Abstract
Coronavirus-19 (COVID-19) can result in irrecoverable acute respiratory distress syndrome (ARDS) or life-limiting fibrosis for which lung transplantation is currently the only viable treatment. COVID-19 lung transplantation has transformed the field of lung transplantation, as before the pandemic, few transplants had been performed in the setting of infectious disease or ARDS. Given the complexities associated with COVID-19 lung transplantation, it requires strict patient selection with an experienced multidisciplinary team in a high-resource hospital setting. Current short-term outcomes of COVID-19 lung transplantation are promising. However, follow-up studies are needed to determine long-term outcomes and whether these patients may be predisposed to unique complications.
Keywords: Lung transplantation evaluation, COVID-19, Acute respiratory distress syndrome
Key points
-
•
Lung transplantation is the only viable treatment option for patients with severe irrecoverable coronavirus-19 (COVID-19)-associated acute respiratory distress syndrome or life-debilitating post-COVID fibrosis and shows promising short-term outcomes.
-
•
COVID-19-related respiratory failure lung transplantation requires strict patient selection and extensive lung transplant evaluation must be done.
-
•
COVID-19-related respiratory failure lung transplants are complex and should be done at high-volume centers with multidisciplinary teams.
Introduction
The novel coronavirus-19 (COVID-19) has infected more than 490 million people since the start of the pandemic in March 2020, causing over 6.1 million deaths.1 Although some patients remain asymptomatic, COVID-19 infection has been shown to cause anywhere from common cold manifestations to severe illness, characterized by respiratory and multi-organ failure.2 In fact, 15.7% of COVID-19 patients are noted to have severe illness manifestations.3 Although several organs may be affected by COVID-19, the lungs are the primary site of disease, with 6% to 10% of patients with COVID-19 ultimately developing severe acute respiratory distress syndrome (ARDS), requiring mechanical ventilation and extracorporeal membrane oxygenation (ECMO).4 , 5 Subsequently, the mortality of patients with COVID-19-associated ARDS requiring mechanical ventilation can exceed 20% to 40%.6 , 7 Furthermore, there is a subset of survivors from COVID-19 acute lung injury who are left with chronic lung disease necessitating supplemental oxygen and impairing their mobility.8 This review article discusses the emerging role of lung transplantation in treatment of patients with COVID-19-associated respiratory failure, both acute and chronic.
Nature of the problem
With the continuously increasing number of COVID-19 cases due to multiple waves of the pandemic, the number of patients developing COVID-19-associated ARDS, requiring prolonged mechanical ventilation and extracorporeal support, or significant morbidity from COVID-19-related chronic lung disease is expected to rise or remain high. Therefore, when medical therapy fails, it is important to consider lung transplantation as a life-saving and life-altering treatment of patients with COVID-19-associated ARDS and post-COVID chronic lung disease, respectively.
Lung transplantation is a well-established therapy for a variety of end-stage lung diseases, such as idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, cystic fibrosis, and pulmonary hypertension.9 Currently, over 4000 lung transplants occur annually worldwide, with a median survival of 6.7 years.10 , 11 However, before the COVID-19 pandemic, lung transplantation was rarely considered for patients with ARDS.9 There are multiple concerns raised when considering COVID-19-associated lung disease patients for lung transplantation. First, there is a concern that COVID-19 or superinfecting pathogens associated with viral or ventilator-associated pneumonia in the native lung may recur in the newly transplanted lungs. Second, due to severe damage caused by COVID-19 infection, there are technical challenges to transplantation, which may increase ischemic times and worsen transplant outcomes. Third, there is a concern regarding transplant recovery as COVID-19 patients are severely deconditioned from prolonged mechanical ventilation, sedation, and neuromuscular blockade. Finally, there is an uncertainty as to whether the native lung may recover, which could result in long-term outcomes preferable to lung transplantation.12
Despite these concerns, lung transplantation was pursued as treatment of irrecoverable COVID-19-associated ARDS and post-COVID fibrosis, which allowed further insight into COVID-19-associated respiratory failure. By examining explanted native lung tissue from COVID-19-associated ARDS transplant recipients and postmortem lung tissue from patients who died of COVID-19-associated ARDS, Bharat and colleagues showed that COVID-19 caused severe and irreversible lung parenchymal damage that is molecularly and pathologically similar to end-stage pulmonary fibrosis.12 When comparing the three-dimensional matrix organization of end-stage lung tissue between patients who underwent transplant for COVID-19-associated ARDS to those who underwent transplant for idiopathic pulmonary fibrosis, they found similar disorganized matrix patterns with punctate islands of cells surrounding fibrotic airway regions. Furthermore, when comparing single-cell Ribonucleic Acid sequencing of COVID-19-associated ARDS lungs to lungs in the end stages of idiopathic pulmonary fibrosis, there were many similarities across cell lineages. Of note, they observed an abnormal population of basaloid-like epithelial cells expressing Keratin-17 (KRT17) in the explanted lung from patients transplanted for COVID-19-associated ARDS, which had previously been observed lining fibroblastic foci in idiopathic pulmonary fibrosis patients.12, 13, 14, 15 In explanted lung tissue from patients transplanted for COVID-19-associated ARDS, Bharat and colleagues found these KRT17-positive cells localized near collagen (COL) 1A1-positive cells, which they report is a potential marker for irrecoverable fibrosis.12
Therefore, like other end-stage lung diseases, lung transplantation is the only solution for patients who develop severe COVID-19 irreversible lung injury, despite optimal medical therapy. Currently, over 200 lung transplants have been performed for COVID-19-related respiratory failure in the United States.16 With the ongoing pandemic, the need for COVID-19 transplants may remain, making it critically important to ensure appropriate patient selection. In addition to patients who have severe COVID-19-associated ARDS, there is a cohort of patients who develop respiratory insufficiency after initially recovering. The acutely ill and those suffering from chronic respiratory failure from COVID-19 should be considered distinct subsets. Although the acutely ill patients should be generally considered for double lung transplantation, those in the latter group may benefit from either double of single, especially if there is no pulmonary hypertension or opportunistic infections in the damage native lungs. As there are several reports describing the acutely ill patients, we discuss a patient in the latter group.12 , 17 , 18
Case report
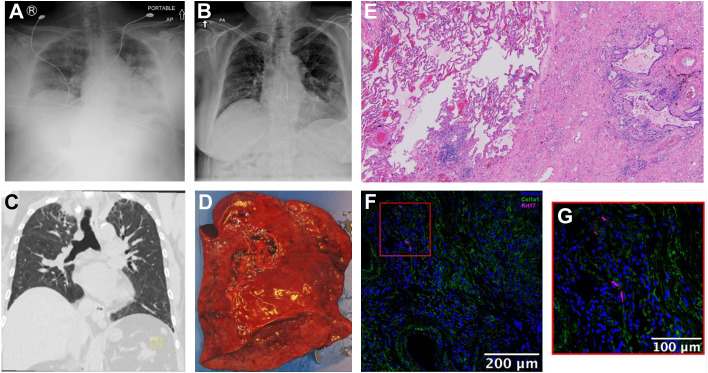
A 59-year-old woman with history of obstructive sleep apnea, hypertension, and hyperlipidemia presented to an outside hospital 3 days after testing positive for COVID-19 with shortness of breath and bilateral pulmonary infiltrates on chest X-ray (CXR) (Fig. 1 A). She was admitted to the Intensive Care Unit for 30 days of high-flow oxygen, with an overall 40-day hospitalization. During her hospital course, she was never intubated and received convalescent plasma, tocilizumab, and intravenous steroids for COVID-19 treatment. Following her discharge, she had shortness of breath with minimal activity and was started on continuous oxygen supplementation.
Fig. 1.
Patient’s chest x-ray (A) in the emergency department, 3 days after testing positive for COVID-19 and (B) in outpatient clinic, POD22 following left single lung transplant. Patient’s computed tomography of chest (C) coronal section. Patient’s explanted left lung (D) with histology using hematoxylin and eosin stain (E) showing relatively uninvolved lung parenchyma directly next to fibrotic lung. (F, G) Immunofluorescence microscopy showing KRT17 positive staining (magenta) cells next to COL1A1 positive cells (green).
She presented to our institution as an outpatient for lung transplant evaluation for her post-COVID fibrosis 11 months from her initial COVID-19 infection. Her echocardiogram showed mild concentric left ventricular hypertrophy (ejection fraction: 69%) and normal size left atrium, right atrium, and right ventricle, with normal right ventricular systolic function. Her right heart catheterization was notable for moderate nonobstructive coronary artery disease, but normal pulmonary capillary wedge pressure and cardiac output with a pulmonary artery pressure of 34/16. Computed tomography (CT) of her chest showed poorly defined areas of ground glass with associated mild bronchiectasis and significant fibrosis in the upper lobes, with traction bronchiectasis and volume loss (Fig. 1C). Her lung ventilation perfusion scan showed grossly equal perfusion to both lungs (right 54.7% vs left 45.3%), without significant segmental or subsegmental perfusion defects. She was listed for left, right, and bilateral lungs with a Lung Allocation Score of 42.
After 39 days of listing, she received an organ offer for a single left lung with a positive virtual crossmatch. She underwent plasma exchange preoperatively before undergoing her left single lung transplant, which was uneventful with a total ischemic time of 3.75 hours for the transplanted left lung. Final pathology of her explanted lung (Fig. 1D) showed uninvolved lung parenchyma next to areas of interstitial fibrosis and capillary congestion (Fig. 1E). Immunofluorescence microscopy showed KRT17-positive cells localized near COL1A1-positive cells (Fig. 1F, G), suggestive of irrecoverable fibrosis. Postoperatively, her hospital course was unremarkable. Given her positive retrospective crossmatch, she completed antibody-mediated rejection treatment with multiple plasmapheresis treatments, antithymocyte globulin, intravenous immunoglobulin, and eculizumab. Postoperative day (POD) 1, she was extubated to high-flow nasal cannula, which was weaned to nasal cannula on POD 3 and to room air on POD12. She did not have primary graft dysfunction. On POD 4, she was transferred out of the Intensive Care Unit. Her last chest tube was removed on POD 18 and she was discharged home with home health services on POD 19. She was first seen in outpatient clinic on POD 22 and was noted to be doing well with no need for supplemental oxygen and improving CXR (Fig. 1B).
Preoperative lung transplant evaluation
Currently, there are two main indications for lung transplantation in the setting of COVID-19-related diseases: irrecoverable COVID-19-associated ARDS and post-COVID fibrosis. The former patients will require an expedited inpatient lung transplant work up due to the nature of their disease and inability to leave the intensive care unit, whereas the latter patients may be evaluated as an inpatient versus outpatient setting. Here, the authors discuss the approaches to inpatient versus outpatient evaluations of patients with COVID-19-related indications for lung transplantation.
Outpatient Lung Transplant Evaluation for Post-Coronavirus Fibrosis
As the COVID-19 pandemic continues, the number of patients with post-COVID fibrosis may continue to increase. In fact, King and colleagues states that history of severe COVID-19 infection should be added to the differential diagnosis or contributory exposure for all fibrotic interstitial lung disease (ILD) and a part of standard history taking when working up ILD patients.8 Thus, this prompts the question, “should post-COVID fibrosis be treated similarly to those patients with ILD?” The most critical branch point when deciding to list patients for lung transplantation involves weighing the current morbidity and mortality of the underlying lung disease against the risk of lung transplantation. Multidisciplinary transplant teams, consisting of pulmonologist, surgeons, and others, must decide where the balance falls between the natural history course of the patient’s lung disease and the likeliness for transplantation to improve a patient’s quality of life and longevity. To assist in this decision-making, in 2014, the International Society of Heart and Lung Transplantation provided consensus guidelines on the appropriate timing of referral and listing of lung transplant candidates.19 However, as the ISHLT criteria predominantly pertain to progressive fibrotic ILD, and the rate of progression and potential for improvement in post-COVID fibrosis is unknown, it remains questionable whether these criteria can be applied to post-COVID fibrosis patients.
Data regarding the progression or improvement of post-COVID fibrosis are limited. From the case report detailed previously, the co-localization of KRT17-postive cells near COL1A1-positive cells on immunofluorescence microscopy from the explanted post-COVID fibrotic lung suggest that the fibrosis seen in some cases of post-COVID fibrosis is, in fact, irrecoverable. However, the pathogenesis of post-COVID pulmonary fibrosis is incompletely understood. Multiple fibrogenic mechanisms have been demonstrated to be engaged by COVID-19 and include viral activation of profibrotic pathways, such as alterations in the renin–angiotensin system and activation of growth factors, direct cellular injury of macrophages, endothelial cells, and alveolar epithelial cells, cytokine-induced injury through immune recruitment, and mechanical injury from barotrauma and extracellular matrix dysregulation.20
Although the previous case report demonstrates sustained irrecoverable post-COVID fibrosis, there are other studies which have reported anywhere from persistent fibrotic abnormalities to complete radiographic resolution of fibrotic changes on chest CT following COVID-19 infection. Han and colleagues prospectively followed 114 patients with severe COVID-19 pneumonia for 6 months following infection where they found that 35% of patients continued to have fibrotic-like changes on chest CT, whereas 38% of patients had complete radiologic resolution, and 27% of patients had residual ground-glass opacifications or interstitial thickening.21 In a study analyzing 3 month follow-up in 52 patients who had COVID-19 with an initial abnormal CT chest, 42% of patients continued to have residual CT chest abnormalities. Although those with residual radiographic abnormalities were also more likely to be symptomatic, with shortness of breath, chest pain, or cough, 33% of those patients with complete radiographic resolution were also symptomatic.22 The persistence of symptoms despite radiographic findings highlights the need to not only consider radiographic findings but also pulmonary function tests (PFT). In fact, in a group of 57 patients who experienced anywhere from mild to severe COVID-19 infection, 75% of patients had abnormalities on PFTs 30 days after discharge.23 Furthermore, Guler and colleagues found that 4 months following mild/moderate COVID-19 infection, average PFT was normal, as opposed to 4 months following severe COVID-19 infection where patients averaged lower lung volumes and abnormal and reduced diffusion capacity.24 Together, these studies show that although there is a subset of patients who will recover, there is a subset of patients who continue to remain symptomatic with clear radiologic lung fibrosis, who are candidates for lung transplantation. Risk factors for the development of those in the latter group are older age, history of smoking or alcohol abuse, severe COVID-19 infection, longer Intensive Care Unit admissions, and need for mechanical ventilation.25
When deciding whether or not those patients with symptomatic post-COVID should be worked up for transplant, one should first carefully assess for previously unrecognized fibrotic lung disease, as an estimated 2% to 7% of nonsmokers and 4% to 9% of smokers have interstitial lung abnormalities which often go undetected in the absence of CT chest imaging.26 This can be done by reviewing any available chest imaging done before COVID-19 infection and by obtaining a thorough history (symptoms before COVID-19 infection, occupational or other exposures associated with ILD, family history of ILD, and associated signs and symptoms of connective tissue disorder). Following, baseline PFT, chest CT, and 6-min walk test (6MWT) should be performed. Those patients who have residual pulmonary sequelae, especially those with significant morbidity, should then subsequently be referred to pulmonary rehabilitation. Furthermore, a trial of corticosteroids should be considered as preliminary studies document a benefit in those patients with radiographic findings of organizing pneumonia.27, 28, 29 Patients should be serially followed with repeat PFT, CT chest, and 6MWT. Those patients who demonstrate disease progression should then be considered for a trial of anti-fibrotic therapy, with either pirfenidone or nintedanib. However, clinical trials investigating the effects of these interventions are ongoing.30, 31, 32 Lung transplantation should then be considered for those patients with progressive or residual disease with substantial morbidity related to the lung disease. These patients undergoing transplant work up should subsequently undergo screening for anxiety, depression, and other mental health disorders which may have developed since their COVID-19 infection, given the substantial and diverse mental health burden noted in COVID-19 patients after hospitalization.33 If any mental health disorders are newly identified, patients should be referred for appropriate treatment before transplant. Of note, for those patients with nonprogressive disease, all efforts for optimal medical management and rehabilitation should be trialed with enough time for recovery before considering lung transplantation. Although it remains uncertain what a sufficient amount of time for recovery for these static post-COVID fibrosis patients would be, the authors of this article suggest a minimum of 6 months to 1 year based off a recent review article investigating treatments for post-COVID fibrosis, recommending medical treatment for 6 months, and historic data of SARS patients with fibrotic lung damage showing recovery within 1 year.34, 35, 36
Inpatient Lung Transplant Evaluation for Coronavirus-19 Patients
In general, there are two types of patients undergoing inpatient lung transplant evaluation for COVID-19-related illness: those with COVID-19-associated ARDS, either on mechanical ventilation or ECMO with inability to wean, or those who have cleared their initial COVID-19 infection, but have high supplemental oxygen requirements, prohibiting them from a safe discharge. Timing of transplant evaluation in these patients is even more critical than those with post-COVID fibrosis patients discussed previously. Clinicians must identify those patients with COVID-19-associated ARDS who are likely to recover, without transplant, while simultaneously not waiting too long to see if a patient will recover allowing time for the patient to develop severe complications and deconditioning which would then preclude them from transplantation.
The first question to ask when evaluating any inpatient COVID-19 patient is, “Do they have any contraindications to lung transplantation?” This serves as an appropriate initial screening question for this population as if the patient already has a well-known contraindication to lung transplantation, the option of salvage lung transplantation can be taken out of consideration. Currently accepted general criteria for the selection of COVID-19 patients for lung transplantation are outlined in Box 1 . Of note, with regard to malignancy, patients should not have an active or recent malignancy. Patients should have a minimum of 2-year disease-free interval from cancer with a low likelihood of cancer recurrence.8 In addition, the last criteria listed in Table 1 brings up another critical issue: “Is the patient agreeable to lung transplantation?” This would require the patient to be off sedation and have the capacity to provide informed consent. However, in many cases of COVID-19-associated ARDS, the patient cannot be taken off sedation without severe hypoxemia and hemodynamic instability. In these instances, every effort should be made to safely wake patients up to discuss lung transplantation, provide education, assess patient interest in the procedure, and obtain informed consent. If sedation is unable to be safely held, it is recommended for clinicians to demonstrate the absence of irreversible brain injury through either physical assessment and brain imaging (ie, CT) or neuropsychological consultation. Furthermore, clinicians then must discuss lung transplantation with the patient’s medical power of attorney who can provide informed decisions in line with the patient’s wishes.17
Box 1. General criteria for lung transplantation in coronavirus-19 patient.
Age less than 65 year old, with extension to less than 70 year old in exceptionally fit patients
Isolated lung failure (single-organ failure), however, in certain cases multi-organ transplantation can be considered
Absence of malignancy
No disabling comorbidities
No substance dependence (alcohol, drugs, and so forth)
Not an active smoker
Body mass index between 17 and 32 kg/m2 (exceptions allowed on case-by-case basis)
Reliable postoperative social support (at minimum: one primary and one secondary caregiver)
Insurance approval and/or financial support identified for transplant associated care
Patient is participating in physical therapy while hospitalized (exceptions allowed for select cases undergoing urgent transplant evaluation, with a high potential for posttransplant recovery, and rehabilitation is hindered predominantly due to pulmonary failure from COVID-19)
Patient and social support in agreement to lung transplantation and willing to relocate close to transplant center for a period established by the transplant center
Table 1.
Summary of published literature on coronavirus-19-related lung transplantation
| Authors | Title | Month-Year Published | Summary |
|---|---|---|---|
| King et al,8 2010 | Lung transplantation for patients with COVID-19 | Aug-21 | Review paper discussing approaches to lung transplantation in the setting of post-COVID fibrosis or COVID-19-associated ARDS by high-volume lung transplant providers.8 |
| Bharat et al,12 2020 | Lung transplantation for patients with severe COVID-19 | Nov-20 | Case series detailing the approach to/outcomes of three patients with COVID-19-associated ARDS and additional investigation of explanted lung tissue from the transplants and comparison to control lung samples.12 |
| Roach et al,16 2022 | Lung transplantation for COVID-19-related respiratory failure in the United States | Mar-22 | A retrospective review of lung transplants performed between August 2020 and September 2021 reported in the United Network for Organ Sharing (UNOS) registry, investigating the survival and clinical outcomes of those transplants performed for COVID-19 respiratory failure.8 |
| Bharat et al,17 2021 | Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries | Mar-21 | International, multicenter case series of 12 patients who underwent bilateral lung transplantation for COVID-19-associated ARDS from May to September 2020.17 |
| Kurihara et al,18 2022 | Clinical characteristics and outcomes of patients with COVID-19-associated acute respiratory distress syndrome who underwent lung transplant | Jan-22 | A retrospective, single-center, case series of 102 consecutive lung transplants, from January 2020 to September 2021, which analyzed the clinical outcomes of patients undergoing lung transplantation for COVID-19-associated ARDS and compared them with the outcomes of lung transplant patients without COVID during that time.18 |
Once patients have passed these first screening criteria, the next question to ask is: “Will the patient’s lungs recover?” As much is still unknown regarding the timeline or possibility of recovery following acute lung injury from COVID-19, this question requires the best judgment of a multidisciplinary lung transplant team. Patients should undergo an appropriate trial of standard-of-care medical therapy for their COVID-19 infection to optimize any possibility of lung recovery. Although pharmaceutical approaches to COVID-19 are evolving, this trial should otherwise include lung-protective ventilation and negative fluid balance as these clinical practices are critical to prevent worsening lung injury.8 Currently, the literature supports a minimum of 4 to 6 weeks’ time to allow for lung recovery from the onset of COVID-19-associated ARDS before lung transplantation; however, many centers wait for ≥8 weeks.8 , 12 , 17 Specifically for COVID-19-associated ARDS patients supported on ECMO, Kurihara and colleagues showed that patients should be supported for at least 4 weeks before consideration of lung transplantation.18 Exceptions can be made to the minimum 4 week wait period if a life-threatening pulmonary complication arises first, which cannot be managed medically or with extracorporeal life support (ECMO) and requires lung transplantation. Furthermore, it is recommended that two physicians, from two different specialties (surgery, critical care, or pulmonology), agree that lung recovery is unlikely despite optimal medical management. Of note, lung transplantation should not be considered in cases where patients are showing ongoing lung improvement, regardless of time elapsed in COVID-19-associated ARDS.17
CT imaging may have a possible role in aiding physicians in assessing the progression/recovery of lung injury in COVID-19 patients and distinguishing patients who have irrecoverable lung damage. King and colleagues report that CT chest findings such as traction bronchiectasis and subpleural fibrosis are suggestive of irreversible injury in COVID-19 patients, whereas ground-glass infiltrates are potentially reversible.8 Furthermore, we have previously discussed how other studies have seen resolution of fibrotic changes on CT scan following COVID-19 infection, showing the potential utility for CT imaging to discern these two patient populations.21 , 22 CT chest imaging can additionally assist in defining potentially treatable causes of lung dysfunction, such as organizing pneumonia, pulmonary edema, or pleural disease.8
The final essential question to ask when working up inpatient COVID-19 patients for lung transplantation is, “Has the patient cleared their COVID-19 infection?” Current literature states that immunocompetent patients with severe COVID-19 infection have cleared all active virus by 20 days from symptom onset, whereas severely immunocompromised patients may continue to have active virus for significantly longer.37 , 38 As transplantation will require immunosuppression, especially in the early posttransplant period, any residual active virus is a major threat to the transplant patient. Therefore, experts in COVID-19-related lung transplantation suggest a very conservative approach when proving clearance of the virus. Most patients are initially diagnosed with COVID-19 through real-time polymerase chain reaction (RT-PCR) testing, which detects COVID-19 Ribonucleic Acid on upper respiratory tract samples, although lower respiratory tract samples have higher viral loads and are therefore less likely to have a false negative result.39 However, positive RT-PCR results are limited as this does not necessarily indicate actively replicating virus as the Ribonucleic Acid from viral fragments can cause persistent positive RT-PCR results. Viral culture is the gold standard test to establish active virus; however, given the potential infectious control issues with performing viral cultures, these are not widely available.40 Therefore, to prove clearance of COVID-19 infection in lung transplantation evaluation, expert consensus recommends two negative RT-PCR tests, obtained at least 24 hours apart from bronchial alveolar lavage samples. In cases where patients are not on the ventilator and without tracheostomy, the two negative RT-PCR tests may be obtained from a nasopharyngeal swab.17 Although this approach may delay lung transplantation unnecessarily in those patients who have persistent positive testing, given the severe potential morbidity of transplanting patients with active infection, this conservative approach has been accepted.
Single Versus Bilateral Lung Transplant Listing
Initially, only bilateral lung transplantation was recommended for COVID-19-related lung transplantation as many COVID-19 patients being evaluated for lung transplant had developed significant pulmonary hypertension, which would only be addressed by bilateral lung transplantation.39 , 40 Furthermore, as explants from the initial COVID-19 lung transplant recipients had cavitary areas of pneumonia, there was concern that these could serve as nidus of future infection if single lung transplantation was pursued, especially once postoperative immunosuppression was started.40 However, as more lung transplants are performed for post-COVID fibrosis, we believe that there will be a utility for single lung transplantation with careful patient selection, as highlighted in our previously discussed case report. We propose if when patients undergo extensive outpatient lung transplant workup, there is no pulmonary hypertension, and only mild–moderate, but not severe fibrosis, on CT chest, single lung transplantation may be considered. A lung ventilation perfusion scan would subsequently be needed to assess the ideal side for single lung transplantation, and if even, the patient may receive either laterality.
Operative approach
Performing lung transplantation in patients with COVID-19-related ARDS is not the same as performing lung transplants in those with non-COVID-19-related indications. Kurihara and colleagues performed a study comparing clinical characteristics and outcomes of patients undergoing lung transplant for COVID-19-associated ARDS to those undergoing lung transplant for chronic end-stage lung disease without COVID-19, where they demonstrated the complexity and challenges associated with COVID-19-associated ARDS lung transplants. They noted patients undergoing lung transplantation for COVID-19-associated ARDS had nearly double the lung allocation scores compared with those with chronic end-stage lung disease without COVID-19 (85.8 vs 46.7). Furthermore, they reported that 56.7% of COVID-19-associated ARDS lung transplant recipients were on preoperative ECMO, compared with 1.4% of non-COVID-19 transplants, highlighting just how sicker the COVID-19-associated ARDS lung transplant patient is compared with standard lung transplant recipient. Intraoperatively, they found COVID-19-associated ARDS lung transplants had higher rates of veno-arterial ECMO use (96.7% vs 62.5%) and required more intraoperative red blood cell transfusions (6.5 units vs 0 units) and longer operative time (8.5 vs 7.4 hours), demonstrating the technical challenges associated with performing transplants in this population.18
When performing lung transplantation for COVID-19-associated ARDS, there are a couple critical operative factors to note. First, surgeons should prepare to perform the lung transplantation on cardiopulmonary bypass or veno-arterial ECMO support.18 Although the use of cardiopulmonary bypass is potentially associated with worse outcomes in lung transplantation because of the high incidence of pulmonary hypertension, right ventricular dysfunction, and pleural adhesions in patients with COVID-19-associated ARDS, surgeons should use intraoperative cardiopulmonary support when performing these transplants.17 , 41 VA-ECMO can be used in place of cardiopulmonary bypass to reduce total blood loss and need for blood transfusion which are anticipated with cardiopulmonary bypass.42, 43, 44 However, in patients that have extensive and thick pleural adhesions, there is a possibility of entraining air in the ECMO circuit during the dissection. Hence, we suggest that either cardiopulmonary bypass be considered in those patients or at least be available for immediate conversion in case of emergency. Second, it is important to expect high intraoperative blood loss and be prepared to give a large number of blood products. This is likely secondary to the increased complexity of lung transplantation in COVID-19-associated ARDS patients due to factors such as pleural adhesions, fragile tissue quality, and platelet dysfunction form preoperative ECMO support.40 , 42 When the blood loss is too extensive, the use of cardiopulmonary bypass may be required, although it would have the attendant risk of disseminating pulmonary pathogens through the systemic circulation.
Postoperative management
Following transplantation, these COVID-19 lung transplant patients must be followed closely. King and colleagues report that COVID-19 lung transplant recipients had no different course or risk of specific posttransplantation complications compared with a general transplant population, but they attributed this to the patients being closely monitored and evaluated before transplant.8 Conversely, Kurihara and colleagues report COVID-19-associated ARDS lung transplant patients had higher rates of Primary Graft Dysfunction (grades 1–3) and permanent hemodialysis use and longer postoperative ventilator requirements, Intensive Care Unit stays, and overall hospital admissions compared with non-COVID-19-related transplants. However, they additionally reported that COVID-19 lung transplant recipients had a higher rate of improvement in their Karnofsky Performance Status after lung transplant compared with the non-COVID-19 transplants.18 Bharat and colleagues note that this prolonged ventilation and difficult initial recovery are expected as this COVID-19 patient population are generally deconditioned before transplant and that this highlights the importance of appropriate selection of patients with a healthy baseline status before their COVID-19 infection, so they may safely tolerate the posttransplant rehabilitation.17 Taken together, the increased complexity of both the lung transplant operation and postoperative management highlights the need for COVID-19 lung transplantation to be performed in high-volume centers with access to many hospital resources.
Outcomes
As it has only been 2 years since the first COVID-19-related lung transplant was performed in June 2020, there is no long-term data on COVID-19 lung transplant outcomes (see Table 1). However, studies have shown short-term survival greater than 90% at both 3 months and 1 year.17 Roach and colleagues performed a retrospective review of the United Network for Organ Sharing (UNOS) registry, analyzing lung transplants performed between August 2020 and September 2021, where they found COVID-19-related lung transplants made up 7% of all lung transplants. Of the 214 COVID-19-related lung transplants identified, 65% were performed for COVID-19-associated ARDS with the remaining performed for post-COVID fibrosis. This is the largest study analyzing COVID-19-related lung transplant outcomes published and they report a 2.2% 30-day mortality with 95.6% 3-month survival rate, which rivals the survival rate among patients undergoing lung transplantation for non-COVID-19-related indications.16 Overall, lung transplant for COVID-19-related indications is becoming more common and current outcomes of COVID-19-related lung transplantation are promising.
Future directions
As the COVID-19 pandemic continues with more waves and new subvariants, lung transplantation for COVID-19-related respiratory failure will become increasingly more common. As we get farther out from the initial COVID-19 lung transplants, further studies will be needed to analyze long-term outcomes of these transplant recipients and see how they compare to non-COVID-19-related lung transplants. Further studies will also be needed to investigate the outcomes of COVID-19 single lung transplants and how they compare to those who received bilateral lung transplants. As COVID-19-related lung transplantation demand increases, especially as more patients develop post-COVID fibrosis, expanding the donor pool of lungs through the use of single lung transplantation must be considered. Finally, as we learn more about the COVID-19 virus and the natural course of disease, we must continuously revise our guidelines and criteria for lung transplant evaluation and patient selection.
Summary
COVID-19 can result in severe irrecoverable ARDS or life-limiting fibrosis for which lung transplantation is currently the only viable treatment. COVID-19 lung transplantation has transformed the field of lung transplantation, as before the pandemic, few transplants had ever been performed in the setting of infectious disease or ARDS. Given the complexities associated with COVID-19 lung transplantation, it requires strict patient selection with an experienced multidisciplinary team in a high-resource hospital setting. Current short-term outcomes of COVID-19 lung transplantation are promising; however, further follow-up studies are needed to determine long-term outcomes and whether this subset of lung transplant patients may be predisposed to unique complications.
Clinics care points
-
•
Lung transplantation is the only viable treatment option for patients with severe irrecoverable COVID-19-associated ARDS or life-debilitating post-COVID fibrosis and shows promising short-term outcomes.
-
•
COVID-19-related respiratory failure lung transplantation requires strict patient selection and extensive lung transplant evaluation must be done.
-
•
COVID-19-related respiratory failure lung transplants are complex and should be done at high-volume centers with multidisciplinary teams.
Disclosure
E.J. Cerier is supported by the National Institutes of Health, United States Grant T32AI083216 and Thoracic Surgery Foundation, United States, A. Bharat is supported by the National Institutes of Health Grants: HL145478, HL147290, and HL147575. The authors declare they have no conflict of interest.
References
- 1.WHO COVID-19 dashboard. https://covid19.who.int/ Available at: Accessed April 11, 2022.
- 2.Mokhtari T., Hassani F., Ghaffari N., et al. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol. 2020;51(6):613–628. doi: 10.1007/s10735-020-09915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baud D., Qi X., Nielsen-Saines K., et al. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20(7):773. doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geleris J., Sun Y., Platt J., et al. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of covid-19 - preliminary report reply. N Engl J Med. 2020;383(10):994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 8.King C.S., Mannem H., Kukreja J., et al. Lung transplantation for patients with COVID-19. Chest. Jan 2022;161(1):169–178. doi: 10.1016/j.chest.2021.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Mark S.C., Hoek R.A.S., Hellemons M.E. Developments in lung transplantation over the past decade. Eur Respir Rev. 2020;29(157) doi: 10.1183/16000617.0132-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers D.C., Cherikh W.S., Harhay M.O., et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: thirty-sixth adult lung and heart-lung transplantation report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1042–1055. doi: 10.1016/j.healun.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erasmus M.E., van der Bij W. Death after lung transplantation: improving long term survival despite perilous early postoperative years. Transpl Int. 2020;33(2):128–129. doi: 10.1111/tri.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharat A., Querrey M., Markov N.S., et al. Lung transplantation for patients with severe COVID-19. Sci Translational Med. 2020;12(574):eabe4282. doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams T.S., Schupp J.C., Poli S., et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. 2020;6(28):eaba1983. doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habermann A.C., Gutierrez A.J., Bui L.T., et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv. 2020;6(28):eaba1972. doi: 10.1126/sciadv.aba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyfman P.A., Walter J.M., Joshi N., et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199(12):1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roach A., Chikwe J., Catarino P., et al. Lung transplantation for covid-19–related respiratory failure in the United States. N Engl J Med. 2022;386(12):1187–1188. doi: 10.1056/NEJMc2117024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharat A., Machuca T.N., Querrey M., et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9(5):487–497. doi: 10.1016/S2213-2600(21)00077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurihara C., Manerikar A., Querrey M., et al. Clinical characteristics and outcomes of patients with COVID-19–associated acute respiratory distress syndrome who underwent lung transplant. JAMA. 2022;327(7):652–661. doi: 10.1001/jama.2022.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weill D., Benden C., Corris P.A., et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the pulmonary transplantation council of the international society for heart and lung transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 20.McDonald L.T. Healing after COVID-19: are survivors at risk for pulmonary fibrosis? Am J Physiol Lung Cell Mol Physiol. 2021;320(2):L257–L265. doi: 10.1152/ajplung.00238.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han X., Fan Y., Alwalid O., et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299(1):E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabatabaei S.M.H., Rajebi H., Moghaddas F., et al. Chest CT in COVID-19 pneumonia: what are the findings in mid-term follow-up? Emerg Radiol. 2020;27(6):711–719. doi: 10.1007/s10140-020-01869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y., Tan C., Wu J., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guler S.A., Ebner L., Aubry-Beigelman C., et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021;57(4) doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojo A.S., Balogun S.A., Williams O.T., et al. Pulmonary fibrosis in COVID-19 survivors: predictive factors and risk reduction strategies. Pulm Med. 2020;2020:6175964. doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatabu H., Hunninghake G.M., Richeldi L., et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the fleischner society. Lancet Respir Med. 2020;8(7):726–737. doi: 10.1016/S2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.González J., Benítez I.D., Carmona P., et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160(1):187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myall K.J., Mukherjee B., Castanheira A.M., et al. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18(5):799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vadász I., Husain-Syed F., Dorfmüller P., et al. Severe organising pneumonia following COVID-19. Thorax. 2021;76(2):201–204. doi: 10.1136/thoraxjnl-2020-216088. [DOI] [PubMed] [Google Scholar]
- 30.National Institutes of Health Clinical Center Pirfenidone compared to placebo in post-COVID19 pulmonary fibrosis COVID-19 (FIBRO-COVID). National Institutes of health. https://clinicaltrials.gov/ct2/show/NCT04607928?term=pirfenidone&cond=Covid19&draw=2 Available at: Updated 12/30/21. Accessed 05/10/22, 2022.
- 31.Pirfenidone vs. Nintedanib for fibrotic lung disease after coronavirus disease-19 pneumonia (PINCER). National Institutes of health. https://clinicaltrials.gov/ct2/show/NCT04856111?term=pirfenidone&cond=Covid19&draw=2 Available at: Updated 04/26/22. Accessed 05/10/22, 2022.
- 32.The study of the use of nintedanib in slowing lung disease in patients with fibrotic or non-fibrotic interstitial lung disease related to COVID-19 (ENDCOV-I). National Institutes of health. https://clinicaltrials.gov/ct2/show/NCT04619680?term=nintedanib&cond=Covid19&draw=2 Available at: Updated 4/14/22. Accessed 05/10/22, 2022.
- 33.Evans R.A., McAuley H., Harrison E.M., et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9(11):1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui D.S., Joynt G.M., Wong K.T., et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong K.T., Antonio G.E., Hui D.S., et al. Severe acute respiratory syndrome: thin-section computed tomography features, temporal changes, and clinicoradiologic correlation during the convalescent period. J Comput Assist Tomogr. 2004;28(6):790–795. doi: 10.1097/00004728-200411000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Bazdyrev E., Rusina P., Panova M., et al. Lung fibrosis after COVID-19: treatment prospects. Pharmaceuticals (Basel) 2021;14(8) doi: 10.3390/ph14080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ending isolation and precautions for people with COVID-19: interim guidance. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html Available at: Updated 01/14/22. Accessed 05/17/22, 2022.
- 38.Aydillo T., Gonzalez-Reiche A.S., Aslam S., et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai C.K.C., Lam W. Laboratory testing for the diagnosis of COVID-19. Biochem Biophys Res Commun. 2021;538:226–230. doi: 10.1016/j.bbrc.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jefferson T., Spencer E.A., Brassey J., et al. Viral cultures for coronavirus disease 2019 infectivity assessment: a systematic review. Clin Infect Dis. 2020;73(11):e3884–e3899. doi: 10.1093/cid/ciaa1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weingarten N., Schraufnagel D., Plitt G., et al. Comparison of mechanical cardiopulmonary support strategies during lung transplantation. Expert Rev Med Devices. 2020;17(10):1075–1093. doi: 10.1080/17434440.2020.1841630. [DOI] [PubMed] [Google Scholar]
- 42.Bermudez C.A., Shiose A., Esper S.A., et al. Outcomes of intraoperative venoarterial extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation. Ann Thorac Surg. 2014;98(6):1936–1942. doi: 10.1016/j.athoracsur.2014.06.072. discussion 1942-3. [DOI] [PubMed] [Google Scholar]
- 43.Moreno Garijo J., Cypel M., McRae K., et al. The evolving role of extracorporeal membrane oxygenation in lung transplantation: implications for anesthetic management. J Cardiothorac Vasc Anesth. 2019;33(7):1995–2006. doi: 10.1053/j.jvca.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto K., Hoetzenecker K., Yeung J.C., et al. Intraoperative extracorporeal support during lung transplantation in patients bridged with venovenous extracorporeal membrane oxygenation. J Heart Lung Transplant. 2018;37(12):1418–1424. doi: 10.1016/j.healun.2018.07.003. [DOI] [PubMed] [Google Scholar]