occurrence of irAEs, but it is still in the exploratory stage.[7] Therefore, we conducted a study, recorded and counted the concentrations of IL-6 and IFN-γ in patients with malignant tumors in the China-Japan Friendship Hospital during the treatment of ICIs and when irAEs occurred to explore the IL-6, IFN-γ levels and the changes in them before and after the occurrence of irAEs, and explore its clinical significance.
2. Materials and methods
2.1. Patients
Patients who were hospitalized in the China-Japan Friendship Hospital from January 2017 to August 2021, were treated with ICIs and developed irAEs.
2.2. Collection and review of clinical information
All patients’ diagnosis and treatment information come from the China-Japan Friendship Hospital medical record system and hospital information management system. This study mainly recorded indicators: basic demographic characteristics: gender and age. Clinical diagnosis and treatment characteristics: time of diagnosis, tumor type, ICIs drugs, irAEs type, and treatment plan. Serological indicators: IL-6 and IFN-γ.
2.3. Inclusion and exclusion criteria
Inclusion criteria: pathologically diagnosed with malignant tumor; treated with ICIs and developed irAEs; no previous immune system-related diseases; complete medical records and examination data.
Exclusion criteria: combined with immune system-related diseases, including but not limited to autoimmune thyroiditis, autoimmune hepatitis, scleroderma; combined with severe liver and kidney damage; missing medical records, such as incomplete test results or lost.
2.4. Statistical analysis
SPSS 26 software was used for statistical analysis of data, and measurement data were expressed as mean ± standard deviation. When the overall data represented by the sample obeys the normal distribution or approximately normal distribution, the paired t test is used for the comparison of a single group before and after; the nonparametric test is used for the data not conforming to the normal distribution.
2.5. Ethical approval
This study has been approved by the hospital ethics committee (No.2021-122-K80).
3. Results
3.1. Basic information of the participants
A total of 10 patients were included in this study, including 7 males and 3 females, with an average age of 54.1 years. The tumor types involved 5 cancer types, including lung cancer, gastric cancer, colon cancer, liver cancer, and kidney cancer, among which lung cancer accounted for 60% (6/10). ICIs include tislelizumab, toripalizumab, pembrolizumab, sintilimab, and nivolumab. The irAEs mainly include thyroiditis, pneumonia, liver injury, and dermatitis, among which thyroiditis has the highest incidence, accounting for about 40% (4/10) (Table 1).
Table 1.
Clinical characters of patients.
| Characters | Number (n = 10) |
|---|---|
| Gender | |
| Male | 7 |
| Female | 3 |
| Age | |
| Mean (range) | 54.1 (35–64) |
| Cancer type | |
| Lung cancer | 6 |
| Stomach cancer | 1 |
| Colon cancer | 1 |
| Liver cancer | 1 |
| Kidney cancer | 1 |
| ICI | |
| Tislelizumab | 3 |
| Toripalimab | 2 |
| Pembrolizumab | 2 |
| Sintilimab | 2 |
| Nivolumab | 1 |
| irAEs type | |
| Thyroiditis | 4 |
| Pneumonia | 3 |
| Liver damage | 2 |
| Dermatitis | 1 |
irAEs = inhibitors-related adverse events, ICI = immune checkpoint inhibitors.
3.2. Cytokine levels in each participant
As can be seen, before the occurrence of irAEs, 8 of the 10 patients had IL-6 concentrations within the normal range; when irAEs occurred, 8 of them were above the normal range, and only 2 of them were below the normal range. And the 2 patients had lower IL-6 concentrations than before irAEs. In terms of IFN-γ concentrations, both before and after the occurrence of irAEs, all patients’ IFN-γ concentrations were within the normal range (Table 2).
Table 2.
The cytokine concentration of each patient.
| Cytokine | Time | Patinet 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 (pg/mL) | Before irAEs | 3.05 | 3.96 | 5.35 | 3.10 | 3.44 | 5.19 | 0.00 | 5.17 | 15.43 | 5.46 |
| irAEs | 23.03 | 10.45 | 5.05 | 5.89 | 7.72 | 8.87 | 8.95 | 2.64 | 25.00 | 11.01 | |
| IFN-γ (pg/mL) | Before irAEs | 7.51 | 0.00 | 13.41 | 8.51 | 2.76 | 3.83 | 6.70 | 0.00 | 21.58 | 0.00 |
| irAEs | 5.82 | 2.52 | 6.82 | 2.59 | 4.45 | 9.54 | 4.25 | 2.81 | 17.45 | 5.87 |
The normal range of IL-6 is < 5.4 pg/mL, and the normal range of IFN-γ is < 23.01 pg/mL. IL-6 = interleukin-6, INF-γ = interferon-γ, irAEs = inhibitors-related adverse events.
3.3. Changes and levels of cytokines before and after irAEs
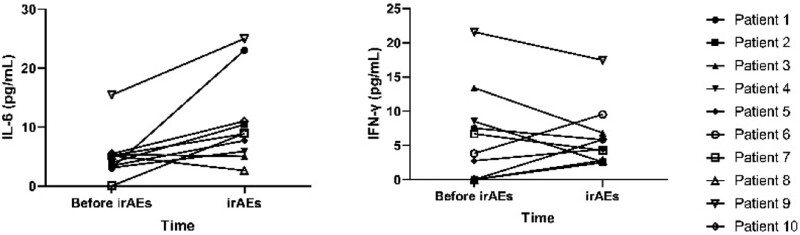
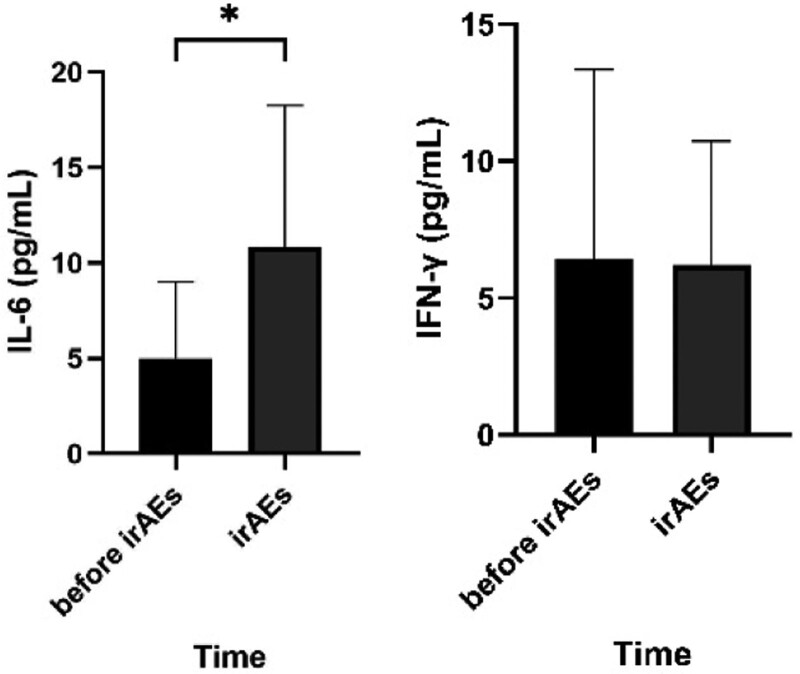
Among the above 10 patients, when irAEs occurred, the IL-6 concentration in 8 patients increased compared with that before the irAEs occurred, and all of them were higher than normal levels; otherwise, IL-6 concentration in 2 patients was lower than before and lower than the normal high value. As for the changing trend of IFN-γ, 5 cases increased compared with those before irAEs, and 5 cases decreased, but all were within the normal range (Fig. 1). Statistical analysis showed that when irAEs occurred, the concentration of IL-6 increased significantly compared with that before irAEs, and the difference was statistically significant (P = .023). There was no significant change in IFN-γ compared with that before the occurrence of irAEs, and the difference was not statistically significant (P = .853) (Fig. 2).
Figure 1.
The tendency of cytokine. Elevations in IL-6 were detected in 8 of 10 individuals after the onset of irAEs and all were above the normal range; elevations in INF-γ were detected in 5 individuals but remained within the normal range. IL-6 = interleukin-6, INF-γ = interferon-γ, irAEs = inhibitors-related adverse events.
Figure 2.
The concentration of IL-6 and IFN-γ at different time. Statistical analysis revealed a statistically significant difference in IL-6 after the occurrence of irAEs compared to that before, in the samples included in the statistics; while no statistically significant difference was observed in INF-γ. (*P ≤ .05). IL-6 = interleukin-6, INF-γ = interferon-γ, irAEs = inhibitors-related adverse events.
4. Discussion
With the wide application of ICIs, the incidence of irAEs occurrence is gradually increasing.[8] The previous literature showed that the symptoms of irAEs are diverse and can occur in multiple organs and systems such as digestion, circulation, endocrine, and skin.[9] In our study, the types of irAEs in our study were mainly thyroiditis, pneumonia, hepatic injury, and dermatitis, which were consistent with the previous studies. What’s more, irAEs can occur at any stage of treatment and are highly unpredictable.[10,11] But so far, the mechanism of irAEs has not been fully elucidated,[12] leading to early prevention with no targeted drugs.[13] It is crucial to explore relevant biomarkers and monitor them regularly in order to predict the occurrence of irAEs in advance and conduct timely intervention. Recent researches suggested that IL-6 is currently promising as a biomarker in predicting irAEs, but lacking data from sufficient clinical trials.[14]
IL-6 is an important proinflammatory cytokine,[15] participates in the body’s inflammatory process through different pathways,[16] plays a role in promoting the development of inflammation, and has a linear relationship with the disease state. Some studies have found that when irAEs occur, the concentration of IL-6 will increase significantly compared with before,[4,7,14,17–19] so it is suggested that IL-6 may be an important biomarker for predicting irAEs.[20] In this study, we found that when irAEs occurred, the IL-6 concentration of 8 people increased compared with the baseline period, which was consistent with the previous literature. However, some studies have also reported that the changes of IL-6 have no significant correlation with the occurrence of irAEs. Just as in this study, the IL-6 level of 2 patients did not increase significantly when irAEs occurred, but decreased. Therefore, the conclusions of this study still need to be demonstrated by large-scale clinical studies and more frequent detection of IL-6 in the whole ICIs treatment to finally clarify the predictive value of IL-6.
IFN-γ is mainly produced by NK cells and has functions such as promoting the occurrence and development of inflammation and immune regulation.[21] Because IFN-γ can change the tumor microenvironment, and thus improve the efficacy of ICIs therapy to a certain extent,[19,22] it is believed that the level of IFN-γ has a certain correlation with the efficacy of ICIs.[23] In terms of irAEs, studies have found that a low level of IFN-γ after treatment may be associated with the occurrence of ICI-related pneumonia.[24] In this study, although the IFN-γ concentration of 5 patients decreased after the occurrence of irAEs, 2 of them developed ICI-related pneumonia, and the incidence rate was 40%, which was consistent with the literature. In another 5 patients, the level of IFN-γ increased after the occurrence of irAEs, which was inconsistent with the reports in the literature. The reason may be related to the small sample size and selection bias of this study.
From the results of this study, we can conclude that there is a certain correlation between high levels of IL-6 after treatment and the occurrence of irAEs, which is consistent with the literature; while the changes in IFN-γ have no clear correlation with the occurrence of irAEs, which is inconsistent with the literature. However, due to the small number of samples included in this study, and the fact that this study is a retrospective study, there may be selection bias, the conclusions of this study still need to be verified and further explored by large-scale prospective clinical studies.
Author contributions
Xu Zhang brewed and designed experiments, carried out research, and drafted articles. Xingyu Lu conducted data statistics analysis and revised the content of the article. Yixuan Yu and Kexin Tan collected data and reviewed the data. Huijuan Cui provided research ideas, designed experiments, and revised the content of the article.
Conceptualization: Xu Zhang, Xingyu Lu, Huijuan Cui.
Data curation: Xu Zhang, Xingyu Lu, Yixuan Yu, Kexin Tan.
Formal analysis: Kexin Tan.
Funding acquisition: Huijuan Cui.
Methodology: Xu Zhang, Xingyu Lu, Yixuan Yu, Kexin Tan.
Writing—original draft: Xu Zhang.
Writing—review and editing: Xu Zhang, Xingyu Lu, Huijuan Cui.
Abbreviations:
- ICIs =
- immune checkpoint inhibitors
- IFN-γ =
- interferon-γ
- IL-6 =
- interleukin-6
- irAEs =
- immune checkpoint inhibitors-related adverse events
XZ and XL contributed equally to this work.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
This project was funded by the Capital Health Development Research Project of China (shoufa-2022-2-4065) and the National High Level Hospital Clinical Research Funding (2022-NHLHCRF-LX-02-0111).
The authors declare that no competing interest.
This study has been approved by the hospital ethics committee. All participants informed and agreed to participate in this study.
How to cite this article: Zhang X, Lu X, Yu Y, Tan K, Cui H. Changes of IL-6 And IFN-γ before and after the adverse events related to immune checkpoint inhibitors: A retrospective study. Medicine 2022;101:46(e31761).
Contributor Information
Xu Zhang, Email: zx9300h@163.com.
Xingyu Lu, Email: 826102274@qq.com.
Yixuan Yu, Email: yuyixuankx@163.com.
Kexin Tan, Email: 547929045@qq.com.
References
- [1].Zhou C, Cheng XJ, Tu SP. Current status and future perspective of immune checkpoint inhibitors in colorectal cancer. Cancer Lett. 2021;521:119–29. [DOI] [PubMed] [Google Scholar]
- [2].He MY, Yang T, Wang YH, et al. Immune checkpoint inhibitor-based strategies for synergistic cancer therapy. Adv Healthcare Mater. 2021;10:2002104. [DOI] [PubMed] [Google Scholar]
- [3].Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49. [DOI] [PubMed] [Google Scholar]
- [4].Khan S, Khan SA, Luo X, et al. Immune dysregulation in cancer patients developing immune-related adverse events. Br J Cancer. 2019;120:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tanaka R, Okiyama N, Okune M, et al. Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-α is a biomarker of nivolumab reactivity. J Dermatol Sci. 2017;86:71–3. [DOI] [PubMed] [Google Scholar]
- [7].Hommes JW, Verheijden RJ, Suijkerbuijk KPM, et al. Biomarkers of checkpoint inhibitor induced immune-related adverse events: a comprehensive review. Front Oncol. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xu C, Chen YP, Du XJ, et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lewis AL, Chaft J, Girotra M, et al. Immune checkpoint inhibitors: a narrative review of considerations for the anaesthesiologist. Br J Anaesth. 2020;124:251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48. [DOI] [PubMed] [Google Scholar]
- [11].Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov. 2021. [DOI] [PubMed] [Google Scholar]
- [12].Lee DJ, Lee HJ, Farmer JR, et al. Mechanisms driving immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Curr Cardiol Rep. 2021;23. [DOI] [PubMed] [Google Scholar]
- [13].Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv264–6. [DOI] [PubMed] [Google Scholar]
- [14].Ke W, Zhang L, Dai Y. The role of IL-6 in immunotherapy of non-small cell lung cancer (NSCLC) with immune-related adverse events (irAEs). Thorac Cancer. 2020;11:835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yao X, Huang J, Zhong H, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125–39. [DOI] [PubMed] [Google Scholar]
- [16].Jones BE, Maerz MD, Buckner JH. IL-6: a cytokine at the crossroads of autoimmunity. Curr Opin Immunol. 2018;55:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Keegan A, Ricciuti B, Garden P, et al. Plasma IL-6 changes correlate to PD-1 inhibitor responses in NSCLC. J ImmunoTher Cancer. 2020;8:e000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Okiyama N, Tanaka R. Varied immuno-related adverse events induced by immune-check point inhibitors - nivolumab-associated psoriasiform dermatitis related with increased serum level of interleukin-6. Nihon Rinsho Meneki Gakkai Kaishi. 2017;40:95–101. [DOI] [PubMed] [Google Scholar]
- [19].Wang M, Zhai X, Li J, et al. The role of cytokines in predicting the response and adverse events related to immune checkpoint inhibitors. Front Immunol. 2021;12:670391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jia XH, Geng LY, Jiang PP, et al. The biomarkers related to immune related adverse events caused by immune checkpoint inhibitors. J Exp Clin Cancer Res. 2020;39:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Enzler T, Gillessen S, Manis JP, et al. Deficiencies of GM-CSF and interferon gamma link inflammation and cancer. J Exp Med. 2003;197:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Teng F, Meng X, Kong L, et al. Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: a systematic review. Cancer Lett. 2018;414:166–73. [DOI] [PubMed] [Google Scholar]
- [23].Boutsikou E, Domvri K, Hardavella G, et al. Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:1758835918768238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hirashima T, Kanai T, Suzuki H, et al. The levels of interferon-gamma release as a biomarker for non-small-cell lung cancer patients receiving immune checkpoint inhibitors. Anticancer Res. 2019;39:6231–40. [DOI] [PubMed] [Google Scholar]