Abstract
Endowing materials with catalytic activities analogous to those of the natural endothelium to thus enhance their biological performance has become an option for constructing advanced blood-contact materials. The electron transfer between Cu2+ and Cu+ in the porphyrin center can catalyze the reaction of GSH and GSNO to generate NO, and this electron transfer can also catalyze the decomposition of ROS. Based on this, we created a dual-catalytic surface possessing NO-generating and ROS-scavenging activities to better mimic the versatile catalytic abilities of the endothelium. Copper tetraphenylporphyrin/titanium dioxide nanoparticles (CuTPP/TiO2-NPs) exhibiting excellent NO-generating and ROS-scavenging activities were synthesized and immobilized on the material surface to form a dual-catalytic film (CuTPP/TiO2-film) with the help of the catechol chemistry technique. Unlike most single catalytic surfaces, the dual-catalytic CuTPP/TiO2-film effectively regulated the microenvironment surrounding the implanted device by releasing NO signaling molecules and scavenging harmful ROS. This dual-catalytic film exhibited excellent biosafety and biocompatibility with anti-thrombosis, vascular wall cells (ECs and SMCs) modulation, and anti-inflammatory properties. We envision that this dual-catalytic endothelial bionic strategy may provide a promising solution to the clinical problems plaguing blood-contact devices and provide a novel basis for the further development of surface catalytic-engineered biomaterials.
Keywords: Dual-catalytic surface, Copper tetraphenylporphyrin (CuTPP), NO-Generating and ROS-Scavenging activities, Cardiovascular materials
Graphical abstract

1. Introduction
Cardiovascular medical devices (e.g., vascular stents and artificial blood vessels) provide essential support for the modern treatment of cardiovascular diseases [1]. However, current cardiovascular medical devices are plagued by side effects such as coagulation, oxidative stress, inflammation, and tissue proliferation after implantation. Particularly for long-term implant devices, these harmful side effects typically lead to severe complications such as thrombosis, stenosis, and blockage, ultimately leading to treatment failure and even endangering patient lives [2,3].
To protect cardiovascular-implanted devices from these side effects, inspiration from the natural endothelial layer was sought. Researchers have determined that the excellent biocompatibility of the natural endothelium is based on its catalytic activities [[4], [5], [6], [7]]. In particular, Nitric Oxide (NO) that is catalyzed by endothelial cells exhibits promising properties, including anticoagulation, anti-inflammation, and anti-hyperplasia properties [[8], [9], [10]]. Due to its high efficiency and versatility, NO is now known to be the guardian of cardiovascular-implanted devices. Therefore, the loading of NO catalytic molecules (e.g., selenocysteine [SeCA] and copper [Cu]) onto the surface of cardiovascular devices through surface catalytic engineering has become a hot research topic [[11], [12], [13], [14], [15]].
However, the majority of the current studies examining the surface catalytic engineering of cardiovascular devices only focus on the NO generation efficiency of the materials while ignoring the modulation of the oxidative stress microenvironment in which the NO-generating devices typically function. The oxidative stress microenvironment leads to a tendency for generated NO to react with various oxygen radicals (ROS) to form secondary radicals, and these may not only diminish the benefits of NO but may also pose additional hazards [16,17]. For example, NO reacts with superoxide anions (O2−) to form nitrite radicals (ONOO−). ONOO− is incredibly harmful to the cardiovascular system and is an essential factor for inflammation, hyperplasia, and endothelial apoptosis. The generation of ONOO− radicals is undoubtedly a process that must be avoided [[18], [19], [20]].
| NO + O2−→ONOO− | (1) |
In-depth studies have demonstrated that Endothelial cells (ECs) possess multi-catalytic activity. On the one hand, ECs catalyze NO generation through NO synthase (eNOS). Additionally, the glycocalyx structure of the ECs adsorbs many antioxidant stress enzymes such as superoxide anion dismutase (SOD) and catalase (CAT) that scavenge excess free radicals through catalytic catabolism [16,17,[21], [22], [23]]. Therefore, the excellent biocompatibility and microenvironment-stabilizing ability of the natural endothelium could be closely related to its multi-catalytic activities.
Inspired by the above knowledge, we proposed the development of nanoparticles possessing multi-catalytic activities to mimic the numerous beneficial enzymes utilized by ECs for the surface catalytic engineering of cardiovascular devices. The key for this nanoparticle was the use of copper porphyrin (5,10,15,20-Tetraphenyl-21H, 23H-porphine copper [II] [CuTPP]), a biomolecule possessing catalytic functions of multiple enzymes such as eNOS and SOD/CAT can occur simultaneously. CuTPP has been widely used in the context of tumor therapy, and its biosafety has been well acknowledged. However, its application in the context of cardiovascular devices is currently rare. We hypothesized that the chelated Cu ion in the porphyrin ring of CuTPP could sustainably generate NO in vivo by catalyzing the endogenous NO donor nitroso-glutathione (GSNO). Concurrently, CuTPP acts as a mimetic enzyme for SOD and CAT (both SOD and CAT possess iron porphyrins as their catalytic cores) and exhibits the ability to decompose H2O2 and O2−. Therefore, CuTPP as an SOD/CAT mimetic enzyme capable of catalyzing NO generation is expected to be used to construct a multi-catalytic surface that mimics the natural endothelium.
TiO2 is a semiconductor material that plays an important role in the development of antibacterial and antitumor therapy due to its excellent photocatalysis [24]. Additionally, TiO2 has an oxide layer that spontaneously generated on its surface. This layer is very biocompatible and is frequently utilized in blood contact devices, particularly stents [25]. However, TiO2 implants were also insufficient in anticoagulant [26]. The modification of TiO2 by copper porphyrin can produce NO in situ in vivo, which can further improve the biocompatibility.
In this study, CuTPP/TiO2 nanoparticles (CuTPP/TiO2-NPs) possessing good biosafety as a carrier were prepared by adsorption using TiO2 nanoparticles (TiO2-NPs, P25). Subsequently, using polydopamine as a bioadhesive, CuTPP/TiO2 nanoparticles were immobilized onto the surface of the cardiovascular material (Fig. 1). This CuTPP/TiO2-modified surface exhibited favorable multi-catalytic activities for facilitating NO generation and ROS decomposition. This multi-catalytic surface possessed excellent biocompatibility by effectively inhibiting a series of adverse reactions (coagulation, inflammation, tissue proliferation, and others) and by promoting EC growth and repair, increases the expression of contractile SMCs, and inhibits intimal hyperplasia, thus representing an effective strategy to address in-stent restenosis and late thrombosis. We envision that this multi-catalytic surface that produces NO and decomposes ROS will provide a new reference for the future design of surface catalytic-engineered cardiovascular devices.
Fig. 1.
Schematic of CuTPP/TiO2-NPs-immobilized coating function.
2. Material and methods
2.1. Materials
The 5,10,15,20-Tetraphenyl-21H,23H-porphine copper (II) (CuTPP) (Mw = 676.27 Da, CAS 14172-91-9) was purchased from HEOWNS. P25 TiO2 particles, dopamine hydrochloride, S-nitrosoglutathione (GSNO), and l-glutathione (GSH) were purchased from Sigma-Aldrich. Dichloromethane was purchased from the Chengdu Kelon Chemical Reagent Factory.
2.2. Synthesis of CuTPP/TiO2-NPs and CuTPP/TiO2-films
CuTPP/TiO2-NPs were synthesized in the following steps: (1) copper porphyrin (3.85 mg) was dissolved in CH2Cl2 (20 mL); (2) TiO2 nanoparticles (P25, 0.5 g) were then added to the solution. The mixed suspension was magnetically stirred at room temperature for 4 h (3) The solvent was removed by rotary evaporation at 60 °C to collect the CuTPP and TiO2 composite particles that are referred to as CuTPP/TiO2-NPs.
Synthesis of CuTPP/TiO2-films was achieved using the following steps: (1) the synthesized CuTPP/TiO2-NPs were mixed in 1 mg/mL of PDA solution at a concentration of 2 mg/mL and ultrasonically dispersed; (2) stainless steel (SS), polyvinyl chloride (PVC), silicon (Si), and glass substrates were immersed in the prepared solution and allowed to stand for 6 h to avoid light; (3) the substrate was dried in an oven at 37 °C to obtain CuTPP/TiO2-films on different substrates.
2.3. Materials characterization
2.3.1. Characterization of CuTPP/TiO2-NPs
A dynamic light scattering particle size analyzer (MALVERN Nano-2S90, Malvern Ltd., Malvern, UK) was used to determine the average particle size, and the surface chemical properties of the samples were detected using ST-IR20SX Fourier transform infrared spectroscopy (FTIR) and a UV-via-NIR spectrophotometer (Shimadzu, Japan). X-ray diffraction (XRD) was performed by the X'Pert Pro MPD (Philips, the Netherlands) to detect the crystalline structure of the samples. Under the acceleration voltage of 200 kV, the transmission electron microscope image of the material was observed using the JEM-2011 Transmission Electron Microscope (TEM). An X-ray photoelectron spectrometer (Thermo SCIENTIFIC ESCALAB 250Xi, Thermo Fisher, US) was used to test and analyze the chemical composition of the sample surface.
2.3.2. Characterization of the CuTPP/TiO2-films
A water contact angle (WCA) tester was used to determine the hydrophobicity of the material surface. The morphologies of these films were observed using scanning electron microscopy (SEM, Quanta 200, FEI, Holland). Composition analyses were performed using energy dispersive spectroscopy (EDS, Quanta 200, FEI, Holland).
2.3.3. Hydrogen peroxide (H2O2) decomposition experiment
The ability of CuTPP/TiO2-NPs to decompose H2O2 was determined using an H2O2 kit (Sigma-Aldrich, USA). The principle of this test is that H2O2 reacts with molybdate to form a complex with a specific absorbance peak at 406 nm. In this test, a hydrogen peroxide standard solution was added dropwise to the CuTPP/TiO2-NPs solutions (0.0625, 0.125, 0.25, 0.5, 1, and 2 mg/mL) and reacted for 30 min at 37 °C. Then, liquids A and B from the kit were added to the solution. After the reaction at 37 °C for 15 min, the solution was transferred into a 96-well plate, and the absorbance of the mixed liquid at 406 nm was determined using a microplate reader.
2.3.4. Catalytic NO-releasing test
The catalytic activity of the CuTPP/TiO2-films in regard to NO generation was determined in real time using a chemiluminescence NO analyzer (NOA 280i, Sievers, Boulder, CO, USA). The detailed procedure for this test has been reported elsewhere [12]. First, 5 mL of PBS (pH 7.4) containing 10 μM GSNO and 10 μM of GSH was used to run the baseline. After the NO-generating baseline was stabilized for 10 min, a stainless-steel foil (0.3 × 1 cm) with CuTPP/TiO2-film was immersed in the reaction chamber for 30 min, and the generated NO was purged from the solution and delivered to the NOA reaction chamber by a nitrogen gas carrier where it reacted with O3 to produce NO2∗ in its excited state. Upon relaxation to NO2∗, a photon was emitted that was then detected to calculate the NO generated from the sample. The NO generation curve catalyzed by the CuTPP/TiO2-film was monitored in real-time.
2.4. Biocompatibility characterization
2.4.1. Hemolysis test
First, the experimental samples (e.g., CuTPP/TiO2-film on an SS disc, Diameter = 10 mm) were placed in centrifuge tubes with 10 mL of physiological saline. Then, we preheated the tubes in a 37 °C water bath for 30 min. Subsequently, 4 mL of whole blood was diluted with 5 mL of normal saline. We pipetted 0.2 mL of the diluted whole blood into the centrifuge tube where the sample was placed in the previous step, shook and mixed the tube, and incubated the mixture in a 37 °C incubator for 1 h. After pouring the liquid into the centrifuge tube, it was centrifuged at 3000 rpm for 5 min. After centrifugation, the supernatant was carefully collected and transferred to a 96-well plate. We added 150 μL to each well, measured the absorbance value at a wavelength of 540 nm, measured three parallel samples for each sample, and finally calculated the average value. For the diluted experiment, 0.2 mL of diluted blood +10 mL of normal saline was used as the negative control group, and 0.2 mL of diluted blood +10 mL of deionized water was used as the positive control group. The hemolysis rate of each sample was calculated using the following formula:
2.4.2. In vitro anti-platelet test
Human blood containing an anticoagulant (sodium citrate [3.8 wt%]) was centrifuged at 1500 rpm for 15 min to obtain plasma-rich platelets (PRP). After adding 70 μL of PRP to cover the surface of each sample, the samples were divided into two groups. One group was treated with nitric oxide donors, including 10 μM GSNO and 10 μM glutathione. Then, the samples were incubated for 30 min at 37 °C. Subsequently, all of the samples were washed with PBS and fixed with 2.5% glutaraldehyde. Finally, the adhered platelets were stained with rhodamine and observed under a fluorescence microscope.
2.4.3. Anti-thrombogenicity test in ex-vivo blood circulation
We followed the ethical guidelines of the China Animal Protection Committee and the Southwest Jiaotong University Animal Use Protocol. New Zealand white rabbits (2.5–3.5 kg) were used in this study. General anesthesia was administered to all experimental animals before the experiments were conducted. The left carotid artery and right jugular vein of rabbits were isolated and connected to an arteriovenous (AV) extracorporeal circuit (ECC). In the middle of the ECC, an SS foil with CuTPP/TiO2-film was pre-placed and tightly attached to the inner wall. The ECC was then loosened on both sides to allow for the blood to flow. After blood circulation for 2 h, the flow rate in the PVC pipe loop was evaluated. Subsequently, ECC was disconnected and removed from the animals. Cross-sections of the tubes and photographs of residual thrombus formation were acquired to analyze the percentage of obstruction in the circuit. Specimens were then rinsed with PBS (pH 7.4), stored overnight in 2.5% glutaraldehyde solution (with PBS), dehydrated, and finally critical point dried for SEM analysis.
2.4.4. Vascular cell growth
To study the growth behavior of cells on the surface of the material, this experiment utilized static cultures of human venous endothelial cells (ECs) and arterial smooth muscle cells (SMCs). In these experiments, human venous ECs and human arterial SMCs were divided into two groups. One group lacked NO donors in the cell culture medium. The other group contained 10 μM of the NO donor (GSNO) and 10 μM of glutathione in the cell culture medium. The medium containing the NO donor was changed every day. Briefly, we placed the sample in a 24-well cell culture plate, added 1 mL of cell culture medium containing 2 × 104 ECs or SMCs, and incubated the endothelium statically in a cell incubator containing 5% CO2 at 37 °C. ECs were cultured for 4 h, 1 d, and 3 d, while SMCs were cultured for 1 d. The samples were then washed with PBS (pH 7.4) and fixed with 2.5% glutaraldehyde. After rhodamine staining, samples were observed under a fluorescence microscope.
Cell Counting Kit-8 (CCK-8) was used to estimate the proliferation of ECs and SMCs. Briefly, after the old medium was removed, it was replaced with a new medium supplemented with CCK-8 reagent (10%). After incubation at 37 °C for 3 h, the absorbance of the medium was measured at 450 nm.
2.4.5. Culturing of macrophages
The static culture method for macrophages (from SD suckling mice) was similar to those used for ECs and SMCs. The planting density of the cells was 6 × 104 cells/mL, and the culture time was one day.
2.4.6. In vitro cytotoxicity
The cytotoxicity of CuTPP/TiO2-NPs against ECs was evaluated using a CCK-8 assay. The cells were incubated in cell culture medium with CuTPP/TiO2-NPs at concentrations of 0.03125, 0.0625, 0.125, or 0.25 mg mL−1. After 20 h of culture, the cell culture medium containing nanoparticles was aspirated. Then, we added medium containing 10% CCK-8 to incubate the ECs for 4 h more. Finally, the absorbance of the cell culture medium was measured at 450 nm using a microplate reader.
2.5. Wire implantation in vivo
In vivo animal implantation experiments were conducted to further investigate the biological properties of the modified stents. All procedures were performed in accordance with the China Council on Animal Care and Southwest Jiaotong University animal use protocols and in accordance with the ethical rules for experimental animals.
The samples were prepared as described in section 2.2, and the prepared wire was sterilized by ozonation and implanted into the abdominal aorta of male SD rats weighing 250–300 g. After the samples were implanted and the rats were fed for one month, the rats were anesthetized by intraperitoneal injection of 3% sodium pentobarbital, the abdominal aorta was stripped, and the samples were removed from the vessels as a whole and fixed in 4% paraformaldehyde for longer than 24 h. Finally, the samples were carefully withdrawn from the paraformaldehyde-fixed vascular tissues that were subsequently paraffin-embedded and stained in sections for observation.
2.6. Statistical analysis
The results are expressed as mean ± standard deviation of the mean for each sample. Three independent samples were used for each experimental sample group unless otherwise indicated. One-way ANOVA of variance (SPSS 17.0, IBM, USA) was used to compare the data from different groups. ∗p < 0.05 indicated significance.
3. Results and discussion
3.1. Characterizations of CuTPP/TiO2-NPs
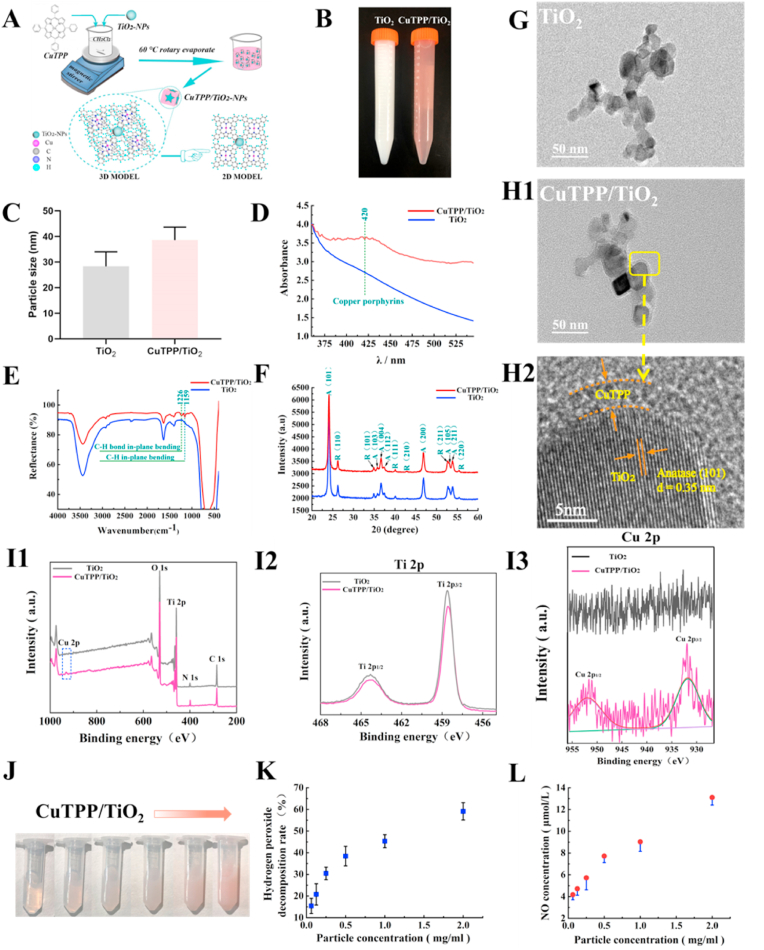
Fig. 2A illustrates the preparation process and ideal particle model of CuTPP/TiO2-NPs. As presented in Fig. 2B, a noticeable color change (from white to red) was observed during sample preparation. Particle size analysis revealed that the average particle size of TiO2-NPs was 25–35 nm. After adsorption of copper tetraphenylporphyrin (CuTPP), the average particle size of the CuTPP/TiO2-NPs increased to 35–45 nm (Fig. 2C). Fig. 2D presents the UV–vis spectra of TiO2-NPs and CuTPP/TiO2-NPs. Compared to TiO2-NPs, a new absorption peak appeared at approximately 420 nm that could be attributed to the characteristic peak of CuTPP. As presented in Fig. 2E, the functional groups in the samples were detected using FITR. For TiO2-NPs and CuTPP/TiO2-NPs, the peaks at 3446 cm−1, 2925 cm−1, and 400-821 cm−1 could be attributed to the stretching vibration of surface O–H, C–H stretching vibration, and the stretching vibration of Ti–O, respectively. Compared to TiO2-NPs, CuTPP/TiO2-NPs exhibits two additional absorption peaks at 1226 cm−1 and 1159 cm−1. The peak at 1226 cm−1 can be attributed to the C–H bond in-plane bending vibration absorption peak of the benzene ring structure in CuTPP. The peak at 1159 cm−1 can be attributed to the C–H in-plane bending vibration absorption peak of the pyridine ring in CuTPP. The FT-IR results indicate that CuTPP was successfully adsorbed on the surface of TiO2, and this is consistent with the UV–Vis results.
Fig. 2.
(A) The preparation process and ideal particle model of CuTPP/TiO2-NPs. (B) Color changes in the samples during sample preparation. (C) The average particle size of TiO2-NPs and CuTPP/TiO2-NPs. (D) UV–vis spectra of the nanoparticles. (E) FT-IR spectra of the nanoparticles. (F) XRD patterns of TiO2-NPs and CuTPP/TiO2-NPs (A: Anatase; R: Rutile). TEM images of (G) TiO2-NPs and (H1) CuTPP/TiO2-NPs and (H2) HR-TEM images of CuTPP/TiO2-NPs. XPS spectra of TiO2-NPs and CuTPP/TiO2-NPs: (I1) survey, (I2) Ti 2p, (I3) Cu 2p. (J) Photo images of CuTPP/TiO2-NPs at scaled-up concentration. (K) The decomposition activity of samples against H2O2. (L) Activity of samples in regard to the catalytic generation of NO.
As presented in Fig. 2F, X-ray diffraction (XRD) was employed to investigate the differences in the crystal phases of TiO2 and CuTPP/TiO2. High-intensity peaks of anatase and low-intensity peaks of rutile were observed, thus suggesting that the nanoparticles were primarily composed of anatase crystals and a small amount of rutile crystals. No differences were observed in this pattern, thus indicating that the adsorption of CuTPP did not alter the crystal structure of TiO2.
As presented in Figure 2 (G, H1, and H2), transmission electron microscopy (TEM) was used to determine the shape and structure of CuTPP/TiO2-NPs. Morphologically, TiO2-NPs were predominantly round, while square nanoparticles were observed for CuTPP/TiO2-NPs (Figure 2 (G and H1). As presented in Fig. 2 H2, in the high-resolution TEM photograph the CuTPP/TiO2-NPs possessed lattice fringes with a pitch of 0.35 nm that could be attributed to the (101) crystal plane of anatase TiO2. Furthermore, we observed that surrounding TiO2, there is an amorphous adsorption layer possessing a thickness of approximately 3 nm that could be the CuTPP adsorption layer on the TiO2 surface. These results indicated that CuTPP was well distributed on the surface of TiO2 to form CuTPP/TiO2-NPs.
The composition and chemical state of the CuTPP/TiO2-NPs composites were investigated by XPS. It can be observed in Fig. 2 I1 that the total spectrum of CuTPP/TiO2-NPs exhibits a signal in the Cu 2p region relative to that of TiO2-NPs, and it can be clearly observed from the Cu 2p high-resolution map in Fig. 2 I3 that the signal in the Cu 2p region of CuTPP/TiO2-NPs can be anti-folded into two peaks, Cu 2p3/2 and 2p1/2 are located at 934.2 eV and 953.8 eV27-29. The Ti high-resolution map in Fig. 2 I2 indicates that the Ti signal of CuTPP/TiO2-NPs was lower than was that of TiO2-NPs, as it was wrapped by CuTPP. According to the XPS analysis, molecular CuTPP was successfully loaded onto the TiO2-NPs.
The catalytic activities of CuTPP/TiO2-NPs in regard to H2O2 decomposition and NO generation are presented in Figure 2 (J, K, and L). Different concentrations of CuTPP/TiO2-NPs were prepared in physiological saline solutions (Fig. 2J). For H2O2, CuTPP/TiO2-NPs exhibited significant catalytic decomposition activity (Fig. 2K). After 1 h of reaction conditions, the catalytic decomposition rate of H2O2 increased linearly from approximately 16% for 0.0625 mg/mL of CuTPP/TiO2-NPs to approximately 58% for 2 mg/mL of CuTPP/TiO2-NPs. The catalytic decomposition of the ROS (e.g., H2O2 and O2−) of CuTPP in vivo relies on the redox reaction between Cu (I) and Cu (II) in the presence of a large amount of reducing agents such as GSH. These results indicate that CuTPP/TiO2-NPs may exert considerable anti-oxidative stress activity in vivo.
Conversely, as presented in Fig. 2L, CuTPP/TiO2-NPs exhibited excellent catalytic activity to promote NO generation. In the presence of sufficient GSH and GSNO, after 1 h of reaction conditions the NO generation rate increased from the 4 nmol/cm2 for 0.0625 mg/mL of CuTPP/TiO2-NPs to 13 nmol/cm2 for 2 mg/mL of CuTPP/TiO2-NPs, in a nearly linear manner. These results suggest the NO-generating catalytic activity of CuTPP that has rarely been previously reported.
The above results indicate that CuTPP/TiO2-NPs could effectively catalyze both NO generation and H2O2 decomposition, and this could be applied to construct endothelial layer-like multi-catalytic surfaces.
3.2. Characterizations of CuTPP/TiO2-film
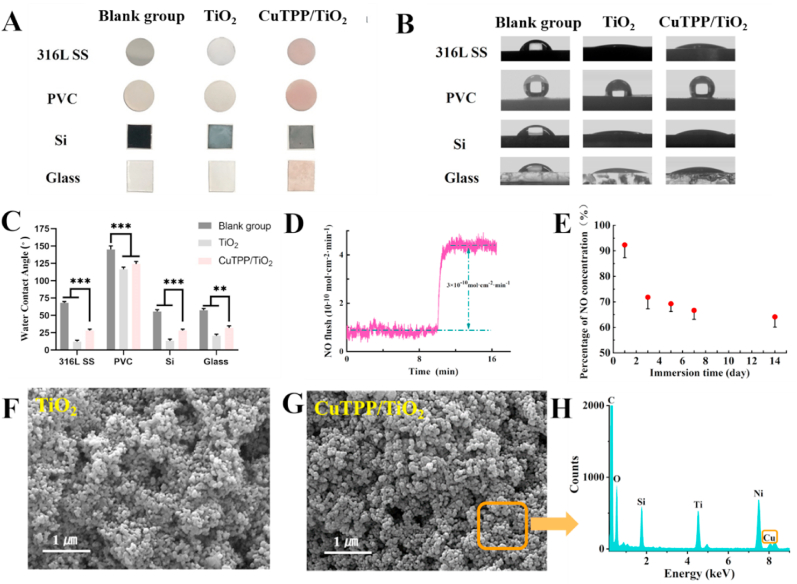
By using polydopamine as a bio-adhesive, TiO2-NPs and CuTPP/TiO2-NPs were immobilized on the surface of various biomedical materials, including stainless steel (SS), polyvinyl chloride (PVC), silicon (Si), and glass. As presented in Fig. 3A, the color of all of the materials changed to pink after immobilization of CuTPP/TiO2-NPs. Figure 3 (B and C) indicates that the hydrophilicity of all materials significantly increased after the deposition of the TiO2-film and CuTPP/TiO2-film. This could be primarily due to the introduction of numerous hydrophilic groups (e.g., –NH3 and phenolic hydroxyl groups) into the bio-adhesive PDA. Moreover, for the same materials, the hydrophilicity of the CuTPP/TiO2-film was lower than was that of the TiO2-film, and this could have resulted from the hydrophobic properties of CuTPP that contained a large number of hydrophobic benzene rings.
Fig. 3.
(A) Photographs of different material substrates after deposition of the films. (B) and (C) Photographs and results of the water contact angle test. (D) The NO-generating catalytic activity of CuTPP/TiO2-film. (E) The stability of NO-generating catalytic activity of the CuTPP/TiO2-film after 1, 3, 5, 7, and 14 days of immersion in PBS (pH = 7.4). SEM images of (F) TiO2-film and (G) CuTPP/TiO2-film. (H) EDS results for the CuTPP/TiO2-film.
The ability of the CuTPP/TiO2-film to catalyze NO generation was tested by real-time chemiluminescence measurements in a PBS environment. The concentrations of GSH and GSNO were both 10 μM. Fig. 3D indicates that the NO generation rate in the CuTPP/TiO2-film tended to be stable at approximately 3 × 10−10 mol cm−2·min−1 after the sample was placed in PBS solution for 2 min. The NO generation rate of the natural endothelium layer is approximately 1–4 × 10−10 mol cm−2∙min−1. Thus, the NO generation rate of the CuTPP/TiO2-film fell into the physiological range and was therefore expected to exert the physiological functions of NO gas. Notably, the CuTPP/TiO2 coating catalyses the generation of NO at a relatively constant rate without a burst generation during this process, and this could be attributed to the chelation of the copper element by the porphyrin ring that causes copper to become stably immobilized on materials. Furthermore, we soaked the samples in PBS (pH = 7.4) and shook them at 37 °C for 1, 3, 5, 7, and 14 days to examine the catalytic activity stability of CuTPP/TiO2-film. As presented in Fig. 3E, the NO-generating catalytic activity of the CuTPP/TiO2-film decreased rapidly by approximately 17% during the first 3 days of soaking. However, during the subsequent 3–14 days of soaking, the decrease in the CuTPP/TiO2-film NO-generating catalytic activity became slow and finally stabilized at approximately 65% of the original samples (presumably approximately 1.95 × 10−10 mol cm−2∙min−1).
The morphologies of the TiO2-film and CuTPP/TiO2-film were examined by scanning electron microscopy (SEM). Compared to the small spherical TiO2 particles, the CuTPP/TiO2-NPs were larger (Figure 3 (F and G)). Additionally, the energy dispersive spectrometer (EDS) results (Fig. 3 H) revealed that copper was present on the CuTPP/TiO2-NPs, thus indicating that CuTPP adhered well to the surface of the TiO2-NPs.
These results indicated that the CuTPP/TiO2-film possessed good stability in regard to catalytic NO generation, and this could be favorable for long-term implantation.
3.3. Blood compatibility test
Favorable hemocompatibility includes excellent resistance to thrombosis. Platelet adhesion, spreading, and aggregation on the material surface play important roles in the initial formation of the thrombus. The process of platelet adhesion and activation is regulated by both intravascular oxygen radicals and nitrogen radicals: (1) ROS leads to NO dysfunction that in turn inhibits platelet disaggregation to thereby promote platelet activation and recruitment [27]; (2) NO inhibits platelet activation through the upregulation of cGMP. In healthy blood vessels, ECs continuously produce NO and simultaneously break down ROS using multiple enzymes. Accordingly, we speculated that constructing dual-catalytic surfaces with catalytic breakdown of ROS and catalytic generation of NO may achieve excellent anti-thrombogenic ability.
In this study, we explored the ability of the CuTPP/TiO2-film to resist platelet adhesion and activation. As presented in Figure 4(A and B), in the donor (−) groups there was a large amount of platelet adhesion and spreading on SS. Meanwhile, on TiO2-film we observed a high degree of platelet aggregation. In contrast, on the CuTPP/TiO2-film the platelet adhesion area ratio was substantially reduced, and only a small number of platelets appeared to be aggregated.
Fig. 4.
(A) Fluorescence staining of platelet adhesion of the sample. (B) Statistical results for sample platelet adhesion area ratio. (C) Hemolysis test sample comparison chart. (D) Statistical graph of the sample hemolysis rate. Data are presented as mean ± SD (n = 6) and were analyzed using a one–way ANOVA, ∗p < 0.05, ∗∗p < 0.01,∗∗∗p < 0.001.
Conversely, the platelet adhesion area ratio was slightly reduced in the SS and TiO2-film in donor (+) groups relative to that of the donor (−) groups, and this could be related to the generation of a small amount of NO by spontaneous decomposition of the donor. In particular, on the CuTPP/TiO2-film (donor [+]) the addition of the NO donor significantly reduced platelet adhesion and aggregation. This result suggests that the CuTPP/TiO2-film film possesses excellent anti-thrombogenic ability by catalyzing NO generation.
In addition to its excellent antithrombogenic ability, this film does not trigger a hemolysis reaction, and this is an essential quality for cardiovascular materials. As presented in Figure 4 (C and D), the hemolysis rate for all samples was less than 1%, and this is far lower than the international standard for the hemolysis rate of cardiovascular materials (5%). In particular, the hemolysis rate of CuTPP/TiO2-film was less than 0.1%, and this is significantly lower than that of TiO2-film (approximately 0.4%). The lower hemolysis rate of the CuTPP/TiO2-film could be related to the observation that CuTPP/TiO2-NPs possess a larger particle size than do TiO2-NPs.
3.4. Ex vivo blood circulation test
Metal implants and polymer catheters are two typical types of cardiovascular devices. Here, we deposited a CuTPP/TiO2-film on an SS foil and inside a PVC catheter. We then performed ex vivo blood circulation tests to investigate the antithrombotic properties of this dual-catalytic film in an environment that was as similar as possible to the actual application (Fig. 5A). Due to the large size of the samples and the small rabbit blood banks, GSNO was injected intravenously as a supplement to compensate for the rapid breakdown of the endogenous NO donor.
Fig. 5.
(A) The ex vivo circulation thrombogenicity model of rabbits. (B) Photographs of thrombi formed on various samples and cross-sectional photographs of various samples exposed to blood flow for 2 h in a rapid AV shunt model without external heparin. (C) The occlusion rate of the sample is calculated by measuring the cross-sectional diameter of the sample. (D) SEM micrographs of the thrombi. (E) Quantitative analysis of the thrombus formation on the surfaces. (F) Blood flow rates in different PVC tubing circuits at the end of circulation experiments. Data are presented as mean ± SD and were analyzed using a one-way ANOVA, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
As presented in Figure 5 (B and C), after 30 min of blood circulation in the arteriovenous shunt model the SS, PVC, and TiO2-film surfaces triggered severe thrombosis, ultimately resulting in acute occlusion. In contrast, no severe thrombosis or occlusion was observed on the CuTPP/TiO2-film. As presented in Fig. 5D, further observation by SEM revealed that the thrombus formed on the surfaces of the SS, PVC, and TiO2-films consisted of numerous erythrocytes, platelets, and fibrin networks, while few blood components adhered to the CuTPP/TiO2-film. As presented in Fig. 5E, the thrombus weight on the sample surface was reduced by more than 80% after coating with CuTPP/TiO2-films relative to that of the control samples (SS and PVC). Additionally, Fig. 5F indicates that the blood flow rate of the CuTPP/TiO2-film was 3-fold higher than that of the SS and PVC samples, thus indicating that the blood could flow through unimpeded. These results suggest that dual-catalytic CuTPP/TiO2-films films are desirable for use in cardiovascular devices with high antithrombotic requirements.
3.5. In vitro evaluation of vascular cell growth behavior
3.5.1. Endothelial cells (ECs) culture test
Endothelial cells (ECs) play an important role in regard to inhibiting restenosis and advanced thrombosis after intervention with cardiovascular materials. Here, the affinity of the CuTPP/TiO2-NPs film for ECs was studied.
As presented in Fig. 6 (A1 and A2), after the cells were cultured for 4 h the number of ECs on the CuTPP/TiO2-films with added NO donors was significantly increased, thus indicating that the NO catalyzed by the CuTPP/TiO2-film promoted cell adhesion. After the ECs were cultured statically for 1 d, in the donor (−) group more ECs adhered to the TiO2-film and CuTPP/TiO2-film than to SS. After the addition of the NO donor, the CuTPP/TiO2-film exhibited the highest number of EC adhesions among all samples due to the effective catalytic NO generation. After the ECs were cultured for 3 days, the majority of the ECs were observed on the CuTPP/TiO2-film with the addition of the NO donor, and this indicated that the continuous catalytic generation of NO from the CuTPP/TiO2-film contributes to the proliferation of ECs.
Fig. 6.
(A1) Fluorescence staining of ECs on 316L SS, TiO2-NPs, and CuTPP/TiO2-NPs surfaces after culture for 4, 24, and 72 h with or without NO donor. (A2) Cell counting of ECs on the samples. Proliferation of HUVECs after culture for 24 (B1) and 72 h (B2) as evaluated by CCK-8 tests. (C1) Schematic diagram of in vitro cytotoxicity. (C2) EC activity (CCK-8) in cell culture media with different concentrations of nanoparticles. Data are presented as mean ± SD (n = 6) and were analyzed using a one–way ANOVA, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
As presented in Fig. 6 (B1, B2), the results of the CCK-8 cell activity assay demonstrated that after 1 d of culture, both TiO2-films and CuTPP/TiO2-films with or without the addition of donor exhibited higher affinity for ECs compared to SS, and this may be related to the coverage of the PDA coating with higher affinity for ECs. After 3 days of incubation, ECs exhibited the greatest increase in activity on the CuTPP/TiO2-film (donor [+]). These results suggest that the devices covered with CuTPP/TiO2-film could catalytically degrade endogenous NO donors to generate NO, ultimately promoting the adhesion, growth, and proliferation of ECs. The high EC affinity of the CuTPP/TiO2-film was promising for enhancing the endothelialization of implanted devices.
The cytotoxicity of nanoparticles is an ongoing concern in regard to their use for practical applications. The CuTPP/TiO2-film may shed some CuTPP/TiO2-NPs in vivo despite its good stability. Therefore, we further evaluated the toxicity of CuTPP/TiO2-NPs against ECs to further evaluate the in vivo biosafety of the CuTPP/TiO2-film. Here, we cultured ECs in a medium containing 0.03125, 0.0625, 0.125, and 0.25 mg/mL of nanoparticles. As presented in Fig. 6C1, after 1 d of incubation, the activity of the cultured ECs with nanoparticles was better than was that of the cultured ECs without nanoparticles, and this indicates that TiO2-NPs and CuTPP/TiO2 -NPs were both non-toxic to cell growth at the above concentrations. Furthermore, the activity of ECs cultured with the addition of CuTPP/TiO2-NPs was higher than was that of ECs cultured with the addition of TiO2-NPs at the same concentrations, thus suggesting that CuTPP/TiO2-NPs possess a superior safety profile compared to that of TiO2-NPs. As presented in Fig. 6C2, these results imply that the small number of nanoparticles shed from the CuTPP/TiO2-film may promote growth rather than damage the repair of ECs when used in practice.
3.5.2. Smooth muscle cell (SMC) culture test
Excessive proliferation of smooth muscle cells (SMCs) triggers vascular stenosis that is a significant cause of failure of blood-implanted devices such as vascular stents [28,29]. Therefore, the inhibition of SMC growth is considered beneficial for the repair of blood-implanted devices in vivo. The growth behavior of SMCs is closely linked to free radicals in the vascular microenvironment. Healthy vessels typically benefit from the nitric oxide (NO) generated by the intact EC layer. NO can inhibit SMC proliferation by upregulating the pathway of cGMP [[30], [31], [32]], thus playing an essential role in maintaining vascular homeostasis. Additionally, injured vessels often experience oxidative stress. Excessive reactive oxygen species (ROS) such as O2−and H2O2 activate resting SMCs and enhance their growth and proliferation [33]. In this context, constructing a surface with dual catalytic activities for ROS decomposition and NO generation may result in an excellent ability to inhibit the growth of SMCs.
In this study, we explored the ability of the dual-catalytic CuTPP/TiO2-film to regulate the adhesion and growth of SMCs. As presented in Fig. 7 (A, B), after 24 h of incubation SMCs adhered to the SS in large amounts and spread out fully. Relative to SS, the adhesion, spreading, and activity of SMCs decreased on TiO2-film, and this may be related to the introduction of PDA coating and the change in surface roughness [34,35]. In contrast, after the addition of donor, CuTPP/TiO2-film (donor [+]) exhibited the most potent inhibition of SMC adhesion and spreading, and the cellular activity decreased to 36% of that of SS (donor (−)). This result implies that the CuTPP/TiO2-film with the dual-catalytic function of NO generation exerts a strong inhibitory effect on the growth of SMCs in vivo.
Fig. 7.
(A) Fluorescence staining of SMCs on 316L SS, TiO2-NPs surfaces, and CuTPP/TiO2-film surfaces after culture for 24 h with or without NO donor. (B) Proliferation of SMCs after culture for 24 h as evaluated by CCK-8 tests. (C) Fluorescence staining of macrophages on 316L SS, TiO2-NPs surfaces, and CuTPP/TiO2-NPs surfaces after culture for 24 h. (D) Cell counting of macrophages on the samples. Data are presented as mean ± SD (n = 6) and were analyzed using a one–way ANOVA, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Macrophages are critical inflammatory cells in vessels. The macrophage-mediated inflammatory response triggered by device implantation can promote SMC proliferation and delay EC repair [[36], [37], [38], [39]]. Similar to SMCs, macrophages are regulated by both ROS and NO. (1) ROS can lead to macrophage accumulation and inflammatory expression [[40], [41], [42]]. (2) Physiological concentrations of NO alleviated macrophage accumulation and promoted macrophage expression of an anti-inflammatory phenotype [43,44].
As presented in Figure 7 (C, D), on CuTPP/TiO2-film (donor [−]) samples the number of macrophages was reduced to 57% of that on SS (donor [−]), thus implying that the catabolic effect of CuTPP/TiO2-film on ROS could have reduced the accumulation of macrophages on the sample surface. Additionally, the number of macrophages on the CuTPP/TiO2-film (donor [+]) decreased to 45% of that of SS (donor [−]). This result implied that the CuTPP/TiO2-film that possessed a dual-catalytic function for NO generating and ROS scavenging may exert a powerful inhibitory effect against the inflammatory response triggered by macrophage accumulation.
3.6. Evaluation of in vivo implantation experiments
The CuTPP/TiO2 coating was prepared on a stainless-steel wire and implanted into the abdominal aortas of Sprague Dawley rats (Fig. 8A). The results of HE staining after one month revealed that the hyperplasia tissue surrounding the CuTPP/TiO2 was thinner, and the cross sectional area of tissue hyperplasia was reduced by 78% compared to that of SS (Figure 8B and C). This is due to the observation that after the sample was implanted into the abdominal aorta, the dual-catalytic CuTPP/TiO2-film inhibited the excessive proliferation of SMCs by catalyzing the generation of NO to upregulate cGMP and decompose the increased ROS.
Fig. 8.
(A) Schematic of sample wire implantation in Sprague Dawley rat abdominal aortas. (B) Hematoxylin and eosin (H&E) staining and (C) quantitative analysis of abdominal aortic tissue hyperplasia. (D) Platelet endothelial cell adhesion molecule-1 (CD31), Osteopontin (OPN), Alpha smooth muscle cell actin (α-SMA), and Tumor Necrosis Factor-α (TNF-α) in abdominal aortic tissue hyperplasia and (E–H) quantitative analysis. Data are presented as mean ± SD (n = 6) and were analyzed using a one–way ANOVA, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
CD31 staining of endothelial cells indicated that CD31 was sporadically expressed on the surface of proliferative tissues of SS and CuTPP/TiO2, and the endothelialization rates were 10% and 11%, respectively, with no significant difference (Figure 8D and E). This may be due to the observation that the tissue environment is relatively stable at the later stage of implantation and the sample exerts a relatively weak effect on endothelial repair.
Ostepontin (OPN) and α-smooth muscle actin (α-SMA) are classic markers of synthetic and contractile SMCs, respectively. OPN and α-SMA staining indicated that a large number of OPN-positive cells gathered around the SS and that the number of peripheral α-SMA-positive cells was lower. However, SMA was strongly expressed in the tissues surrounding CuTPP/TiO2 (Fig. 8C). For quantification of OPN and α-SMA, the area ratios of OPN expression in hyperplastic tissue of SS and CuTPP/TiO2 were 35% and 8%, while the area of α-SMA expression accounted for 19% and 65%, respectively (Figure 8F and G). The results indicated that the dual-catalytic CuTPP/TiO2-film reduced the expression of synthetic SMCs, maintained the contractile SMC phenotype, and maintained tissue stability.
Tumor Necrosis Factor-α (TNF-α) is a key regulator of inflammation [45]. Elevated TNF-α expression causes oxidative stress and EC dysfunction [46]. TNF-α staining revealed that more TNF-α-positive cells gathered in new tissues surrounding the SS. The area ratios of TNF-α expression in hyperplastic tissue of SS and CuTPP/TiO2 were 16% and 7%, respectively. The results indicated that dual-catalytic CuTPP/TiO2-film could inhibit TNF-α expression in neonatal tissues and indirectly reduce oxidative stress in ECs.
4. Conclusions
In summary, using a combination of chemical synthesis and surface engineering techniques, we constructed an endothelial-biomimetic film possessing dual-catalytic properties based on the ability of CuTPP to catalyze NO generation and scavenge ROS. CuTPP/TiO2-NPs with dual-catalytic activity were successfully prepared by adsorbing CuTPP onto TiO2-NPs as carriers. The obtained CuTPP/TiO2-NPs could be easily immobilized onto common cardiovascular materials such as SS and PVC using PDA as a bioadhesive to construct CuTPP/TiO2-film. Due to the synergistic effect of NO-releasing and ROS scavenging, this dual-catalytic film exhibited excellent biosafety and anti-thrombosis, vascular wall cell (ECs and SMCs) modulation, and anti-inflammatory properties. In addition to its potential to address the clinical complications of cardiovascular devices, this dual-catalytic film may provide new insights for the development of surface catalytic engineering of biomaterials.
Credit author statement
Luying Liu & Peng Liu: Experiment, Writing- Original draft preparation. Youhe Yang & Sheng Dai & Zhixing Wang: Experiment, Original draft preparation, Data curation. Ansha Zhao: Supervision and language polishment. Nan Huang: Writing- Reviewing. Jiang Chen: manuscript revision. Ping Yang: Conceptualization and manuscript revision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos., 31870958 (Ping Yang)).
Contributor Information
Jiang Chen, Email: 283876533@qq.com.
Ping Yang, Email: yangping8@263.net.
Data availability
The data that has been used is confidential.
References
- 1.Soehnlein O., et al. Sci. Transl. Med. 2011;3(103):103ra98. doi: 10.1126/scitranslmed.3002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otsuka F., et al. Nat. Rev. Cardiol. 2012;9(8):439. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- 3.Liang C., et al. Biomaterials. 2016;103:170. doi: 10.1016/j.biomaterials.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 4.Hauser S., et al. Trends Biotechnol. 2017;35(3):265. doi: 10.1016/j.tibtech.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor I., Wall J. Functionalised Cardiovascular Stents. Elsevier; 2018. Immobilization of antibodies on cardiovascular stents; p. 319. [Google Scholar]
- 6.Jansen F., et al. Basic to Translational Science. 2017;2(6):790. doi: 10.1016/j.jacbts.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganz P., Hsue P.Y. vol. 34. Oxford University Press; 2013. p. 2025. (Endothelial Dysfunction in Coronary Heart Disease Is More than a Systemic Process). [DOI] [PubMed] [Google Scholar]
- 8.Jourde-Chiche N., et al. Nat. Rev. Nephrol. 2019;15(2):87. doi: 10.1038/s41581-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 9.Gresele P., et al. Biochem. Pharmacol. 2019;166:300. doi: 10.1016/j.bcp.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Pober J.S., Sessa W.C. Nat. Rev. Immunol. 2007;7(10):803. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z., et al. Biomaterials. 2018;178:1. [Google Scholar]
- 12.Qiu H., et al. Biomaterials. 2019;207:10. doi: 10.1016/j.biomaterials.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y., et al. Research. 2020:2020. [Google Scholar]
- 14.Hunt A.P., et al. ACS Catal. 2019;9(9):7746. doi: 10.1021/acscatal.9b01520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konopińska K.K., et al. ACS Appl. Mater. Interfaces. 2018;10(30) doi: 10.1021/acsami.8b05917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touyz R.M., Briones A.M. Hypertens. Res. 2011;34(1):5. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 17.Li J.-M., Shah A.M. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287(5):R1014. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 18.Beckman J.S., Koppenol W.H. Am. J. Physiol. Cell Physiol. 1996;271(5):C1424. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 19.Pryor W.A., Squadrito G.L. Am. J. Physiol. Lung Cell Mol. Physiol. 1995;268(5):L699. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 20.Xie Z., et al. J. Biol. Chem. 2006;281(10):6366. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 21.Muller-Delp J.M., et al. Microcirculation. 2012;19(1):19. doi: 10.1111/j.1549-8719.2011.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foerstermann, U., (2006).
- 23.Förstermann U., Li H. Br. J. Pharmacol. 2011;164(2):213. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiva Samhitha S., et al. Mater. Today Proc. 2022;54:765. [Google Scholar]
- 25.Weng Y., et al. Biomaterials. 2011;32(5):1253. doi: 10.1016/j.biomaterials.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 26.Benčina M., et al. Materiali in tehnologije. 2019;53(6):791. [Google Scholar]
- 27.Xu F., et al. Appl. Catal. B Environ. 2018;230:194. [Google Scholar]
- 28.Jain V., et al. Surf. Sci. Spectra. 2019;26(1) [Google Scholar]
- 29.Pashkow F.J. Int. J. Inflamm. 2011:2011. doi: 10.4061/2011/514623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douglas G., et al. Eur. Heart J. 2013;34(43):3378. doi: 10.1093/eurheartj/ehs240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colombo A., Jabbour R.J. Restenosis in a bare-metal stent: drug-eluting balloon or drug-eluting stent? Am Heart Assoc. 2016;9:e003829. doi: 10.1161/CIRCINTERVENTIONS.116.003829. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter A.W., Schoenfisch M.H. Chem. Soc. Rev. 2012;41(10):3742. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu S.-M., et al. Circulation. 1997;95(5):1269. doi: 10.1161/01.cir.95.5.1269. [DOI] [PubMed] [Google Scholar]
- 34.Wu F., et al. ACS Appl. Mater. Interfaces. 2016;8(1):109. doi: 10.1021/acsami.5b07427. [DOI] [PubMed] [Google Scholar]
- 35.Li H., et al. Materials. 2021;14(21):6447. [Google Scholar]
- 36.Napoli C., et al. J. Am. Coll. Cardiol. 2013;62(2):89. doi: 10.1016/j.jacc.2013.03.070. [DOI] [PubMed] [Google Scholar]
- 37.Papaharalambus C.A., Griendling K.K. Trends Cardiovasc. Med. 2007;17(2):48. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis C., et al. J. Thromb. Haemostasis. 2003;1(8):1699. doi: 10.1046/j.1538-7836.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- 39.Mosser D.M., Edwards J.P. Nat. Rev. Immunol. 2008;8(12):958. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka H., et al. Circulation. 1993;88(4):1788. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- 41.Welt F.G., Rogers C. Arterioscler. Thromb. Vasc. Biol. 2002;22(11):1769. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- 42.Schieber M., Chandel N.S. Curr. Biol. 2014;24(10):R453. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittal M., et al. Antioxidants Redox Signal. 2014;20(7):1126. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bulua A.C., et al. J. Exp. Med. 2011;208(3):519. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Rashed F., et al. Sci. Rep. 2020;10(1):1. [Google Scholar]
- 46.Li X., et al. Cytokine. 2020;129 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.