Abstract
Triglyceride–glucose (TyG) index has been proposed to be a simple, economical, and reliable marker of insulin resistance. We aimed to investigate whether TyG is an independent predictor of hyperuricemia in diabetic kidney disease (DKD) populations by conducting a cross-sectional and longitudinal study. A total of 6,471 patients were enrolled in cross-sectional analysis, and 3,634 patients without hyperuricemia at the baseline were included in longitudinal analysis and were followed up for a median of 23.0 months. Hyperuricemia was categorized as a serum uric acid level ≥ 420 umol/L (7 mg/dL). In this study, 19.58% of participants had hyperuricemia. In the cross-sectional analysis, multivariate logistics regression analysis showed that the ORs (95% CI) for hyperuricemia in the second, third, and fourth TyG quartiles were 1.40 (95% CI 0.73–2.65), 1.69 (95% CI 0.90–3.18), and 4.53 (95% CI 2.39–8.57), respectively, compared with the first quartile. Longitudinally, the Kaplan–Meier survival analysis showed that higher TyG levels predicted higher incidence of hyperuricemia. Multivariate Cox regression model revealed that the hazard ratios for hyperuricemia in the upper quartiles of the TyG index were 1.69 (95% CI 0.97–2.93), 2.23 (95% CI 1.33–3.75), and 2.50 (95% CI 1.46–4.27), respectively, compared with the first quartile. Moreover, the subgroup analyses revealed that the relationship between TyG levels and hyperuricemia was robust in DKD patients. Our findings indicate a significant independent correlation between the TyG index and the risk of hyperuricemia in DKD patients.
Subject terms: Biomarkers, Endocrinology
Introduction
Hyperuricemia has become a global health problem due to an improving standard of living and rapid economic growth1,2. Recent epidemiological investigations have estimated the incidence of hyperuricemia in adults is approximately 21% in the United States3, 13.3% in mainland China4, 20% in the urban areas of Mexico5, and 25.8% in Japan6. It has been shown that hyperuricemia not only plays an important role in the development of gout, which can impair patient quality of life7, but is also an independent risk factor for many diseases, including hypertension8, type 2 diabetes9, dyslipidemia10, stroke11, chronic kidney disease12, and cardiovascular events13, which increase the risk of morbidity and mortality. However, due to the complexity of metabolic regulation, the pathogenesis of hyperuricemia remains unclear. Therefore, early identification of high-risk groups of hyperuricemia can enable them to receive early intervention, which is very meaningful for improving patient quality of life and alleviate the burden placed on the healthcare system and the economy.
The triglyceride-glucose (TyG) index is combination of fasting plasma glucose and triglycerides14, and can be used as a simple, economical, and reliable substitute maker of insulin resistance, compared with HOMA-IR15,16. Studies have shown that insulin resistance is a pathophysiological process that is closely associated with hyperuricemia17,and hyperinsulinemia caused by insulin resistance can cause renal urate excretion reduce, resulting in blood urate accumulation18. Recent studies have revealed the correlation between TyG index and hyperuricemia, and the correlation was stronger than that between obesity indices and hyperuricemia among general Chinese populations19–22. In addition, in a cross-sectional study, the TyG index and hyperuricemia were also positively correlated in hypertensive adults23.
Research has shown that patients with diabetes are more insulin resistant (IR) in the context of renal disease24. However, in patients with diabetic kidney disease (DKD), studies on the relationship between the TyG index and hyperuricemia are limited. Thus, the aim of our study was to explore the relationship between the TyG and the risk of hyperuricemia, after adjusting for known influencing factors, to evaluate the value of TyG for optimizing the risk stratification and prevention of hyperuricemia in the DKD population.
Results
Baseline characteristics
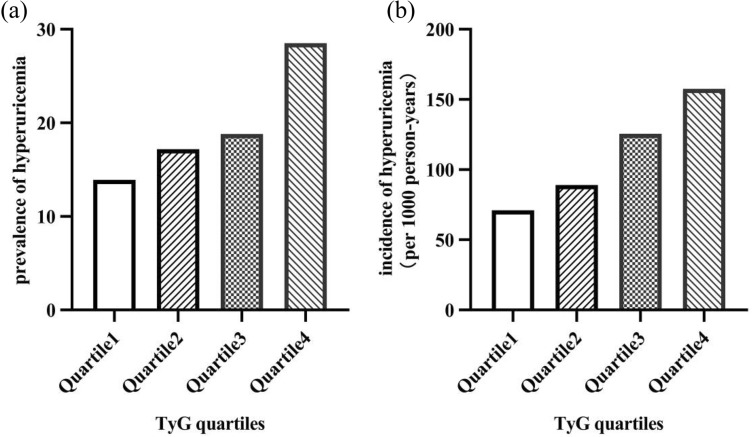
A total of 6,471 participants were enrolled in the cross-sectional study. The clinical characteristics of the participants were stratified using TyG quartiles, as shown in Table 1. The mean age at the baseline was 59.11 ± 10.53 years, and included 3,780 men (58.41%; age, 57.45 ± 10.99 years) and 2,691 women (41.59%; age, 61.44 ± 9.36 years). The prevalence of hyperuricemia was 19.58% (males, 23.99%; females, 13.38%). The prevalence of hyperuricemia was significantly increased with increasing TyG quartile (13.9%, 17.2%, 18.8%, and 28.5%, respectively, for the first, second, third, and fourth quartiles; P < 0.05 for the trend) (Fig. 1a). In comparison with patients with lower TyG levels, patients with higher TyG levels had higher DBP, BMI, HbA1c, TC, LDL-C, BUN, eGFR, Blood uric acid, 24hTP and ALT levels but lower levels of Bilirubin, HDL-C, and LDH (P < 0.05 for each).
Table 1.
Baseline characteristics of the subjects stratified using TyG quartiles.
| Characteristic | TyG quartiles | P value | |||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| N | 1604 | 1625 | 1636 | 1606 | |
| Age (y) | 61.38 ± 9.59 | 60.36 ± 9.57 | 59.32 ± 10.38 | 55.36 ± 11.48 | 0.000 |
| Male, n (%) | 990 (61.7) | 893 (55) | 922 (56.4) | 975 (60.7) | 0.000 |
| Hypertension, n (%) | 284 (76.1) | 363 (77.4) | 393 (73.5) | 487 (76.6) | 0.478 |
| SBP (mmHg) | 138.08 ± 24.33 | 140.07 ± 23.32 | 141.91 ± 20.52 | 140.43 ± 22.75 | 0.055 |
| DBP (mmHg) | 79.69 ± 13.30 | 81.42 ± 12.85 | 82.77 ± 12.31 | 83.53 ± 13.15 | 0.000 |
| BMI (kg/m2) | 26.41 ± 3.75 | 26.96 ± 3.86 | 27.60 ± 3.87 | 28.07 ± 4.11 | 0.000 |
| FPG (mmol/L) | 7.54 ± 1.80 | 9.02 ± 2.35 | 10.43 ± 2.92 | 13.05 ± 4.10 | 0.000 |
| HbA1c (%) | 7.45 ± 1.48 | 7.97 ± 1.60 | 8.51 ± 1.75 | 9.39 ± 1.87 | 0.000 |
| TG (mmol/L) | 0.99 (0.80–1.20) | 1.48 (1.25–1.75) | 2.02 (1.67–2.45) | 3.51 (2.62–5.16) | 0.000 |
| TC (mmol/L) | 4.73 ± 1.10 | 5.22 ± 1.17 | 5.49 ± 1.23 | 6.08 ± 1.79 | 0.000 |
| LDL-C (mmol/L) | 3.02 (2.43–3.60) | 3.39 (2.80–4.00) | 3.53 (2.86–4.16) | 3.43 (2.81–4.25) | 0.000 |
| HDL-C (mmol/L) | 1.37 ± 0.34 | 1.29 ± 0.28 | 1.23 ± 0.26 | 1.18 ± 0.24 | 0.000 |
| BUN (mmol/L) | 5.80 (4.84–7.14) | 5.80 (4.82–7.13) | 5.82 (4.77–7.12) | 6.01 (4.93–7.43) | 0.002 |
| SCR (umol/L) | 70.30 (57.50–86.68) | 69.10 (57.15–85.10) | 69.10 (57.00–86.18) | 69.70 (57.80–88.40) | 0.574 |
| eGFR (mL/min/1.73 m2) | 95.18 ± 30.22 | 95.20 ± 30.73 | 95.66 ± 31.68 | 98.36 ± 34.33 | 0.018 |
| SUA (umol/L) | 325.75 ± 91.31 | 337.82 ± 96.87 | 344.46 ± 97.55 | 374.36 ± 104.63 | 0.000 |
| Hyperuricemia, n (%) | 223 (13.9) | 279 (17.2) | 308 (18.8) | 457 (28.5) | 0.000 |
| TBIL (umol/L) | 12.50 (9.50–16.10) | 12.50 (9.80–16.50) | 12.30 (9.60–15.80) | 12.00 (9.15–15.20) | 0.001 |
| DBIL (umol/L) | 3.80 (2.70–5.30) | 3.50 (2.60–4.90) | 3.30 (2.38–4.60) | 3.00 (2.10–4.10) | 0.000 |
| AST (U/L) | 18.60 (15.60–22.60) | 18.90 (15.90–23.30) | 18.60 (15.50–23.80) | 18.60 (15.10–24.20) | 0.326 |
| ALT (U/L) | 18.20 (13.10–25.05) | 19.60 (14.20–27.60) | 20.30 (14.50–29.45) | 21.10 (14.60–31.95) | 0.000 |
| LDH (U/L) | 187.11 ± 47.88 | 181.56 ± 36.90 | 182.63 ± 41.85 | 175.98 ± 36.57 | 0.001 |
| ACR (mg/g) | 99.56(28.19–415.34) | 77.05(29.93–439.65) | 117.26(26.53–408.94) | 169.05 (51.80–464.05) | 0.007 |
| 24hTP (g/d) | 0.23(0.13–0.92) | 0.24(0.13–0.96) | 0.28(0.13–1.15) | 0.35(0.15–1.50) | 0.000 |
| TyG | 8.65 ± 0.29 | 9.24 ± 0.12 | 9.69 ± 0.15 | 10.56 ± 0.57 | 0.000 |
TyG triglyceride-glucose index; SBP systolic blood pressure; DBP diastolic blood pressure; BMI body mass index; FPG fasting plasma glucose; HbA1c glycated hemoglobin; TG plasma triglyceride level; TC total cholesterol; LDL-C low-density lipoprotein cholesterol; HDL-C high-density lipoprotein cholesterol; BUN blood urea nitrogen; SCR serum creatinine; eGFR estimated glomerular filtration rate; SUA serum uric acid; TBIL total bilirubin; DBIL direct bilirubin; AST aspartate aminotransferase; ALT alanine aminotransferase; LDH lactic dehydrogenase; ACR albumin-creatinine ratio; 24hTP 24 h total urine protein;
Data are presented as the mean ± standard error or median (interquartile range) for continuous variables, and the numbers (percentage) for categorical variables.
P < 0.05 was considered statistically significant.
Figure 1.
Relationship between baseline TyG and the hyperuricemia (a) the prevalence of hyperuricemia in the cross-sectional analysis, by baseline TyG quartile: < 9.02; quartile 2, 9.02–9.45 quartile 3, 9.45–9.96; quartile 4, ≥ 9.96; (b) the incidence of hyperuricemia ( per 1,000 person-years) in the cohort analysis, by baseline TyG quartile: quartile 1, < 9.00; quartile 2, 9.00–9.42; quartile 3, 9.42–9.90; quartile 4, ≥ 9.90;
The associations of the TyG index and hyperuricemia
We conducted multivariate logistic regression to show the odds ratios (ORs) and 95% CI for hyperuricemia based on the TyG index quartiles (Table 2). In unadjusted model (model 1), the ORs (95% CI) for hyperuricemia in the second, third, and fourth TyG quartiles were 1.28 (95% CI 1.06–1.55), 1.44 (95% CI 1.19–1.73), and 2.46 (95% CI 2.06–2.94), respectively, compared with the first quartile. After adjustment for age and sex (model 2), the ORs (95% CI) were 1.33 (95% CI 1.10–1.62), 1.47 (95% CI 1.21–1.78), and 2.40 (95% CI 1.99–2.88), respectively. After adjusting for age, sex, HDL-C, LDL-C, BMI, eGFR, SBP, DBP, 24hTP, HbA1c (model 3), the relationship was still significant, with ORs (95% CIs) of 1.40 (95% CI 0.73–2.65), 1.69 (95% CI 0.90–3.18), and 4.53 (95% CI 2.39–8.57), respectively. Our findings revealed that the TyG index and hyperuricemia were independently correlated in DKD patients.
Table 2.
Cross-sectional analysis of the association between the baseline TyG index and the incidence of hyperuricemia.
| Variables | Odds ratio (95% CI) | |||||
|---|---|---|---|---|---|---|
| Model1 | P value | Model 2 | P value | Model 3 | P value | |
| Quartiles of TyG | 0.000 | 0.000 | 0.000 | |||
| Quartile 1 | Reference | Reference | Reference | |||
| Quartile 2 | 1.28 (1.06–1.55) | 0.011 | 1.33 (1.10–1.62) | 0.003 | 1.40 (0.73–2.65) | 0.310 |
| Quartile 3 | 1.44 (1.19–1.73) | 0.000 | 1.47 (1.21–1.78) | 0.000 | 1.69 (0.90–3.18) | 0.105 |
| Quartile 4 | 2.46 (2.06–2.94) | 0.000 | 2.40 (1.99–2.88) | 0.000 | 4.53 (2.39–8.57) | 0.000 |
CI confidence interval.
Model 1: no adjustment; Model 2: adjusted for age, gender.
Model 3: adjusted for all the factors in model 1 and HDL-C, LDL-C, BMI, eGFR, 24hTP, SBP, DBP, HbA1c.
The associations of the TyG index and the incidence of hyperuricemia
During the median follow-up of 23.0 months (IQR 8.0, 49.25), 970 of the 3,634 participants developed hyperuricemia. We revealed that an increased TyG index was associated with an increased risk of hyperuricemia. The incidence of the hyperuricemia was 71.08 per 1000 person-years (20.0%), 89.15 per 1000 person-years (23.4%), 125.53 per 1000 person-years (28.9%), 157.63 per 1000 person-years (34.2%), respectively, for the first, second, third, and fourth quartiles. (P < 0.05 for the trend) (Fig. 1b).
The Kaplan–Meier survival analysis (Fig. 2) revealed that the risk of hyperuricemia was based on TyG quartiles category (P for log-rank test < 0.001). The lowest quartile of the 4 groups indicated the lowest level disease hazard for hyperuricemia, while the highest quartile indicated the highest level of disease hazard.
Figure 2.
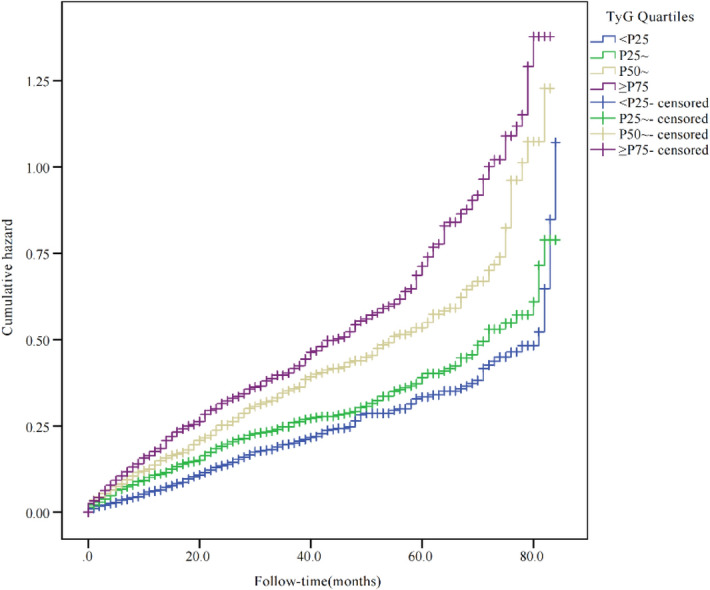
Kaplan–Meier curves for hyperuricemia by TyG quartile: quartile 1 (< 25), < 9.00; quartile 2 (25 ~), 9.00–9.42; quartile 3 (50 ~), 9.42–9.90; and quartile 4 (≥ 75), ≥ 9.90. P value < 0.05 for log-rank test, other quartiles versus first quartile.
We further used the multivariate Cox regression model to test the associations of the TyG index and the incidence of hyperuricemia in all models (Table 3). After full adjustment based on age, sex, LDL-C, HDL-C, BMI, eGFR, 24hTP, SBP, DBP, and HbA1c (model 3), the hazard ratios for hyperuricemia in the upper quartiles of the TyG index were 1.69 (95% CI 0.97–2.93), 2.23 (95% CI 1.33–3.75), and 2.50 (95% CI 1.46–4.27), respectively, compared with the first quartile.
Table 3.
Cohort analysis of the association between the baseline TyG index and the incidence of hyperuricemia.
| Variables | Hazard ratio (95% CI) | |||||
|---|---|---|---|---|---|---|
| Model 1 | P value | Model 2 | P value | Model 3 | P value | |
| Quartiles of TyG | 0.000 | 0.000 | 0.006 | |||
| Quartile 1 | Reference | Reference | Reference | |||
| Quartile 2 | 1.25 (1.03–1.53) | 0.028 | 1.29 (1.05–1.57) | 0.014 | 1.69 (0.97–2.93) | 0.062 |
| Quartile 3 | 1.80 (1.48–2.18) | 0.000 | 1.82 (1.50–2.21) | 0.000 | 2.23 (1.33–3.75) | 0.002 |
| Quartile 4 | 2.27 (1.88–2.73) | 0.000 | 2.32 (1.92–2.80) | 0.000 | 2.50 (1.46–4.27) | 0.001 |
CI confidence interval.
Model 1: no adjustment; Model 2: adjusted for age, gender.
Model 3: adjusted for all the factors in model 1 and HDL-C, LDL-C, BMI, eGFR, 24hTP, SBP, DBP, HbA1c.
Subgroup analyses
We further assessed the relationships of TyG index and the incidence of hyperuricemia stratified by age, sex, BMI, eGFR, SBP, and DBP (Fig. 3). No significant heterogeneity was found in the following stratified analysis, including age (< 50 vs. 50 ~ 60 vs. > 60 years), sex (Male vs. Female), BMI (< 24 vs.24 ~ 28 ≥ 28 kg/m2), eGFR (< 60 vs. ≥ 60 mL/min/1.73 m2), SBP (< 140 vs. ≥ 140 mmHg), and DBP (< 90 vs. ≥ 90 mmHg) (P values > 0.05 for all). The association between the TyG index and the incidence of hyperuricemia was consistent within all subgroups.
Figure 3.
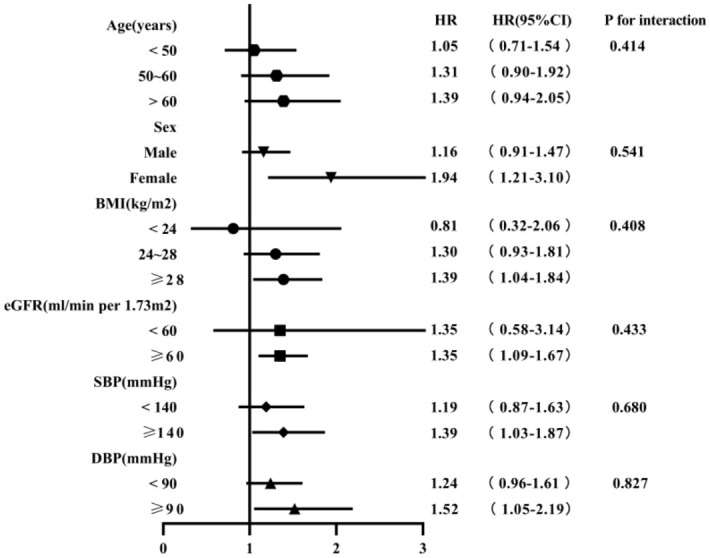
Stratified analyses of the association between the TyG index and the incidence of hyperuricemia. Adjusted for age, sex,HDL-C, LDL-C, BMI, eGFR, 24hTP, SBP, DBP and HbA1c.
Discussion
As far as we know, this is the first cross-sectional and longitudinal study to examine the relationship between TyG index and hyperuricemia in DKD populations. After adjusting for potential confounding factors of hyperuricemia, our study revealed that the TyG index could independently predict the incidence of hyperuricemia. In addition, our findings were robust across major demographic and clinical subgroups.
In recent years, researchers have explored the relationships between the TyG index and hyperuricemia in the general and hypertensive populations. Shi et al.21 performed cross-sectional studies on 6,466 subjects and revealed that TyG was positively related to hyperuricemia after adjusting for potential confounding factors. Kahaer et al.22 performed a cross-sectional analysis on 2,243 patients in a medical check-up population from Xinjiang, China, and the results revealed that the TyG index was more closely associate with hyperuricemia than obesity indices. Yu et al.23 performed a cross-sectional study on adults with hypertension, and showed that a higher level of TyG was associated with a higher risk of hyperuricemia. A cohort study conducted by Qing et al.19 on a general Chinese population found an increase in the risk of hyperuricemia for the upper TyG quartiles, after multivariate adjustment. Consistent with the results of the above-mentioned studies, our findings further revealed that TyG index levels are stable and independently related with the incidence of hyperuricemia in the DKD population through cross-sectional and longitudinal analysis. Taken together, these results suggest that the TyG index can predict hyperuricemia in a larger population as well.
Epidemiologically, it is widely known that the prevalence of hyperuricemia varies between different populations and geological locations, and may even vary a lot between different provinces25. According to our study, 19.58% of the participants had hyperuricemia, which is similar to that reported in T2DM populations in Tianjin (17.24%)26, Shanghai (18.7%)27 and in patients with early stage DKD (20.69%)28. Our study population was DKD patients not only accompanied by insulin resistance but also hyperinsulinemia due to insulin resistance and also included patients with impaired kidney function. Studies have shown that uric acid levels are dependent on purine production, excretion, and absorption29, and that 70% of daily UA production is eliminated by the kidney30. Thus, due to the effects of kidney injury on uric acid excretion in our study population, the prevalence of hyperuricemia should be higher than that of T2DM populations. However, in our study, the incidence of hyperuricemia was similar to that reported in previous studies conducted on T2DM populations in a geographically-matched rural area26,28. One of the reasons may be that we used newer diagnostic criteria that were higher than that used in previous studies. Another reason maybe that only a small section of our population (13.1%) had significantly impaired kidney function (eGFR < 60 mL/min/1.73 m2). There was a significant difference in hyperuricemia prevalence between men and women in our population, and the prevalence of hyperuricemia in males was nearly twice as high as in females, which is largely consistent with previous studies4.
The mechanism behind the association between the TyG index and hyperuricemia is still unclear. The TyG index is calculated from fasting plasma glucose and triglycerides, and has been proposed as a simple and reliable clinical surrogate marker for metabolic syndrome and insulin resistance31–33. Thus, a potential mechanism that associates the TyG index with hyperuricemia may involve insulin resistance. Studies has confirmed that glycolysis intermediates are transferred to 5-phosphoribose and phosphoric acid ribose pyrophosphate under insulin resistance, which leads to an increase in the production of serum uric acid34. Moreover, high levels of insulin caused by insulin resistance stimulate Na+-H+ exchange in renal tubules, increase H+ excretion, and increase uric acid reabsorption35, while the activation of the renin-angiotensin system by hyperinsulinemia decreases renal blood flow, increases urate reabsorption, and produces xanthine oxidase, which then leads to an increase in uric acid production36, while McCormick et al. provided robust evidence that insulin resistance has a positive causal effect on serum urate concentrations37. Another potential mechanism may be through blood glucose and lipid levels. On the one hand, it has been reported that hyperglycemia and hyperlipidemia decrease glyceraldehyde 3-phosphate dehydrogenase activity, which in turn increases UA synthesis28. On the other hand, TG can result in the renal small arteries to become stenotic or even occlusive as a result of long-term dyslipidemia, which ultimately cause urate excretion disorders. Studies have shown that the TG level is a independent and important risk factor for hyperuricemia38.
Our study has the following strengths. First, previous studies that reported on the associations of the TyG index and hyperuricemia were mostly cross-sectional. Our study is the first to provide a cross-sectional and longitudinal analysis to confirm an independent association between the TyG index and hyperuricemia, and included a relatively long follow-up period. Second, we were also the first study to investigate the relationship between the TyG index and hyperuricemia in the patients with DKD.
However, some limitations of this study should also be mentioned. First, although our model adjusted for many covariates, data on smoking, alcohol consumption, dietary habits, application of uric acid lowering drugs and other medications that may lower uric acid levels, duration of diabetes and socioeconomic factors, which are known to affect uric acid levels, were not available. Second, the sample size was relatively small. Third, all enrolled subjects were from a single center and only a Chinese population with DKD. Therefore, the findings of this research study may be influenced by a certain level of bias. Fourth, this is an observational study and as such cannot be used to confirm a causal relationship between the TyG index and hyperuricemia.
Conclusion
In summary, we showed a significant independent association of the TyG index and the risk of hyperuricemia in DKD patients. Along with other known risk factors, providing simpler and more economical options that can distinguish high-risk populations to implement early stage management may help reduce the occurrence of hyperuricemia and related diseases. In addition, rigorous clinical studies will be required to further explore the relationship and mechanism involved in the association of the TyG index and hyperuricemia.
Methods
Study population
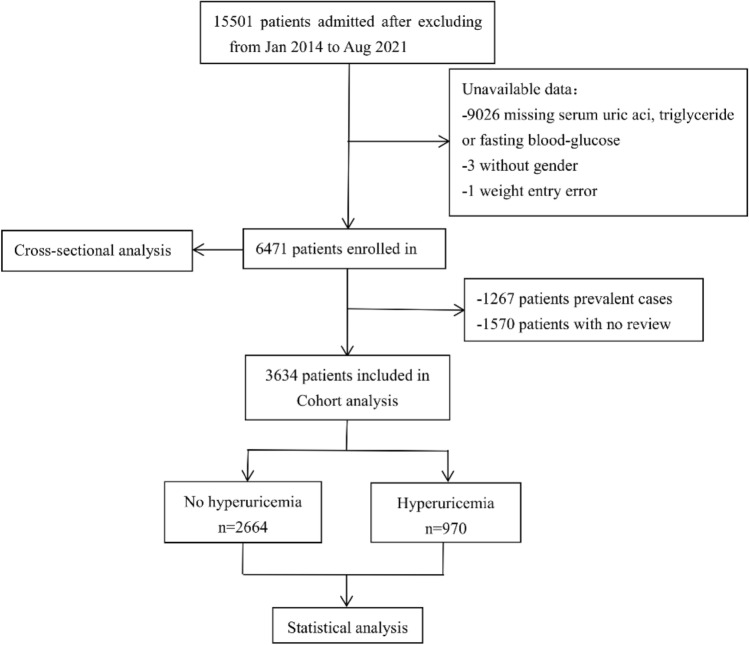
All participants were selected among patients admitted to the Tianjin Medical University Chu Hsien-I Memorial Hospital from January 2014 to August 2021. DKD patients aged over 18 years old were included in our present study. DKD was defined as eGFR < 60 mL/min/ 1.73 m2 or continuously increasing UACR (> 30 mg/g) over a period of 3 months in type 2 diabetes mellitus (T2DM) patients. The exclusion criteria used was : (1) patients with type 1 or other special types of diabetes; (2) hepatitis B, hepatitis C, or liver function test abnormalities (alanine aminotransferase or aspartate aminotransferase value 2.5 times higher the normal level); (3) Cushing’s syndrome, hyperthyroidism, or other endocrine metabolic diseases; (4) serious diseases, such as acute cardiovascular and cerebrovascular diseases or severe infections; (5) serious complications, such as diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome, or other acute metabolic disorders; (xi) pregnancy or a malignancy (xii) hypoglycemia (fasting plasma glucose (FPG) < 4 mmol/L, < 72 mg/dL). In this study, 15,501 participants were admitted after excluding. After further screening, a total of 6,471 patients were enrolled in the cross-sectional study and 3,634 patients were enrolled in the cohort study. The detailed research procedures for selecting participants was shown in Fig. 4. This study was complied with the Helsinki Declaration of 1964, as amended in 2008 and was approved by the Ethics Committee of Tianjin Medical University Chu Hsien-I Memorial Hospital.
Figure 4.
Flow chart of subject selection for this study.
Data collection and laboratory assays
Height, weight, and blood pressure were obtained through medical examination. BMI is calculated by dividing the body weight in kilograms by the height in meters squared. FPG, glycosylated hemoglobin (HbA1c), lipid profiles (triacylglycerol (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C)), liver function (aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactic dehydrogenase (LDH), total bilirubin (TBIL) and direct bilirubin (DBIL)) and renal function (blood urea nitrogen, and serum creatinine, serum uric acid (SUA), albumin-creatinine ratio (ACR), 24 h total urine protein(24hTP)) were evaluated through laboratory tests. All samples were processed at the Clinical Laboratory Center of Tianjin Medical University Chu Hsien-I Memorial Hospital. The estimated glomerular filtration rate (eGFR (mL/min/1.73 m2)) was calculated using the MDRD formula (eGFR = 186 × (Scr/88.4)−1.154 × (Age)−0.203 × (0.742 if female)). The TyG index was calculated as: TyG = ln [TG (mg/dL) × FPG (mg/dL)/2].
Definition of covariates
Hyperuricemia was defined as serum uric acid levels of ≥ 420 umol/L (7 mg/dL) in both men and women based on the guidelines for the diagnosis and treatment of hyperuricemia and gout in China. Hypertension was defined as systolic blood pressure (SBP) of ≥ 140 mmHg or diastolic blood pressure (DBP) of ≥ 90 mmHg, measured in a resting and sitting position, or a self-reported diagnosis, or current use of an antihypertension medications.
Statistical analysis
The baseline characteristics were showed as the mean ± standard deviation (SD) when the sample distribution was approximately normal. If the data showed a skewed distribution, the median and interquartile range (IQR) were used for continuous variables. Categorical variables were summarized as a number (percentage). Comparing baseline characteristics between different groups based on the TyG index quartiles was conducted using the analysis of variance (ANOVA) or Kruskal–Wallis test for continuous variables and was evaluated using chi-square (x2) tests for group variables, respectively. Multivariate logistic regression models (results presented as odds ratios (ORs) and 95% confidence interval (95% CI)) were performed to assess the relationship of TyG levels and hyperuricemia. For the time-to-event analyses, we used multivariate Cox proportional hazards regression to estimate the hazard ratios (HRs) and 95% CI of incident hyperuricemia using TyG quartiles. The cumulative risk of hyperuricemia among TyG quartiles was compared using the Kaplan–Meier survival. Furthermore, subgroup analyses were carried out to identify potential factors that could be used to evaluate the stability of our main findings. Potential interactions were tested by including interaction terms.
All data were analyzed using SPSS 24.0 software (SPSS Inc., Chicago, IL, USA), GraphPad Prism version 8.0 software (San Diego, CA, USA) and R software, version 4.2.1(http://www.R-project.org/). Two-tailed P value < 0.05 were considered statistical significance.
Ethics approval and consent to participate
This study was complied with the Helsinki Declaration of 1964, as amended in 2008 and was reviewed and approved by the ethics committee of Tianjin Medical University Chu Hsien-I Memorial Hospital. Verbal informed consent was obtained from each participant and was recorded by the physician who explained the study procedures. Written informed consent was waived because the data were anonymous and observational, which was approved by the ethics committee of Tianjin Medical University Chu Hsien-I Memorial Hospital.
Acknowledgements
The authors thank all subjects who took part in this study and have worked hard to ensure the reliability and accuracy of the data.
Author contributions
Q.L. prepared the figures and wrote the manuscript; Q.L., X.S., Z.C. and H.L. analyzed the data and performed the statistical analyses; P.Y. designed this study; Z.C., T.W., X.F. and S.Z. checked the data, S.Z. and P.Y. edited and revised the manuscript. All authors read and approved the final manuscript. Written informed consent for publication was obtained from all participants.
Funding
This study was supported by research grants as follows:1. China International Medical Exchange Foundation Key Fund Project No. Z-2017–26-1902; 2. Whitehorn Diabetes Research Fund Project No. G-X-2019–56; 3. Tianjin Municipal Health Care Commission Scientific Research Fund Project No. ZC20128; 4. Scientific Research Funding of Tianjin Medical University Chu Hsien-I Memorial Hospital No. ZXY-ZDSYSZD-1; 5. Tianjin Science and Technology Plan Project Public Health Science and Technology Major Special Project No. 21ZXGWSY00100; 6. Tianjin Health Research Project No. RC20175.
Data availability
The raw data are not available. However, the data are available from the corresponding author upon reasonable individual request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choi HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr. Opin. Rheumatol. 2010;22:165–172. doi: 10.1097/BOR.0b013e328335ef38. [DOI] [PubMed] [Google Scholar]
- 2.Rai SK, et al. The dietary approaches to stop hypertension (DASH) diet, western diet, and risk of gout in men: Prospective cohort study. BMJ. 2017;357:j1794. doi: 10.1136/bmj.j1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: The national health and nutrition examination survey 2007–2008. Arthritis Rheum. 2011;63:3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 4.Liu R, et al. Prevalence of hyperuricemia and gout in mainland china from 2000 to 2014: A systematic review and meta-analysis. Biomed. Res. Int. 2015;2015:762820. doi: 10.1155/2015/762820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meneses-Leon J, et al. Sweetened beverage consumption and the risk of hyperuricemia in mexican adults: A cross-sectional study. BMC Public Health. 2014;14:445. doi: 10.1186/1471-2458-14-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagahama K, et al. Hyperuricemia and cardiovascular risk factor clustering in a screened cohort in Okinawa Japan. Hypertens. Res. 2004;27:227–233. doi: 10.1291/hypres.27.227. [DOI] [PubMed] [Google Scholar]
- 7.Scire CA, et al. Gout impacts on function and health-related quality of life beyond associated risk factors and medical conditions: Results from the KING observational study of the Italian society for rheumatology (SIR) Arthritis Res. Ther. 2013;15:R101. doi: 10.1186/ar4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feig DI. Uric acid and hypertension. Semin. Nephrol. 2011;31:441–446. doi: 10.1016/j.semnephrol.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, et al. Two-year changes in hyperuricemia and risk of diabetes: A five-year prospective cohort study. J. Diabetes Res. 2018;2018:6905720. doi: 10.1155/2018/6905720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng TC, et al. Relationship between hyperuricemia and lipid profiles in US adults. Biomed. Res. Int. 2015;2015:127596. doi: 10.1155/2015/127596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Hou W, Zhang X, Hu L, Tang Z. Hyperuricemia and risk of stroke: A systematic review and meta-analysis of prospective studies. Atherosclerosis. 2014;232:265–270. doi: 10.1016/j.atherosclerosis.2013.11.051. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava A, Kaze AD, McMullan CJ, Isakova T, Waikar SS. Uric acid and the risks of kidney failure and death in individuals with CKD. Am. J. Kidney Dis. 2018;71:362–370. doi: 10.1053/j.ajkd.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto I, Moriya S, Kurozumi M, Namba T, Takagi Y. Relationship between serum uric acid levels and the incidence of cardiovascular events after percutaneous coronary intervention. J. Cardiol. 2021;78:550–557. doi: 10.1016/j.jjcc.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Li M, et al. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: The China H-type hypertension registry study. Cardiovasc. Diabetol. 2020;19:139. doi: 10.1186/s12933-020-01124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unger G, Benozzi SF, Perruzza F, Pennacchiotti GL. Triglycerides and glucose index: A useful indicator of insulin resistance. Endocrinol. Nutr. 2014;61:533–540. doi: 10.1016/j.endonu.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Garcia A, et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: A systematic review. Int. J. Endocrinol. 2020;2020:4678526. doi: 10.1155/2020/4678526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013;25:210–216. doi: 10.1097/BOR.0b013e32835d951e. [DOI] [PubMed] [Google Scholar]
- 18.Toyoki D, et al. Insulin stimulates uric acid reabsorption via regulating urate transporter 1 and ATP-binding cassette subfamily G member 2. Am. J. Physiol. Renal Physiol. 2017;313:F826–F834. doi: 10.1152/ajprenal.00012.2017. [DOI] [PubMed] [Google Scholar]
- 19.Gu Q, et al. Associations of triglyceride-glucose index and its derivatives with hyperuricemia risk: A cohort study in Chinese general population. Int. J. Endocrinol. 2020;2020:3214716. doi: 10.1155/2020/3214716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu XZ, Xu X, Zhu JQ, Zhao DB. Association between three non-insulin-based indexes of insulin resistance and hyperuricemia. Clin. Rheumatol. 2019;38:3227–3233. doi: 10.1007/s10067-019-04671-6. [DOI] [PubMed] [Google Scholar]
- 21.Shi W, Xing L, Jing L, Tian Y, Liu S. Usefulness of triglyceride-glucose index for estimating hyperuricemia risk: Insights from a general population. Postgrad. Med. 2019;131:348–356. doi: 10.1080/00325481.2019.1624581. [DOI] [PubMed] [Google Scholar]
- 22.Kahaer M, et al. Triglyceride glucose index is more closely related to hyperuricemia than obesity indices in the medical checkup population in Xinjiang China. Front. Endocrinol. (Lausanne) 2022;13:861760. doi: 10.3389/fendo.2022.861760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu C, et al. Positive association between the triglyceride-glucose index and hyperuricemia in Chinese adults with hypertension: An insight from the China H-type hypertension registry study. Int. J. Endocrinol. 2022;2022:4272715. doi: 10.1155/2022/4272715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: A systematic review. Am. J. Physiol. Renal Physiol. 2016;311:F1087–F1108. doi: 10.1152/ajprenal.00340.2016. [DOI] [PubMed] [Google Scholar]
- 25.Liu B, et al. The prevalence of hyperuricemia in China: A meta-analysis. BMC Public Health. 2011;11:1–10. doi: 10.1186/1471-2458-11-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei F, et al. Serum uric acid levels were dynamically coupled with hemoglobin A1c in the development of type 2 diabetes. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan D, et al. Uric acid is independently associated with diabetic kidney disease: A cross-sectional study in a Chinese population. PLoS ONE. 2015;10:e0129797. doi: 10.1371/journal.pone.0129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Z, et al. Renal impairment markers in type 2 diabetes patients with different types of hyperuricemia. J. Diabetes Investig. 2019;10:118–123. doi: 10.1111/jdi.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima WG, Martins-Santos ME, Chaves VE. Uric acid as a modulator of glucose and lipid metabolism. Biochimie. 2015;116:17–23. doi: 10.1016/j.biochi.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Maesaka JK, Fishbane S. Regulation of renal urate excretion: A critical review. Am. J. Kidney Dis. 1998;32:917–933. doi: 10.1016/s0272-6386(98)70067-8. [DOI] [PubMed] [Google Scholar]
- 31.Mohd Nor NS, Lee S, Bacha F, Tfayli H, Arslanian S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic-euglycemic clamp. Pediatr. Diabetes. 2016;17:458–465. doi: 10.1111/pedi.12303. [DOI] [PubMed] [Google Scholar]
- 32.Khan SH, et al. Metabolic clustering of risk factors: Evaluation of triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol. Metab. Syndr. 2018;10:74. doi: 10.1186/s13098-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R, et al. Clinical surrogate markers for predicting metabolic syndrome in middle-aged and elderly Chinese. J. Diabetes Investig. 2018;9:411–418. doi: 10.1111/jdi.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leyva F, Wingrove CS, Godsland IF, Stevenson JC. The glycolytic pathway to coronary heart disease: A hypothesis. Metabolism. 1998;47:657–662. doi: 10.1016/s0026-0495(98)90026-9. [DOI] [PubMed] [Google Scholar]
- 35.Tsunoda S, Kamide K, Minami J, Kawano Y. Decreases in serum uric acid by amelioration of insulin resistance in overweight hypertensive patients: Effect of a low-energy diet and an insulin-sensitizing agent. Am. J. Hypertens. 2002;15:697–701. doi: 10.1016/s0895-7061(02)02953-9. [DOI] [PubMed] [Google Scholar]
- 36.Katsiki N, Dimitriadis GD, Mikhailidis DP. Serum uric acid and diabetes: From pathophysiology to cardiovascular disease. Curr. Pharm. Des. 2021;27:1941–1951. doi: 10.2174/1381612827666210104124320. [DOI] [PubMed] [Google Scholar]
- 37.McCormick N, et al. Assessing the causal relationships between insulin resistance and hyperuricemia and gout using bidirectional mendelian randomization. Arthritis Rheumatol. 2021;73:2096–2104. doi: 10.1002/art.41779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, et al. Higher triglyceride level predicts hyperuricemia: A prospective study of 6-year follow-up. J. Clin. Lipidol. 2018;12:185–192. doi: 10.1016/j.jacl.2017.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data are not available. However, the data are available from the corresponding author upon reasonable individual request.