Abstract
Serotypes A and B of the relapsing fever spirochete Borrelia turicatae produce different disease manifestations in infected mice. Whereas serotype B causes more severe arthritis and reaches higher densities in the blood of mice than serotype A, serotype A invades the central nervous system earlier than serotype B during infection. These differences between serotypes A and B in mice are associated with the expression of different surface proteins, VspA and VspB, respectively, in the culture medium. To determine whether these proteins, in particular, VspB, are also expressed in vivo, scid mice infected with B. turicatae were studied. The expression of VspB by spirochetes in the blood was demonstrated in Coomassie blue-stained polyacrylamide gels and Western blots with a specific monoclonal antibody. Indirect immunofluorescence and immunoperoxidase studies confirmed the expression of VspB in the blood and also demonstrated VspB expression in the joints and heart. The gene for VspB was next identified and cloned by using partial amino acid sequencing, reverse transcriptase PCR, and a specific monoclonal antibody. The vspB gene encodes a protein of 216 amino acids that is 68% identical to VspA of B. turicatae and 44 to 56% identical to representative Vsp and OspC lipoproteins of other Borrelia spp. The processed VspB protein was distinguished from 26 other Vsp and OspC proteins by a high predicted isoelectric point at 9.39. The promoter region for vspB was similar to the promoter region for the vsp33 gene of Borrelia hermsii and for the ospC gene of Borrelia burgdorferi, two genes known to be environmentally regulated. These studies established that the virulence-associated VspB protein is expressed by spirochetes in the mouse and that VspB is a novel member of the Vsp-OspC family of proteins.
Relapsing fever occurs in a louse-borne form that is often epidemic and a tick-borne form that is usually sporadic in occurrence (reviewed in reference 7). In North America relapsing fever is caused by the tick-borne species Borrelia turicatae and Borrelia hermsii. In comparison to B. hermsii and most other species of relapsing fever Borrelia, B. turicatae is more neurotropic in experimental infections and humans (16). In addition, mice infected with B. turicatae have manifestations that resemble those of disseminated Lyme disease (19, 37).
In infected animals the successive relapse populations, or serotypes, of spirochetes are antigenically distinct from both the infecting serotype and from serotype populations that will follow them (7). This fundamental feature of relapsing fever was demonstrated by several investigators using immune sera and some form of in vitro assay, e.g., immunofluorescence of blood smears, before any Borrelia species was cultivated (reviewed in reference 2). Once in vitro cultivation from single cells was achieved (48), the different serotypes of this species were found to be distinguishable by a single abundant outer membrane protein, originally called a variable major protein (10). These serotype-specific proteins were characterized by their migration in polyacrylamide gels, their reactivity with serotype-specific antibodies, and their primary sequence (4, 10, 11, 15, 42, 43).
The serotype-specific proteins of relapsing fever Borrelia spp. have recently been divided into two groups on the basis of size and primary sequence: the variable small proteins (Vsp), which are 20 to 23 kDa, and the variable large proteins (Vlp), which are 36 to 40 kDa (18, 25, 43). Both sets of proteins have signal peptidase II sites, are processed to lipoproteins at a cysteine, and, by an unknown mechanism, are transported to the outer membrane and the spirochete’s surface (15, 18, 20). Within each group, processed Vsp and Vlp proteins are similar to one another at their N and C termini and are more variable in their central regions. There are two possible copies of the genes for Vsp and Vlp proteins in B. hermsii: a silent form which is found in all serotypes and an extra expression-linked copy in cells producing the given Vsp or Vlp (27, 28, 33, 40–43).
The genetic basis of antigenic variation has been most extensively studied with B. hermsii, but another important aspect of relapsing fever pathogenesis was first observed with experimental B. turicatae infections. Cadavid et al. (19) and Pennington et al. (37), using the scid mouse model of B. turicatae infection, showed that variation between two serotypes, A and B, was associated not only with immune evasion but also with differential tissue localization and disease manifestations. While serotype A of the Oz1 strain of B. turicatae invades and persistently infects the brain, serotype B causes more severe arthritis in adult mice and higher mortality in infant mice. The greater arthritogenicity and overall greater virulence of serotype B appeared to be the consequence of 10-fold higher levels of serotype B spirochetes in the blood and joints than those for serotype A infections (37). Paradoxically, in the culture medium the opposite is observed: serotype A grows to higher cell densities than serotype B (39).
B. turicatae serotype A expresses VspA, and serotype B expresses VspB; these proteins differ in electrophoretic migration and reactivity with monoclonal antibodies (18, 19). The expression-linked copy of vspA, the gene for VspA, has been cloned and sequenced (18). VspA is part of a larger family of proteins that includes not only the Vsp of B. hermsii and Borrelia miyamotoi, a related species isolated from ixodid ticks in Japan, but also the OspC proteins of the Lyme disease agents Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii (18, 20, 30, 31). Another member of the Vsp-OspC family, Vsp33 of B. hermsii, is expressed from a different expression site than the expression-linked locus for other vsp and vlp genes in that species (20).
Although these past studies provided the insights into the molecular mechanisms of serotype switching and an extensive catalog of the vsp-vlp genes, Vsp and Vlp function is not well understood. These polymorphic proteins are associated with immune evasion (10, 17, 48), and our studies with B. turicatae further suggest that they determine infection outcome (19, 37). Nevertheless, there has been to date only indirect evidence that Vsp or Vlp proteins are expressed in vivo (17, 38, 45, 48). Given the environmental determinants of OspA (12, 22, 34), OspC (34, 46), and OspF (1) expression in B. burgdorferi, one cannot assume that phenotypes in the culture medium or in ticks accurately represent spirochete expression in the mammalian host.
The present study had two major goals. The first was to determine whether the virulence-associated VspB protein was actually expressed during infections of a mammalian host. We did this by using infected mice and directly examining spirochetes in their blood and tissues for VspB expression. The second goal was to identify the expressed gene for VspB from the several paralogous Vsp genes in the B. turicatae genome and then clone it. The sequence of the expressed VspB gene confirmed that it was member of the Vsp-OspC family, and it revealed distinctive features as well.
MATERIALS AND METHODS
Strains and culture conditions.
The clonal populations of serotypes A and B of the Oz1 strain of B. turicatae were described previously (19). Serotype identity was confirmed by polyacrylamide gel electrophoresis (PAGE), reactivity with monoclonal antibodies in Western blots, and indirect immunofluorescence assay (10, 43). Spirochetes in the blood were cultured at 34°C in BSK II medium supplemented with 12% rabbit serum (3) and counted in a Petroff-Hauser counting chamber under phase contrast microscopy (48). Culture harvests were prepared and frozen as concentrated cell suspensions at −80°C with 10% (vol/vol) dimethyl sulfoxide as previously described (37). Escherichia coli strains were grown in Luria-Bertani medium (Difco, Detroit, Mich.).
Mouse infections.
Four- to six-week-old male or female CB-17 scid mice (Charles River Laboratories, Wilmington, Mass.) were inoculated intraperitoneally with 0.1 ml of phosphate-buffered saline (PBS), pH 7.4, alone or containing 103 spirochetes. Tissues were obtained after euthanization with methoxyflurane and whole body perfusion with PBS (37) and processed as described below. Blood was terminally collected from anesthetized mice by cardiac puncture, the citrated blood was centrifuged for 2 s at 12,000 × g, and the plasma supernatant was stored in aliquots with 10% dimethyl sulfoxide at −80°C.
Immunofluorescence and immunohistochemistry.
The origins of VspA- and VspB-specific murine monoclonal antibodies 1H12 and 5F12 (18) and Borrelia spp. flagellin-specific monoclonal antibody H9724 (8) have been described. Plasma aliquots or culture aliquots were thawed and centrifuged (12,000 × g for 3 min). The cell pellet was immediately resuspended with an equal volume of packed, washed rat erythrocytes in 50% fetal calf serum in PBS. Thin smears of the spirochete and blood suspension were fixed with methanol for 15 min (10). Tibiotarsal joints of mice were obtained after 15 days of infection and were decalcified, fixed with formaldehyde, embedded in paraffin, cut as 5-μm sections with a microtome, deparaffinized, and prepared for antibody incubations as described previously (37). Blood smears and joint tissue sections were incubated with hybridoma supernatant diluted 1:10 in 1% bovine serum albumin (BSA) in PBS for 20 min or in 1% ovalbumin in 3% Brij35–0.1 M Tris (pH 7.3) for 1 h, respectively. The specimens were then washed with PBS, incubated with fluorescein-conjugated F(ab′)2 fragment of goat anti-mouse immunoglobulin (Kirkegaard & Perry) that was diluted 1:15 in BSA-PBS or 1:50 in ovalbumin-Tris, and then washed again with PBS.
For immunohistochemistry, hearts were removed en bloc, immediately placed in a cryostat at −20°C, rapidly frozen with Ultrafreeze spray (Fisher Scientific), and stored at −80°C until sectioning. Frozen hearts were embedded in OCT (optimum cutting temperature) compound (Tissue-Tek) at −20°C, sectioned (15 μm) in a Reichert-Jung cryostat, mounted on glass slides precoated with gelatin, and stored at 4°C until use. All slides were fixed with 100% methanol for 10 min prior to immunostaining by using a three-step streptavidin-peroxidase technique at room temperature with a Biogenex kit and reagents (San Ramon, Calif.). Nonspecific binding was reduced by blocking slides with 10% mouse serum in 1× blocking solution for 1 h, and endogenous peroxidase activity was reduced by preincubation with 3% H2O2 for 30 min. Monoclonal antibody in ascites fluids and diluted 1:500 in Biogenex diluent buffer was the primary reagent. The secondary reagent was a biotinylated goat anti-mouse polyclonal antibody, and the tertiary reagent was streptavidin labeled with horseradish peroxidase. The primary reagent was incubated for 1 h, and the secondary and tertiary reagents were incubated for 30 min. The slides were then incubated in chromogen solution (3,3-diaminobenzidine tetrahydrochloride in 0.24% H2O2) for 5 min and Mayer’s hematoxylin counterstain for 2 min. Each incubation was separated by three washes with 1× OptiMax wash buffer (Biogenex).
Tissue sections and smears from uninfected animals were included as negative controls, and sections from known positive tissues were included as positive controls in each run. Slides of smears and sections were examined on an Olympus model BX50 microscope with transmitted UV light or standard light. The microscopist did not know the identity of the infecting serotype. Photomicrographs were made with Kodak TMAX 400 film.
PAGE and Western blot analysis.
Cell aliquots were thawed, centrifuged at 12,000 × g at 4°C for 3 min, resuspended in PBS with 5 mM MgCl2, and centrifuged again. The whole cell pellets were immediately resuspended in a sample buffer with 50 mM dithiothreitol and subjected to PAGE with 12% acrylamide as described previously (10). The gel was stained with Coomassie blue. For Western blot analysis, proteins were transferred onto 0.45-μm Immobilon membranes (Millipore Corp., Bedford, Mass.), and the membranes were blocked in 3% milk in 10 mM Tris (pH 7.4)–150 mM NaCl (TS). After washing in 0.3% milk–TS, the membranes were incubated with a 1:100 dilution of monoclonal antibody in ascites fluid in 0.3% milk–TS. Alkaline phosphatase-conjugated protein A/G (Pierce) at a 1:5,000 dilution was used as a second ligand, and the binding was detected with tetrazolium chloride–5-bromo-4-chloro-3-indolylphosphatase p-toluidine salt (Pierce).
Protein purification, digestion, and amino acid sequencing.
A Vsp-enriched extract was obtained by differential solubilization in the detergent octyl-glucopyranoside and subjected to high-performance liquid chromatography (HPLC) with a C-4 column essentially as described previously (11). Purified VspB was digested with endoproteinase Glu-C (Boehringer Mannheim, Indianapolis, Ind.) in 25 mM ammonium bicarbonate–0.05% sodium dodecyl phosphate (SDS). Proteolytic products were separated either (i) by PAGE (20% acrylamide) followed by electrophoretic transfer to polyvinyl difluoride membranes (Millipore) as described previously (18) or (ii) by HPLC as described (11). N-terminal amino acid sequences of purified peptides were determined by Richard Cook at the Protein Chemistry Core Facility, Baylor College of Medicine.
Nucleic acid methods.
Total DNA or total RNA were prepared from spirochetes as described previously (33). Plasmid DNA from E. coli was extracted with a plasmid extraction kit (Qiagen, Chatsworth, Calif.). Oligonucleotides for probes and primers were synthesized on an Applied Biosystems DNA synthesizer. Restriction enzymes were from Boehringer Mannheim. DNA restriction fragments were isolated from Pure Elute agarose (Invitrogen, San Diego, Calif.) with an electroelution instrument (International Biotechnologies, Inc., New Haven, Conn.). Oligonucleotides were 5′ end labeled with [γ-32P]ATP by using polynucleotide T4 kinase (Boehringer Mannheim) and purified in Nensorb-20 columns (DuPont, Boston, Mass.). Alternatively, oligonucleotides were 3′ end labeled by using dUTP-digoxigenin and deoxyterminal transferase with the Genius kit (Boehringer Mannheim). Southern and Northern blots were performed as described before (33). Nucleotides were transferred onto 0.45-μm-pore-size Nytran membranes (Schleicher & Schuell). Hybridizations were done in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and washes were done in 0.1× SSC–0.1% SDS–1 mM EDTA. A plasmid containing the flaB gene of B. turicatae has been described previously (37).
RT-PCR.
Total RNA was treated with RNase-free DNase I (Boehringer Mannheim) at 1 U of RNA per μg for 15 min at 23°C. Reverse transcriptase (RT) PCR was performed with the rTth reverse transcriptase RNA PCR kit (Perkin-Elmer) with the following conditions for a Perkin-Elmer thermal cycler: 30 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 2 min, with a final extension of 7 min at 72°C. As controls, RT-PCR was performed on RNA in the absence of Mg2+ and on RNA that had been treated with DNase-free RNase (Boehringer Mannheim).
DNA cloning and sequencing.
Eluted DNA was ligated into pUC19 digested with EcoRI and PstI, pUC18 digested with SmaI, or pBR322 digested with PstI. The ligation products were transformed into E. coli SURE or XL-1 MRF′ Blue-Kan cells (Stratagene). RT-PCR products were ligated into the pCRII vector and transformed into E. coli INVαF′ cells (Invitrogen). Transformants were identified by hybridization with oligonucleotides. Sequences of both strands of the inserts were determined by the dideoxy chain termination method on double-stranded templates with Sequenase (United States Biochemical, Cleveland, Ohio) and custom primers. The vspB gene was cloned into the pET-15b (Novagen, Madison, Wis.) plasmid vector in E. coli BL21(DE3), and recombinant VspB was expressed as a fusion protein after induction with isopropyl-β-d-thiogalactopyranoside as described previously (18).
Sequence analysis.
Sequences were analyzed with Genetics Computer Group Sequence Analysis software, version 7.3 (24a), MacDNASIS (Hitachi, San Bruno, Calif.), and PHYLIP, version 3.5c (23, 49). Overall hydrophobicity values were determined by the Kyte-Doolittle procedure (29).
Nucleotide sequence accession numbers.
The sequence of vspB and its 5′ and 3′ flanking sequences has been assigned GenBank accession no. AF049852. vspD, a gene that is nearly identical to vspB in its first 200 nucleotides, has been assigned GenBank accession no. AF129737 (38). For the comparison of protein sequences, the following Borrelia sp. sequences (with accession numbers in parentheses) were used: B. burgdorferi sensu stricto OspC proteins from strains B31 (X69596), 297 (U08284), DN127 (U04280), 25015 (U04282), 2591 (U01892), and CA11 (L25413); B. afzelii OspC proteins from strains PKo (X62162) and ACA1 (L42892); B. garinii OspC proteins from strains PHei (X83553), Ip90 (L42886), SL14 (X84784), and HT37 (D49381); B. hermsii Vmp proteins 1 (L33870), 11 (L33900), 13 (L33901), 2 (L33897), 26 (L26497), 27 (L33903), 3 (L04789), 33 (L24911), and 8 (L33899); B. turicatae VspA (U85413); and B. miyamotoi Vmp protein (D78201).
RESULTS
VspB is expressed in vivo.
B. turicatae produced abundant amounts of VspB while growing in the culture medium (19, 37), but it was not known whether this protein was expressed by spirochetes inside their mammalian hosts. To determine this, we directly examined spirochetes in the blood and in tissues and compared the results with those of spirochetes grown in vitro.
(i) PAGE and Western blot analysis.
Mice in groups of five were inoculated on day 0 with 103 serotype B cells or with PBS alone as a control. By day 5 the infected mice had approximately 4 × 107 spirochetes per milliliter of blood, and at this time infected and control mice were euthanized and exsanguinated. A brief centrifugation of the citrated blood from infected mice yielded a supernatant rich in spirochetes as well as platelets. Equal volumes of blood from uninfected mice yielded plasma with platelets alone. After further centrifugation at a higher speed, the spirochetes and platelets in the pellets were washed with PBS and resuspended in a sample buffer. Cultured bacteria were prepared by inoculating BSK II medium with serotype B-infected mouse blood; after two serial passages, or approximately 15 generations, cells were harvested by the same procedures. Equivalent numbers of spirochetes in each sample were subjected to PAGE and Western blot analysis.
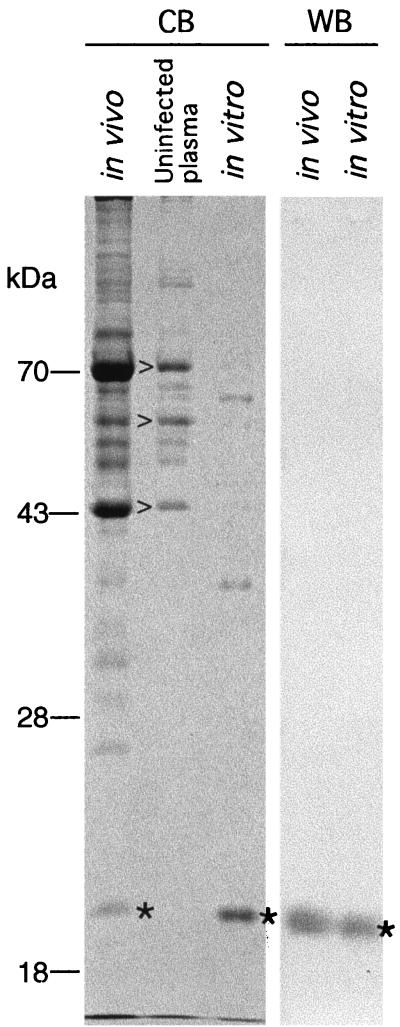
Figure 1 shows Coomassie blue-stained proteins of samples of uninfected plasma, infected plasma, and culture medium. The lane of infected plasma is overloaded in comparison to the lane of uninfected plasma in this experiment. Several of the proteins with apparent sizes of greater than 43 kDa were present in noninfected plasma as well as in the plasma of infected mice; these proteins are most likely derived from platelets. Present only in the samples containing spirochetes in the plasma or the broth medium was a protein with an apparent size of 21 kDa. Western blotting with the monoclonal antibody showed that this protein was VspB. In a separate experiment, a comparably overloaded lane of uninfected plasma did not reveal an immunoreactive protein of 21 kDa by Western blotting (data not shown).
FIG. 1.
Expression of VspB of B. turicatae in mouse blood and in culture. Plasma from uninfected mice (uninfected) or from mice infected with serotype B (in vivo) and cells from a culture of serotype B (in vitro) were subjected to PAGE (12% acrylamide) with Coomassie blue staining (CB) and to Western blot (WB) analysis with a monoclonal antibody against VspB. The location of VspB is designated by an asterisk, and the locations of major proteins in the uninfected plasma fraction are marked by open arrowheads. Molecular size standards are shown to the left (in kilodaltons).
(ii) Immunofluorescence and immunohistochemistry.
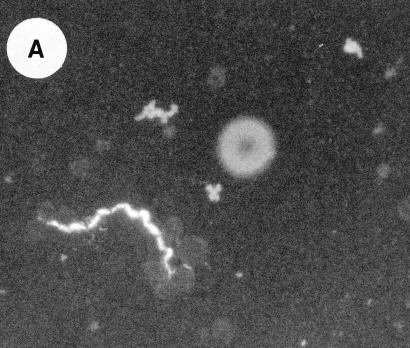
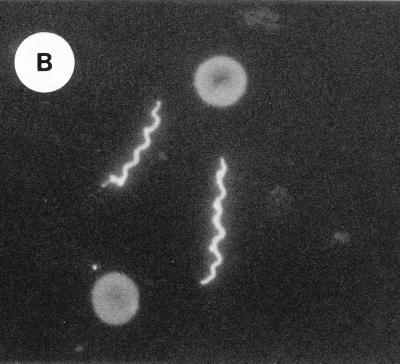
In a previous study of mice infected with serotype B, spirochetes in joints were identified by direct immunofluorescence with a conjugated Borrelia genus-specific polyclonal antiserum (37), which is evidence of the spirochetes’ presence in tissue but not of Vsp expression. For the present study we used indirect immunofluorescence with a monoclonal antibody directed against VspB of serotype B cells. The positive control was an antibody to the flagellin of borrelias; this antibody had been shown to identify spirochetes in fixed smears and sections (8, 36). The negative control was the antibody to VspA of B. turicatae (18).
On day 15 of infection of serotype B, four scid mice were bled and euthanized; their joints were taken after perfusion. Smears of blood spirochetes and the joint tissue sections were examined by indirect immunofluorescence with the monoclonal antibodies. The results of these studies are summarized in Table 1. Figure 2 shows the binding of blood spirochetes and cultured spirochetes by monoclonal antibodies to VspB (Fig. 2A) and to flagellin (Fig. 2B). The anti-VspB and anti-flagellin monoclonal antibodies were applied to fixed, paraffin-embedded joint tissue sections of the mice infected with serotype A or B. The antibodies gave the same results as what had previously been obtained with the more broadly reactive polyclonal antiserum (37; Table 1). The monoclonal antibody to VspA did not bind to the spirochetes in the blood or joints of the serotype B-infected mice but did bind to spirochetes in tissues of mice infected with serotype A.
TABLE 1.
Reactivities of monoclonal antibodies determined by immunofluorescence and immunohistochemistry
| Specimen | Infection | Reactivity of monoclonal antibody raised against:
|
||
|---|---|---|---|---|
| VspB | VspA | Flagellin | ||
| Blooda | None | − | − | − |
| Serotype B | + | − | + | |
| Serotype A | − | + | + | |
| Jointsa | None | − | − | − |
| Serotype B | + | − | + | |
| Serotype A | − | + | + | |
| Heartb | None | − | − | NDc |
| Serotype B | + | − | ND | |
| Serotype A | − | + | ND | |
Indirect immunofluorescence.
Immunoperoxidase.
ND, not done.
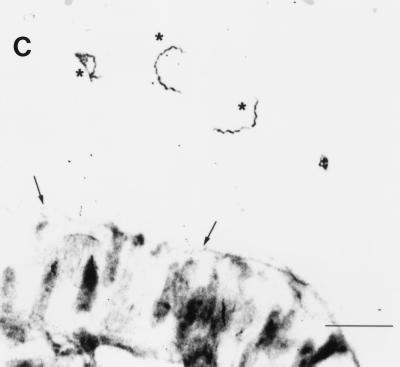
FIG. 2.
VspB is expressed by B. turicatae spirochetes in the blood and hearts of infected mice. Thin smears of spirochetes in the blood were examined by indirect immunofluorescence with a monoclonal antibody to flagellin (A) or VspB (B); magnification, ×1,200. A single spirochete and a single erythrocyte are shown in panel A, and two spirochetes and two erythrocytes are shown in panel B. (C) Immunoperoxidase reaction of serotype B cells in the pericardial space of the heart of an infected mouse. The counterstained tissue is the mesothelium of the epicardium (arrows). Three spirochetes bound by the monoclonal antibody to VspB are indicated by asterisks. Bar, 10 μm.
We also used anti-VspB antibody and immunoperoxidase staining to examine the hearts of two mice infected with serotype B. The antibody bound to the numerous spirochetes in the pericardial space (Fig. 2C). Occasional immunoreactive spirochetes were also noted in the myocardium. The anti-VspA monoclonal antibody did not detectably bind to spirochetes in the pericardium or myocardium of mice infected with serotype B but did bind to spirochetes in the hearts of two mice infected with serotype A (Table 1).
Characterization of VspB and expressed vspB.
Having demonstrated the expression of VspB in the blood, joints, and hearts of infected mice, we next sought the gene coding for this protein. Given the large number of paralogous vsp genes in B. hermsii and, thus, the possibility of mistaken identification, we used three independent means to confirm that the cloned gene was vspB. The first was partial peptide sequencing of purified native VspB. The second strategy was to use RT-PCR to identify transcribed vsp sequences. The third method was to confirm the antigenic identity of the cloned gene with the VspB-specific monoclonal antibody.
(i) Amino acid sequencing.
Glu-C endoproteinase fragments of purified VspB were separated by electrophoresis or HPLC, and these sequences were obtained from three peptides: peptide 1, LAKAIKKKIQAGGLQDDTDN; peptide 2, TAFLNKLKSE; and peptide 3, NATLGAASAAVS. Peptide 1 at its start was nearly identical to a smaller peptide that was previously shown to be common to VspA and VspB (18). Peptides 2 and 3 conjoined were found to be highly similar to Vsp27 of B. hermsii (GenBank accession no. L33903; AFIIKLKNQHATLGAADGAAT) and to an OspC protein of B. afzelii (accession no. AF098942; AFTNKLKNSHAELGAANHATT).
(ii) Identification of a serotype B-specific transcript.
Given the similarity of VspB to known Vsp and OspC proteins, we used sequences common to several different vsp genes to amplify the vsp transcript in serotype B cells by RT-PCR. The reverse primer (5′CCGATAGCTTTAGCAAGC3′) was based on an internal sequence that is about 210 bp downstream of several vsp start sites in B. hermsii (43) and in vspA of B. turicatae (positions 273 to 290 of GenBank accession no. U85413). The forward primer (5′AAGTGCGATAATAATGACTTTATT3′) represented the conserved signal peptide sequence of Vsp proteins (positions 76 to 99 of GenBank accession no. LO4789). By using these primers a 210-bp product was obtained by RT-PCR and cloned. Sequence analysis revealed that it was similar to but distinct from the 5′ end of vspA (not shown).
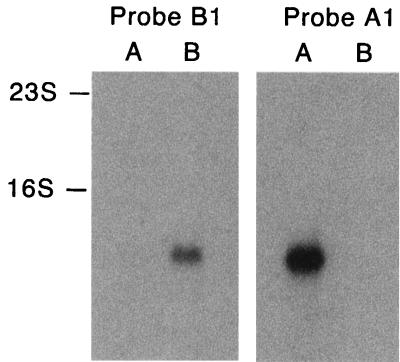
The differences between vspA and the RT-PCR product from serotype B cells allowed the design of probes specific for VspA-expressing cells (A1; 5′AACAGCATCGGTAATGTTTTTAGT3′) and VspB-expressing cells (B1; 5′CAAAAGCAACAGTGTCTTTTATGT3′). Northern blots of RNA from serotype A and B cells were probed with these two oligonucleotides (Fig. 3). The flagellin transcript was probed as a positive control with the B. turicatae flagellin gene under the same hybridization and washing conditions described previously (37). The blot showed that the RT-PCR-derived sequence was specific for a transcript in serotype B cells. Opposite results were obtained with the vspA-specific probe. To verify that the serotype B-specific transcript was vspB, we designed an oligonucleotide probe based on the sequence of peptide 1 (B2; 5′CATTCTTGAAGACCACCTGCTTGAA3′). Probe B2 hybridized to the same-sized transcript as probe B1 in serotype B RNA (Fig. 3) but not serotype A RNA (not shown).
FIG. 3.
Transcription of vspB in serotype B but not in serotype A cells of B. turicatae. Shown is a Northern blot of serotype A and serotype B RNA hybridized with an oligonucleotide probe derived from RT-PCR of a vsp sequence from serotype B RNA (Probe B1) and a corresponding oligonucleotide based on the vspA cloned sequence (Probe A1). The migrations of the 16S and 23S ribosomal RNAs are indicated to the left of the blot. The hybridization of both oligonucleotides was performed at 47°C in 6× SSC, and washes were done at up to 45°C in 0.1× SSC.
(iii) Expression of vspB is associated with a unique restriction fragment.
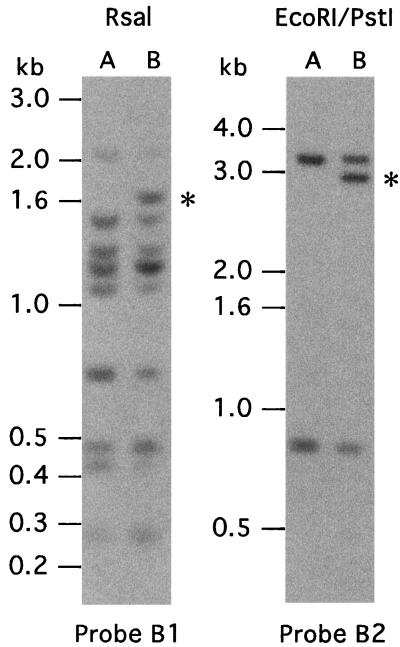
Probes B1 and B2 were next used to probe Southern blots of restriction enzyme digests of total DNA of serotypes A and B. Probe B1 hybridized to a 1.7-kb RsaI fragment in serotype B DNA but not serotype A DNA (Fig. 4). The probe hybridized to seven other RsaI fragments that were common to both serotypes, an indication that identical or near-identical sequences were at several locations in the genome. Probe B2 hybridized to fewer fragments of an EcoRI-PstI digest: serotypes A and B both had hybridizing fragments of 0.7 and 3 kb, and serotype B had a unique 2.7-kb fragment (Fig. 4). This latter fragment, as well as the overlapping 1.7-kb RsaI expression-linked fragment of serotype B, was cloned and sequenced.
FIG. 4.
Restriction fragment length polymorphisms associated with vspB expression. Serotype A and B total DNA was digested with the enzyme RsaI alone or EcoRI and PstI in combination. The fragments were transferred to a membrane for Southern blot analysis with oligonucleotide probes B1 and B2 (see the text). The vspB expression-linked fragments are indicated by asterisks. Hybridizations were done in 6× SSC, and washes were done in 0.1× SSC–0.1% SDS–1 mM EDTA. The B1 hybridization was done at 47°C, and washes were done at 45°C. The B2 hybridization was done at 50°C, and washes were done at 45°C.
(iv) Sequence of the expressed vspB expression site.
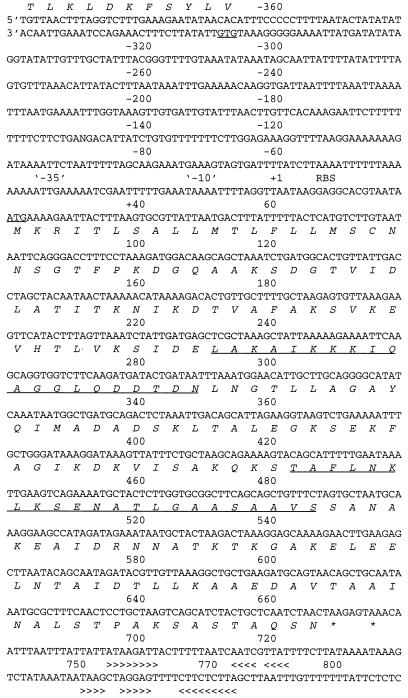
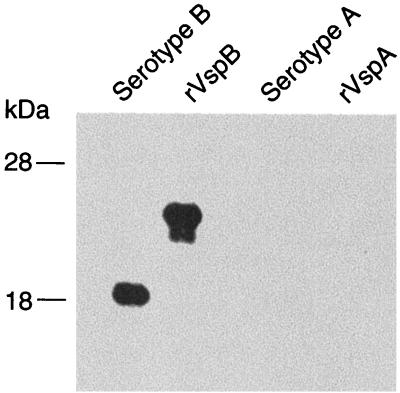
Figure 5 shows the sequence of an open reading frame (ORF) of 664 nucleotides (positions +21 to 667) and it 5′ and 3′ flanking regions. This would encode a protein of 216 amino acids. The evidence that this ORF encoded VspB was the following: (i) the deduced protein encoded by the ORF contained all of the partial peptide sequences obtained from VspB; (ii) the genomic clone matched in sequence the partial sequence obtained by RT-PCR (not shown); (iii) when the gene was subcloned into an expression vector the fusion protein was bound by a monoclonal antibody specific for VspB but not by antibody to VspA (Fig. 6). The fusion partner for the vspB gene accounts for the larger size of the recombinant protein (18).
FIG. 5.
DNA sequence and deduced amino acid sequence of the expressed vspB gene of B. turicatae. Nucleotides are numbered relative to the predicted transcription initiation site (+1). Putative −35 and −10 elements, ribosomal binding site (RBS), in-frame termination codons (asterisks), and complementary regions consistent with hairpin loop formation (>>>> and <<<<) are indicated. The predicted vspB translation product is shown below the nucleotide sequence. The underlined amino acid sequence corresponds to the sequences obtained from proteolytic cleavage fragments of the VspB protein. An ORF on the minus-sense strand starts with a GTG codon at position −367. The full sequence of this divergent ORF is available in GenBank under accession no. AF049852.
FIG. 6.
Western blot analysis of whole cell lysates of B. turicatae serotypes B and A and E. coli with the recombinant vspB (rVspB) gene (this study) or the recombinant vspA gene (rVspA) (18). The blot was incubated with a monoclonal antibody to VspB. The locations of molecular mass standards (in kilodaltons) are shown on the left. Neither the native VspB nor recombinant VspB fusion protein was bound by the monoclonal antibody to VspA (not shown).
We placed the transcriptional start site (+1) at the same locus that was identified by primer extension analysis of serotype A mRNA (18). The expression site for vspB contained the same −10 (TAAAAT) and −35 sequences (TTGAAA), which are consistent with a ς70 promoter and which were found upstream of expressed vspA (18). The N-terminal amino acid sequence of the ORF is highly similar to the signal peptide of Borrelia sp. lipoproteins (13–15, 20). Following the stop codon at position +668 were another stop codon and two potential rho-independent terminators.
Comparison of the vspB expression site with other Borrelia promoters.
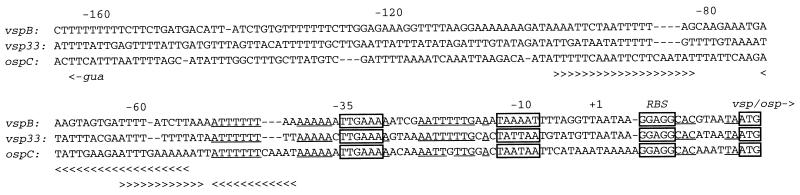
Figure 5 shows an additional 0.4 kb of sequence upstream of the expressed vspB gene. In the previous study of the expressed vspA gene, the 5′ sequence obtained extended only to the −35 element of the promoter (18). With the additional upstream sequence obtained in the present study, we compared the vspB promoter region with known promoters for two orthologous proteins: OspC of B. burgdorferi and Vsp33 of B. hermsii (Fig. 7). The sequences around the −35 and −10 elements of the three ς70-type promoters are very similar. The spacing between the promoter elements, the ribosomal binding sequence, and start codons are the same or nearly identical among the sequences. Upstream of the −35 elements the three sequences are highly AT-rich. The major difference between the promoter regions is the presence of two inverted repeats upstream of the ospC promoter but not either the vsp33 or vspB promoter.
FIG. 7.
Comparison of the promoter regions for the vspB gene of B. turicatae (Fig. 5), the vsp33 gene of B. hermsii (GenBank accession no. L24911), and ospC of B. burgdorferi (X69596). The promoters of vspB, vsp33, and ospC were aligned to identify common elements at the expression sites. Nucleotides are numbered relative to the predicted transcriptional start site (+1) for vspB. The putative −35 and −10 elements, the ribosomal binding sequences (RBS), and the start codons (vsp/osp−>) are boxed. Two inverted repeats (>>>> and <<<<) are uniquely found upstream of the ospC promoter. Selected nucleotide identities between the three sequences are underlined. At position −161 is the start codon for the guaA gene (gua) upstream of the ospC gene on the minus-sense strand.
Figure 7 also shows the start of the guaAB operon of B. burgdorferi, which is oriented oppositely and upstream of the ospC gene on a circular plasmid (32). Figure 5 shows the start of an ORF on the minus-sense strand and about 400 bp upstream of vspB. The sequence of this ORF of B. turicatae, which is presented in full in GenBank as accession no. AF049852, was 51% identical over 141 amino acids to an ORF of unknown function of B. burgdorferi (24). This orthologous ORF of B. burgdorferi is separated from the ospC gene on the plasmid by the guaAB operon and a gene for an oligopeptide permease.
Comparison of VspB with other Vsp-OspC proteins.
Overall, VspB and VspA of B. turicatae were 68% identical in amino acid sequence; the vspA and vspB genes were 76% identical in nucleotide sequence. The predicted VspB protein was found to be highly similar in overall sequence to the following selected Vsp and OspC proteins (with the percent identity in amino acid sequence): Vsp33 (56%), Vsp13 (55%), Vsp1 (54%), Vsp2 (52%), Vsp26 (50%), and Vsp11 (48%) of strain HS1 of B. hermsii; OspC (47%) of strain B31 of B. burgdorferi; OspC (46%) of strain PKo of B. afzelii; and OspC (44%) of strain Ip90 of B. garinii.
As expected, VspA and VspB were most similar at their N and C termini (18, 20). The more variable central regions observed in VspB, VspA, and other Vsp-OspC proteins confer different properties on these proteins; among these are hydrophobicity and protein charge. For the hydrophobicity and isoelectric point predictions, we omitted the signal peptide sequence which is highly hydrophobic and is posttranslationally removed. By the Kyte-Doolittle algorithm, processed VspB, with an average hydrophobicity value of −0.2, was less hydrophobic than VspA, which has a predicted hydrophobicity value of −0.1 (18). In this respect VspB resembled other Vsp and OspC proteins. VspB stood apart from other Vsp-OspC proteins in another characteristic. Table 2 shows the predicted isoelectric points of 25 known Vsp and selected OspC proteins. With a predicted pI of 9.39, VspB is more positively charged than the Vsp-OspC proteins of other Borrelia spp. Vsp-OspC proteins, which ranged in pI from 5.12 to 9.12 and had a mean pI of 6.68.
TABLE 2.
Predicted isoelectric points of the processed Vsp-OspC proteins of Borrelia species
| Proteina | pIb |
|---|---|
| Vsp1c | 7.31 |
| Vsp2c | 5.24 |
| Vsp3c | 5.63 |
| Vsp8c | 5.60 |
| Vsp11c | 5.12 |
| Vsp13c | 9.12 |
| Vsp22c | 5.68 |
| Vsp24c | 6.24 |
| Vsp26c | 5.32 |
| Vsp27c | 7.46 |
| Vsp33c | 8.58 |
| VspAd | 8.57 |
| Vmpe | 6.20 |
| OspC (B31)f | 7.25 |
| OspC (297)f | 6.24 |
| OspC (2591)f | 7.06 |
| OspC (CA11)f | 6.21 |
| OspC (25015)f | 8.19 |
| OspC (PKO)g | 7.25 |
| OspC (ACA1)g | 8.19 |
| OspC (PHei)h | 5.81 |
| OspC (Ip90)h | 5.72 |
| OspC (HT37)h | 5.92 |
| OspC (SL14)h | 7.25 |
| OspC (DN127)i | 7.18 |
| Mean (±2 SD) | 6.68 (4.34–9.02) |
| VspBd | 9.39 |
Strain names are in parentheses.
pI, predicted isoelectric point.
B. hermsii.
B. turicatae.
B. miyamotoi.
B. burgdorferi.
B. afzelii.
B. garinii.
Borrelia bissettii sp. nov.
DISCUSSION
Immunodeficient mice infected with B. turicatae serotype B have a persistent spirochetemia accompanied by severe arthritis and carditis, as well as cranial neuritis and eye disease (19, 37). Serotype B infections are also fatal for newborn immunocompetent mice (37). The greater virulence of serotype B in comparison to serotype A is associated with expression by the spirochetes of VspB instead of VspA. But this conclusion was based on phenotypes of spirochetes under in vitro growth conditions. It was possible that what appeared to be a key determinant of virulence was not expressed in the mouse environment. Accordingly, we first investigated whether VspB was produced by spirochetes in infected mice. The PAGE and Western blot experiments indicated that VspB was an abundant in spirochetes in the blood as it was in cells grown in culture medium. Although we used scid mice to minimize immune selection of other serotypes in these studies, there is evidence that VspB is also expressed in more immunocompetent mice: BALB/c and CBA/N mice produced antibodies to VspB in response to infection and spirochetes in the blood of infant C3H/HeN mice were identified as VspB positive by indirect immunofluorescence (18, 39).
The study further showed that VspB was expressed in two tissues with particularly marked involvement during the infection: the joints and heart. Although a vspA gene was present in serotype B cells (18), there was no evidence that vspA was expressed by the serotype B cells in either the culture medium or the mouse. Although we cannot rule out the action of another protein besides VspB in affecting the virulence of serotype B, the direct demonstration of in vivo expression justifies further study of VspB structure and function.
As has been the case in other studies of Vsp-OspC and Vlp proteins of Borrelia spp., the identification of the gene that encodes the protein of interest is not straightforward. This is primarily because there are numerous vsp and vlp genes in the genome, and two or more genes can share short regions of sequence over their lengths (18, 20, 43). Thus, probes may hybridize to more than one fragment or cloned sequence (33). In the present study, an oligonucleotide probe for the vspB sequence hybridized to several RsaI fragments. The extra hybridizing fragment unique to serotype B cells in Southern blots allowed the identification of the expressed gene. One of the hybridizing bands in both serotype A and B DNA is probably the silent or archived form of the vspB gene, as has been found for vlp and vsp genes of B. hermsii (5, 42). The identification and characterization of the archived versions of vspA and vspB are the object of another study. The other hybridizing bands may represent genes or pseudogenes that have a sequence similar to the probe. The encoded product of the vspD gene was distinguished from VspB by the amino acid sequences obtained by peptide sequencing of the VspB protein.
The present study also allowed a more extensive comparison of the promoter region for the vspA and vspB genes of B. turicatae to other well-defined Borrelia sp. promoters. The promoter region for vspB was not like the vlp-vsp promoter at the telomeric expression site of 28- to 32-kb linear plasmids B. hermsii (5, 6) or the promoter for the ospAB operon of B. burgdorferi (26, 47). One notable difference was the lack in B. turicatae of a T-rich region, which characterizes the latter two promoter regions, upstream of the −35 element (5, 6, 47). The B. turicatae vsp promoter resembled instead the promoters for the ospC gene of B. burgdorferi and vsp33 gene of B. hermsii (Fig. 6). From the −35 element to the start codon the vspB, vsp33, and ospC promoters are highly similar in sequence.
The ospC gene is located on a circular plasmid in B. burgdorferi (24, 35, 44), and the vsp33 gene is located on an approximately 50-kb linear plasmid of B. hermsii (9, 20). The ospC gene is expressed in ticks after blood feeding begins and in mammalian hosts during infection (21, 34, 46). OspC is expressed in vitro but only above a certain temperature or when other plasmids are missing (44, 46). The expression of vsp33 occurs in vitro; indeed, its original designation (VmpC) derives from its identification first as a “culture serotype” (10, 20, 48). More recently, Schwan and Hinnebusch have shown that the Vsp33 protein of infectious B. hermsii is produced in the salivary glands of the tick vector but not in the mouse host (45). In contrast, VspB of B. turicatae is expressed in vivo in the mouse, as well as in vitro. It is not known whether VspB or VspA is expressed by B. turicatae in the tick. Although VspB is expressed in vivo as well as in vitro, the similarity in promoter regions suggests that there may be environmental determinants of vsp expression in B. turicatae as well.
Finally, the predicted sequence of VspB provides some insights for the design of structure-function studies of Vsp and OspC proteins. The differences between serotypes A and B in tissue localization and virulence indicate an important role of Vsp proteins in interactions with the host. At an early stage of these studies, we saw that the neurotropism of serotype A is associated with a particularly hydrophobic Vsp protein (18). Serotype A appears to be more suited for leaving the vascular space than serotype B (19). This migration may be aided by the greater hydrophobicity of VspA in comparison to VspB. In contrast, VspB is notable for its exceptionally basic pI (Table 2). This higher charge may serve the pathogen population because of the retention of spirochetes circulating in the blood or by an association with endothelial cells, thus increasing the probability of transmission by a blood-feeding vector. According to this hypothesis, the serotype A phenotype represents an alternative strategy for survival through the persistence of the pathogen in the brain and other immunoprivileged sites of a host.
ACKNOWLEDGMENTS
We thank Lynda Bonewald for performing chromatography, Tatiana Kerentseva for assistance in producing recombinant proteins, and Carol Carter for technical assistance and advice.
This work was supported by grant AI24424 from the National Institutes of Health and a grant from the Arthritis Foundation.
REFERENCES
- 1.Akins D R, Porcella S F, Popova T G, Shevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 2.Barbour A G. Immunobiology of relapsing fever. Contrib Microbiol Immunol. 1987;8:125–137. [PubMed] [Google Scholar]
- 3.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour A G, Barrera O, Judd R C. Structural analysis of the variable major proteins of Borrelia hermsii. J Exp Med. 1983;158:2127–2140. doi: 10.1084/jem.158.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour A G, Burman N, Carter C J, Kitten T, Bergström S. Variable antigen genes of the relapsing fever agent Borrelia hermsii are activated by promoter addition. Mol Microbiol. 1991;5:489–493. doi: 10.1111/j.1365-2958.1991.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 6.Barbour A G, Carter C J, Burman N, Freitag C S, Garon C F, Bergström S. Tandem insertion sequence-like elements define the expression site for variable antigen genes of Borrelia hermsii. Infect Immun. 1991;59:390–397. doi: 10.1128/iai.59.1.390-397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour A G, Hayes S F. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour A G, Hayes S F, Heiland R A, Schrumpf M E, Tessier S L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbour, A. G., M. Schrumpf, and C. Sohaskey. Antigenic variation by expression site switching in the relapsing fever agent Borrelia hermsii. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 10.Barbour A G, Tessier S L, Stoenner H G. Variable major proteins of Borrelia hermsii. J Exp Med. 1982;156:1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barstad P A, Coligan J E, Raum M G, Barbour A G. Variable major proteins of Borrelia hermsii. Epitope mapping and partial sequence analysis of CNBr peptides. J Exp Med. 1985;161:1302–1314. doi: 10.1084/jem.161.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergström S, Bundoc V G, Barbour A G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989;3:479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 14.Brandt M E, Riley B S, Radolf J D, Norgard M V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990;58:983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burman N, Bergström S, Restrepo B I, Barbour A G. The variable antigens Vmp7 and Vmp21 of the relapsing fever bacterium Borrelia hermsii are structurally analogous to the VSG proteins of the African trypanosome. Mol Microbiol. 1990;4:1715–1726. doi: 10.1111/j.1365-2958.1990.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 16.Cadavid D, Barbour A G. Neuroborreliosis during relapsing fever: review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clin Infect Dis. 1998;26:151–164. doi: 10.1086/516276. [DOI] [PubMed] [Google Scholar]
- 17.Cadavid D, Bundoc V, Barbour A. Experimental infection of the mouse brain by a relapsing fever Borrelia species: a molecular analysis. J Infect Dis. 1993;168:143–151. doi: 10.1093/infdis/168.1.143. [DOI] [PubMed] [Google Scholar]
- 18.Cadavid D, Pennington P M, Kerentseva T A, Bergström S, Barbour A G. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect Immun. 1997;65:3352–3360. doi: 10.1128/iai.65.8.3352-3360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cadavid D, Thomas D D, Crawley R, Barbour A G. Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J Exp Med. 1994;179:631–642. doi: 10.1084/jem.179.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter C J, Bergström S, Norris S J, Barbour A G. A family of surface-exposed proteins of 20 kilodaltons in the genus Borrelia. Infect Immun. 1994;62:2792–2799. doi: 10.1128/iai.62.7.2792-2799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Silva A M, Fikrig E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J Clin Investig. 1997;99:377–379. doi: 10.1172/JCI119169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Silva A M, Telford S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felsentein J. Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol. 1996;266:418–427. doi: 10.1016/s0076-6879(96)66026-1. [DOI] [PubMed] [Google Scholar]
- 24.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 24a.Genetics Computer Group. Sequence analysis software, version 7.3. Madison, Wis: Genetics Computer Group; 1991. [Google Scholar]
- 25.Hinnebusch B J, Barbour A G, Restrepo B I, Schwan T G. Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect Immun. 1998;66:432–440. doi: 10.1128/iai.66.2.432-440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsson M, Noppa L, Barbour A G, Bergström S. Heterogeneity of outer membrane proteins in Borrelia burgdorferi: comparison of osp operons of three isolates of different geographic origins. Infect Immun. 1992;60:1845–1853. doi: 10.1128/iai.60.5.1845-1853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitten T, Barbour A G. Juxtaposition of expressed variable antigen genes with a conserved telomere in the bacterium Borrelia hermsii. Proc Natl Acad Sci USA. 1990;87:6077–6081. doi: 10.1073/pnas.87.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitten T, Barrera A V, Barbour A G. Intragenic recombination and a chimeric outer membrane protein in the relapsing fever agent Borrelia hermsii. J Bacteriol. 1993;175:2516–2522. doi: 10.1128/jb.175.9.2516-2522.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 30.Marconi R T, Samuels D S, Schwan T G, Garon C F. Identification of a protein in several Borrelia species which is related to OspC of the Lyme disease spirochetes. J Clin Microbiol. 1993;31:2577–2583. doi: 10.1128/jcm.31.10.2577-2583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margolis N, Hogan D, Cieplak W, Schwan T, Rosa P. Homology between Borrelia burgdorferi OspC and members of the family of Borrelia hermsii variable major proteins. Gene. 1994;143:105–110. doi: 10.1016/0378-1119(94)90613-0. [DOI] [PubMed] [Google Scholar]
- 32.Margolis N, Hogan D, Tilly K, Rosa P A. Plasmid location of Borrelia purine biosynthesis gene homologs. J Bacteriol. 1994;176:6427–6432. doi: 10.1128/jb.176.21.6427-6432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier J, Simon M, Barbour A G. Antigenic variation is associated with DNA rearrangement in a relapsing fever borrelia. Cell. 1985;41:403–407. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery R R, Malawista S E, Feen K J, Bockenstedt L K. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padula S J, Sampieri A, Dias F, Szczepanski A, Ryan R W. Molecular characterization and expression of p23 (OspC) from a North American strain of Borrelia burgdorferi. Infect Immun. 1993;61:5097–5105. doi: 10.1128/iai.61.12.5097-5105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park H K, Jones B E, Barbour A G. Erythema chronicum migrans of Lyme disease: diagnosis by monoclonal antibodies. J Am Acad Dermatol. 1986;15:406–410. doi: 10.1016/s0190-9622(86)70190-4. [DOI] [PubMed] [Google Scholar]
- 37.Pennington P M, Allred C D, West C S, Alvarez R, Barbour A G. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65:285–292. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pennington P M. Ph.D. thesis. San Antonio: University of Texas Health Science Center at San Antonio; 1998. [Google Scholar]
- 39.Pennington, P. M., D. Cadavid, and A. G. Barbour. 1997. Unpublished observations.
- 40.Plasterk R H, Simon M I, Barbour A G. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985;318:257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- 41.Restrepo B I, Barbour A G. Antigen diversity in the bacterium Borrelia hermsii through “somatic” mutations in rearranged vmp genes. Cell. 1994;78:867–876. doi: 10.1016/s0092-8674(94)90642-4. [DOI] [PubMed] [Google Scholar]
- 42.Restrepo B I, Carter C J, Barbour A G. Activation of a vmp pseudogene in Borrelia hermsii: an alternate mechanism of antigenic variation during relapsing fever. Mol Microbiol. 1994;13:287–299. doi: 10.1111/j.1365-2958.1994.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 43.Restrepo B I, Carter C J, Kitten T, Barbour A G. Subtelomeric expression regions of Borrelia hermsii linear plasmids are highly polymorphic. Mol Microbiol. 1992;6:3299–3311. doi: 10.1111/j.1365-2958.1992.tb02198.x. [DOI] [PubMed] [Google Scholar]
- 44.Šadžiene A, Wilske B, Ferdows M S, Barbour A G. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993;61:2192–2195. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwan T G, Hinnebusch B J. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science. 1998;280:1938–1940. doi: 10.1126/science.280.5371.1938. [DOI] [PubMed] [Google Scholar]
- 46.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sohaskey C D, Arnold C, Barbour A G. Analysis of promoters in Borrelia burgdorferi by use of a transiently expressed reporter gene. J Bacteriol. 1997;179:6837–6842. doi: 10.1128/jb.179.21.6837-6842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoenner H G, Dodd T, Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982;156:1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Department of Genetics, University of Washington. PHYLIP, version 3.5c. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]