Dear Editor:
Acquired thrombotic thrombocytopenic purpura (aTTP) is caused by an autoantibody-mediated deficiency of a disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13), a von Willebrand factor (VWF)-cleaving protease. This alteration results in ultra-large circulating VWF multimers that cause platelet aggregation and systemic microvascular thrombi [1]. Patients with TTP frequently develop neurological manifestations caused by cerebral microcirculatory dysfunction, and several cases of status epilepticus (SE) secondary to TTP have been reported [2,3].
Perfusion-based magnetic resonance imaging (MRI) using arterial spin labeling (ASL) sequences has become a useful imaging tool for the clinical diagnosis of different diseases such as epilepsy, strokes, tumors, or dementia. Herein, we report the case of a 59-year-old man with refractory aTTP who developed SE in addition to severe thrombocytopenia and a marked decrease in ADAMTS13 activity due to a massive increase in ADAMTS13 inhibitor levels. In this patient, ASL was useful in diagnosing SE-related cerebral hyperperfusion and evaluating the perfusion normalization after treatment.
This patient was admitted to our hospital with shortness of breath and dizziness. His temperature was 37.5 °C; blood pressure, 109/74 mmHg; respiratory rate, 19 breaths/min; and heart rate, 94/min, and he was alert. The laboratory findings were as follows: hemoglobin, 6.3 g/dL; white blood cell count, 12,500/mm3; platelet count, 17,000/mm3; creatinine, 1.13 mg/dL; lactate dehydrogenase, 1429 U/L; indirect bilirubin, 5.28 mg/dL; prothrombin time-international normalized ratio, 1.16; and reticulocyte count, 21.7%. A peripheral blood smear revealed schistocytes. After hospitalization, his consciousness level decreased to 13 points (E4V4M5) on the Glasgow coma scale (GCS), but no other neurological deficits or abnormal findings were detected upon brain computed tomography (CT). The patient was diagnosed with aTTP based on the typical symptoms, with low (< 1.0%) ADAMTS13 activity and a positive test result for ADAMTS13 inhibitor (5.7 BU/mL). Immediate treatment with plasma exchange (PE) three times a week and prednisolone (1.5 mg/kg/day) helped to improve the patient's consciousness level and platelet count (79,000/mm3) by day 7. However, he became restless and aggressive, and the thrombocytopenia worsened (8000/mm3) again on day 10. An increase in ADAMTS13 inhibitor levels (22.0 BU/mL) after PE initiation supported the diagnosis of refractory aTTP, and weekly intravenous rituximab infusion at 375 mg/m2 was started from day 11. The patient's consciousness level decreased to 10 points (E3V3M4) on the GCS on day 15, although brain CT revealed no acute stroke lesions.
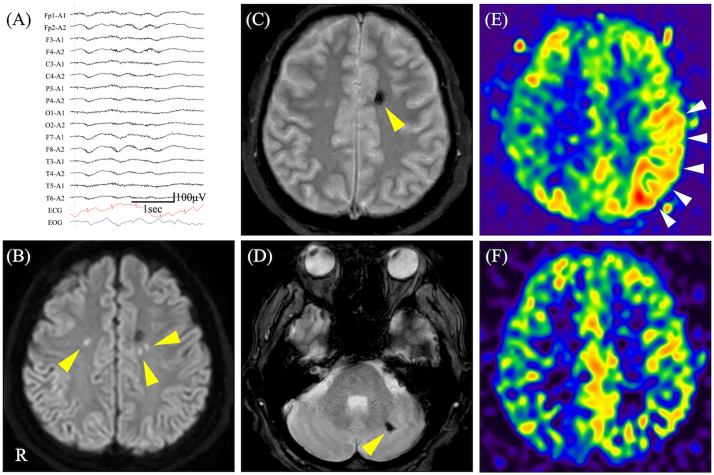
As the patient experienced repeated seizures consisting of right-arm tonic extension with secondary generalization, intravenous diazepam was administered on day 18. An electroencephalogram (EEG) showed moderately diffuse slowing with a low-amplitude background, without epileptiform activity (Fig. 1A). Brain MRI, including diffusion-weighted imaging and T2*-weighted imaging, showed only minor acute ischemic strokes and hemorrhages (Fig. 1B-D). However, ASL revealed cerebral hyperperfusion in the left parietal-temporal region (Fig. 1E). The patient was diagnosed with “new-onset refractory SE (NORSE)” [4] because the seizures were refractory to diazepam and levetiracetam (1000 mg/day); hence, we additionally started intravenous midazolam infusion (0.05 mg/kg/h). Daily PE in addition to rituximab and prednisolone gradually reduced the patient's seizures and normalized his consciousness level to 15 points (E4V5M6) on the GCS, followed by disappearance of the hyperperfusion upon ASL by day 25 (Fig. 1F). Consistent with his recovery from the neurological symptoms, blood test results were negative for ADAMTS13 inhibitor (< 0.5 BU/mL), and his blood counts returned to normal. After treatment with prednisolone alone was tapered to 0.25 mg/kg, ADAMTS13 inhibitor levels remained negative without relapse of aTTP-related symptoms.
Fig. 1.
Electroencephalogram (EEG), brain magnetic resonance imaging (MRI), and arterial spin labeling (ASL). (A) EEG on day 18 showed diffuse slowing with a low-amplitude background, without epileptiform activity. (B) Diffusion-weighted imaging on day 18 revealed small hyperintense areas in both parietal lobes (arrowheads). (C, D) T2*-weighted imaging on day 18 demonstrated small hypointense areas in the left parietal lobe and left cerebellar cortex (arrowheads). (E) ASL on day 18 revealed hyperperfusion in the left parietal lobe (arrowheads). (F) ASL on day 25 showed an absence of the hyperperfusion in the left parietal lobe. ECG, electrocardiogram; EOG, electrooculogram; R, right.
Thrombotic microangiopathy (TMA) is characterized by thrombocytopenia, microangiopathic hemolytic anemia, and organ injury. TTP, classified as a TMA, is associated with severe ADAMTS13 deficiency (< 10%) due to either a genetic defect in the ADAMTS13 gene or, in most cases, anti-ADAMTS13 autoantibodies (aTTP). Other TMAs (with ADAMTS13 activity >10%) include pathologies associated with underlying conditions, such as drug use, malignancies, systemic lupus erythematosus, malignant hypertension, transplantations, and pregnancy. Neurological involvement in TTP has been reported in 83% of patients [5]. Stroke is a major complication of TTP; however, stroke lesions upon radiological examinations often do not correlate with the neurological manifestations. Brief attacks of focal ischemia caused by microthrombi may cause transient and fluctuating neurological signs, such as an altered mental status [6]. However, several cases of patients with SE secondary to TTP have been reported [2,3]. A possible pathophysiological mechanism is endothelial dysfunction induced by microthrombi, causing blood-brain barrier (BBB) impairment, hyperperfusion, and vasogenic edema [7]. Furthermore, ADAMTS13 deficiency after ischemic damage results in BBB disruption, loss of cerebrovascular integrity, and impaired capillary perfusion [8]. Routine EEG alterations are rare in patients with TTP, and epileptic discharge is often undetectable [5]. Recently, perfusion-based ASL has become a useful imaging tool for the early clinical diagnosis of epilepsy. If routine EEG findings are inconclusive in patients with SE, ASL may help detect ictal and post-ictal hyperperfusion, even following antiepileptic treatment [9], with high sensitivity, for identification of the seizure source.
PE has typically been the first line of treatment for aTTP, often in combination with corticosteroid therapy; however, large increases in ADAMTS13 inhibitor titers frequently occur after PE initiation (“inhibitor boosting”) in patients with aTTP, rendering PE alone insufficient to control the disease [10]. In this case, although immediate PE helped to slightly lower the thrombocytopenia and consciousness disturbance, the patient relapsed with an increase in the ADAMTS13 inhibitor titers. Additional treatment with rituximab and management of SE with antiepileptic and anticonvulsive drugs allowed patient recovery without neurological sequelae.
The most frequently identified cause of NORSE among adult patients is autoimmunity, but vascular disorders have also been reported as the cause [4]. The clinical course of our patient was consistent with a diagnosis of NORSE, which often requires immunotherapy in addition to antiepileptics.
To our knowledge, this is the first report on the usefulness of ASL for aberrant cerebral hyperperfusion in a patient with SE secondary to aTTP. We should be aware of possible SE in a patient with aTTP, and brain MRI including ASL may be beneficial for the diagnosis and treatment of patients with impaired consciousness.
Ethical standards
Informed consent was obtained from the patient for publication of this case report and accompanying images.
Study funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributions
Tomoya Shibahara, MD, PhD; Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; Study concept or design; analysis or interpretation of data.
Keiji Sakamoto, MD, PhD; Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; Study concept or design; analysis or interpretation of data.
Fumitaka Yoshino, MD; Drafting/revision of the manuscript for content, including medical writing for content; Study concept or design; analysis or interpretation of data.
Mikiaki Matsuoka, MD; Drafting/revision of the manuscript for content, including medical writing for content; Study concept or design; analysis or interpretation of data.
Masaki Tachibana, MD, PhD; Drafting/revision of the manuscript for content, including medical writing for content; Study concept or design; analysis or interpretation of data.
Kenjiro Kamezaki, MD, PhD; Drafting/revision of the manuscript for content, including medical writing for content.
Junya Kuroda, MD, PhD; Drafting/revision of the manuscript for content, including medical writing for content; Study concept or design; analysis or interpretation of data.
Mika Kuroiwa, MD, PhD; Drafting/revision of the manuscript for content, including medical writing for content.
and Hiroshi Nakane, MD, PhD; Drafting/revision of the manuscript for content, including medical writing for content.
Declaration of Competing Interest
None.
Acknowledgments
We thank Editage (www.editage.com) for the English language editing.
References
- 1.Tsai H.M., Lian E.C. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N. Engl. J. Med. 1998;339:1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrett W.T., Chang C.W., Bleck T.P. Altered mental status in thrombotic thrombocytopenic purpura is secondary to nonconvulsive status epilepticus. Ann. Neurol. 1996;40:245–246. doi: 10.1002/ana.410400218. [DOI] [PubMed] [Google Scholar]
- 3.Beydoun A., Vanderzant C., Kutluay E., Drury I. Full neurologic recovery after fulminant thrombotic thrombocytopenic purpura with status epilepticus. Seizure. 2004;13:549–552. doi: 10.1016/j.seizure.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Lattanzi S., Leitinger M., Rocchi C., Salvemini S., Matricardi S., Brigo F., Meletti S., Trinka E. Unraveling the enigma of new-onset refractory status epilepticus: a systematic review of aetiologies. Eur. J. Neurol. 2022;29:626–647. doi: 10.1111/ene.15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirouse A., Legriel S., Dumas G., Labro G., Veyradier A., Zafrani L., Valade S., Hourmant Y., Boutboul D., Darmon M., Coppo P., Mariotte E., Azoulay E. Pattern of brain injury in patients with thrombotic thrombocytopenic purpura in the precaplacizumab era. Crit. Care Med. 2021;49:e931–e940. doi: 10.1097/CCM.0000000000005164. [DOI] [PubMed] [Google Scholar]
- 6.Meloni G., Proia A., Antonini G., De Lena C., Guerrisi V., Capria S., Trisolini S.M., Ferrazza G., Sideri G., Mandelli F. Thrombotic thrombocytopenic purpura: prospective neurologic, neuroimaging and neurophysiologic evaluation. Haematologica. 2001;86:1194–1199. [PubMed] [Google Scholar]
- 7.Burrus T.M., Wijdicks E.F., Rabinstein A.A. Brain lesions are most often reversible in acute thrombotic thrombocytopenic purpura. Neurology. 2009;73:66–70. doi: 10.1212/WNL.0b013e3181aaea1b. [DOI] [PubMed] [Google Scholar]
- 8.Xu H., Cao Y., Yang X., Cai P., Kang L., Zhu X., Luo H., Lu L., Wei L., Bai X., Zhu Y., Zhao B.Q., Fan W. ADAMTS13 controls vascular remodeling by modifying VWF reactivity during stroke recovery. Blood. 2017;130:11–22. doi: 10.1182/blood-2016-10-747089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim T.J., Choi J.W., Han M., Kim B.G., Park S.A., Huh K., Choi J.Y. Usefulness of arterial spin labeling perfusion as an initial evaluation of status epilepticus. Sci. Rep. 2021;11:24218. doi: 10.1038/s41598-021-03698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isonishi A., Bennett C.L., Plaimauer B., Scheiflinger F., Matsumoto M., Fujimura Y. Poor responder to plasma exchange therapy in acquired thrombotic thrombocytopenic purpura is associated with ADAMTS13 inhibitor boosting: visualization of an ADAMTS13 inhibitor complex and its proteolytic clearance from plasma. Transfusion. 2015;55:2321–2330. doi: 10.1111/trf.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]