Abstract
Although vaccination efforts have expanded, there are still gaps in our understanding surrounding the immune response to SARS-CoV-2. Measuring IgG Fc glycosylation provides insight into an infected individual’s inflammatory state, among other functions. We set out to interrogate bulk IgG glycosylation changes from SARS-CoV-2 infection and vaccination, using plasma from mild or hospitalized COVID-19 patients, and from vaccinated individuals. Inflammatory glycans are elevated in hospitalized COVID-19 patients and increase over time, while mild patients have anti-inflammatory glycans that increase over time, including increased sialic acid correlating with RBD antibody levels. Vaccinated individuals with low RBD antibody levels and low neutralization have the same IgG glycan traits as hospitalized COVID-19 patients. In addition, a small vaccinated cohort reveals a decrease in inflammatory glycans associated with peak IgG concentrations and neutralization. This report characterizes the bulk IgG glycome associated with COVID-19 severity and vaccine responsiveness and can help guide future studies into SARS-CoV-2 protective immunity.
Keywords: COVID-19; antibodies; glycosylation; SARS-CoV-2, IgG, RBD, Nucleocapsid, vaccination, infection, neutralization, inflammation
Graphical abstract

In Ash et al., bulk IgG glycosylation is measured in SARS-CoV-2 infected and COVID-19 vaccinated cohorts and is found to predict disease severity and vaccine antibody response. These glycosylation profiles change dynamically over time, with similarities found between hospitalized COVID-19 patients and vaccinees with low antibody responses.
Introduction
The COVID-19 pandemic has claimed the lives of more than 6 million people as of September 2022 and placed a great burden on hospital systems worldwide. COVID-19 manifests clinically with symptoms of loss of taste and smell, fever, and persistent cough, which are early predictors of COVID-19.1 In severe disease, COVID-19 patients present with clinical symptoms including saturated oxygen at rest and pneumonia confirmed by X-ray and computed tomography scan.2 In addition, a number of severe patients also exhibit systemic inflammation inducing cytokine storm and lymphopenia.3 Lymphopenia of CD4+ and CD8+ T cells also coincides with interferon-γ increase, indicating the reduction of lymphocytes may drive inflammation seen in severe disease.4 Identifying the root cause of this inflammatory response is paramount to alleviating severe symptoms and predicting disease outcomes. Although vaccination efforts against SARS-CoV-2, the etiologic agent of COVID-19, have expanded, many questions remain relevant to the characteristics of a potent anti-SARS-CoV-2 immune response that may differentiate between mild and severe cases of COVID-19.
Human immunoglobulin (Ig)Gs have a glycosylation site on the Fc that is post-translationally modified at a single asparagine-297 residue (Figure S1), which in addition to contributing to immune activity, is also important for antibody stability and half-life.5 Addition of N-glycans on the Fc of the antibody can predict which aspects of the immune system an antibody activates (Figure S1), including: fucosylation, removal of which increases Fc affinity for FcγRIIIa; bisection, addition of which can increase activity of antibody-dependent cellular cytotoxicity (ADCC); galactosylation, lack of which is associated with autoimmunity and inflammation; and sialylation, which generally has an anti-inflammatory effect and increases antibody half-life.6 , 7 Degrees of disease severity are often marked by changes in glycan structure, including increased levels of agalactosylated and asialylated IgGs.6 , 8 , 9 Decreased galactosylation and sialylation have also been shown on autoantibodies in autoimmune diseases, correlating with exacerbated disease states.10 Furthermore, IgG glycosylation traits are altered in chronic disease, such as in type 2 diabetes where IgGs tend to be less galactosylated and sialylated and have more bisection, indicating an overall inflammatory state.11
In this study we set out to characterize the glycosylation of bulk IgG between mild and hospitalized COVID-19 patients as well as vaccinated individuals. We measured antibody binding and neutralization from vaccinated individuals and correlated with the glycosylation patterns found in their bulk IgG. We found that hospitalized COVID-19 patients have similar glycosylation patterns to vaccinated individuals with a low neutralizing antibody response. Collectively, our findings point to a key shift in IgG glycosylation of COVID-19 patients and vaccinated individuals over time that can predict disease severity and vaccine responsiveness, adding prognostic value to investigating the antibody structure of bulk IgG of COVID-19 patients and vaccinated individuals.
Results
SARS-CoV-2 infected and COVID-19 vaccinated cohort
We received 98 patient samples from the Rush COVID-19 Biorepository and 27 patient samples from the Rush Infectious Disease Clinic. These were individuals who had tested RT-qPCR positive for SARS-CoV-2 and included 69 men and 56 women, with grouped ages ranging from 15 to 49, 50 to 64, and >65 years old (median 50–64), all of which were collected between April and May 2020, non-vaccinated, and were not previously infected. Forty-six of these patients were not hospitalized and classified as mild cases, the majority of which included health care workers who were mildly symptomatic but not hospitalized, collected at the same institution during the same time as the hospitalized patients. The other 79 patients were considered hospitalized as characterized by hospital admission, and 61 required admission to the Rush Intensive Care Unit, of whom 14 died (Table S1). These individuals included a combination of moderate, severe, and critical patients, according to the World Health Organization (WHO) guidelines for COVID-19 severity.12 The vaccinated cohort included 228 individuals, ranging in age from 15 to >65 (median 50–64) with 66 male and 162 female individuals immunized with SARS-CoV-2 Spike protein mRNA (Pfizer/BioNTech or Moderna, Table S2). Within the vaccinated cohort, 13 individuals reported previous infection; however, the actual prevalence of previous infection within the cohort included 72 individuals. Subsequent grouping of high versus low neutralizers can be found in Table S3, and further grouping by high neutralization/high receptor binding domain (RBD) binding and low neutralization/low RBD binding can be found in Table S4.
Hospitalized COVID-19 patients have increased inflammatory glycans
We first set out to understand how IgG glycan traits differ between mild and hospitalized COVID-19 patients to investigate a possible correlation between glycosylation traits and disease severity. To assess IgG glycosylation patterns of COVID-19 patients, IgG heavy chains from mild and hospitalized COVID-19 patients (Table S1) were isolated and assessed via mass spectrometry. In total, we assessed 13 IgG glycan traits, which can be seen stratified by hospitalized or mildly symptomatic status (Figure 1 ). Analysis of patient glycans stratified into critical, severe, moderate, and mild categories based on WHO classification guidelines12 via one-way ANOVA revealed a marked increase in G0F (Figure 1A, p < 0.01) and agalactosylation (Figure 1G, p < 0.0001) in critical COVID-19 patients compared with those with mild disease, with higher levels of agalactosylated glycans in severe patients compared with mild as well (Figure 1G, p < 0.05). There was a significantly lower mean level of G2F (Figure 1C, p < 0.01), G2 (Figure 1D, p < 0.01), galactosylated (Figure 1E, p < 0.0001), sialylated (Figure 1F, p < 0.0001), and fucosylated bisecting (Figure 1H, p < 0.0001) glycans among critical COVID-19 patients compared with mildly symptomatic individuals. There was also a decrease of G1F (Figure 1B, p < 0.05) in severe compared with mild patients, as well as a decrease of sialylation (Figure 1F, p < 0.05) between critical compared to moderate patients, and a decrease in fucosylated bisecting glycans (Figure 1H, p < 0.05) in critical compared with severe patients. Fucosylated, bisecting, afucosylated bisecting, G0, and G1 glycans (Figures 1I-1M) did not obtain significant levels of difference when stratified by hospitalization, although G0 was significantly increased in hospitalized patients compared with mild individuals (Figure 1L, ∗p < 0.05). We also performed receiver operating characteristic (ROC) analysis of increased G0F (Figure 2A, area under the curve [AUC] = 0.73, p < 0.0001), reduced G2F (Figure 2B, AUC = 0.72, p < 0.0001), and reduced sialylation (Figure 2C, AUC = 0.74, p < 0.0001) as biomarkers of severe COVID-19 (Figure 2). Last, we observed decreases in sialylation in hospitalized individuals also correlates with RBD binding in mild patients compared with hospitalized (p < 0.01, Figures 2D and 2E). These data demonstrate that inflammatory glycans are elevated among hospitalized individuals, especially in critical patients, in comparison to mild COVID-19, and that mild COVID-19 patients may benefit from the increased half-life and protection from inflammation that sialylation and galactosylation confers.
Figure 1.
Bulk IgG glycosylation analysis reveals an anti-inflammatory profile in mild and an inflammatory profile in hospitalized COVID-19 patients
Glycosylation analysis of 46 mild (blue) versus 79 hospitalized (stratified by disease severity, critical = orange, severe = yellow, moderate = green) COVID-19 patient glycans were analyzed using one-way ANOVA (A–M). Data points illustrate the observed distribution for % glycosylation measured from digested, excised IgG heavy chain isolated from each individual (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
Figure 2.
Bulk IgG glycosylation predicts disease severity and correlates with increased RBD antibody levels
Hospitalized COVID-19 patients were assessed for the presence of G0F (A, AUC = 0.73, p < 0.0001), G2F (B, AUC = 0.72, p < 0.0001), and sialylation (C, AUC = 0.74, p < 0.0001) as a biomarker for severe disease compared with mild patients as controls, assessed via receiver operating characteristic (ROC) analysis. IgG Fcs were assessed for RBD binding via Pearson correlations between RBD binding and percent sialylation for hospitalized (D), mild (E), and combined (F) groups. RBD binding ELISA was performed in technical duplicate for each individual, and data points illustrate the observed distribution for % glycosylation measured from digested, excised IgG heavy chain isolated from each individual.
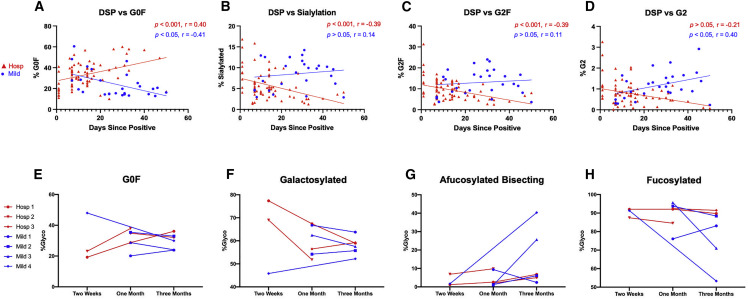
Bulk IgG glycosylation traits change over time and are predictive of COVID-19 disease state
We next wanted to know if the observed difference in glycosylation traits between mild and hospitalized COVID-19 patients remained constant over time. We assessed patient plasma from mild and hospitalized COVID-19 patients up to 50 days after a SARS-CoV-2 positive PCR test using stratified Pearson correlations between glycans and days, and Spearman correlations for non-normal datasets (Figure 3 ). Based on simple linear regression modeling (group, days, group∗days), hospitalized individuals showed a positive correlation between the presence of G0F glycans (Figure 3A, r = 0.4, 95% CI 0.2–0.6, p < 0.001) and the time since positivity, while G1F (Figure S2G, p < 0.05, r = −0.27) and G2F glycans (Figure 3C, r = −0.39, 95% CI 0.2–0.6, p < 0.001) revealed a negative correlation, indicating a shifting IgG glycan phenotype. Observed differences in the glycosylation pattern of IgG among mild individuals were opposite of changes seen in hospitalized patients, with G0F correlating negatively (Figure 3A, r = −0.41, 95% CI 0.1–0.7, p < 0.05) and G2 correlating positively with increased days since positive (Figure 3D, r = 0.40, 95% CI 0.02–0.7, p < 0.05). In addition, there was a negative correlation between days since positive and sialylation within hospitalized individuals (Figure 3B, r = −0.39, 95% CI −0.6 to −0.2, p < 0.001). This phenomenon is reversed within mild individuals, which show increased sialylation correlating with elevated anti-RBD levels (Figure 2E). Analysis of additional glycosylation traits can be seen in Figure S2, with significance returning for mild patients in fucosylation (Figure S2B), fucosylated bisection (Figure S2C), bisection (Figure S2D), and G1 (Figure S2E), while hospitalized patients showed significance for fucosylated bisection (Figure S2C) and G1F (Figure S2G). These data demonstrate a skewing of inflammatory glycans over time among hospitalized individuals that is opposite from the glycan changes observed among mild patients.
Figure 3.
Bulk IgG glycosylation of mild and hospitalized COVID-19 patients changes over time following SARS-CoV-2 infection
COVID-19 patient IgG Fc glycosylation was analyzed using simple linear regression to compare slopes between groups (A–D). Pearson correlations were performed for all normally distributed groups. Anderson-Darling tests for normality revealed non-normal distribution in hospitalized G2F, sialylation, and G2, and in mild G0F, for which Spearman correlations were performed. Longitudinal hospitalized (n = 3) and mild (n = 4) COVID-19 patients were assessed for the percentage of IgG Fc glycosylation over time, including G0F (E–H). Data Points illustrate the observed distribution for % glycosylation measured from digested, excised IgG heavy chain isolated from each individual (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
We also tested a longitudinal cohort of mild and hospitalized COVID-19 patients for changes in IgG glycosylation over 3 months. Hospitalized individuals showed an increase in G0F glycans (Figure 3E) and decrease in global galactosylation (Figure 3F) over time. Conversely, we observed an increase of afucosylated bisecting glycans in mild individuals (Figure 3G), as well as a decrease in global fucosylation (Figure 3H). Additional glycosylation trait analysis revealed a drop in G2 and G2F glycans over time in the hospitalized group (Figures S3C, S3E). Taken together, these two cohorts show a significant increase in inflammatory agalactosylated glycans over time in hospitalized individuals, while mild patients see a decrease in the same glycan traits.
COVID-19 vaccine responsiveness corresponds with IgG glycosylation traits
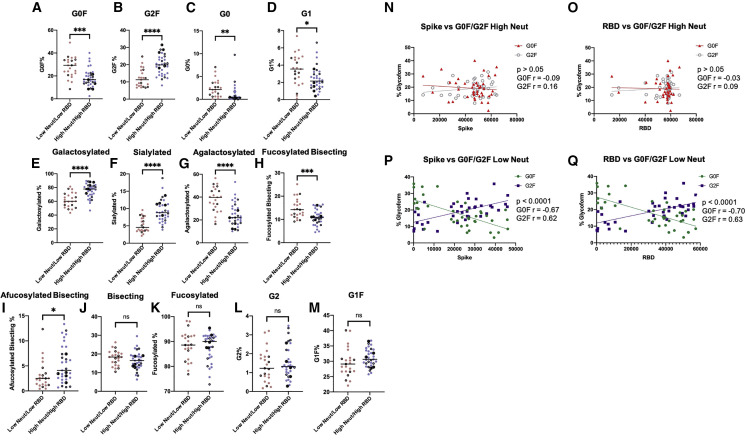
Because of the differences observed in naturally infected individuals, we wanted to further investigate the IgG traits in a large cohort of COVID-19 vaccinated individuals. We screened 228 Spike mRNA vaccinated samples (Table S2) for SARS-CoV-2 Spike pseudovirus neutralization potential, as measured by blocking fusion to the cell by pseudoviruses (decreased relative luminescence [RLU], higher neutralization), up to 100 days after full vaccination (e.g., second shot) (Figure 4A). Neutralization of SARS-CoV-2 was stratified into high and low neutralizers (Table S3) binned with neutralization above or below the RLU for virus without antibodies blocking, indicating the absence of neutralization, delineated by the dotted line in Figure 4A. We first assessed binding of plasma to either S1 or RBD, grouped by high and low neutralizers (Figure 4B). As expected, high neutralizers have greater binding to both S1 and RBD (Figure 4B, p < 0.0001) when compared with low neutralizers. Of note was an S1 and RBD high population in the low neutralizer cohort, which represents SARS-CoV-2 elicited non-neutralizing antibodies. Due to the heterogeneity of high and low neutralizers within the vaccinated cohort, we gated a sub-cohort of individuals based on RBD binding and neutralization, creating high neutralizers with high RBD binding and low neutralizers with low RBD binding sub-groups. We also assessed these groups for age differences and found that individuals with high neutralization alone, as well as high neutralization and high RBD binding had a significantly lower age than individuals with low RBD binding alone and low RBD binding/low neutralization (Figure 4C). In the case of nucleocapsid binding, with infection threshold set at nucleocapsid values above 1,500 mean fluorescence intensity (MFI), we observed a significantly increased mean nucleocapsid antibody binding in low neutralizers compared with high neutralizers (Figure 4D, p < 0.05), while there was no observed difference in nucleocapsid binding between low neutralizing, low RBD binding individuals and high neutralizing, high RBD binding individuals (Figure 4D, p > 0.05). We also assessed the significance of stratification by sex within this cohort, but did not determine any significance. We observed a statistically significant difference between high and low neutralizers for days since the second dose of vaccine, while low RBD/low neutralizers and high RBD/high neutralizers showed no difference (Figure 4E). In addition, we assessed individuals with low neutralization and low RBD binding for differences in glycosylation traits and revealed an increased amount of G0F (Figure 5A, p < 0.001), G0, (Figure 5C, p < 0.01), G1 (Figure 5D, p < 0.05), agalactosylation (Figure 5G, p < 0.0001), and fucosylated bisection (Figure 5H, p < 0.001), and decreased G2F (Figure 5B, p < 0.0001), galactosylation (Figure 5E, p < 0.0001), sialylation (Figure 5F, p < 0.001), and afucosylated bisection (Figure 5I, p < 0.05) when compared with individuals with high neutralization and high RBD binding. We also compared four additional glycosylation traits and found that glycosylation traits bisection, fucosylation, G2, and G1F were not significantly altered in low neutralizing, low RBD binding versus high neutralizing, high RBD binding (Figures 5J–5M). High SARS-CoV-2 neutralizers did not present with differences in Spike S1 (Figure 5N, p > 0.05) or RBD (Figure 5O, p > 0.05) levels when stratified between either G0F or G2F glycosylation traits. When comparing Spike and RBD binding of the low neutralization group, there was a positive correlation for G2F and a negative correlation for G0F with antibody binding (Figures 5P and 5Q, p < 0.0001). As an additional facet of analysis, we analyzed all 13 glycosylation traits in the same vaccinated cohort, but with previous infection removed (Figure S4A), as well as the vaccinated cohort showing only previously infected individuals, indicated by either positive PCR test or nucleocapsid binding above 1,500 MFI (Figure S4B). Within the not previously infected group, afucosylated bisection was increased in the high neutralizing/high RBD group (Figure S4A, p < 0.001), while there was a lower mean value of G1 (Figure S4A, p < 0.05) and G2 (Figure S4A, p < 0.05) in the high neutralizing/high RBD group. Among previously infected individuals, galactosylation (Figure S4B, p < 0.05), sialylation (Figure S4B, p < 0.05), and G1F (Figure S4B, p < 0.01) were all significantly increased. Collectively, these findings demonstrate similar glycosylation patterns were observed in hospitalized COVID-19 patients and vaccinated individuals with low neutralization and low elicited antibodies and that glycosylation state of bulk IgG may be a predictor for vaccine-elicited antibody neutralization potential.
Figure 4.
Characterization of COVID-19 vaccinated individuals stratified by neutralization and SARS-CoV-2 antibody binding
Plasma from vaccinated individuals was diluted 1:900 and assessed for neutralization potential via a spike pseudotyped entry (A). Red = low neutralization, blue = high neutralization, outlined circles = previously infected based on nucleocapsid values above 1,500 MFI, black circles = previously reported PCR positive infection. Within each neutralizing group, two-tailed unpaired t tests were used to compare Spike S1 (B) binding and RBD (B) binding of vaccinated individuals. Two-tailed unpaired t tests were performed for high and low neutralizers as well as high neutralizing high RBD binding and low neutralizing low RBD binding for age (C). Nucleocapsid binding was also assessed for high and low neutralizers, and high neutralizing high RBD binding and low neutralizing low RBD binding (D). Two-tailed unpaired t tests were performed for high and low neutralizers, as well a high neutralizer high RBD binding and low neutralizing low RBD binding (E) for days since second vaccination. Neutralization assessment, Spike S1, RBD, and nucleocapsid binding Luminex assays were all performed in technical duplicate for each individual. (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
Figure 5.
Bulk IgG glycosylation analysis of COVID-19 vaccinated individuals stratified by high and low neutralization and RBD binding
Glycosylation analysis between high neutralizers with high RBD binding and low neutralizers low RBD binding was carried out for glycosylation signatures (A–M) using two-tailed unpaired t tests. Vaccinated individuals assessed for binding to both Spike S1 (N) and RBD (O) were correlated for glycosylation traits within the high neutralizing subgroup using Pearson correlations and simple linear regression modeling. Simple linear regression modeling was performed on Spike (P) and RBD (Q) binding within the low neutralizing group. RBD and Spike binding assays were performed in technical duplicate for each individual, and data points illustrate the observed distribution for % glycosylation measured from digested, excised IgG heavy chain isolated from each individual (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
The IgG glycome changes over time in response to COVID-19 vaccination
We next longitudinally assessed individuals vaccinated with SARS-CoV-2 Spike mRNA out to 8 months post-full vaccination. We set out to understand how the antibody repertoire changes in the months after vaccination (Figure 6 ). Assessing four individuals longitudinally revealed a spike in antibody binding to RBD (Figure 6A, red) and NTD (Figure 6B, orange) between 2 weeks and 1 month after second vaccine, which then sharply drops off around 2 to 3 months out, and continues to decrease out to 8 months. Neutralization potential, as readout by a decrease in cell entry and lower RLU, shows highest neutralization weeks after the second shot, which correlates with the highest antibody titers (Figure 6C, green). Interestingly when assessing glycosylation, the percentage of fucosylated agalactosylated (G0F) containing IgG drops between the 1- to 2-month mark and then slowly returns to the pre-vaccine baseline (Figure 6D, Black). Nucleocapsid binding in all four donors was also assessed via ELISA, with all four donors returning positive values for nucleocapsid antibodies, indicating points of infection (Figure S5). Analysis of additional glycosylation traits revealed a sharp decrease in global fucosylation (Figure 6F), and increase in afucosylated bisection (Figure 6G) near the 3-month post full-vaccination mark. There was also a marked decrease in G1F (Figure S6D) and increase in bisection (Figure S6G) near the same time point. These data reinforce changes in G0F observed in a larger vaccinated cohort and in naturally infected individuals, highlighting a protective role when skewed away from this glycosylation trait.
Figure 6.
Longitudinal assessment of SARS-CoV-2 antibody, neutralization, and glycosylation traits of vaccinated individuals
Longitudinal vaccinated samples were assessed for RBD binding (A, E, red), NTD binding (B, E, orange), neutralization (C, E, green), and percent of G0F (D, E, black) over 7 months post second vaccination. Samples were also assessed for differences in glycosylation trait over 7 months, including afucosylated bisecting (F) and fucosylated glycans (G). RBD and NTD binding ELISA as well as neutralization assays were performed in technical duplicate for each individual, and data points illustrate the observed distribution for % glycosylation measured from digested, excised IgG heavy chain isolated from each individual.
Discussion
In this study, we investigated antibody traits of bulk IgGs from mild and hospitalized COVID-19 patients at independent time points and compared these results with COVID-19 vaccinated individuals. Analysis of glycan traits on IgG Fcs of hospitalized patients indicated an elevated level of fucosylated agalactosylated antibodies (G0F) that increased in the days since testing positive for SARS-CoV-2. Further, we observed an association between days since positive and lower sialylation for bulk IgG Fcs, suggesting hospitalized individuals experience a shift toward more inflammatory antibody responses in the weeks and months after testing positive. Interestingly, we detected a decrease in G0F and increase in G1 and G2 glycosylation at independent increasing time points in mild cases, which is mirrored for G0F in our longitudinal COVID-19 patient cohort, indicating the phenomenon of switching to inflammatory glycan structures may be a predictive marker of severe disease. We demonstrate that this inflammatory glycan trait seen in hospitalized COVID-19 patients, namely elevated fucosylated agalactosylated IgG, was also detected in vaccinated individuals with low neutralization and low RBD antibody levels. Last, we detected a distinct increase in afucosylated bisecting glycans, and a decrease in fucosylated and G1F glycans around 3 months post full vaccination within our longitudinal vaccinated cohort, alluding to changes in the antibody repertoire that may occur as a consequence of vaccination and potentially offer protection. We acknowledge that our mild versus hospitalized patient cohort is not balanced, with more patients falling in the hospitalized category; however, we do not believe this affects the strength of our findings, as the increase in G0F in hospitalization, is reflected across hospitalized disease states.
Our findings differ from other studies assessing the SARS-CoV-2 IgG glycome, as it has been shown that advanced disease correlates with afucosylated galactosylated glycans, although these studies evaluated patients in the acute stage of infection (10 days after hospital admission),13 , 14 , 15 alluding to a need for evaluation of the IgG glycome over a larger time frame. Afucosylated IgGs in COVID-19 have been associated with formation of immune complexes activating the FcγRIIIa receptor and production of inflammatory cytokines.13 It has also been suggested that age plays a role in decreased RBD binding depending on glycosylation trait, with older individuals who received the BNT162b2 vaccine showing markedly lower levels of sialylated and galactosylated glycans, and higher levels of fucosylated and bisecting glycans interacting with RBD,16 which is in line with our observations within our large vaccinated cohort. We show that age plays a larger role within the context of neutralization and RBD binding, rather than RBD binding alone, reinforcing that increased levels of sialylated and galactosylated glycans may be more protective against COVID-19 hospitalization, leaving older populations without these glycan characteristics especially vulnerable to severe disease. While these findings have been addressed in a number of studies, ours is notable in its analysis of separate individuals over a larger time course. Our study demonstrates that hospitalized COVID-19 patients may shift toward a G0F trait over time, reinforced by the fact that each data point is a unique patient, showing the trends observed hold for a significant number of individuals. This finding points to a protective nature of G2F antibodies, as the percentage goes up over time in mild individuals while decreases in hospitalized patients. A recent study on the COVID-19 IgG glycome has produced similar results, identifying an increase in agalactosylation and decrease in sialylation and bisection as altered glycosylation traits are responsible for heightened immune activation in severe COVID-19.17 These data suggest that the shift in antibody population dynamics (i.e., glycosylation state) could be crucial to whether or not an individual develops severe or mild COVID-19 and less about the virus itself per se and more about the evolving immune response following viral clearance. These observations are mirrored in our vaccinated cohort where we observe anti-inflammatory antibody population dynamics that could contribute to the high efficacy these vaccines elicit. While this study lacks characterization of SARS-CoV-2 specific IgGs, the shifting total IgG glycosylation observed in our cohorts are comparable to what is seen in specific IgGs, where COVID-19-induced cytokine expression was measured.18 It is likely that both the SARS-CoV-2 specific and the bulk IgG glycome together influence the inflammatory response to COVID-19.
In the acute phase of COVID-19 infection, critically ill patients exhibit high levels of afucosylated galactosylated IgGs, which elicits a strong response against FcγRIIIa and recruits inflammatory alveolar macrophages and NK cells.13 , 14 Interestingly, both neutralizing and non-neutralizing antibodies specific to SARS-CoV-2 can mount an effector response via ADCC, which has been found to primarily function through CD16 found on NK cells.19 , 20 In the past, it has been noted that alteration of Fc glycosylation can directly affect effector function and complement activation for neutralizing antibodies to drive the humoral immune response.21 Fc-dependent complement activation was shown to be decreased in severe COVID-19 patients compared with mildly infected individuals.22 IgGs, especially non-neutralizing antibodies, associated with severe COVID-19 are namely afucosylated and contribute to the massive inflammatory response seen in patients, which may be partially attributed to lower fucosyltransferase 8 (FUT8) levels in some individuals.23 , 24 We found a sizable non-neutralizing antibody response in our vaccinated cohort, defined as low neutralization and high RBD binding, and we are currently investigating these antibodies for non-neutralizing effector function. In addition, our longitudinal vaccinated cohort shows differential timing for increases in glycosylation traits that confer elevated FcγR binding and effector functions, namely a decrease in fucosylation and an increase in bisection, occurring after 2 months post second shot. Therefore, despite waning antibody levels, there are increased non-neutralizing enhancing glycosylation traits that arise after the initial peak of antibody/neutralization response.
An additional protective trait in IgGs is the addition of sialic acid, or sialylation, on the Fc. Our data show mild COVID-19 patients presenting with increased percentages of sialylated IgGs compared with hospitalized patients. The presence of sialylation in mild patients may be protective against immune activation, as well as prolong the lifetime of anti-SARS-CoV-2 antibodies in serum. Similarly, the lack of and/or decrease of sialylation in hospitalized COVID-19 patients may point to a loss of protective antibodies, therefore promoting severe symptoms and cytokine storm. This is not the first report of sialylation differences stratified by COVID-19 disease severity13 , 16 , 25; however, we add to these reports by showing a decrease in sialyation over time in hospitalized COVID-19 patients, and a positive correlation in sialyation with RBD antibody levels in the mildly infected individuals. This finding is mirrored within our large vaccinated cohort, in which low neutralizers/low RBD binders exhibited lower sialylated glycans than their high neutralizing/high RBD binding counterparts. These results have the potential to influence ongoing and future antibody studies, including those investigating anti-inflammatory properties and half-life, within the context of the SARS-CoV-2 infection.
In the case of other viral infections, such as Ebola, severe disease correlates with agalactosylated afucosylated antibodies, while protective antibodies exhibit afucosylated bisecting glycan patterns.26 , 27 Interestingly, however, in a large-scale study probing characteristics of monoclonal antibodies to Ebola, fucosylated agalactosylated antibodies correlated most with protection when used therapeutically.28 These are the same glycosylation traits (G0F) that we identified here to associate with low RBD binding and low neutralization.28 Last, studies on the anti-influenza IgG glycome post-vaccination have indicated a shift toward increased galactosylation and sialylation traits, although no changes in bulk IgG glycosylation were observed.29 Our data specifically identifies bulk IgGs as skewing toward the G0F trait for vaccinated individuals with low viral neutralizing capability, and G2F for those who are high neutralizers.
Interestingly, we detected varying degrees of nucleocapsid protein binding in both our vaccinated and longitudinal vaccinated cohorts. Among our larger vaccinated cohort, we detected a previous infection level of 30%, while reported previous infection was only 5% of individuals. Within our low neutralizing/low RBD binding and high neutralizing/high RBD binding subset of vaccinated individuals, this level of previous infection maintained a level of around 30%, mirroring previous infection distribution seen in our larger cohort. We also detected varying degrees of nucleocapsid antibody binding in our longitudinal vaccinated cohort, with all four individuals showing significant spikes in nucleocapsid antibody production. This is especially pertinent to the current state of COVID-19, wherein the era of Omicron has revealed the prevalence of previous infection in the population is nearly 92%.30 The majority of donors in both cohorts were not previously reported as positive for SARS-CoV-2 infection, meaning there are likely many more individuals who have become infected and remain asymptomatic, promoting asymptomatic transmission within the community.
Our study identifies inflammatory COVID-19 IgG glycosylation traits in the total IgG glycome that change over time in hospitalized COVID-19 patients, while the opposite effect is observed in mildly symptomatic patients. Presence of G0F glycosylated IgGs and skewing toward G0F glycans over time in hospitalized patients may be predictors that a patient will develop advanced disease. Similarly, vaccine antibody responses may be predicted based on the glycan traits of vaccinated patient IgGs, which underscores the clinical relevance of studying glycosylation in context of disease progression and vaccine response.
Limitations of the study
There are a few limitations to this study that the authors would like to disclose. The samples used from infected individuals are remnant samples that reduced the amount of information we had available. In particular, we were not able to stratify our data to include comorbidities nor drug regimens these individuals were on, nor were we provided baseline data for each patient. In addition, we include previously infected individuals in our vaccinated cohort analysis. While removing previously infected individuals from the cohort would remove significant outside confounds, it is more clinically relevant to include previously infected individuals within this analysis, as it is more likely an individual would have previously come into contact with COVID-19-positive individuals given the fast-spreading nature of SARS-CoV-2 infection. Finally, all the glycosylation analysis done in this study was bulk IgG characterization and not specific to SARS-CoV-2. While a limitation, interestingly we were able to show parallels to papers in the literature using antigen-specific glycosylation patterns, highlighting the potential of using bulk IgG as a predictor of inflammatory state and COVID-19 severity.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit Polyclonal anti-ACE protein | ProteinTech | Cat# 21115-1-AP |
| PE-conjugated goat anti-human IgG polyclonal | Southern Biotech | Cat# 2040-09; RRID:AB_2795648 |
| Goat anti-human IgG Fc-HRP | Southern Biotech | Cat# 2048-05 |
| Chemicals, peptides, and recombinant proteins | ||
| SARS-CoV-2 Spike S1 | Sino Biologicals | Cat# 40591-V08H |
| SARS-CoV-2 RBD | Sino Biologicals | Cat# 40592-T62 |
| SARS-CoV-2 Nucleocapsid | Sino Biologicals | Cat# 40588-V08B |
| Puromycin | Invivogen | Cat# ant-pr-5 |
| Protein G Plus Agarose | Pierce | Cat# 22851 |
| IgG Elution Buffer | ThermoFisher | Cat# 21004 |
| 40 kDa Zeba De-Salting Columns | ThermoFisher | Cat# 87766 |
| His-Tagged RBD | Acro Biosystems | Cat# SPD-S52H6 |
| His-tagged NTD | Acro Biosystems | Cat# S1D-C52H6 |
| His-tagged Nucleocapsid | Acro Biosystems | Cat# NUN-C5227 |
| SigmaFAST OPD | Sigma-Aldrich | Cat# P9187 |
| Critical commercial assays | ||
| HIV-1 Gag p24 Quantikine ELISA Kit | R&D Systems | Cat# DHP240B |
| Nano-Glo® Dual-Luciferase® Reporter Assay | Promega | Cat# N1610 |
| Experimental models: Cell lines | ||
| HEK-293T Cells | AIDS Reagents & Reference Program, Frederick, MD | N/A |
| Recombinant DNA | ||
| psPAX2 | Addgene | RRID:Addgene_12260 |
| pCMV-VSVG | AIDS Reagents & Reference Program, Frederick, MD | N/A |
| pRRL.sin.cPPT.SFFV/Ace2.IRES-puro.WPRE | Addgene | RRID:Addgene_145839 |
| NL4-3-nanoluc Delta env | (Ozer et al., 2022)31 | N/A |
| pCAGGS-Spike SARS-CoV-2 plasmid | BEI Resources | Cat# NR-52309 |
| Software and algorithms | ||
| Skyline | https://skyline.ms | Version 21.1 |
| GraphPad Prism 9 | Dotmatics | Version 9.1.2 |
| Other | ||
| Bravo Liquid Handler | Agilent | Cat# G5563AA |
| Plate Washer | BioTek | Cat# 405TSUVSQ; RRID:SCR_019725 |
| FlexMap 3D | Luminex Corp. | N/A |
| Ni-NTA HisSorb 96-Well Plates | Qiagen | Cat# 35061 |
| Cytation3 Plate Reader | Biotek | N/A |
| Dionex UltiMate 3000 Rapid Separation nanoLC | Thermo Fisher Scientific | N/A |
| Q Exactive™ HF Hybrid Quadrupole-Orbitrap™ Mass Spectrometer | Thermo Fisher Scientific | N/A |
| PicoChip, C18 beads | New Objective Inc | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Jeffrey Schneider (jeffrey_schneider@rush.edu).
Materials availability
All plasmids generated in this study are available from the lead contact without restriction.
Experimental model and subject details
Specimens were collected by the Rush COVID-19 Biorepository Core, Rush Cancer Center Biorepository Core, and the Rush Infectious Disease clinic with all subjects enrolled with written, informed consent on protocols approved by the Rush Institutional Review Board. Longitudinal COVID-19 patient biospecimens were provided by Advocate Aurora Research Institute’s Biorepository and Specimen Resource Core. Naturally infected individuals (n = 125) ranged in age from 15 to greater than 65 years and was comprised of 69 males and 56 females. The vaccinated cohort (n = 228) included 66 males and 162 females ranging in age from 15 to greater than 65 years.
To produce HeLa cells (obtained from AIDS reagents and reference program, Fredrick, MD) overexpressing ACE2, HEK-293T cells were transfected with psPAX2, pCMV-VSVG, and pRRL.sin.cPPT.SFFV/Ace2.IRES-puro.WPRE (a gift from Caroline Goujon; Addgene plasmid # 145839; http://n2t.net/addgene:145839; RRID: Addgene_145839). HeLa cells were then transduced with the lentiviral particles and puromycin (Invitrogen) selected as described previously.32 Overexpression was validated by Western blotting to ACE protein (Rabbit Polyclonal Antibody Proteintech 21115-1-AP).
Method details
IgG purification
IgG was isolated from COVID-19 patient plasma as well as vaccinated plasma according to manufacturer instructions for Protein G Plus Agarose Beads (Pierce). In brief IgG was diluted 1:5 in PBS to make 250 μL of sample, and incubated with 150 μL of Protein G Plus agarose bead slurry and put on a rotisserie for 2 h at 4°C. IgG bound to beads were then washed with 1x Triton X-100 wash buffer and spun at 1,000 RCF for 1 min, repeated 3 times. Beads were then incubated for 10 min on a rotisserie at 4°C with IgG elution buffer (Thermo Fisher), followed by centrifugation at 1,000 RCF for 1 min into a tube with 1/10 the elution volume of 1M Tris-HCl pH 8, repeated for two elutions. Eluted IgGs were then buffer exchanged into PBS using 40 kDa Zeba de-salting columns (Thermo Scientific).
Evaluation of SARS CoV-2 antibody titers
Assessment of antibody titers in response to SARS-CoV-2 natural infection or vaccination were performed on the Luminex immunobead platform, consistent with methods we previously defined33 , 34 , 35 , 36 , 37 utilizing His-tagged SARS CoV-2 specific recombinant proteins for Spike S1 (Sino Biologicals, cat. 40591-V08H), RBD (Sino Biologicals,cat. 40592-T62), and Nucleocapsid (Sino Biologicals, cat. 40588-V08B). Assays were performed using a 384-well format in an automated fashion using an Agilent Bravo liquid handler with plate washes achieved on a BioTek 405TSUVSQ. All incubations were performed at room temperature with continuous shaking at 700 RPM. Briefly, 12.5 μL of the 3-plex working bead mixture (1250 beads each) were dispensed into an opaque 384-well plate (Nunc Maxisorp, ThermoFisher) and incubated with 12.5 μL of plasma, diluted at 1000-fold with ‘Assay Buffer’ (1X PBS containing 0.01% Tween 20/1% BSA) for 1 h. Plates were then washed twice with ‘Washing Buffer’ (1xPBS containing 0.01% Tween 20) followed by addition of 50 μL of 4 μg/mL phycoerythrin (PE)-conjugated goat anti-human IgG polyclonal (Southern Biotech) and incubated for 30 min. Plates were again rinsed twice with Washing Buffer and beads resuspended in a total of 75 μL of Assay Buffer prior to reading on a FlexMAP 3D (Luminex Corp.). Each well was read with 60 μL sampling volume and minimum acquisition of 50 beads per assay. Antibody titers were reported as an average median fluorescence intensity (MFI) value. Average net MFI values for nucleocapsid titers from a library of over 500 subjects were used to establish a threshold value for assigning “positive” versus “negative” status for patients enrolled in the study, with positivity determined to be above 1500 MFI. Briefly, a double logarithmic plot was used to display these data and an average and standard deviation of the MFI values for subjects below the apparent inflection point calculated. The threshold was then defined to be the sum of the average and two-fold the standard deviation values.
ELISA for SARS CoV-2 RBD, NTD and nucleocapsid antibody binding
Ni-NTA HisSorb 96-well plates (Qiagen) were coated with 50 μL of either His-tagged RBD (Acro Biosystems, SPD-S52H6, AA 306–527), His-tagged NTD (Acro Biosystems, S1D-C52H6, AA13-303) or His-tagged Nucleocapsid (Acro Biosystems, NUN-C5227, AA1-419) at a concentration of 2 μg/mL overnight at 4°C according to previously published methods.31 , 38 , 39 In brief all subsequent steps were performed at room temperature. Plates were blocked with 3% milk; plasma was diluted 1:500 in PBS for RBD ELISA, 1:1500 for NTD ELISA, and 1:500 for Nucleocapsid ELISA prior to adding 100 μL per well for 2 h. Plates were washed with PBS-T (PBS containing 0.1% Triton X-100) 3 times, followed by incubation with secondary goat anti-human IgG Fc HRP (Southern Biotech) at 1:4000 for 1 h. Plates were washed 3 additional times with PBS-T, followed by addition of SigmaFAST OPD substrate for 10 min. The reaction was stopped with the addition of 3M HCl; and plates were read at an OD 450.
Generation of SARS-CoV-2 pseudovirus
HEK-293T cells cultured with DMEM 10% FBS were transfected with a 3:2 ratio of NL4-3-nanoluc delta env plasmid as described in Mamede et al., 201331 and pCAGGS-Spike SARS-CoV2 plasmid respectively. (The following reagent was produced under HHSN272201400008C and obtained through BEI Resources, NIAID, NIH: Vector pCAGGS Containing the SARS-Related Coronavirus 2, Wuhan-Hu-1 Spike Glycoprotein Receptor Binding Domain (RBD), NR-52309). Media was changed 16 h post transfection and viral particles were harvested at 48 h. Viral particles were concentrated using a 20% sucrose gradient with overnight centrifugation at 5,600 RCF at 4°C and then resuspended into fresh media to an effective 500x concentration. The concentrated Spike pseudoviruses were quantified using HIV-1 Gag p24 Quantikine ELISA Kit (R&D Systems), and the infectivity was determined using the Hela-ACE2 cells and the Nano-Glo® Dual-Luciferase® Reporter Assay (Promega) according to manufacturers standard protocol.
Antibody neutralization assay
HeLa-ACE2 cells were plated in 96-well black wall plates at 105 cells per well and incubated at 37°C, with 5% CO2 for 24 h. Media was then removed, and serum was added using serial dilutions. 1.5 μg of p24 of pseudotyped virus (pCAGGS-Spike SARS-CoV2 and mutants; NL4-3-nanoluc delta env) was added to each well, and cells were returned to the incubator. After a 48hour incubation, reporter gene expression was determined with Nano-Glo® Dual-Luciferase® Reporter Assay (Promega) following the vendor’s protocol using a luminometer (Cytation3, Biotek).
IgG antibody glycan sample preparation
Protein G + Agarose (Pierce) was used to purify IgG from plasma. Purified IgG was next treated with dithiothreitol (DTT) for 5 min at 100°C to break up the heavy and light chains. The heavy chain of IgG was resolved on a 4–15% gradient polyacrylamide gel (Biorad) and the gel band was excised following Coomassie stain. Next, in-gel digestion was performed on the excised heavy chain gel bands. Each gel band was washed in 100 mM ammonium bicarbonate (AmBic)/acetonitrile (ACN) and reduced with 10 mM DTT at room temperature (RT) for 45 min. Cysteines were alkylated with 50 mM iodoacetamide in the dark for 45 min in room temperature. Gel band was washed in 100 mM AmBic/ACN prior to adding 600 ng Lys-C for overnight incubation at RT. Supernatant containing peptides was transferred into a new tube. Peptides were extracted at RT for 10 min with gentle shaking in 50% ACN/5% formic acid (FA), and peptides solution was collected. Further, peptides were extracted by 80% ACN/5% FA, and followed with 100% ACN. The peptides were lyophilized and reconstituted in 5% ACN/0.1% FA.
Peptides were analyzed by LC-MS/MS using a Dionex UltiMate 3000 Rapid Separation nanoLC and a Q Exactive™ HF Hybrid Quadrupole-Orbitrap™ Mass Spectrometer (Thermo Fisher Scientific Inc, San Jose, CA). The peptide samples were loaded onto the trap column, which was 150 μm × 3 cm in-house packed with 3 μm C18 beads. The analytical column was a 75 μm × 10.5 cm PicoChip column packed with 3 μm C18 beads (New Objective, Inc. Woburn, MA). The flow rate was kept at 300 nL/min. Solvent A was 0.1% FA in water and Solvent B was 0.1% FA in ACN. The peptide was separated on a 60-min analytical gradient from 5% to 50% of Solvent B. The mass spectrometer was operated in ‘Full MS scan’ mode. The source voltage was 2.50 kV and the capillary temperature 320°C. Full MS scans were acquired from 400 to 2000 m/z at 60,000 resolving power and automatic gain control (AGC) set to 3 × 10.6
MS data were processed using Skyline (Version 21.1). The integration and correction for the chromatographic peaks of 18 glycans were performed manually. Three of the most intense precursor ions of each glycan were selected, summed, and exported as the quantitative value of corresponding glycan. % of each glycan was measured by dividing individual glycan quantitative values by the sum total of all the glycans’ quantitative values.
Quantification and statistical analysis
Descriptives of the study sample are provided for mild and severe cases. Data were analyzed using the GraphPad Prism 9 software (Version 9.1.2). Frequency counts were used for categorical variables, with mean and standard deviation values provided for numerical variables. In both cohorts (natural infection & vaccinated), mean levels of glycans in mild and severe patients were compared using two-tailed two-sample t-tests. Similar tests were used to compare mean glycan levels in high and low neutralizing vaccinated individuals (determined by ability to block entry of pseudovirus into HeLa ACE2-expressing cells). We assessed the association of the IgG glycome over time in naturally infected individuals, stratified by mild and severe disease. We assessed linear associations between glycans and days since positive in naturally infected individuals using Pearson correlations, with correlations calculated separately in mild and hospitalized patients. To test if the association between glycan and time were associated with disease status we fit a linear regression model with group, days and a group by days interaction term. All tests were calculated using an alpha of 0.05, and were not corrected for multiple testing. To test for normality, we performed Anderson-Darling tests and adjusted analyses to reflect non-normal datasets.
Acknowledgments
This work was supported by the Walder Foundation’s Chicago Coronavirus Assessment Network (Chicago CAN) Initiative grants SCI16 and 21-00147 (J.R.S. and J.A.B.). IgG antibody glycan analysis was performed by the Northwestern Proteomics Core Facility, supported by NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center, instrumentation award (S10OD025194) from NIH Office of Director, and the National Resource for Translational and Developmental Proteomics supported by P41 GM108569, an internal RUSH grant to João Mamede. We thank the Rush COVID-19 Biorepository Core (Dr. Alan Landay, Dr. James Moy, and Cheryl Jennings) and Rush Biomarker Development Core (J.A.B.) for specimen sourcing and antibody titer determinations. Longitudinal COVID-19 patient biospecimens were provided by Advocate Aurora Research Institute’s Biorepository and Specimen Resource Core. We also thank the University of Illinois - Chicago Statistics Core for their independent statistical analysis.
Author contributions
Experiments were carried out by M.K.A., P.P.B., B.B.M., S.G., S.J.W., I.T., and C.L.F. Data were analyzed by J.R.S., P.P.B., M.K.A., S.J.W., B.C., J.F., and F.N. B.E.S., I.T., C.L.F., and A.F.R. helped with sample acquisition. R.G., T.J.H., Y.A.G., L.A., J.I.M., J.A.B., and N.L.K. assisted with overseeing the experiments and data analysis. T.L.B. assisted with statistical analysis. M.K.A. and J.R.S. wrote the manuscript. All authors helped give critique to the manuscript.
Declaration of interests
Dr. Jeffrey Borgia is the inventor of the dual target Luminex assay for SARS-CoV-2 antigen detection.
Published: December 13, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.111799.
Supplemental information
Data and code availability
All data supporting the findings of this study are available within the main manuscript and the supplementary files provided.
This paper does not report any original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Menni C., Valdes A.M., Freidin M.B., Sudre C.H., Nguyen L.H., Drew D.A., Ganesh S., Varsavsky T., Cardoso M.J., El-Sayed Moustafa J.S., et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang H., Cai S., Li Y., Li Y., Fan Y., Li L., Lei C., Tang X., Hu F., Li F., Deng X. Prognostic factors for COVID-19 pneumonia progression to severe symptoms based on earlier clinical features: a retrospective analysis. Front. Med. 2020;7:557453. doi: 10.3389/fmed.2020.557453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., Chen Y., Zhang Y. COVID-19: immunopathogenesis and Immunotherapeutics. Signal. Transduct. Target. Ther. 2020;5:128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold J.N., Wormald M.R., Sim R.B., Rudd P.M., Dwek R.A. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 6.Irvine E.B., Alter G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology. 2020;30:241–253. doi: 10.1093/glycob/cwaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bas M., Terrier A., Jacque E., Dehenne A., Pochet-Béghin V., Beghin C., Dezetter A.-S., Dupont G., Engrand A., Beaufils B., et al. Fc sialylation prolongs serum half-life of therapeutic antibodies. J. Immunol. 2019;202:1582–1594. doi: 10.4049/jimmunol.1800896. [DOI] [PubMed] [Google Scholar]
- 8.Lauc G., Pezer M., Rudan I., Campbell H. Mechanisms of disease: the human N-glycome. Biochim. Biophys. Acta. 2016;1860:1574–1582. doi: 10.1016/j.bbagen.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Moore J.S., Wu X., Kulhavy R., Tomana M., Novak J., Moldoveanu Z., Brown R., Goepfert P.A., Mestecky J. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS. 2005;19:381–389. doi: 10.1097/01.aids.0000161767.21405.68. [DOI] [PubMed] [Google Scholar]
- 10.Sonneveld M.E., de Haas M., Koeleman C., de Haan N., Zeerleder S.S., Ligthart P.C., Wuhrer M., van der Schoot C.E., Vidarsson G. Patients with IgG1-anti-red blood cell autoantibodies show aberrant Fc-glycosylation. Sci. Rep. 2017;7:8187. doi: 10.1038/s41598-017-08654-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemmers R.F.H., Vilaj M., Urda D., Agakov F., Šimurina M., Klaric L., Rudan I., Campbell H., Hayward C., Wilson J.F., et al. IgG glycan patterns are associated with type 2 diabetes in independent European populations. Biochim. Biophys. Acta Gen. Subj. 2017;1861:2240–2249. doi: 10.1016/j.bbagen.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Living Guidance for Clinical Management of COVID-19 (2021). (World Health Organization). [PubMed]
- 13.Chakraborty S., Gonzalez J., Edwards K., Mallajosyula V., Buzzanco A.S., Sherwood R., Buffone C., Kathale N., Providenza S., Xie M.M., et al. Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat. Immunol. 2021;22:67–73. doi: 10.1038/s41590-020-00828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen M.D., de Graaf E.L., Sonneveld M.E., Plomp H.R., Nouta J., Hoepel W., Chen H.-J., Linty F., Visser R., Brinkhaus M., et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science. 2021;371:eabc8378. doi: 10.1126/science.abc8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrović T., Alves I., Bugada D., Pascual J., Vučković F., Skelin A., Gaifem J., Villar-Garcia J., Vicente M.M., Fernandes Â., et al. Composition of the immunoglobulin G glycome associates with the severity of COVID-19. Glycobiology. 2021;31:372–377. doi: 10.1093/glycob/cwaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farkash I., Feferman T., Cohen-Saban N., Avraham Y., Morgenstern D., Mayuni G., Barth N., Lustig Y., Miller L., Shouval D.S., et al. Anti-SARS-CoV-2 antibodies elicited by COVID-19 mRNA vaccine exhibit a unique glycosylation pattern. Cell Rep. 2021;37:110114. doi: 10.1016/j.celrep.2021.110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrović T., Vijay A., Vučković F., Trbojević-Akmačić I., Ollivere B.J., Marjanović D., Bego T., Prnjavorac B., Đerek L., Markotić A., et al. IgG N-glycome changes during the course of severe COVID-19: an observational study. EBioMedicine. 2022;81 doi: 10.1016/j.ebiom.2022.104101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pongracz T., Nouta J., Wang W., van Meijgaarden K.E., Linty F., Vidarsson G., Joosten S.A., Ottenhoff T.H.M., Hokke C.H., de Vries J.J.C., et al. Immunoglobulin G1 Fc glycosylation as an early hallmark of severe COVID-19. EBioMedicine. 2022;78:103957. doi: 10.1016/j.ebiom.2022.103957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tso F.Y., Lidenge S.J., Poppe L.K., Peña P.B., Privatt S.R., Bennett S.J., Ngowi J.R., Mwaiselage J., Belshan M., Siedlik J.A., et al. Presence of antibody-dependent cellular cytotoxicity (ADCC) against SARS-CoV-2 in COVID-19 plasma. PLoS One. 2021;16:e0247640. doi: 10.1371/journal.pone.0247640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X., Rostad C.A., Anderson L.J., Sun H.Y., Lapp S.A., Stephens K., Hussaini L., Gibson T., Rouphael N., Anderson E.J. The development and kinetics of functional antibody-dependent cell-mediated cytotoxicity (ADCC) to SARS-CoV-2 spike protein. Virology. 2021;559:1–9. doi: 10.1016/j.virol.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lofano G., Gorman M.J., Yousif A.S., Yu W.-H., Fox J.M., Dugast A.-S., Ackerman M.E., Suscovich T.J., Weiner J., Barouch D., et al. Antigen-specific antibody Fc glycosylation enhances humoral immunity via the recruitment of complement. Sci. Immunol. 2018;3:eaat7796. doi: 10.1126/sciimmunol.aat7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartsch Y.C., Wang C., Zohar T., Fischinger S., Atyeo C., Burke J.S., Kang J., Edlow A.G., Fasano A., Baden L.R., et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 2021;27:454–462. doi: 10.1038/s41591-021-01263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakraborty S., Gonzalez J.C., Sievers B.L., Mallajosyula V., Chakraborty S., Dubey M., Ashraf U., Cheng B.Y.-L., Kathale N., Tran K.Q.T., et al. Early non-neutralizing, afucosylated antibody responses are associated with COVID-19 severity. Sci. Transl. Med. 2022;14:eabm7853. doi: 10.1126/scitranslmed.abm7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Coillie J., Pongracz T., Rahmöller J., Chen H.-J., Geyer C., van Vlught L.A., Buhre J.S., Šuštić T., van Osch T.L.J., Steenhuis M., et al. 2022. The BNT162b2 mRNA SARS-CoV-2 Vaccine Induces Transient Afucosylated IgG1 in Naive but Not Antigen-Experienced Vaccinees (Immunology) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou H., Yang H., Liu P., Huang C., Wang M., Li Y., Zhu M., Wang J., Xu Y., Wang Y., et al. Profile of immunoglobulin G N-glycome in COVID-19 patients: a case-control study. Front. Immunol. 2021;12:748566. doi: 10.3389/fimmu.2021.748566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunn B.M., Yu W.-H., Karim M.M., Brannan J.M., Herbert A.S., Wec A.Z., Halfmann P.J., Fusco M.L., Schendel S.L., Gangavarapu K., et al. A role for Fc function in therapeutic monoclonal antibody-mediated protection against Ebola virus. Cell Host Microbe. 2018;24:221–233.e5. doi: 10.1016/j.chom.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeitlin L., Pettitt J., Scully C., Bohorova N., Kim D., Pauly M., Hiatt A., Ngo L., Steinkellner H., Whaley K.J., Olinger G.G. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc. Natl. Acad. Sci. USA. 2011;108:20690–20694. doi: 10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saphire E.O., Schendel S.L., Fusco M.L., Gangavarapu K., Gunn B.M., Wec A.Z., Halfmann P.J., Brannan J.M., Herbert A.S., Qiu X., et al. Systematic analysis of monoclonal antibodies against Ebola virus GP defines features that contribute to protection. Cell. 2018;174:938–952.e13. doi: 10.1016/j.cell.2018.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selman M.H.J., de Jong S.E., Soonawala D., Kroon F.P., Adegnika A.A., Deelder A.M., Hokke C.H., Yazdanbakhsh M., Wuhrer M. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol. Cell. Proteomics. 2012;11 doi: 10.1074/mcp.M111.014563. M111.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L., Berger N.A., Kaelber D.C., Davis P.B., Volkow N.D., Xu R. COVID infection rates, clinical outcomes, and racial/ethnic and gender disparities before and after Omicron emerged in the US. medRxiv. 2022 doi: 10.1101/2022.02.21.22271300. Preprint at. [DOI] [Google Scholar]
- 31.Ozer E.A., Simons L.M., Adewumi O.M., Fowotade A.A., Omoruyi E.C., Adeniji J.A., Olayinka O.A., Dean T.J., Zayas J., Bhimalli P.P., et al. Multiple expansions of globally uncommon SARS-CoV-2 lineages in Nigeria. Nat. Commun. 2022;13:688. doi: 10.1038/s41467-022-28317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamede J.I., Sitbon M., Battini J.-L., Courgnaud V. Heterogeneous susceptibility of circulating SIV isolate capsids to HIV-interacting factors. Retrovirology. 2013;10:77. doi: 10.1186/1742-4690-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farlow E.C., Patel K., Basu S., Lee B.-S., Kim A.W., Coon J.S., Faber L.P., Bonomi P., Liptay M.J., Borgia J.A. Development of a multiplexed tumor-associated autoantibody-based blood test for the detection of non-small cell lung cancer. Clin. Cancer Res. 2010;16:3452–3462. doi: 10.1158/1078-0432.CCR-09-3192. [DOI] [PubMed] [Google Scholar]
- 34.Fhied C., Kanangat S., Borgia J.A. Development of a bead-based immunoassay to routinely measure vimentin autoantibodies in the clinical setting. J. Immunol. Methods. 2014;407:9–14. doi: 10.1016/j.jim.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Tarhoni I., Wakefield C.J., Kollipara R., Fidler M.J., Batus M., Bonomi P., Borgia J.A. Relationship between circulating tumor-associated autoantibodies and clinical outcomes in advanced-stage NSCLC patients receiving PD-1/-L1 directed immune checkpoint inhibition. J. Immunol. Methods. 2021;490:112956. doi: 10.1016/j.jim.2021.112956. [DOI] [PubMed] [Google Scholar]
- 36.Tarhoni I., Fhied C.L., Pool M., Liptay M.J., Bonomi P., Seder C.W., Borgia J.A. Development of bead based multiplexed immunoassay for evaluation of midkine, syndecan-1, and ANGPTL4 in patient serum. J. Immunoassay Immunochem. 2018;39:84–98. doi: 10.1080/15321819.2017.1407338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fhied C.L., Tarhoni I., Gerard D., Lewin G.M., Moudgalya H., Schneider J.R., Borgia J.A. Dynamic monitoring of seroconversion using a multianalyte immunobead assay for Covid-19. J. Vis. Exp. 2022 doi: 10.3791/63352. [DOI] [PubMed] [Google Scholar]
- 38.Melani R.D., Des Soye B.J., Kafader J.O., Forte E., Hollas M., Blagojevic V., Negrão F., McGee J.P., Drown B., Lloyd-Jones C., et al. Next-generation serology by mass Spectrometry: readout of the SARS-CoV-2 antibody repertoire. J. Proteome Res. 2022;21:274–288. doi: 10.1021/acs.jproteome.1c00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simons L.M., Ozer E.A., Gambut S., Dean T.J., Zhang L., Bhimalli P., Schneider J.R., Mamede J.I., Ison M.G., Karmali R., et al. De novo emergence of SARS-CoV-2 spike mutations in immunosuppressed patients. Transpl. Infect. Dis. 2022:e13914. doi: 10.1111/tid.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the main manuscript and the supplementary files provided.
This paper does not report any original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.