Abstract
Objectives
To report the safety and immunogenicity profile of a protein subunit vaccine (CovovaxTM) given as a third (booster) dose to individuals primed with different primary vaccine regimens.
Methods
A third dose was administered to individuals with an interval range of 3-10 months after the second dose. The four groups were classified according to their primary vaccine regimens, including two-dose BBIBP-CorV, AZD1222, BNT162b2, and CoronaVac/AZD1222. Immunogenicity analysis was performed to determine binding antibodies, neutralizing activity, and the T-cell responses.
Results
Overall, 210 individuals were enrolled and boosted with the CovovaxTM vaccine. The reactogenicity was mild to moderate. Most participants elicited a high level of binding and neutralizing antibody against Wild-type and Omicron variants after the booster dose. In participants who were antinucleocapsid immunoglobulin G-negative from all groups, a booster dose could elicit neutralizing activity to Wild-type and Omicron variants by more than 95% and 70% inhibition at 28 days, respectively. The CovovaxTM vaccine could elicit a cell-mediated immune response.
Conclusion
The protein subunit vaccine (CovovaxTM) can be proposed as a booster dose after two different priming dose regimens. It has strong immunogenicity and good safety profiles.
Keywords: SARS-CoV-2, Omicron, Side effect, CovovaxTM, Novavax, Booster dose
1. Introduction
The Omicron variant (B.1.1.529) of SARS-CoV-2 was first identified in November 2021 (Viana et al., 2022) and has dramatically increased the transmission of COVID-19 worldwide. The COVID-19 vaccine protects against serious disease, hospitalization, and death. However, vaccination does not entirely prevent infection and transmission to others. Massive two-dose vaccination campaigns cannot prevent breakthrough infections caused by the variants (Cele et al., 2022; Kuhlmann et al., 2022). A third dose is recommended to obtain high immunity against the Omicron variant and its subvariants. In Thailand, several forms of COVID-19 vaccines have been introduced, including inactivated vaccines (CoronaVac, BBIBP-CorV), viral vectored vaccines (AZD1222), and messenger RNA (mRNA) vaccines (BNT162b2), which have resulted in the population receiving two-dose (prime) series of the vaccine. Several two-primed vaccine regimens have been widely used and approved. Previous studies have shown that booster doses after two-dose homologous and heterologous regimes achieved a high level of antibody titers (Munro et al., 2021; Wanlapakorn et al., 2022a) and are associated with neutralizing activity against the Omicron variant (Guirakhoo et al., 2022). Evidence has also indicated that the booster dose improved vaccine effectiveness and reduced severe outcomes of COVID-19 (Grewal et al., 2022).
On April 21, 2022, the Quadrilateral Security Dialogue donated 200,000 doses of CovovaxTM (Serum Institute of India (SII) Pvt Ltd, Pune, India) to Thailand. This vaccine, also known as Novavax vaccine (NVX-CoV2373/ NuvaxovidTM) (Novavax, Inc., Gaithersburg, MD, USA), is manufactured under the brand name CovovaxTM. CovovaxTM is a protein subunit vaccine to prevent COVID-19.
Protein subunit vaccines have been used for decades to prevent viral infectious diseases. To date, recombinant protein subunit vaccines have emerged as candidate vaccines. Moreover, the protein subunit vaccine presents more benefits than the viral vector and mRNA vaccines because they overcome the limitations due to storage conditions and is easier to ship to rural/remote areas (Bhiman et al., 2022; Fiolet et al., 2022).
The US Food and Drug Administration (US-FDA, 2022) has recently authorized the Novavax protein subunit vaccine for primary series vaccination of recipients aged 12 years and older. A phase III trial in the United Kingdom demonstrated that two doses of Novavax showed an efficacy of 86.3% against the Alpha variant and 96.4% against other variants that circulated between September and November 2021 (Heath et al., 2021). Moreover, a phase IIb trial in South Africa showed that the efficacy against symptomatic COVID-19 elicited by two-dose Novavax was 60.1% (Shinde et al., 2021). Furthermore, the United States and Mexico demonstrated 90.4% efficacy against symptomatic and 100% efficacy against severe COVID-19 (Dunkle et al., 2022). However, this vaccine has not been authorized to be used as a booster dose in the United States. Recently, the COV-Boosted trial in the United Kingdom investigated the booster effect of the Novavax vaccine in two-dose individuals primed with AZD1222 and BNT162b2. The results showed that a high level of antispike immunoglobulin (Ig)G could be detected in AZD1222- and BNT162b2-primed individuals, and the neutralizing antibody against the Wild-type and Delta variant was also evident (Munro et al., 2021). However, information is limited on the use of the COVID-19 protein subunit vaccine as the third booster dose after vaccination with different platforms and schedules, especially in combination with a series of inactivated vaccines. This study investigated the reactogenicity and immunogenicity of the protein subunit COVID-19 vaccine CovovaxTM (SII Pvt Ltd, Pune, India) as a third dose (booster dose) after a two-dose primary series COVID-19 vaccine.
2. Materials and methods
2.1. Study design and participants
The cohort study was conducted between May and July 2022 at the Center of Excellence in Clinical Virology, Chulalongkorn University, Bangkok, and at 10 vaccination sites in Chonburi province. The study protocol was approved by the institutional review board (IRB) of the Faculty of Medicine of Chulalongkorn University (IRB 871/64) and was performed under the principles of the Declaration of Helsinki. This trial was registered in the Thai Clinical Trials Registry (TCTR 20210910002). Written informed consent was obtained from participants before enrollment.
A total of 210 healthy adults aged 18 years and older who had previously been immunized with any two doses of the COVID-19 vaccine between 3 and 10 months were enrolled to receive CovovaxTM as a booster dose. The cohorts were assigned to four groups according to their primary vaccine series, including homologous and heterologous regimens. The inclusion criteria were no history of COVID-19 disease with no or well-controlled comorbidity. Serious chronic medical conditions, pregnancy, or participants who are immunocompromised, such as individuals with autoimmune diseases or malignancy, were excluded.
Patients were classified into four groups based on the priming vaccine regimen received: two doses of BBIBP-CorV (hereafter referred to as SPC), two doses of AZD1222 (hereafter referred to as AZC), two doses of BNT162b2 (hereafter referred to as PFC), and heterologously primed with CoronaVac/AZD1222 (hereafter referred to as SAC). Blood samples were collected before vaccination (day 0, baseline) and after the booster dose (day 14 ± 7 and day 28 ± 7). The sera samples were subjected to laboratory evaluation.
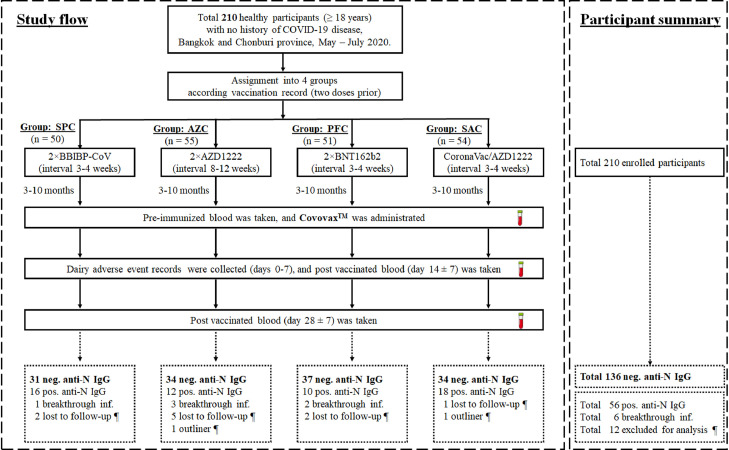
We selected antinucleocapsid (anti-N) immunoglobulin G (IgG) for baseline specimens and divided subjects into seropositive (hereafter referred to as PosN) and seronegative anti-N IgG (hereafter referred to as NegN) groups. Participants who had PosN (≥1.4) before and after vaccination were classified into previous infection and breakthrough infection, respectively. In contrast, individuals who had seronegative anti-N IgG were further assessed for immune responses, as described in Fig. 1 .
Fig. 1.
Study flow diagram of participant enrollment, blood sampling, and participant categorization. Participants who were primed with two doses of vaccines including SPC, AZC, PFC, and SAC at 3-10 months prior were enrolled to receive the protein subunit vaccine (CovovaxTM) as a booster dose. Blood samples were collected on days 0, 14 (± 7) and 28 (± 7) postbooster vaccination. The participants were categorized for further analysis according to the anti-N IgG titers. Only seronegative anti-N IgG participants were eligible for evaluation of immunogenicity analyses after booster vaccination. The 12 exclude for analysis (¶) including 10 lost to follow-up and two outliners. The dotted boxes indicate the participants after categorization using anti-N IgG.
Abbreviations: AZC, AZD1222; Ig, immunoglobulin; Inf, infection; N, nucleocapsid; neg, negative; PFC, BNT162b2; pos, positive; SAC, CoronaVac/AZD1222; SPC, BBIBP-CorV.
2.2. Vaccine used as a booster dose
Covovax™ was used as a booster dose in this trial. One dose (0.5 ml) contains 5 μg of SARS-CoV-2 recombinant spike protein (Prototype Wuhan) with 50 μg Matrix-M1™ adjuvants. A full 0.5 ml dose was intramuscularly administered (Serum Institute of India, 2022).
2.3. Safety assessments
The participants reported reactogenicity using an electronic or paper questionnaire, starting on the day of vaccination and for 7 subsequent days (days 0-7). Local, systemic, and adverse events (AEs) were classified as mild, moderate, and severe, as previously described (Kanokudom et al., 2022a).
2.4. Laboratory assessments
Serum samples were collected to determine binding antibody responses, including total Ig antireceptor binding domain (RBD) of the SARS-CoV-2 spike protein (the anti-RBD Ig), the anti-RBD IgG, and anti-N IgG, as previously described (Kanokudom et al., 2022a). Neutralizing activities against the Wild-type (Euroimmun, Lubeck, Germany) and Omicron (BA.2) (GenScript Biotech, NJ, USA) were analyzed using a surrogate virus neutralization test (sVNT), as previously described (Assawakosri et al., 2022b, Wanlapakorn et al., 2022b). The seropositivity of sVNT against Wild-type and Omicron (BA.2) showed ≥35% and ≥30% inhibition, respectively. The interferon-γ (IFN-γ) release assay measuring the T-cell response to SARS-CoV-2 to antigen 3 was performed using a heparinized whole blood sample. An elevated response was defined as a value of at least 0.20 IU/ml greater than the negative control (Nil), which was used to subtract IFN-γ release not deriving from SARS-CoV-2-specific T-cell stimulation (Busà et al., 2022).
2.5. Statistical analysis
The sample size was calculated using the G* power software version 3.1.9.6 (based on conventional effect size = 0.25, given significance level [α] = 0.05, power [1-β] = 0.8, numerator degree of freedom = 3, and the number of groups = 4). The calculated total sample was 179. Therefore, the desired enrolled sample with 10% dropout coverage was approximately 200 (n = 50/group).
A categorical analysis of sex was performed using the Pearson chi-square test. The difference in age and the time interval between the second and third dose were performed using the Kruskal-Wallis test, followed by the Dunn post hoc test with Bonferroni correction. Anti-RBD Ig and IgG were designated as geometric mean titers (GMT) with a 95% confidence interval (CI). sVNT and IFN-γ values are presented as medians with Is. Differences in the geometric mean ratio (GMR) of anti-RBD Ig and anti-RBD IgG between groups were calculated by analysis of covariance with Bonferroni adjustment. Significant differences between groups in antibody titers and percentage inhibition were calculated by analysis of covariance with Bonferroni adjustment. High IFN-γ values (IU/ml minus Nil) were evaluated using the Mann-Whitney U test. A P-value of <0.05 was considered statistically significant.
3. Results
3.1. Demographic data and baseline characteristics
A total of 210 participants were enrolled to receive CovavaxTM as a booster dose. According to their primary vaccine regimens, participants were classified into four groups: SPC (n = 50), AZC (n = 55), PFC (n = 51), and SAC (n = 54). The demographics and baseline characteristics of enrolled participants, including sex, age, comorbidities, the time interval between the second and third dose, and the time interval between the third dose and blood collection (2 and 4 weeks), are described in Table 1 . The number of females per total (%) participants was similar among groups (frequencies range from 40.0% to 54.9%). The age of participants in the AZC group (49.7 years) was significantly higher than the SPC (39.3 years) and SAC (37.7 years) groups but not different from the PFC (42.0 years) group. The median time interval between the second and third dose in the SAC group (241.0 days) was the longest, followed by the SPC (237.5 days), AZC (221.0 days), and PFC (164.0 days) groups, respectively. The time interval between the second and third doses of the PFC and AZC groups was significantly shorter than the SAC and SPC groups. No significant difference among groups was observed in the time interval between the third dose and blood collection.
Table 1.
Demographics and baseline characteristics of enrolled participants who received CovovaxTM as a booster dose (total 210 enrolled participants).
| BBIBP-CorV | AZD1222 | BNT162b2 | CoronaVac/AZD1222 | |
|---|---|---|---|---|
| Total number | 50 | 55 | 51 | 54 |
| Sex, female/total (%) | 21/50 (42.0) | 29/55 (52.7) | 28/51 (54.9) | 23/54 (42.6) |
| Mean age in year (SD) | 39.3 (12.3) | 49.7 (14.6) | 42.0 (18.7) | 37.7 (13.9) |
| No comorbidity (%) | 38/50 (76.0) | 30/55 (54.5) | 32/51 (62.7) | 45/54 (83.3) |
| Underlying diseases (%) | ||||
| AllergyAsthma | - | 2/55 (36.4) | 1/51 (19.6) | - |
| Cardiovascular diseases | - | 2/55 (36.4) | - | - |
| Diabetes mellitus | 1/50 (2.0) | 1/55 (18.2) | - | - |
| Dyslipidemia | 4/50 (8.0) | 11/55 (20.0) | 10/51 (19.6) | 3/54 (5.6) |
| Hypertension | 4/50 (8.0) | 6/55 (7.3) | 4/51 (7.8) | 2/54 (3.7) |
| Thyroid diseases | 8/50 (16.0) | 13/55 (23.6) | 13/51 (25.5) | 8/54 (14.8) |
| Other | - | 1/55 (18.2) | 2/51 (3.9) | - |
| Time interval between the second and third dose | ||||
| Median, days | 1/50 (2.0) | 3/55 (5.5) | 4/51 (7.8) | 3/54 (5.6) |
| (IQR) | 237.5 | 221.0 | 164.0 | 241.0 |
| (225.8-250.8) | (203.5-238.5) | (129.5-255.5) | (214.5-256.8) | |
| Time interval between the third dose and blood collection (days) | ||||
| Median of visit 2 | 14.0 | 14.0 | 14.0 | 14.0 |
| (IQR) | (14.0-14.0) | (14.0-14.0) | (14.0-14.0) | (13.8-14.0) |
| Median of visit 3 | 28.0 | 28.0 | 28.0 | 28.0 |
| (IQR) | (28.0-29.0) | (28.0-28.0) | (28.0-28.0) | (28.0-29.0) |
Abbreviation: IQR, interquartile range.
Two participants were outliers because their baseline anti-RBD Ig was 18,877 and 15,479 U/ml. A total of 10 participants were lost to follow-up. The outliers and the participants who were lost to follow-up were excluded. A total of 198 participants were classified by anti-N IgG level. Six participants who had breakthrough infections (defined by seroconversion of anti-N IgG) were also excluded from the subgroup analysis. According to the baseline anti-N IgG level, eligible participants (n = 192) were divided into the NegN (n = 136) and PosN (n = 56) populations. The demographics and baseline characteristics of the participants classified by anti-N IgG are shown in Table 2 .
Table 2.
Demographic and baseline characteristics of the participants classified by anti-N IgG serostatus (seronegative and seropositive anti-N IgG).
| Population 1: Seronegative anti-N IgG participants (n = 136) | ||||
|---|---|---|---|---|
| BBIBP-CorV | AZD1222 | BNT162b2 | CoronaVac/AZD1222 | |
| Total number | 31 | 34 | 37 | 34 |
| Sex, Female/total (%) | 14/31 (45.2) | 20/34 (58.8) | 23/37 (62.1) | 11/34 (32.4) |
| Mean age in year (SD) | 42.6 (13.4) | 50.7 (15.4) | 42.9 (19.4) | 38.9 (13.6) |
| Time interval between the second and third dose | ||||
| Median, days | 240.0 | 226.5 | 164.0 | 241.0 |
| (IQR) | (231.0-254.0) | (208.8-250.3) | (131.0-251.0) | (228.0-264.3) |
| Time interval between the third dose and blood collection (days) | ||||
| Median of visit 2 | 14.0 | 14.0 | 14.0 | 14.0 |
| (IQR) | (14.0-14.0) | (14.0-14.0) | (14.0-14.0) | (13.0-14.0) |
| Median of visit 3 | 28.0 | 28.0 | 28.0 | 28.0 |
| (IQR) | (28.0-29.0) | (28.0-28.0) | (28.0-28.0) | (28.0-30.0) |
| Population 2: Seropositive anti-N IgG participants (n=56) | ||||
| BBIBP-CorV | AZD1222 | BNT162b2 | CoronaVac/AZD1222 | |
| Total number | 16 | 12 | 10 | 18 |
| Sex, Female/total (%) | 5/16 (31.3) | 7/12 (58.3) | 4/10 (40.0) | 12/18 (66.7) |
| Mean age in year (SD) | 35.1 (8.1) | 50.9 (12.0) | 41.1 (16.2) | 35.9 (14.8) |
| Time interval between the second and third dose | ||||
| Median, days | 237.0 | 213.5 | 179.0 | 221.5 |
| (IQR) | (197.3-249.3) | (200.5-229.0) | (131.0-261.0) | (188.8-245.0) |
| Time interval between the third dose and blood collection (days) | ||||
| Median of visit 2 | 14.0 | 14.0 | 14.0 | 14.0 |
| (IQR) | (14.0-14.0) | (14.0-14.0) | (13.0-14.0) | (14.0-14.0) |
| Median of visit 3 | 28.0 | 28.0 | 28.0 | 28.0 |
| (IQR) | (28.0-29.0) | (28.0-28.0) | (27.5-29.5) | (28.0-29.0) |
Abbreviations: Ig, immunoglobulin; IQR, interquartile range; N, nucleocapsid
Six participants who had seroconversion of the anti-N IgG after a booster dose were excluded from the analysis.
3.2. A safety and tolerability profile
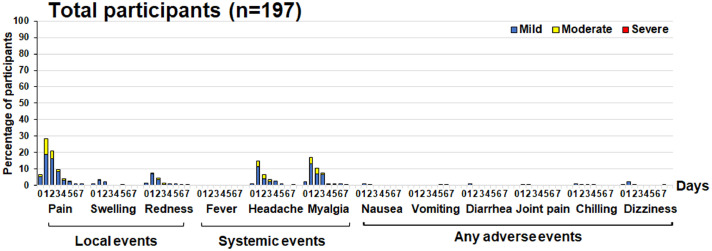
Participants had a similar frequency of mild-to-moderate AEs after receipt of CovovaxTM vaccination in each primed group (Supplementary Fig. S1a-d). A total of 197 of 210 enrolled participants experienced approximately 0.0-32.5% in a range of total AEs. AEs commonly observed were injection site pain (32.5%), followed by myalgia (21.3%), headache (16.8%), and redness (8.1%). In addition, other AEs were observed to be less than 6% and resolved within a few days (Fig. 2 ). None of the participants reported feverish or serious AEs.
Fig. 2.
Local, systemic, and adverse events of total participants experienced 7 days after receiving of a protein subunit vaccine.
3.3. Anti-RBD Ig and IgG responses of all enrolled participants, participants with negative anti-N IgG, and subgroup analysis
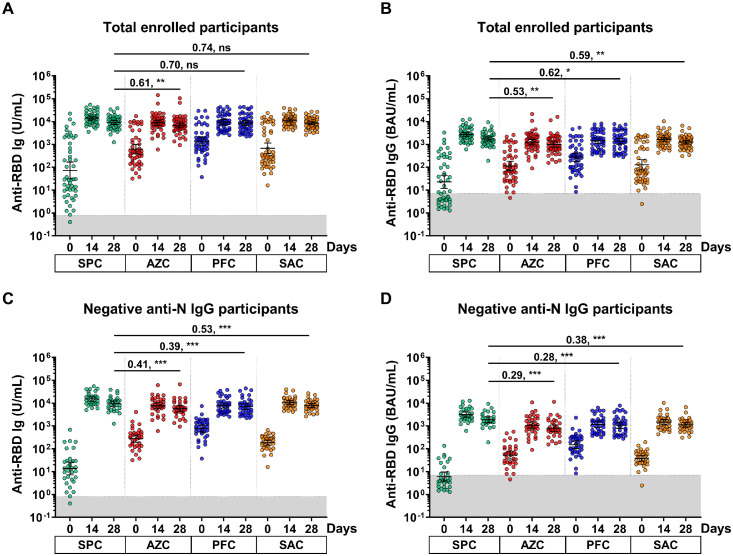
At baseline, the anti-RBD Ig and IgG GMTs of participants in the SPC group were the lowest compared with other groups. After receipt of the booster dose, all enrolled participants elicited anti-RBD Ig and IgG responses at 14 days after vaccination, but the anti-RBD Ig and IgG levels were slightly reduced at 28 days (Fig. 3 a-b).
Fig. 3.
The anti-RBD Ig/IgG responses of the enrolled participants. Sera samples of SPC, AZC, PFC, and SAC groups were monitored at 0, 14 and 28 days. (a) anti-RBD Ig (U/ml) and (b) anti-RBD IgG (BAU/ml) of the total enrolled participants. (c) anti-RBD Ig (U/ml) and (d) anti-RBD IgG (BAU/ml) of the seronegative anti-N IgG participants. The gray area indicates the seronegativity of the anti-RBD Ig (<0.8 U/ml) or anti-RBG IgG (<7.1 BAU/ml). The lines represent GMTs (95% CIs). A pairwise comparisons display (GMR) and significant values including P <0.05 (*), P <0.001 (**), P <0.001 (***).
Abbreviations: AZC, AZD1222; BAU, binding antibody units; CIs, confidence intervals; GMT, geometric mean titer; GMR, geometric mean ratio; Ig, immunoglobulin; N, nucleocapsid; ns, no statistical significance; PFC, BNT162b2; SAC, CoronaVac/AZD1222; SPC, BBIBP-CorV.
All enrolled participants were further classified based on the anti-N IgG serostatus. At 28 days after the booster, participants who were anti-N IgG-negative and were primed with BBIBP-CorV (the SPC group) elicited the highest levels of anti-RBD Ig. Compared with the SPC group, the GMR ratio of AZC, PFC, and SAC groups were 0.41 (95% CI: 0.20-0.81), 0.39 (95% CI: 0.18-0.85), and 0.53 (95% CI: 0.29-0.99), respectively (Fig. 3c and Supplementary Table S1). Similar trends were observed with anti-RBD IgG levels (Fig. 3d and Supplementary Table S1). The patterns were similar between the total enrolled participants and participants who were anti-N IgG-negative (Fig. 3a-d).
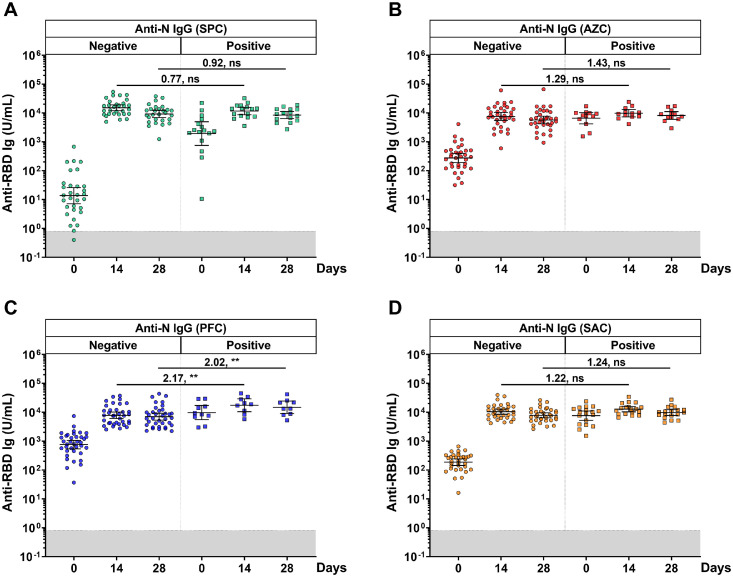
A total of 192 individuals from the SPC, AZC, PFC, and SAC groups were further classified into two groups according to the baseline anti-N IgG levels. In the comparison between the anti-RBD Ig of the PosN and NegN populations, the NegN population was used as a reference group. The anti-RBD Ig of the PosN population in all groups was significantly higher than the NegN population at day 0. Surprisingly, only the PosN population in the PFC group elicited significantly higher GMT of anti-RBD Ig after the booster than in the NegN population, with the GMR ratio of 2.17 (95% CI: 1.32-3.55) and 2.02 (95% CI: 1.23-3.31) at 14 and 28 days, respectively, as shown in Fig. 4 c. There were no differences in the GMT of anti-RBD IgG after the booster among the PosN and NegN populations of other groups (Fig. 4a-b and d and Supplementary Table S1).
Fig. 4.
Comparison of individual anti-RBD Ig values stratified by anti-N IgG titers. Participants were classified by seronegative and seropositive anti-N IgG (cut-off ≥ 1.4 S/C) before a booster dose. (a) SPC, (b) AZC, (c) PFC, (d) SAC regimens. The gray area indicates the seronegativity of the anti-RBD Ig (<0.8 U/ml). The lines represent GMTs (95% CI). A pairwise comparisons display GMR and significant values including P <0.001 (**).
Abbreviations: AZC, AZD1222; CIs, confidence intervals; GMT, geometric mean titer; GMR, geometric mean ratio; Ig, immunoglobulin; N, nucleocapsid; ns, no statistical significance; PFC, BNT162b2; RBD, recptor binding domain; SAC, CoronaVac/AZD1222; SPC, BBIBP-CorV.
3.4. Neutralizing activity against Wild-type and Omicron BA.2 using surrogate virus neutralization test
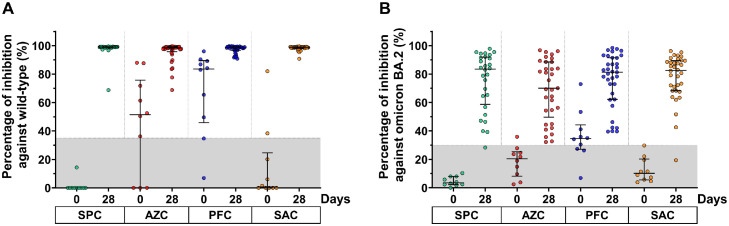
A subgroup of participants was randomly selected to test for the neutralizing activity against the Wild-type and Omicron BA.2 SARS-CoV-2 strain. The frequency of baseline seropositivity against the Wild-type was 7/10 for AZC, 8/10 for PFC, and 2/10 for SAC groups. Nevertheless, all participants in the SPC group were seronegative. At day 28, after a booster dose, most of the boosted participants showed restored neutralizing activity against the Wild-type by more than 95% inhibition, reaching the upper limit of an assay (Fig. 5 a).
Fig. 5.
Neutralizing activity of participants against the Wild-type SARS-CoV-2 virus (a) and BA.2 Omicron variant (b). Lines represent the median (interquartile range [IQR]). The gray area indicates the seronegativity of neutralizing activity of the Wild-type (<35%) and BA.2 Omicron variant (<30%).
Abbreviations: AZC, AZD1222; PFC, BNT162b2; SAC, CoronaVac/AZD1222; SPC, BBIBP-CorV.
Regarding the neutralizing activity against the BA.2 Omicron variant, a baseline seropositivity rate was observed in the AZC (1/10) and PFC (7/10) groups. In contrast, none of the participants in the SPC and SAC groups were seropositive for neutralizing activity against the BA.2 at baseline. After the booster dose (at 28 days), the median percent inhibition of neutralizing activity against the Omicron BA.2 variant was 83.5%, 70.1%, 81.3%, and 82.6% for the SPC, AZC, PFC, and SAC groups, respectively. Only two participants showed no seroconversion of neutralizing activity against Omicron BA.2. Furthermore, the median percent inhibition against BA.2 Omicron variant elicited by all regimens was comparable (Fig. 5b).
3.5. Total IFN-γ release induced by the Antigen 3 QuantiFERON assay
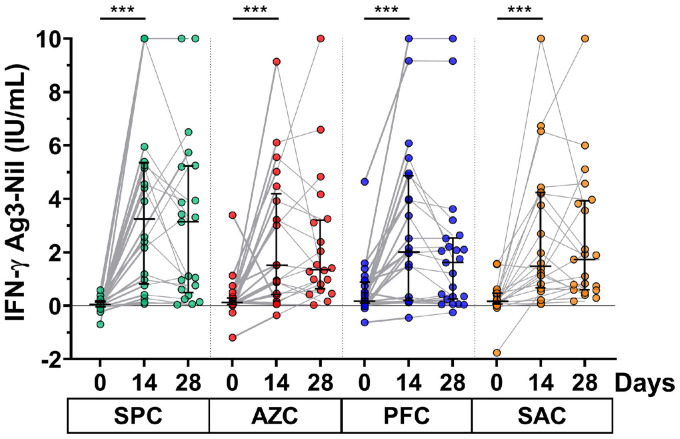
The T-cell response was assessed by measuring total IFN-γ release in whole blood samples of participants who received a booster dose. At baseline, the remaining IFN-γ level after the second dose was less than 0.2 IU/ml for all participants. The results showed that the IFN-γ level after vaccination of all participants was significantly increased at 14 days and slightly reduced at the 28-day follow-up, except for those receiving the SAC regimen. The group comparison indicated that the IFN-γ level was comparable at 14 and 28 days (Fig. 6 ). The elevated response at 14 days was 90.4% for SPC, 85.7% for AZC, 70.8% for PFC, and 81.8% for SAC groups.
Fig. 6.
IFN-γ release assay. Whole blood samples from the participants were heparinized and then transferred to the Antigen 3-QuantiFERON blood collection tube for 21 hours. The IFN-γ release was monitored by enzyme-linked immunosorbent assay. Lines represent the median (interquartile range). A pairwise comparisons display significant values including P <0.001 (***).
Abbreviations: AZC, AZD1222; IFN, interferon; PFC, BNT162b2; SAC, CoronaVac/AZD1222; SPC, BBIBP-CorV.
4. Discussion
Herein, we report the first study evaluating immune responses to the CovovaxTM protein subunit vaccine as a booster dose in individuals who had received a two-dose vaccination (priming) with either homologous inactivated or heterologous inactivated/viral vector vaccine regimens. Moreover, we also compared the vaccine-induced immune response after the CovovaxTM booster dose in individuals primed with homologous viral vector- and mRNA-based vaccines. In addition, the reactogenicity of the protein subunit vaccine had been explored. The AEs record revealed that the common effects were injection site pain, myalgia, headache, and redness at the injection site. A few incidences of AEs were observed but were assessed as mild to moderate in severity. The AEs reported herein were similar to those of pain, myalgia, and headache described in a previous Novavax study in Australia (Keech et al., 2020; Mallory et al., 2022), South Africa (Shinde et al., 2021), and the United States (Mallory et al., 2022). Furthermore, our findings demonstrated that the CovovaxTM vaccine elicited fewer frequency of pain (32.5% vs 71-87%), myalgia (21.9% vs 34-62%), and headache (16.8% vs 39-50%) than the viral vector or the mRNA COVID-19 vaccines (Assawakosri et al., 2022a; Munro et al., 2021). Furthermore, the AEs after the protein subunit vaccine were similar among individuals who previously received different primary COVID-19 vaccine platforms. This is in agreement with a previous study, which found that there was no correlation between the severity of AEs after the booster dose and the types of primary vaccinations (Munro et al., 2021).
Before receiving booster doses, the anti-RBD Ig/IgG titer in participants who were immunized was notably low. Our finding showed that individuals who received the homologous BBIBP-CorV vaccine (SPC group) exhibited lower binding antibodies than other groups at baseline. The anti-RBD Ig and IgG responses after the booster dose indicated that the levels of binding antibodies were significantly increased, regardless of the primary series vaccine regimen received. Consistent with a previous study (Munro et al., 2021), the Novavax subunit protein vaccine elicited a high antispike protein IgG titer in individuals who had received two doses of AZD1222 or BNT162b2. Compared with previous studies evaluating three doses of vaccination in healthy adults, we observed that using the CovovaxTM vaccine after the primary series of the inactivated vaccine group achieved higher antibody levels than three doses of the inactivated vaccine (Ai et al., 2022; Assawakosri et al., 2022b). Furthermore, a heterologous booster dose including CovovaxTM after the primary series of viral vector vaccine (AZD1222) achieved a robust immune response comparable to three doses of AZD1222 (Assawakosri et al., 2022a). However, a heterologous booster dose with CovovaxTM in individuals receiving a primary series of mRNA vaccine (BNT162b2) showed a lower anti-RBD Ig than three doses of BNT62b2 (Wanlapakorn et al., 2022a).
Consistent with the anti-RBD Ig and IgG responses observed in this study, the booster dose elicited high neutralizing activity against the Wild-type and Omicron BA.2 variants of SARS-CoV-2. However, neutralizing antibodies against the Omicron variant were slightly lower than that toward the prototype variant (Bhiman et al., 2022). Several mutations in the RBD could alter the binding affinity of antibodies produced from Wild-type vaccines (Cao et al., 2022). The current study observed IFN-γ releasing after 14 days among all regimens. In agreement with previous studies, the protein subunit vaccine and Matrix-M1 adjuvant could induce high levels of cellular immunity (Keech et al., 2020; Tian et al., 2021). Other than the humoral response, the CovovaxTM vaccine could also elicit a robust cellular response.
The Com-COV trial in the United Kingdom examined sera for anti-N IgG levels at baseline and used seropositivity to define SARS-CoV-2 infection (Liu et al., 2021). Recent studies found that the individuals (n = 645) who had received two doses of the inactivated vaccine (CoronaVac or BBIBP-CorV) and inactivated vaccine, followed by viral vector vaccine (CoronaVac/AZD1222) for longer than 5 months were 95.3 % seronegative of anti-N IgG (Assawakosri et al., 2022b; Chansaenroj et al., 2022; Kanokudom et al., 2022b;Suntronwong et al., 2022). Thus, this study used anti-N IgG as an indicator for recent SARS-CoV-2 infection. Nearly one-third of the participants were suspected of exposure to SARS-CoV-2 infection before enrollment. These individuals were associated with asymptomatic infection or mild/transient symptoms, indicating an underestimate of SARS-CoV-2 infection (Tande et al., 2022). The subgroup analysis suggested that the anti-RBD Ig response of the PosN population in the BNT162b2-primed group (PFC group) was higher than the NegN population. The findings supported that hybrid immunity contributed to a better immune response than the vaccine alone (Moghnieh et al., 2022). In contrast, the PosN SPC population, among other groups, exhibited approximately equal anti-RBD Ig levels as the NegN population, similar to the previous study with BNT162b2 boosted (Zuo et al., 2022). Furthermore, participants in this study had experienced SARS-CoV-2 infection within a few weeks of sampling, despite having received a booster vaccination.
Our study was subject to certain limitations. First, the surrogate virus neutralization test against the Wild-type reached the upper limit of >95% inhibition (Kanokudom et al., 2022a). The pseudovirus reduction neutralization test will be used in further studies to overcome these limitations. In addition, we were unable to obtain the results of COVID-19 virus testing of the seropositive anti-N IgG population to establish the confirmation of SARS-CoV-2 infection. Lastly, long‐term immunity and durability of immune responses after a booster dose against SARS‐CoV‐2 variants should be further monitored.
5. Conclusion
The protein subunit vaccine as a booster dose is safe and elicits a high level of binding and neutralizing antibodies against SARS-CoV-2, as well as a good cellular response. Thus, the CovovaxTM vaccine can be recommended for use as a booster in individuals receiving variable primary vaccine regimens. Our data indicate the CovovaxTM vaccine will be beneficial and convenient as a heterologous booster dose and will be of value for public health vaccine implementation guidelines.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This research was financially supported by the Health Systems Research Institute, National Research Council of Thailand, the Center of Excellence in Clinical Virology, Chulalongkorn University, and King Chulalongkorn Memorial Hospital, and partially supported by the Second Century Fund of Sitthichai Kanokudom, Chulalongkorn University.
Acknowledgments
The authors would like to thank all Center of Excellence in Clinical Virology personnel and all participants for contributing to and supporting this project.
Author contributions
Conceptualization, S.K. (Sitthichai Kanokudom), P.N., S.H., and Y.P.; data curation, S.K. (Sitthichai Kanokudom), R.Y., N.S. (Nungruthai Suntronwong), S.A., A.K., W.T., J.A., W.W., D.S., T.T. (Thaksaporn Thatsanatorn), N.S. (Natthinee Sudhinaraset), and N.W.; formal analysis, S.K. (Sitthichai Kanokudom); methodology, S.K. (Sitthichai Kanokudom), J.C., R.A., N.K., P.V., S.K. (Sirapa Klinfueng), and T.T (Thanunrat Thongmee); project administration, Y.P.; writing—original draft, S.K. (Sitthichai Kanokudom); writing—review and editing, S.K. (Sitthichai Kanokudom), S.H., and Y.P. All authors have read and agreed to the published version of the manuscript.
IRB statement
The study protocol was approved by the IRB, Faculty of Medicine, Chulalongkorn University (IRB number 871/64).
Informed consent statement
Written informed consent was obtained before participant enrollment. The study was conducted according to the Declaration of Helsinki and the Good Clinical Practice Guidelines principles.
Data availability statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.11.022.
Appendix. Supplementary materials
References
- Ai J, Zhang Y, Zhang H, Zhang Q, Fu Z, Lin K, et al. Safety and immunogenicity of a third-dose homologous BBIBP-CorV boosting vaccination: interim results from a prospective open-label study. Emerg Microbes Infect. 2022;11:639–647. doi: 10.1080/22221751.2022.2025746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assawakosri S, Kanokudom S, Chansaenroj J, Suntronwong N, Auphimai C, Nilyanimit P, et al. Persistence of immunity against Omicron BA.1 and BA.2 variants following homologous and heterologous COVID-19 booster vaccines in healthy adults after a two-dose AZD1222 vaccination. Int J Infect Dis. 2022;122:793–801. doi: 10.1016/j.ijid.2022.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assawakosri S, Kanokudom S, Suntronwong N, Auphimai C, Nilyanimit P, Vichaiwattana P, et al. Neutralizing activities against the omicron variant after a heterologous booster in healthy adults receiving two doses of CoronaVac vaccination. J Infect Dis. 2022;226:1372–1381. doi: 10.1093/infdis/jiac092. [DOI] [PubMed] [Google Scholar]
- Bhiman JN, Richardson SI, Lambson BE, Kgagudi P, Mzindle N, Kaldine H, et al. Novavax NVX-COV2373 triggers potent neutralization of Omicron sub-lineages. bioRxiv. 2022 doi: 10.1038/s41598-023-27698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; https://www.biorxiv.org/content/10.1101/2022.07.14.500148v1. [Accessed 17 July 2022].
- Busà R, Sorrentino MC, Russelli G, Amico G, Miceli V, Miele M, et al. Specific anti-SARS-CoV-2 humoral and cellular immune responses after booster dose of BNT162b2 Pfizer-BioNTech mRNA-based vaccine: integrated study of adaptive immune system components. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.856657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–656. doi: 10.1038/s41586-021-04387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chansaenroj J, Suntronwong N, Kanokudom S, Assawakosri S, Yorsaeng R, Vichaiwattana P, et al. Immunogenicity following two doses of the BBIBP-CorV vaccine and a third booster dose with a viral vector and mRNA COVID-19 vaccines against delta and omicron variants in prime immunized adults with two doses of the BBIBP-CorV vaccine. Vaccines (Basel) 2022;10:1071. doi: 10.3390/vaccines10071071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle LM, Kotloff KL, Gay CL, Áñez G, Adelglass JM, Barrat Hernández AQ, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386:531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28:202–221. doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal R, Kitchen SA, Nguyen L, Buchan SA, Wilson SE, Costa AP, et al. Effectiveness of a fourth dose of Covid-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ. 2022;378 doi: 10.1136/bmj-2022-071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirakhoo F, Wang S, Wang CY, Kuo HK, Peng WJ, Liu H, et al. High neutralizing antibody levels against severe acute respiratory syndrome coronavirus 2 omicron BA.1 and BA.2 After UB-612 Vaccine Booster. J Infect Dis. 2022;226:1401–1406. doi: 10.1093/infdis/jiac241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanokudom S, Assawakosri S, Suntronwong N, Auphimai C, Nilyanimit P, Vichaiwattana P, et al. Safety and immunogenicity of the third booster dose with inactivated, viral vector, and mRNA COVID-19 vaccines in fully immunized healthy adults with inactivated vaccine. Vaccines (Basel) 2022;10:86. doi: 10.3390/vaccines10010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanokudom S, Assawakosri S, Suntronwong N, Chansaenroj J, Auphimai C, Nilyanimit P, et al. Comparison of the reactogenicity and immunogenicity of a reduced and standard booster dose of the mRNA COVID-19 vaccine in healthy adults after two doses of inactivated vaccine. Vaccine. 2022;40:5657–5663. doi: 10.1016/j.vaccine.2022.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann C, Mayer CK, Claassen M, Maponga T, Burgers WA, Keeton R, et al. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet. 2022;399:625–626. doi: 10.1016/S0140-6736(22)00090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory RM, Formica N, Pfeiffer S, Wilkinson B, Marcheschi A, Albert G, et al. Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): a secondary analysis of a randomised, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2022;22:1565–1576. doi: 10.1016/S1473-3099(22)00420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghnieh R, El Hajj C, Abdallah D, Jbeily N, Bizri AR, Sayegh MH. Immunogenicity and effectiveness of primary and booster vaccine combination strategies during periods of SARS-CoV-2 delta and omicron variants. Vaccines. 2022;10:1596. doi: 10.3390/vaccines10101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serum Institute of India. SARS-CoV-2 rS Protein (COVID-19) recombinant spike protein Nanoparticle Vaccine (CovovaxTM). Date updated June 2022. https://www.seruminstitute.com/pdf/COVOVAX_Insert.pdf, 2022 (accessed 04 October 2022).
- Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1899–1909. doi: 10.1056/NEJMoa2103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntronwong N, Kanokudom S, Auphimai C, Assawakosri S, Thongmee T, Vichaiwattana P, et al. Effects of boosted mRNA and adenoviral-vectored vaccines on immune responses to omicron BA.1 and BA.2 following the heterologous CoronaVac/AZD1222 vaccination. J Med Virol. 2022;94:5713–5722. doi: 10.1002/jmv.28044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tande AJ, Pollock BD, Shah ND, Farrugia G, Virk A, Swift M, et al. Impact of the coronavirus disease 2019 (COVID-19) vaccine on asymptomatic infection among patients undergoing preprocedural COVID-19 molecular screening. Clin Infect Dis. 2022;74:59–65. doi: 10.1093/cid/ciab229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The United States-Food and Drug Administration (US-FDA). Novavax letter of authorization 08192022 - FDA. Date updated 19 August 2022, https://www.fda.gov/media/159902/download, 2022 (accessed 06 September 2022).
- Tian JH, Patel N, Haupt R, Zhou H, Weston S, Hammond H, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. 2021;12:372. doi: 10.1038/s41467-020-20653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603:679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanlapakorn N, Suntronwong N, Kanokudom S, Assawakosri S, Nilyanimit P, Yorsaeng R, et al. Immunogenicity of the BNT162b2 COVID-19 vaccine as a third dose (booster) following two doses of different primary series regimens in Thailand. Pathog Glob Health. 2022;116:395–397. doi: 10.1080/20477724.2022.2108646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanlapakorn N, Suntronwong N, Phowatthanasathian H, Yorsaeng R, Thongmee T, Vichaiwattana P, et al. Immunogenicity of heterologous inactivated and adenoviral-vectored COVID-19 vaccine: real-world data. Vaccine. 2022;40:32. doi: 10.1016/j.vaccine.2022.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo F, Abolhassani H, Du L, Piralla A, Bertoglio F, de Campos-Mata L, et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat Commun. 2022;13:2670. doi: 10.1038/s41467-022-30340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.