Abstract
Objective: Borderline oligohydramnios always produces a dilemma of management and counseling among obstetricians. This study was designed to compare the effect of sildenafil plus fluid therapy versus fluid therapy alone on pregnancy outcomes and AFI improvement in pregnant women complicated by idiopathic borderline oligohydramnios.
Materials and methods : This randomized clinical trial was conducted in Arash Women’s Hospital, Tehran, Iran from 2017 to 2020. Fifty-one pregnant women with idiopathic borderline oligohydramnios were allocated to two groups. Group 1 received fluid therapy and Group 2 received fluid therapy and Sildenafil 25 mg three times daily for six weeks. AFI was measured at the time of randomization, 24 h after treatment and then weekly for six weeks. The changes in AFI, type of delivery, gestational age at delivery, and neonatal outcomes were compared between the two groups.
Results: After the intervention, the change in AFI between two groups was not statistically significant. Maternal and fetal outcomes are compared between two groups and there was no significant difference between them. The median (Inter-quartile range) AFI after intervention, in Sildenafil group compared with hydration group, were in 24 hours (8.5 vs. 8, p=0.27), first (9.5 vs. 9.1, p=0.74), second (9 vs 10, p=0.12) third (10.4 vs. 9.4, p=0.33), fourth (10.8 vs 9.1, p=0.1) and Fifth week (10 vs 9.3, p=0.5) of follow-up respectively, but none of them were statistically significant.
Conclusion: The findings showed that sildenafil plus fluid therapy do not improve the pregnancy outcomes in women with isolated borderline oligohydramnios compared to fluid therapy alone.
Key Words: Oligohydramnios, Pregnancy Outcome, Sildenafil
Introduction
Amniotic fluid is a clear liquid that surrounds the fetus during pregnancy, contained within amniotic sac and provides a supportive environment for the fetus throughout the pregnancy for normal growth and development. Amniotic fluid volume abnormalities may reflect fetal or placental pathology. Abnormally decreased fluid volume is termed oligohydramnios. The amniotic fluid volume may decrease in 3-5% of pregnancies. A common method for evaluating the volume of the amniotic fluid is to estimate the amniotic fluid index (AFI)using obstetric ultrasound examination (1). Amniotic fluid volume evaluation is essential component of every second or third trimester sonogram. It is assessed by measuring either a single pocket or the amniotic fluid index (AFI). AFI is a quantitative estimate of amniotic fluid. An AFI of 8 or above is normal, while an AFI of 5-8 is considered borderline oligohydramnios (low normal) and an AFI below 5 indicates oligohydramnios.
Oligohydramnios can be accompanied with a non-complicated pregnancy or a finding in a complicated pregnancy (with hypertensive disorders or fetal anomalies) (2). The pathophysiology of oligohydramnios is not clearly established, but when detected in the absence of rupture of membranes, could be a sign of chronic suboptimal placental function. Idiopathic types of oligohydramnios account for about 7% of all cases of oligohydramnios in pregnancy and are associated with adverse fetal outcomes (3). In these cases, it is often assumed that the underlying etiology is associated with placental uterine insufficiency, which can impair the fetal growth and decrease the fetal urine output. The incidence of borderline Oligohydramnios is about 6% to 44% in term pregnancy, with average incidence being 12% (4). Borderline Oligohydramnios is associated with a higher rate of preterm delivery, cesarean delivery for non-reassuring Non-Stress Test (NST), fetal growth restriction, congenital anomalies, perinatal morbidity and mortality (4). There is no definitive treatment for idiopathic oligohydramnios, however, there are some therapeutic methods in the literature such as maternal hydration (5-8) using desmopressin, nitric oxide donors or amnioinfusion (9-11), maternal resting in left lateral decubitus position (12), and usually, the effect of these methods continue for a short time.
Recently Sildenafil is suggested for treatment of isolated oligohydramnios. Sildenafil is a class B drug that causes vascular relaxation and increases uterine blood flow. Several studies investigate the effect of sildenafil on fetal growth restriction and have some conflicting results and there are few studies that investigated the role of this drug on isolated oligohydramnios (13, 14). Sildenafil citrate (Viagra) is a selective inhibitor of the type V cyclic guanosine monophosphate-specific phosphodiesterase. Sildenafil increases the effect of nitric oxide by inhibiting phosphodiesterase type 5, which is responsible for degradation of cyclic guanosine monophosphate. Using sildenafil causes cyclic guanosine monophosphate levels to remain elevated which leads to vascular relaxation and increased uterine blood flow (15). The use of sildenafil is associated with several side effects in pregnant women such as headache, dyspepsia, flushing, nasal congestion, diarrhea and urinary tract infections (16). There is not any report of teratogenicity or fetotoxic effect of sildenafil in the literature (17). Therefore, due to the importance of health in pregnant women and fetuses, and lack of appropriate data for treatment of oligohydramnios, this study was designed to compare the effect of sildenafil plus fluid therapy versus fluid therapy alone on pregnancy outcomes and AFI improvement in pregnant women complicated by idiopathic borderline oligohydramnios.
Materials and methods
This single-blind single-center clinical trial was conducted in Arash Women’s Hospital, Tehran, Iran from 2017 to 2020. The Institutional Review Board and the Ethics Committee of Tehran University of Medical Sciences approved this study (IR.TUMS.MEDICINE.REC.1396.3070). Also this study is registered and approved at Iranian Registry of Clinical Trials (IRCT number: IRCT20170917036227N3). Informed consent was obtained from all participants.
Pregnant women with reduced volumes of amniotic fluid at 28 weeks gestation and above diagnosed during routine trimester ultrasound examination in the third trimester were recruited in this study based on a set of inclusion and exclusion criteria. Inclusion criteria were singleton pregnancy, an AFI of 5-8 cm, and unexplained oligohydramnios. Women with fetal growth restriction or fetal abnormalities, abnormal fetal Doppler ultrasound findings, impaired NST, treatment with prostaglandin synthase inhibitors, active immunodeficiency, rupture of membranes, chronic hypertension, gestational diabetes, and contraindication to fluid therapy such as renal or heart disease were excluded from this study.
First, abdominal ultrasonography was done and the AFI was calculated for the patients. One expert radiologist evaluated all patients (to eliminate the interobserver variation) using WS80 Elite Ultrasound equipment with a1-7MHz convex probe (Samsung Madison, Hongcheon, Korea). This radiologist was blinded to the treatment groups and performed AFI measurements in a standardized fashion.
Random allocation was performed by a methodologist using the Stata V.13 with a block size of four. The patients were randomized into sildenafil or control group. Medicines were placed in similar packets and the sequence of medicines and list of random allocation of patients were not disclosed to dispensing practitioners. To conceal the random allocation process, a random 10- digit code corresponding to the identification number of the patient was written on each medicine packet. These packets were given to the dispensing nurse, who was unaware of the contents of each packet. When the doctor declared the eligibility of patients, the nurse gave packets to them according to the identification numbering.
The participants completed a questionnaire including demographic data, history of pregnancy, and medical history. Then, they were admitted to the hospital to receive intravenous fluid therapy for 24 hours. For each patient, 2 liters of Ringer's solution were administered in the first 24 hours. In addition to liquid therapy, the intervention group received sildenafil 25 mg tablets (Zahravi Pharmaceutical Company, Iran) every 8 hours for 6 weeks. The index of amniotic fluid was measured and recorded after 24 hours of hospitalization. The patients with at least 20% increase in the amniotic fluid volume were discharged from the hospital.
For those who did not show any improvement in AFI, the same regimen to which they were assigned was repeated. The discharged patients in the intervention group were advised to drink 2 liters of water every day and take sildenafil 25 mg every 8 hours for 6 weeks, while the patients in the control group only drank only 2 liters of water every day for 6 weeks.
All patients underwent NST twice a week and ultrasound every week for AFI examination and biophysical testing. Maternal resting in left lateral decubitus position and fetal movement counts were advised and educated. Treatment continued for 6 weeks in both groups and the AFI was measured every week until 6 weeks or at delivery (whichever occurred earlier).
According to our pilot study, the mean ± SD AFI in 5 patients of intervention group (hydration therapy combined with sildenafil) was 11.5 ± 8 and in 5 control group (without sildenafil) was 5.35 ± 4. with the study power of 90% and two-sided α level 0.05, the total sample size after considering 10% drop out, was 52 (26 in each group).
All statistical analysis was performed by using SPSS 24. Categorical and continuous variables are summarized as proportions (percent) and mean ± SD, respectively. X2 test or Fisher exact test used to test the difference between categorical variable, Student’s t-test and non-parametric test of Mann-Whitney U test, used to assess baseline demographic and clinical characteristics between the two groups. We used multiple logistic regression to assess the effect of type of intervention on pregnancy outcome.
Results
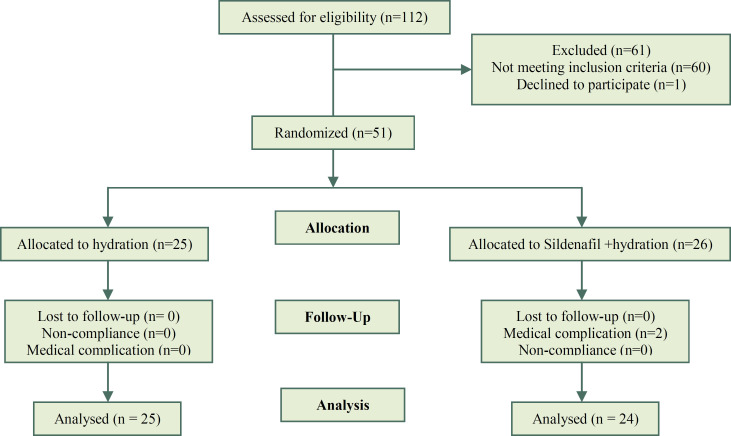
We evaluated 112 pregnant women for this study. 52 eligible women participated and 1 woman declined to participate so 51 participants were randomized. According to allocation 25 participants were in hydration group and 26 participants were in hydration plus sildenafil group. Two participants were withdrawed from the study because of medical complications (Figure 1).
Figure 1.
Summary of patients flow in the study
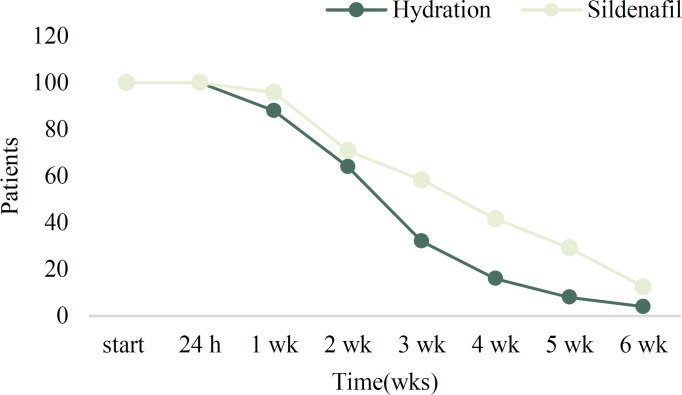
The data of 24 women in the sildenafil +hydration (S+H) group and 25 women in the hydration (H) group were compared. The patients were followed up for 6 weeks. The number of participants in each follow-up visit was as follows: first 24 hours [n=49 (100%)], first week [n=45 (92%)], second week [n=33 (67%)], third week [n=22 (45%)], fourth week [n=14 (29%)], fifth week [n=9 (18%)] and sixth week [n=4 (8%)]. After the first 24 hours, 2 women left the study in the (S+H) because of medical complications. One woman had complained of nausea and the other had headache. Others remained in the study. There was not any loss to follow up in this study and pregnancy termination was the cause of the gradual decrease in the study population.
Demographic and baseline characteristics of the patients in the two groups are presented in Table 1. Patients in both groups were matched in terms of maternal age, gravidity and parity, and gestational age at the beginning of the study (all P values> 0.05).
Table 1.
Baseline characteristics of two study groups
| Variable |
Group
|
P-Value | |
|
Hydration
(mean±SD) |
Sildenafil
(mean±SD) |
||
| Age | 29.7(6) | 30.1(4.9) | 0.83* |
| Gravidity | 2.1(1.1) | 1.7(1.1) | 0.24* |
| Parity | 0.8(0.9) | 0.5(0.7) | 0.24* |
| Gestational age at randomization (week) |
34.5(3) | 33.4(2.4) | 0.10* |
Student t test.
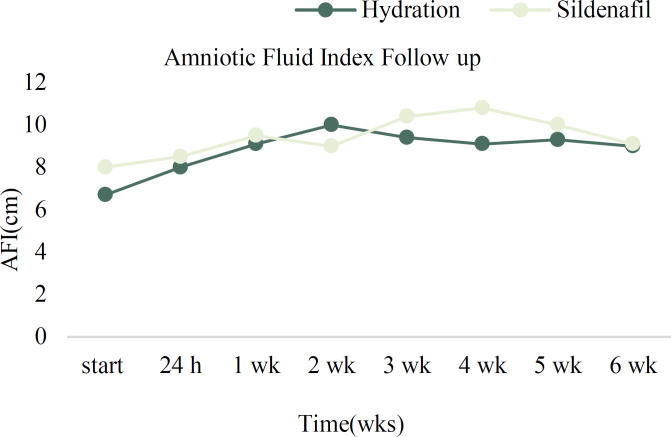
The median AFI before treatment was significantly higher in the S+H group compared to the H group [8(0.7) vs 6.7(1.5) respectively, P value<0.001] therefore multiple logistic regression used for controlling and adjusting initial AFI. The median (interquartile range) AFI was higher in S+H group compared to the H group in all follow-up visits except for the second one but the difference was not statistically significant (all p values> 0.05). After the intervention, the AFI improved in all participants (Table 2).
Table 2.
Baseline characteristics of two study groups
| Hydration group | Sildenafil group | Df ** | P Value # | ||
|---|---|---|---|---|---|
| At randomization | - | 25(100) | 24(100) | - | |
| AFI (cm)* | 6.7(1.5) | 8(7) | <0.001 | ||
| After 24 h | No. continued (%) | 25(100) | 24 (100) | S: +0.5 | - |
| AFI (cm)* | 8(2) | 8.5(2.1) | H: +1.3 | 0.27 | |
| After 1 week | No. continued (%) | 22(88) | 23(95.8) | S: +1.0 | - |
| AFI (cm)* | 9.1(3) | 9.5(28.5) | H: +1.1 | 0.74 | |
| After 2 week | No. continued (%) | 16(64) | 17(70.8) | S: -0.5 | - |
| AFI (cm)* | 10(2.8) | 9(2.7) | H: +.9 | 0.12 | |
| After 3 week | No. continued (%) | 8(32) | 14(58.3) | S: +1.4 | - |
| AFI (cm)* | 9.4(3.8) | 10.4(48) | H: -0.6 | 0.33 | |
| After 4 week | No. continued (%) | 4(16) | 10(41.7) | S: +0.4 | - |
| AFI (cm)* | 9.1(3.2) | 10.8(2) | H: -0.3 | 0.10 | |
| After 5 week | No. continued (%) | 2(8) | 7(29.2) | S: -0.8 | - |
| AFI (cm)* | †9.3(9.5-5) | 10(4.5) | H: +0.26 | 0.5 | |
| After 6 week | No. continued (%) | 1(4) | 3(12.5) | S: -0.9 | - |
| AFI (cm)* | 9(-) | †9.1(4.5-14) | H: -0.3 | - |
DF: Difference
Median (interquartile range (IQR))
Mann-Whitney U test
(Min – Max)
The change of median number in each follow-up period relative to the previous period.
The AFI decreased after 2 and 4 weeks of treatment in the H and S+H group respectively (Figure 2), but the difference was not statistically significant. The percentage of people left each week for each group is demonstrated in figure 3. Maternal and fetal outcomes are compared between H and S+H groups in Table 3. There was no significant difference in the frequency of NICU admission, Apgar score at 1 and 5 minutes, birthweight, and gestational age at birth between the two groups (p values >0.05).
Figure 2.
The Average of amniotic fluid index at the beginning of treatment and during follow-up for 6 weeks in the two groups. (AFI: amniotic Fluid Index)
Figure 3.
The percentage of people left each week for each group
Table 3.
Maternal and neonatal outcomes between the two groups
| Variable | Sildenafil group | Hydration group | P Value | |
|---|---|---|---|---|
| Gestational age at delivery (week) | 37.5(1.1) | 37.2(1.4) | 0.41 | |
| Indications of cesarean delivery | Breach presentation | 1(6) | 2(13) | 0.08 |
| Previous cesarean section | 8(47) | 10(67) | ||
| Meconium | 0 (0) | 1(7) | ||
| Fetal Heart failure | 3(18) | 1(7) | ||
| Genital herpes | 0 (0) | 1(7) | ||
| Mother's request | 5(29) | 0 (0) | ||
| Mode of delivery | Cesarean section | 17(71) | 15(60) | 0.42 |
| Vaginal delivery | 7(29) | 10(40) | ||
| Apgar score < 7 | 1-min | 1(4) | 0 | 0.96 |
| 5-min | 0 (0) | 0 (0) | 0.99 | |
| Weight birth | 2923(347) | 2781(393) | 0.18 | |
| NICU administration | 3(13) | 2(8) | 0.66 |
Data presented as number (%), median (minimum-maximum) or mean ± standard deviation.
Cesarean section was done in 17 patients (71%) in the S+H group and 15 patients (60%) in the H group.
The most common cause of cesarean section was a positive history of cesarean section in both groups. Other causes are shown in Table 3. There was no significant difference in the frequency and causes of cesarean section between the two groups. Pregnancy was terminated at completion of 37 weeks in the majority of the cases in the H+S group while the most common cause of pregnancy termination was a low AFI in the H group; however, there was no significant difference between the two groups in this regard. Although 2 pregnant women left the study because of drug side effects; no adverse effects of the drug were observed in infants.
Multivariate analysis using multiple logistic regression showed that after adjusting for initial AFI, the OR (95%CI) of AFI improvement during 24 hours of intervention was 8% (0-1.92) in the S+H group compared to the H group but the difference was not statistically significant.
Discussion
This study found no difference between the effect of oral hydration therapy plus sildenafil and hydration alone on AFI changes in pregnant women with idiopathic oligohydramnios. Moreover, the maternal and fetal outcomes were similar between the two groups.
Few studies have evaluated the effect of sildenafil citrate in pregnancies complicated by oligohydramnios, and most of the participants in these studies were pregnant women with early and late onset fetal growth restriction (FGR). The results of these studies are conflicting (18). Sildenafil inhibits phosphodiesterase type 1 enzyme and increases the effect of nitric oxide by inhibiting phosphodiesterase type 5 and disrupting cyclic guanosine monophosphate degradation.
As a result, high levels of guanosine monophosphate cause vascular relaxation and increase the uterine blood flow (15). Some studies found that sildenafil citrate increased the fetoplacental perfusion in fetal growth restricted pregnancies and significantly improved the umbilical and middle cerebral artery Doppler velocimetry (19). The STRIDER study (a double-blind RCT) in UK found no significant difference in pregnancy and neonatal outcomes in 135 pregnancies complicated by IUGR starting at 22-28 weeks' gestation between sildenafil citrate and placebo (18).
The exact cause of isolated oligohydramnios is still unknown but the chronic suboptimal placental function is one of the probable reasons (20). In line with the present study, Gizzeo et al. conducted a systematic review and meta-analysis and found that maternal oral hydration was an effective method for increasing AFI in pregnant women with isolated oligohydramnios although its effect only lasted for a short time (6). However, the effect of this treatment is temporary and lasts for a short time ranging from less than 24 hours to up to one week (10). In our study, AFI improvement continued for three weeks in the S+H and for two weeks in the H group.
The mechanism of the AFI change after maternal hydration and its duration is still unclear. Some studies suggest that the fetus can respond to AFI changes after maternal hydration (21).
The results of observational studies regarding the association of isolated and borderline oligohydramnios and maternal and prenatal outcomes are inconsistent. Some studies found that isolated oligohydramnios at term was associated with adverse obstetrical outcomes (22-26) while some others did not find any association (24, 27). The difference in the results could be due to heterogeneity in the study design, study population, and physicians’ anxiety about decreased amniotic fluid volume (28).
The maternal and fetal outcomes were not different between the two groups in this study. This finding was expected due to the similar AFI changes in both groups. The birth weight was similar in both groups. A systematic review evaluated the data of eight studies and found that sildenafil citrate increased the birthweight in pregnancies complicated by placenta insufficiencies such as preeclampsia and FGR. However, most of the participants were pregnant women with IUGR in this systematic review (13). Similarly, Paauw et al. evaluated 22 animal studies and 2 human randomized trials and found that sildenafil increased fetal growth in pregnancies complicated by preeclampsia and FGR while it did not effect on fetal growth in healthy pregnancies (29).
The side effects associated with the use of sildenafil during pregnancy include headache, visual disturbances, dyspepsia, epigastric pain, hypotension, vomiting, dizziness, diarrhea, neurological symptoms, facial flushing, arthralgia and myalgia in mother and neonatal nursery admission, Apgar score <7 at 5 min, and cord arterial pH <7.1 in infants (30). Based on the available evidence, administration of sildenafil citrate during pregnancy is not associated with severe maternal and neonatal adverse effects (31). Our study had a low power for determining the rare adverse events, however, one complaint of nausea and one complaint of headache was seen in pregnant women during our study and no adverse effect on infants was seen.
The Dutch STRIDER trial was initiated to evaluate the effect of sildenafil in pregnancies complicated by early-onset IUGR but this study was suspended because of increased neonatal death (31) but One of the exclusion criteria of our study was pregnancy complicated by IUGR.
The results of this study may have been affected by the insufficient sildenafil dose, timing of sildenafil consumption, and the small sample size, or may actually be true. It is not clear whether the optimal timing of using sildenafil in pregnancies complicated by placental insufficiency is after observing the complication or during the placentation (31). One study found that sildenafil was metabolized more rapidly in pregnancy, and some authors suggest that pregnant women need higher doses of sildenafil because of the altered plasma volume and pH during pregnancy (30), however, low-dose sildenafil was used in the present study.
The strengths of this study included random allocation to study groups, repeated AFI measurement from baseline until delivery, and a wide range of gestational age (30 weeks or more). A limitation of this study was that amniotic fluid characteristics such as the post-treatment solute content were not evaluated. Also only AFI indices and not the actual amniotic fluid volume were used to measure improvement after treatment. Moreover, this study had a single-blind design and the hydration group did not receive a placebo, and the sample size was very small, too.
Conclusion
Overall, the findings showed that sildenafil combined with fluid therapy might not improve the pregnancy outcomes in women with isolated oligohydramnios (5<AFI<8) compared to fluid therapy alone. Larger studies are needed to confirm the results.
Acknowledgments
The authors of this study would like to deeply appreciate the patients who entered the study and fully cooperated with the people involved in the project. Also, the authors would like to thank the Clinical Research Development Center of Arash Women’s Hospital, Tehran University of Medical Sciences, Tehran, Iran for their supports, cooperation and assistance through the period of study.
Conflict of Interests
Authors have no conflict of interests.
Notes:
Citation: Vahid Dastjerdi M, Ghahghaei-Nezamabadi A, Tehranian A, Mesgaran M. The Effect of Sildenafil on Pregnancy Outcomes in Pregnant Women With Idiopathic Borderline Oligohydramnios: A Randomized Controlled Trial. J Family Reprod Health 2022; 16(2): 124-31.
References
- 1.Rosati P, Guariglia L, Cavaliere AF, Ciliberti P, Buongiorno S, Ciardulli A et al. A comparison between amniotic fluid index and the single deepest vertical pocket technique in predicting adverse outcome in prolonged pregnancy. J Prenat Med. 2015;9:12–5. doi: 10.11138/jpm/2015.9.1.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabie N, Magann E, Steelman S, Ounpraseuth S. Oligohydramnios in complicated and uncomplicated pregnancy: a systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2017;49:442–9. doi: 10.1002/uog.15929. [DOI] [PubMed] [Google Scholar]
- 3.HalaAbd El-fttah A, AliSabahRamadan HA. The effect of oral versus intravenous fluid therapy on maternal and neonatal outcomes for women with oligohydramnios. Egyptian Nursing Jouurnal. 2018;15:228. [Google Scholar]
- 4.Yadav P, Basnet T, Yadav SP. Maternal and perinatal outcome in borderline oligohydramnios: a hospital-based studY. International Journal of Medical Science And Diagnosis. 2021;5:19–21. [Google Scholar]
- 5.Ghafarnejad M, Tehrani MB, Anaraki FB, Mood NI, Nasehi L. Oral hydration therapy in oligohydramnios. J Obstet Gynaecol Res. 2009;35:895–900. doi: 10.1111/j.1447-0756.2009.01043.x. [DOI] [PubMed] [Google Scholar]
- 6.Gizzo S, Noventa M, Vitagliano A, Dall’Asta A, D’Antona D, Aldrich CJ, et al. An update on maternal hydration strategies for amniotic fluid improvement in isolated oligohydramnios and normohydramnios: evidence from a systematic review of literature and meta-analysis. PloS one. 2015;10:e0144334. doi: 10.1371/journal.pone.0144334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malhotra B, Deka D. Duration of the increase in amniotic fluid index (AFI) after acute maternal hydration. Arch Gynecol Obstet. 2004;269:173–5. doi: 10.1007/s00404-002-0346-z. [DOI] [PubMed] [Google Scholar]
- 8.Wolman I, Groutz A, Gull I, Gordon D, Geva E, Lessing JB, et al. Is amniotic fluid volume influenced by a 24-hour fast? The Journal of reproductive medicine. 2000;45:685–7. [PubMed] [Google Scholar]
- 9.Ross MG, Cedars L, Nijland MJ, Ogundipe A. Treatment of oligohydramnios with maternal 1-deamino-[8-D-arginine] vasopressin–induced plasma hypoosmolality. Am J Obstet Gynecol. 1996;174:1608–13. doi: 10.1016/s0002-9378(96)70615-4. [DOI] [PubMed] [Google Scholar]
- 10.Morad AWA, Abdelhamid AA. Nitric oxide donors for treatment of isolated oligohydramnios: A randomized controlled trial. Middle East Fertility Society Journal. 2018;23:310–4. [Google Scholar]
- 11.Fisk NM, Ronderos-Dumit D, Soliani A, Nicolini U, Vaughan J, Rodeck CH. Diagnostic and therapeutic transabdominal amnioinfusion in oligohydramnios. Obstet Gynecol. 1991;78:270–8. [PubMed] [Google Scholar]
- 12.Ülker K, Çiçek M. Effect of maternal hydration on the amniotic fluid volume during maternal rest in the left lateral decubitus position: a randomized prospective study. J Ultrasound Med. 2013;32:955–61. doi: 10.7863/ultra.32.6.955. [DOI] [PubMed] [Google Scholar]
- 13.da Silva Ferreira RD, Negrini R, Bernardo WM, Simoes R, Piato S. The effects of sildenafil in maternal and fetal outcomes in pregnancy: A systematic review and meta-analysis. PloS one. 2019;14:e0219732. doi: 10.1371/journal.pone.0219732. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Choudhary R, Desai K, Parekh H, Ganla K. Sildenafil citrate for the management of fetal growth restriction and oligohydramnios. Int J Womens Health. 2016;8:367–72. doi: 10.2147/IJWH.S108370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wareing M, Myers JE, O’Hara M, Baker PN. Sildenafil citrate (Viagra) enhances vasodilatation in fetal growth restriction. J Clin Endocrinol Metab. 2005;90:2550–5. doi: 10.1210/jc.2004-1831. [DOI] [PubMed] [Google Scholar]
- 16.Boyce EG, Umland EM. Sildenafil citrate: a therapeutic update. Clin Ther. 2001;23:2–23. doi: 10.1016/s0149-2918(01)80027-8. [DOI] [PubMed] [Google Scholar]
- 17.Villanueva-Garcia D, Mota-Rojas D, Hernandez-Gonzalez R, Sanchez-Aparicio P, Alonso-Spilsbury M, Trujillo-Ortega ME, et al. A systematic review of experimental and clinical studies of sildenafil citrate for intrauterine growth restriction and pre-term labour. J Obstet Gynaecol. 2007;27:255–9. doi: 10.1080/01443610701194978. [DOI] [PubMed] [Google Scholar]
- 18.Sharp A, Cornforth C, Jackson R, Harrold J, Turner MA, Kenny LC et al. Maternal sildenafil for severe fetal growth restriction (STRIDER): a multicentre, randomised, placebo-controlled, double-blind trial. Lancet Child Adolesc Health. 2018;2:93–102. doi: 10.1016/S2352-4642(17)30173-6. [DOI] [PubMed] [Google Scholar]
- 19.Dastjerdi MV, Hosseini S, Bayani L. Sildenafil citrate and uteroplacental perfusion in fetal growth restriction. J Res Med Sci. 2012;17:632–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25:341–8. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- 21.Kiran S, Ameen A, Akram A, Jamil M. Comparison of effects of oral maternal hydration and intravenous infusions on Amniotic Fluid Index in third trimester isolated Oligohydramnios. The Professional Medical Journal. 2019;26:2064–9. [Google Scholar]
- 22.Kwon JY, Kwon HS, Kim YH, Park YW. Abnormal Doppler velocimetry is related to adverse perinatal outcome for borderline amniotic fluid index during third trimester. J Obstet Gynaecol Res. 2006;32:545–9. doi: 10.1111/j.1447-0756.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 23.Asgharnia M, Faraji R, Salamat F, Ashrafkhani B, Dalil Heirati SF, Naimian S. Perinatal outcomes of pregnancies with borderline versus normal amniotic fluid index. Iran J Reprod Med. 2013;11:705–10. [PMC free article] [PubMed] [Google Scholar]
- 24.Ashwal E, Hiersch L, Melamed N, Aviram A, Wiznitzer A, Yogev Y. The association between isolated oligohydramnios at term and pregnancy outcome. Arch Gynecol Obstet. 2014;290:875–81. doi: 10.1007/s00404-014-3292-7. [DOI] [PubMed] [Google Scholar]
- 25.Patel PK, Pitre DS, Gupta H. Pregnancy outcome in isolated oligohydramnios at term. National Journal of Community Medicine. 2015;6:84–8. [Google Scholar]
- 26.Petrozella LN, Dashe JS, McIntire DD, Leveno KJ. Clinical significance of borderline amniotic fluid index and oligohydramnios in preterm pregnancy. Obstet Gynecol. 2011;117:338–42. doi: 10.1097/AOG.0b013e3182056766. [DOI] [PubMed] [Google Scholar]
- 27.Yenigul NN, Asicioglu O. The Effects of Isolated Oligohydramnios in Term Pregnancies on Labor, elivery Mode, and Neonatal Outcomes. Eurasian Journal of Medical Investigation. 2019;3:59–64. [Google Scholar]
- 28.Jamal A, Kazemi M, Marsoosi V, Eslamian L. Adverse perinatal outcomes in borderline amniotic fluid index. Int J Reprod Biomed. 2016;14:705–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Paauw ND, Terstappen F, Ganzevoort W, Joles JA, Gremmels H, Lely AT. Sildenafil during pregnancy: a preclinical meta-analysis on fetal growth and maternal blood pressure. Hypertension. 2017;70:998–1006. doi: 10.1161/HYPERTENSIONAHA.117.09690. [DOI] [PubMed] [Google Scholar]
- 30.Dunn L, Greer R, Flenady V, Kumar S. Sildenafil in pregnancy: a systematic review of maternal tolerance and obstetric and perinatal outcomes. Fetal Diagn Ther. 2017;41:81–8. doi: 10.1159/000453062. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira RDDS, Negrini R, Bernardo WM, Simões R, Piato S. The effects of sildenafil in maternal and fetal outcomes in pregnancy: A systematic review and meta-analysis. PLoS One. 2019;14:e0219732. doi: 10.1371/journal.pone.0219732. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]