Summary
Background
The effects of socio-economic status on mortality in patients with multiple sclerosis is not well known. The objective was to examine mortality due to multiple sclerosis according to socio-economic status.
Methods
A retrospective observational cohort design was used with recruitment from 18 French multiple sclerosis expert centers participating in the Observatoire Français de la Sclérose en Plaques. All patients lived in metropolitan France and had a definite or probable diagnosis of multiple sclerosis according to either Poser or McDonald criteria with an onset of disease between 1960 and 2015. Initial phenotype was either relapsing-onset or primary progressive onset. Vital status was updated on January 1st 2016. Socio-economic status was measured by an ecological index, the European Deprivation Index and was attributed to each patient according to their home address. Excess death rates were studied according to socio-economic status using additive excess hazard models with multidimensional penalised splines. The initial hypothesis was a potential socio-economic gradient in excess mortality.
Findings
A total of 34,169 multiple sclerosis patients were included (88% relapsing onset (n = 30,083), 12% progressive onset (n = 4086)), female/male sex ratio 2.7 for relapsing-onset and 1.3 for progressive-onset). Mean age at disease onset was 31.6 (SD = 9.8) for relapsing-onset and 42.7 (SD = 10.8) for progressive-onset. At the end of follow-up, 1849 patients had died (4.4% for relapsing-onset (n = 1311) and 13.2% for progressive-onset (n = 538)). A socio-economic gradient was found for relapsing-onset patients; more deprived patients had a greater excess death rate. At thirty years of disease duration and a year of onset of symptoms of 1980, survival probability difference (or deprivation gap) between less deprived relapsing-onset patients (EDI = −6) and more deprived relapsing-onset patients (EDI = 12) was 16.6% (95% confidence interval (CI) [10.3%–22.9%]) for men and 12.3% (95%CI [7.6%–17.0%]) for women. No clear socio-economic mortality gradient was found in progressive-onset patients.
Interpretation
Socio-economic status was associated with mortality due to multiple sclerosis in relapsing-onset patients. Improvements in overall care of more socio-economically deprived patients with multiple sclerosis could help reduce these socio-economic inequalities in multiple sclerosis-related mortality.
Funding
This study was funded by the ARSEP foundation “Fondation pour l'aide à la recherche sur la Sclérose en Plaques” (Grant Reference Number 1122). Data collection has been supported by a grant provided by the French State and handled by the “Agence Nationale de la Recherche,” within the framework of the “Investments for the Future” programme, under the reference ANR-10-COHO-002, Observatoire Français de la Sclérose en Plaques (OFSEP).
Keywords: Multiple sclerosis, Excess mortality, Net survival, Socio-economic status, Flexible model, Observational cohort study
List of abbreviations: AIC, Akaike Information Criteria; CI, confidence interval; DMT, disease modifying therapy; EDR, excess death rate; MS, multiple sclerosis; OFSEP, Observatoire Français de la Sclérose en Plaques; PPMS, primary progressive multiple sclerosis; R-MS, relapsing onset multiple sclerosis; SES, socio-economic status
Research in context.
Evidence before this study
We searched PubMed for articles published until May 1, 2022, using the following search terms: (“multiple sclerosis” [Title/Abstract]) AND (“socioeconomic” [Title/Abstract] OR “socio-economic” [Title/Abstract]) AND (“survival” [Title/Abstract] OR “mortality” [Title/Abstract]). The search was not restricted by date or language, and retrieved 36 articles. Five articles were unrelated to the topic. One article was a review. 26 articles did not study survival in people with MS in relation to socio-economic status. Four studies examined the association between mortality in patients with MS and socio-economic status. Three of the four studies only examined all-cause mortality, and not excess mortality. The one article studying relative survival found a higher mortality risk for patients with a higher socio-economic status. However, this study focussed on a cohort of veterans and did not use population-based data.
Added value of this study
Our study is to our knowledge the first to investigate the association between socio-economic status and excess mortality in an observatory-based MS cohort. The socio-economic indicator used is a comprehensive measure of the neighbourhood level socio-economic environment using ten weighted variables. The cohort included a large number of patients with both relapsing-onset MS and primary progressive MS, and a large number of both men and women. For patients with relapsing-onset MS, a lower socio-economic status was associated with a higher excess death rate than for less socio-economically disadvantaged patients. This deprivation gap was apparent from the first decade from disease onset. The study of excess mortality is paramount to avoid highlighting the existing differences in all-cause mortality in the general population, with more disadvantaged people having a shorter life expectancy than more socio-economically advantaged people. The differences found in our study account only for deaths due to MS, directly or indirectly whilst bypassing the need to have knowledge of the cause of death.
Implications of all the available evidence
This study reveals the deprivation gap in excess mortality in a large cohort of patients with MS. Our findings highlight the need for focus on equality in health outcomes for both policy makers and clinicians in day-to-day practice when treating patients with MS. Measures such as patient navigators or longer and/or more frequent consultations with both the patient's general practitioner and their neurologist to ensure correct understanding of treatment plans could be beneficial. Further research is needed to establish the fields in which these inequalities are the most present.
Introduction
Multiple sclerosis (MS) affects 2.8 million people worldwide with reduced life expectancy for MS patients by 6–14 years compared to the general population matched for age and sex.1, 2, 3, 4 Few risk factors for mortality in MS are known, the main ones being the age at MS onset and sex, both non-modifiable risk factors.5,6 Inconclusive results regarding risk of MS development and socio-economic factors have been found so far.7 Magyari et al. found no effect of educational level, occupational exposures or housing conditions in youth using individual level data on risk of developing MS.8 The most conclusive results so far are from Scandinavian registries which provide individual level data on MS, comorbidities, mortality and socio-economic data.9,10 Socio-economic deprivation has been linked to shorter survival probabilities in several chronic diseases including cancer and diabetes.11,12 This association has been studied in MS with time to disability using the Expanded Disability Status Scale (EDSS). Calocer et al. and Harding et al. both showed that patients with a lower socio-economic status (SES) had a higher risk of reaching EDSS milestones than less deprived patients.13,14
Previous studies have used all-cause mortality to study the influence of SES on mortality in MS patients.3,15 However, it is impossible to know whether the differences in mortality between lower and higher SES result from mortality due to MS or from differences in other-cause mortality, since lower SES is associated with shorter life expectancy in the general population.16 Mortality due to MS can either be estimated by disease-specific mortality framework, requiring knowledge of cause of death, or by excess hazard framework, where survival of the study population is compared to that of the general population matched by demographic variables.
In disease-specific mortality framework, obtaining cause of death can be difficult due to unavailable or sometimes unreliable data.3,17,18 In contrast, excess hazard framework avoids the prerequisite of knowing cause of death by considering that mortality due to MS can be estimated by the excess mortality observed between two populations. In this framework, a way to account for the expected mortality is to use an additive model, which assumes that the mortality rate of MS patients is the sum of their expected mortality rate and of an excess mortality rate due to the disease.19 Excess mortality can be used to derive net survival, which is the survival that would be observed if the disease studied were the only possible cause of death in the population.20 To our knowledge, no research on socioeconomic predictive factors has been published so far studying excess mortality of MS patients from the general population.
The objective of this study was to examine mortality due to MS according to socio-economic deprivation using excess hazard framework and additive models to estimate excess mortality.
Material and methods
Standard protocol approvals, registration, and patient consents
Patients enrolled in OFSEP (registered to clinicaltrials.gov [NCT04028232]) provide written informed consent for participation. In accordance with the French legislation, the present study was approved by both the national data protection agency (Commission Nationale Informatique et Libertés [CNIL]; approval DR-2019-132) and the French expert committee for research, studies and evaluations on health (Comité d'expertise pour les recherches, les études et les évaluations dans le domaine de la santé [CEREES]; approval TPS 216966).
Study population
The data was provided from a retrospective cohort from 18 of the 36 French MS expert centers participating in the French MS database “Observatoire Français de la Sclérose En Plaques (OFSEP www.ofsep.org)”.5,21 The 18 centers were selected for their inclusion of patients for serveal decades, allowing sufficient follow-up to study mortality. For each patient, a neurologist from each center entered clinical and imaging data during routine follow-up visits using dedicated software (European Database on Multiple Sclerosis [EDMUS]).22 The same cohort has been used for a previous study on excess mortality in MS, using the same data and the same inclusion criteria.5 These data were retrospectively collected at the time of the first visit after the center's systematic registration date, and prospectively thereafter, in other words ambispectively. Because a patient has to be alive to attend a visit, the retrospective follow-up before this first visit should not be considered since no death can be observed during this follow-up. To deal with this left truncation, the entry time in the cohort was thus set at the first visit posterior to the center's systematic registration date.
Socio-economic indicator
Socio-economic deprivation was based on the patient's address as available in the OFSEP database, geolocated by the Geographic Information System (GIS ARCGIS 10.2®), which was then assigned to an “Ilot Regroupé pour l’Information Statistique (IRIS)”, the smallest French geographic unit for which census data are available. Each patient's IRIS was then attributed to an EDI (European Deprivation Index) score, an ecological deprivation index based on 10 weighted variables (low level of education, no access to a car, overcrowding, no access to a system of central or electric heating, non-owner, unemployment, foreign nationality, unskilled worker – farm worker, household with more than six persons, single-parent household).23 This score was kept as a continuous variable ranging from −11 to +32 (median value: −0.6); the higher the index, the greater the deprivation in the IRIS. The 2011 version of the EDI score was used.
Mortality in the cohort and in the general population
Linkage to the National Repertory for the Identification of Physical Persons (“Répertoire National d’Identification des Personnes Physiques” (RNIPP)) was performed at an individual level by sex, surname or maiden name for women, date and place of birth and vital status update was obtained for 92.2% of patients. Deaths that occurred between 1976, the minimum date of systematic registration and 2016 were registered, and follow-up was censored beyond 30 years. Censoring at 30 years was performed in order to limit the time-frame between the attribution of EDI scores (2011) and address collection (possibly dating back to 1976) whilst allowing for sufficient follow-up time for a survival study. Patients were followed from MS onset (symptom onset), until death, last clinical visit, the maximum of 30 years of follow-up or end-of-study date, whichever occurred first, defining disease duration or time since disease onset. Patients without any follow-up data (n = 1116) or EDI data (n = 3355) were excluded, leading to a study population of 34,169 patients. Information regarding initial MS phenotype, age and year at MS onset and sex was present in the database. The method used to obtain all-cause mortality rates is described in the study on the same cohort by Rollot et al.5
Statistical analyses
The additive framework used is based on the following: the observed mortality rate due to MS (h0) is defined as the excess mortality due to MS (hE) added to the expected death rate of the general French population (hP):
These mortality rates are defined for a given time, and for each individual according to their age at MS onset, and matched to the mortality rate of the general population by sex, age and year at death and area of residence (“Département”). Excess death rate (hE) (EDR) is then considered to be mortality due to MS, whether directly or indirectly, and is expressed in number of deaths per 1 person-year. When EDR is <0.10, its value is close to the probability of death within 1 year. For example, a rate of 0.02 death per person-year over 1 year can be approximated to a probability of death of 2% within the year. Net survival is then derived from the EDR, and is based upon the hypothesis that mortality due to MS is negligible in the general population.
Multidimensional splines were used to model the logarithm of the EDR, allowing for flexible dynamics of the EDR by accounting for potential non-linear and non-proportional effects of covariables.24,25 The risk of over-fitting of the model was reduced by using penalized one-dimensional splines and penalized tensor product splines (“tensors”) for interactions.
Models were computed separately for each initial phenotype of the disease, based on the clinical differences between the two phenotypes. They were constructed step by step by adding the covariates of interest and potential interactions, and the model with the lowest corrected Akaike information criterion (AIC) was selected as the best fitting model, considering a difference of at least 4 units between two AIC values.26 The first model (M1) was constructed following the results of previous work, then the second model (M2) was obtained by adding year of onset of symptoms (allowing for non-linearity).5 The third model (M3) was constructed by adding EDI to the first model (M1) also allowing for non-linearity. The fourth model (M4) was constructed by adding both EDI and year of onset of symptoms (allowing for non-linearity). The fifth model (M5) was obtained by adding a non-proportional effect of EDI to the third model (M3). The sixth model (M6) was constructed by adding an interaction term between EDI and year of onset of symptoms. Age at onset and year of onset of symptoms were centred. Six models were obtained:
The term s stands for a one-dimensional penalised spline and the term tensor for a penalized tensor product spline. We used 6 knots for time (p0, p20, p40, p60, p80 and p100) and 5 knots for age at onset, EDI and year of onset of symptoms (p0, p2, p50, p75, p100). The locations of these knots corresponded to the percentiles of the distribution of time and age among deceased patients, and the overall percentiles of the distribution of EDI and year of onset of symptoms in the study population. A reading guide for model building strategy is available in Supplemental Materials.
If M1 was selected, this meant that the effects of EDI and year of onset of symptoms on EDR were considered non-significant. If M2 was selected, the effect of year of onset of symptoms on the EDR was considered significant and proportional (potentially non-linear). If M3 was selected, the effect of EDI was considered significant and proportional (potentially non-linear) If M4 was selected, the effects of EDI and year of onset of symptoms were considered significant and proportional (potentially non-linear). If M5 was selected, the effect of EDI was considered significant and time-dependant. If M6 was selected, the effect of year of onset of symptoms depended on the EDI score. The effects of EDI and year of onset of symptoms were considered to be non-linear if the effective degrees of freedom of the splines s (EDI) or s (year of onset of symptoms) were higher than 1.27
Testing for an interaction between sex and EDI was also performed.
In order to allow a two-dimensional representation of results, values of the variables sex, age at onset, year of onset of symptoms and EDI had to be set. Graphs were constructed for an age at onset of 30 years for R-MS patients, approximately the median age at onset, and for years 1980 and 1990 for onset of symptoms, in order to account for two different periods of MS care (before and after introduction of effective treatments) whilst allowing for sufficient follow-up for a survival study. Curves for percentiles 2.5, 97.5 and median value of EDI were shown. The statistical significance of these variables did not depend on these values chosen for graphical representation.
The deprivation gap, corresponding to the difference in net survival between least deprived and most deprived patients, was calculated. The confidence interval of this deprivation gap was obtained using the Delta method (online-only supplements eMethods (Delta method)). The deprivation gap was considered to be statistically significant if the associated 95% confidence interval did not include 0.
Socioeconomic position has an influence on mortality in the general population, however French life tables provided by INSEE are not stratified on deprivation.16 The EDR obtained with the excess hazard additive models may be due to socially determined comorbidities, resulting in an overestimation of the effect of EDI on EDR. Therefore, a sensitivity analysis was led using a mortality table containing data on SES (online-only supplements eMethods (Sensitivity analysis)).
All analyses were performed using R software (4.1.2) with the “survPen” package (1.0.1).28
Role of the funding sources
This study was funded by the ARSEP foundation “Fondation pour l'aide à la recherche sur la Sclérose en Plaques” (Grant Reference Number 1122). Data collection has been supported by a grant provided by the French State and handled by the “Agence Nationale de la Recherche,” within the framework of the “Investments for the Future” programme, under the reference ANR-10-COHO-002, Observatoire Français de la Sclérose en Plaques (OFSEP). The funding sources had no role in the writing of the manuscript or the decision to submit it for publication.
Results
R-MS accounted for 88% of the total number, and PPMS for 12%. Table 1 shows the characteristics of the study population, overall and according to initial phenotype.
Table 1.
Description of vital status, sex, EDI score and age at onset of MS overall and according to MS phenotype.
| Overall (n = 34,169) n (%) | R-MS (n = 30,083) n (%) | PPMS (n = 4086) n (%) | |
|---|---|---|---|
| Vital status as of 2016/01/01 | |||
| Deceased | 1849 (5.4%) | 1311 (4.4%) | 538 (13.2%) |
| Lost to follow-up | 2350 (6.9%) | 2054 (6.8%) | 296 (7‧2%) |
| Alive | 29,970 (87.7%) | 26,718 (88.8%) | 3252 (79.6%) |
| Sex | |||
| Men | 9848 (28.8%) | 8057 (26.8%) | 1791 (43.8%) |
| Women | 24,321 (71.2%) | 22,026 (73.2%) | 2295 (56.2%) |
| EDI | |||
| Median [percentiles 2.5; 97.5] | −0.6 [−5.6; 11.9] | −0.6 [−5.6; 11.8] | −0.6 [−5.5; 12.6] |
| Age at MS onset | |||
| Median [quartiles 1–3] | 31.7 [24.9–40.0] | 30.4 [24.3–38.1] | 43.2 [35.0–50.3] |
| Year of onset of symptoms | |||
| [1960–1970] | 655 (1.9%) | 602 (2.0%) | 53 (1.3%) |
| [1970–1980] | 2114 (6.2%) | 1926 (6.4%) | 188 (4.6%) |
| [1980–1990] | 5025 (14.7%) | 4449 (14.8%) | 576 (14.1%) |
| [1990–2000] | 9917 (29.0%) | 8628 (28.7%) | 1289 (31.6%) |
| [2000–2010] | 12,155 (35.6%) | 10,545 (35.1%) | 1610 (39.4%) |
| [2010–2015] | 4303 (12.6%) | 3933 (13.1%) | 370 (9.1%) |
| Follow-up (years) | |||
| Median [quartiles 1–3] | 15.7 [9.1–23.7] | 15.8 [9.0–24.0] | 15.2 [9.6–22.0] |
EDR dynamics according to EDI for R-MS phenotype
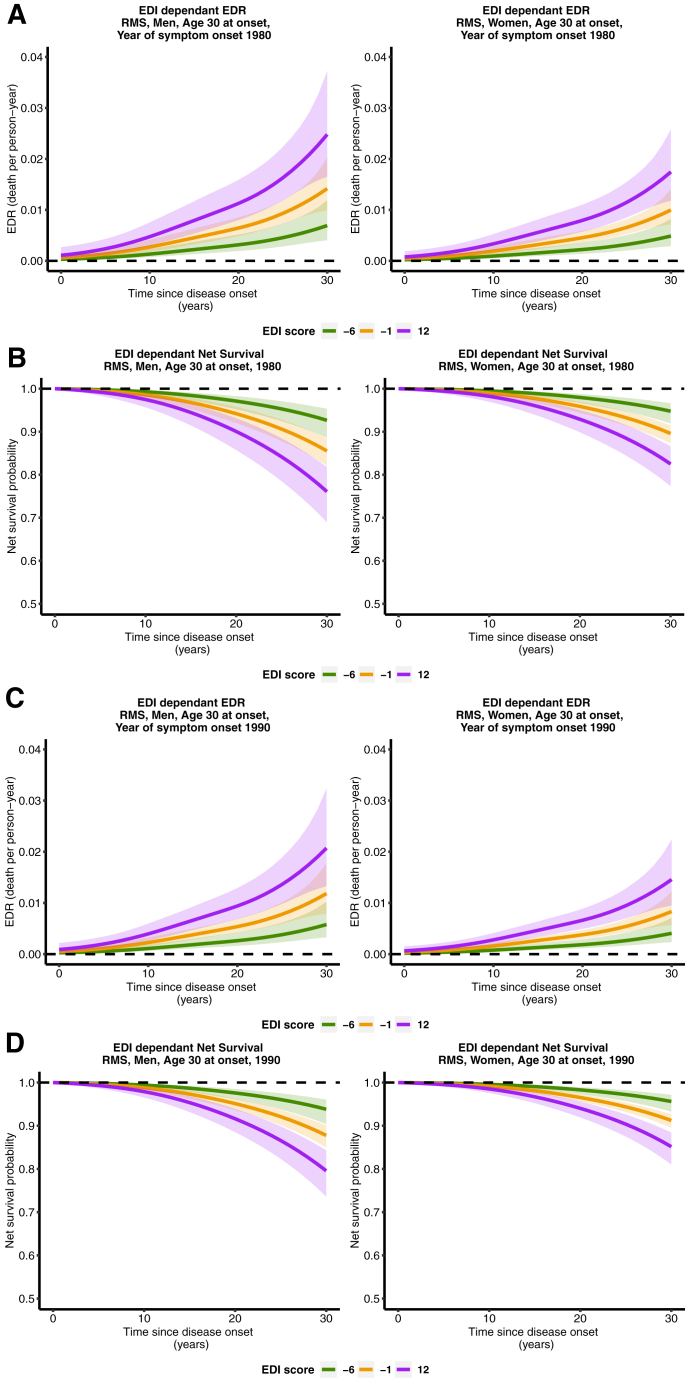
Model 4 was selected for R-MS patients (Table 2). EDI and year of onset of symptoms were associated with EDR, with a non-linear effect (since the effective degrees of freedom for the spline for EDI and for year of onset of symptoms (M4) were >1). For an age at MS onset of 30 years, EDR was higher for patients with higher EDI scores (more deprived patients), and this dynamic was observed from the start of disease onset (Fig. 1A and 1C). EDR for EDI scores −6 (least deprived) and −1 (median) became apparent around 10 years since disease onset. At thirty years of disease duration, for a year of onset of symptoms of 1980, EDR for least deprived R-MS patients (EDI = −6) was 0.7 deaths per 100 person-years for men, and 0.5 for women. For more deprived R-MS patients (EDI = 12), EDR was 2.5 deaths per 100 person-years for men and 1.7 for women. For the same parameters and a year of onset of 1990, EDR for least deprived R-MS patients (EDI = −6) was 0.6 deaths per 100 person-years for men, and 0.4 for women. For more deprived R-MS patients (EDI = 12), EDR was 2.1 deaths per 100 person-years for men and 1.6 for women.
Table 2.
| Model | Formula | Number of regression parameters | Number of smoothing parameters | Effective degrees of freedom | Corrected Akaike Information Criteria |
|---|---|---|---|---|---|
| R-MS | |||||
| M1 | tensor(t,age) + sex | 31 | 2 | 13.27 | 13,826.47 |
| M2 | tensor(t,age) + sex + s2(year of onset of symptoms) | 35 | 3 | 16.10 | 13,811.33 |
| M3 | tensor(t,age) + sex + s1(EDI) | 35 | 3 | 16.42 | 13,796.10 |
| M4 | tensor(t,age) + sex + s1(EDI) + s2(year of onset of symptoms) | 39 | 4 | 18.61 | 13,780.76 |
| M5 | tensor(t,age) + sex + s1(EDI) + s2(t)∗EDI | 39 | 4 | 17.59 | 13,792.95 |
| M6 | tensor(t,age) + sex + s1(EDI) + s2(year of onset of symptoms) + s3(year of onset of symptoms)∗EDI | 43 | 5 | 21.07 | 13,780.19 |
| PPMS | |||||
| M1 | tensor(t,age) + sex | 31 | 2 | 11.44 | 4923.41 |
| M2 | tensor(t,age) + sex + s2(year of onset of symptoms) | 35 | 3 | 12.14 | 4921.56 |
| M3 | tensor(t,age) + sex + s1(EDI) | 35 | 3 | 14.17 | 4922.70 |
| M4 | tensor(t,age) + sex + s1(EDI) + s2(year of onset of symptoms) | 39 | 4 | 14.93 | 4920.28 |
| M5 | tensor(t,age) + sex + s1(EDI) + s2(t)∗EDI | 39 | 4 | 15.55 | 4924.43 |
| M6 | tensor(t,age) + sex + s1(EDI) + s2(year of onset of symptoms) + s3(year of onset of symptoms)∗EDI | 43 | 5 | 16.02 | 4922.18 |
For R-MS, the knots' locations for time were (0.1, 12.1, 16.8, 21.3, 25.6, 30.0), for age were (−19.5, −2.3, 5.2, 13.3, 39.2), for EDI were (−11.1, −2.6, −0.6, 2.0, 32.1) and for year of onset of symptoms were (−39, −9, 0, 7, 16). For PPMS, the knots' locations for time were (2.3, 10.1, 14.2, 18.6, 23.3, 29.9), for age were (−28.7, −7.3, 1.4, 7.8, 29.5), for EDI were (−8.3, −2.5, −0.6, 1.9, 28.6) and for year of onset were (−39, −8, 0, 6, 15).
A reading guide accompanying table to describe model building strategy is available in the Supplemental Materials.
Fig. 1.
Excess death rate for patients with R-MS, aged 30 at onset (A), net survival probability for patients with R-MS, aged 30 at onset (B) both for a year of onset of symptoms of 1980. Excess death rate for patients with R-MS, aged 30 at onset (C), net survival probability for patients with R-MS (D), aged 30 at onset, both for a year of onset of symptoms of 1990.
At thirty years of disease duration and a year of onset of symptoms of 1980 (Fig. 1B), net survival for least deprived R-MS patients (EDI = −6) was 92.7% (95% confidence interval [88.6%; 95.3%]) for men and 94.8% (95% CI [91.9%; 96.6%]) for women. For most deprived R-MS patients (EDI = 12), net survival was 76.1% (95% CI [69.0%; 81.8%]) for men and 82.5% (95% CI [77.3%; 86.6%]) for women. For the same parameters and a year of onset of symptoms of 1990 (Fig. 1D), net survival for least deprived R-MS patients (EDI = −6) was 93.8% (95% confidence interval [90.4%; 96.1%]) for men and 95.6% (95% CI [93.3%; 97.2%]) for women. For most deprived R-MS patients (EDI = 12), net survival was 79.6% (95% CI [73.6%; 84.3%]) for men and 85.2% (95% CI [81.0%; 88.4%]) for women.
A potential interaction between EDI and sex was tested and was not significant (data not shown).
EDR dynamics according to EDI for PPMS phenotype
No clear effect of EDI on EDR was found for PPMS, with a very small difference of AIC between all the models tested.
Deprivation gap
The “deprivation gap” (net survival difference between more socio-economically deprived patients (EDI = 12) and less deprived patients (EDI = −6) (Tables 3 and 4) was significant for both men and women for R-MS throughout disease duration, but not for PPMS patients. For men with R-MS after thirty years of disease duration (for a year of MS onset of 1980), there was a difference of 16.57% of net survival probability between least and most deprived patients.
Table 3.
Deprivation gap by sex, disease duration and MS phenotype (year of onset of symptoms 1980).
| Deprivation Gap (% [CI95%]) |
||||
|---|---|---|---|---|
| Survival(EDI = −6) − Survival(EDI = 12) |
Survival(EDI = −6) − Survival(EDI = 13) |
|||
| Remitting forms |
Progressive forms |
|||
| Men | Women | Men | Women | |
| Disease duration | ||||
| 10 years | 1.81 [0.70; 2.91] | 1.28 [0.50; 2.06] | 0.12 [−1.14; 1.39] | 0.11 [−1.03; 1.26] |
| 20 years | 7.06 [3.85; 10.28] | 5.07 [2.77; 7.37] | 0.53 [−4.85; 5.92] | 0.49 [−4.42; 5.40] |
| 30 years | 16.57 [10.27; 22.88] | 12.27 [7.59; 16.95] | 1.32 [−11.89; 14.53] | 1.22 [−10.98; 13.41] |
Table 4.
Deprivation gap by sex, disease duration and MS phenotype (year of onset of symptoms 1990).
| Deprivation Gap (% [CI 95%]) |
||||
|---|---|---|---|---|
| Survival(EDI = −6) − Survival(EDI = 12) |
Survival(EDI = −6) − Survival(EDI = 13) |
|||
| Remitting forms |
Progressive forms |
|||
| Men | Women | Men | Women | |
| Disease duration | ||||
| 10 years | 1.52 [0.65; 2.38] | 1.07 [0.47; 1.67] | 0.16 [−1.44; 1.76] | 0.14 [−1.31; 1.60] |
| 20 years | 5.97 [3.47; 8.46] | 4.27 [2.52; 6.02] | 0.67 [−6.07; 7.41] | 0.61 [−5.55; 6.77] |
| 30 years | 14.25 [8.90; 19.60] | 10.46 [6.59; 14.34] | 1.59 [−14.37; 17.55] | 1.48 [−13.44; 16.29] |
Sensitivity analysis
In this analysis, 34,164 patients were included, of which 30,078 patients had R-MS and 4086 had PPMS. Five patients were excluded from the analysis, due to lack of data on patients below the age of 15 in the mortality table used. The effect of EDI and year of onset of symptoms were still statistically significant (M4) (etable 1 online-only supplements).
Missing data
In order to control for differences between patients with and without missing data on EDI (n = 3355 vs n = 34,169), descriptive analysis between the two groups was performed (etable 2a and b online-only supplements). This description revealed a higher proportion of deaths (7.2% vs 4.4% in R-MS, 22.4% vs 13.2% in PPMS) and over double the percentage of lost to follow-up patients in the group with missing data on EDI (proportion of patients lost to follow-up: 17.1% vs 6.8% in R-MS, 16.9% vs 7.2% in PPMS). There were statistically significant differences between the two groups for age at onset (R-MS) and year of onset of MS (PPMS), but these differences did not appear to be clinically significant (etable 2a and b online-only supplements). There were differences between centers, with one center representing over 50% of all missing data on EDI for both R-MS and PPMS patients. The main analysis was repeated excluding all patients from this center, and no difference was found in the selection of models for R-MS and PPMS (data not shown).
Discussion
In R-MS, more deprived patients had a higher EDR. EDR for more deprived R-MS patients was apparent from the very start of disease. In other words, socio-economic inequalities were associated with higher mortality due to MS, and these inequalities affected excess mortality of most deprived patients from the onset of symptoms. In PPMS, no clear effect of EDI on EDR was found. The year of onset of symptoms was statistically significant (higher net survival for more recent years), and the deprivation gap for 1980 was greater than the deprivation gap for 1990.
To our knowledge, this is the first study to estimate excess mortality according to SES using observatory-based data. The study of the effect of SES on overall mortality may simply reveal other-cause mortality in MS patients, who survive long enough to develop other health conditions.3,4 Other causes of death in MS patients are those of the general population such as cancer and cardio-vascular diseases,18,29 in which low SES has been associated with worse survival.12,30, 31, 32 The risk of estimating overall survival according to SES would be to highlight existing differences in all-cause mortality between patients with a high and a low SES, and not in mortality due to MS. Moreover, excess mortality models do not require knowledge of the cause of death to estimate mortality due MS. Lunde et al. and Harding et al. both discussed the limitations of using death certificate information, a sometimes unreliable information source.17,29 In addition, Schaffar et al.33 showed that even a small amount of misclassifications in causes of death in cancer patients led to a large change in net survival estimation, whereas use of inappropriate life tables did not modify net survival estimations. Thus, use of a method that does not depend on death certificate information is advantageous, especially in low lethality diseases such as MS where physicians may not always state MS as an underlying cause of death despite a potential association.
Deaths due to MS can occur either by acute death due to brainstem involvement or to respiratory failure, or as a consequence of chronic disabilities leading to bronchopneumonia, sepsis, urinary tract infections or complications from decubitus ulcers.34 Early access to DMTs in R-MS, rehabilitation programs, physiotherapy and support care are the main tools to delay onset of disability. Inequalities in access to these factors could explain the higher EDR found in more deprived patients. Calocer et al.35 showed that prescription of second-line DMTs is more frequent in patients with higher SES. French state health insurance offers universal coverage for all citizens regardless of age or economic situation, therefore access to a sturdy care network for daily management of MS should be equal.
Thankfully, MS is a disease with a relatively long life expectancy for the majority of patients, which presents a challenge when choosing indicators to measure SES. Two options are possible. The first option is to use individual data, with a recommended combination of three variables, education level, income and profession to measure socio-economic status.36 The collection of individual data encompassing the three aforementioned variables could however be complex, due to the collection of data over several decades needed for a survival study of patients with MS. One issue is comparability over time of SES measures throughout the study period necessary, with another issue being the potential for a large proportion of missing data. Even though the majority of the burden MS can have on employment occurs after diagnosis,37 we cannot exclude that the socio-economic level could be influenced by the disease before MS is diagnosed, on account of prodromal symptoms of MS.38
The second option is to use ecological data, based on neighbourhood level census data. Krieger studied the use of census level data to approximate individual data to compensate for lack of individual socio-economic data in medical records in the United States, and found satisfying results.39 A study in Belgium found that both individual SES and neighbourhood deprivation were linked to cause-specific mortality in cancer, and neighbourhood SES measure remained associated with this mortality after controlling for individual socio-economic position.40 Using a neighbourhood level proxy could thus provide additional information compared to individual level measures of SES. However ecological level SES measures are necessarily subject to ecological bias. A further potential bias resides in the fact that linkage to the IRIS was performed according to the address available for each patient in the OFSEP database and that this address could have been recorded at the first clinical visit following inclusion, with first inclusions dating back to 1976 in the OFSEP database, or have been updated throughout follow-up. No date corresponding to the address in the database was available. This exposes to the risk of reverse causation, whereby patients may live in more socio-economically deprived areas as a consequence of disease progression due to lower income.41 Updated addresses for each patient with the date of each address could have prevented the afore mentioned issue or at least allowed to measure the extent of the potential reverse causation occurring. In addition, the socio-economic environment of an IRIS could have evolved between 1976 and 2011, year for which the EDI score was developed. To restrict the implications of this evolution, follow-up was censored at 30 years to limit the timescale between address collection and attribution of the EDI score whilst also allowing sufficient follow-up for the survival study (data for 55 years of follow-up shown in etable 3 online-only supplements). It is conceivable in our study that the more deprived patients of deprived areas died first, leaving the least deprived patients of this area alive, resulting in an accentuation of ecological fallacy, in which living in a deprived area does not necessarily mean every individual within this area is deprived. A prospective cohort with recording of dates corresponding to each change of address for each participant would allow correct attribution of neighbourhood socio-economic status specific de each time period of each address. So far, few studies have examined the effect of neighbourhood-level socio-economic status on neurological disease outcomes; this data has therefore rarely been recorded in past years. Scandinavian population-based registries demonstrate the benefit of having such data, however such registries do not currently exist in France or in many other European countries.
The ideal situation would therefore have been to use both individual and ecological data, the first to avoid using a proxy and therefore ecological bias, and the latter, if based on census data as is the case in this study, to avoid large proportions of missing data and to provide contextual information.42 Individual level data is however not currently available in France for the full cohort of nearly 35,000 patients with MS present in this study. Population-based registries such as in Scandinavian countries providing both individual-level socio-economic data and morbimortality data are an example of the information that could have been used instead of a contextual measure of SES using composite indexes such as the EDI score.9,10 However, it has been shown that the smaller the statistical unit measured, the greater the magnitude effect.43 Therefore, the results found in this study would likely be amplified if measure of SES had been performed using individual-level data. In addition, area-level data can take into account contextual effects of deprivation that individual-level data cannot.40
In order to implement public health policies to reduce these socio-economic inequalities, it is necessary to examine which components of SES are the most responsible for these inequalities. The EDI score is a composite score and separating the ten individual components of the EDI score is not recommended, since the weighting of these ten variables is chosen according to the correlation between variables and according to subjective and objective poverty, and the robustness of the score is only guaranteed when the score is used in its entirety.44 A study by Marrie et al. using individual-level data found that lower health literacy in MS patients was linked to an increase in the risk of smoking, obesity, as well as increased risk of visits to the emergency room and overnight hospitalizations,45 suggesting that preventive behaviours were less adopted by patients with lower health literacy. Further studies such as this study by Marrie et al., examining individual-level data to study the effect of education level, income, and occupation are necessary to adapt public health policies and preventive practices to try to reduce the deprivation gap.
The analysis of missing data on EDI in this study suggests that data is not missing completely at random, however imputation techniques are not yet available in the survPen package used.28 One center was responsible for over 50% of missing data on EDI, however when the analysis was repeated excluding all patients from this center, no difference in selection of models was found.
The OFSEP database provides a large cohort of MS patients with a long enough follow-up to enable decent statistical power for an excess mortality study.21 However, the database is not provided from a national registry but from an observatory, based on MS expert centers. Patients are recruited in the OFSEP database by neurologists in these expert centers. Recruitment bias is possible with an overrepresentation of more active MS forms than in the general MS population since patients with more active MS tend to be treated in expert centers. In order to measure the magnitude of this bias, a previous study on the same cohort provided a sensitivity analysis with censorship of follow-up in 2000, before widespread access to DMTs.5 The results found negligible differences compared to the main analysis, suggesting that this recruitment bias of more active MS patients was limited.
An SES gradient is present in mortality in the general population,16 thus the lack of SES data in the lifetables used could lead to a possible overestimation of the socio-economic gradient in the excess mortality rate of the study population. In the sensitivity analysis using simulated life tables based on individual SES,11,46 EDI score remained significant in the models for both phenotypes, suggesting that the overestimation of EDR due to the lack of socio-economic data in the general population tables does not question the overall results of the study, being that excess mortality is associated with SES in MS patients.
Data on comorbidities, disability stage and DMTs could have been informative to help explain the socio-economic mortality gradient found in R-MS patients. Nevertheless, it is necessary to begin with measuring the total effect of a variable on an outcome of interest before measuring mediating effects of other covariables.47 Year of onset of symptoms was included in the models to measure era effect. Even though no information on treatment status was available, the smaller deprivation gap for a later year of onset of symptoms could be due to the beginning of some effective treatments. Studies including these factors, especially treatment status, are necessary to further explain the association between socio-economic status and mortality in MS.
Conclusion
This study reveals socio-economic inequalities in R-MS patients in excess mortality, therefore accounting only for deaths related directly or indirectly to MS and not in overall mortality, with differences between less deprived and more deprived patients occurring within the first decades of disease duration. Socio-economic deprivation should be considered as a complexifying factor in managing MS from the very beginning of symptoms throughout disease duration. Focus on improving equality in health outcomes should be a priority for both health policy makers and clinicians in day-to-day practice when treating patients with MS.
Contributors
Sarah Wilson: analysis and interpretation, writing of the original draft and reviewing and editing. Floriane Calocer: study concept and design, acquisition of data, analysis, interpretation and critical revision of the manuscript for important intellectual detail. Fabien Rollot: acquisition of data, analysis, interpretation and critical revision of the manuscript for important intellectual content. Mathieu Fauvernier: Analysis, interpretation and critical revision of the manuscript for important intellectual content. Laurent Remontet: Analysis, interpretation and critical revision of the manuscript for important intellectual content. Laure Tron: Analysis and critical revision of the manuscript for important intellectual content. Sandra Vukusic, Emmanuelle Le Page, Marc Debouverie, Jonathan Ciron, Aurélie Ruet, Jérôme De Sèze, Hélène Zephir, Thibault Moreau, Christine Lebrun-Frénay, David-Axel Laplaud, Pierre Clavelou, Pierre Labauge, Eric Berger, Jean Pelletier, Olivier Heinzlef, Eric Thouvenot, Jean Philippe Camdessanché: acquisition of data and critical revision of the manuscript for important intellectual content. Emmanuelle Leray: Study concept and design, acquisition of data, interpretation and critical revision of the manuscript for important intellectual content. Olivier Dejardin: Study concept and design, analysis and interpretation, writing and critical revision of the manuscript for important intellectual content. Gilles Defer: Study concept and design, acquisition of data, analysis and interpretation, writing and critical revision of the manuscript for important intellectual content. Olivier Dejardin and Gilles Defer contributed equally to this work. Sarah Wilson, Floriane Calocer, Emmanuelle Leray and Olivier Dejardin have accessed and verified the data. Sarah Wilson, Olivier Dejardin and Gilles Defer are responsible for the decision to submit the manuscript.
Data sharing statement
Data that support the findings of this study are available upon a motivated request to the OFSEP coordinator, and will be evaluated by the study coordinator and the OFSEP scientific committee for approval according to OFSEP bylaws and access to data procedures.
Declaration of interests
Sarah Wilson, Fabien Rollot, Mathieu Fauvernier, Laurent Remontet, Laure Tron, Marc Debouverie, Jérôme de Sèze, Thibault Moreau, Christine Lebrun Frenay, Pierre Labauge, Jean Pelletier and Olivier Dejardin report no disclosures.
Floriane Calocer: received funding for the present research from the ARSEP foundation for a Postdoctoral Fellowship (payment to the institution), from the “Réseau Bas-Normand pour la SEP” for a Postdoctoral Fellowship (payment to the institution), from the Regional Council of Normandy (payment to the institution), from the Ecole Doctorale of Caen University for a training in LSHTD to conduct this research (payment to the institution). She received support for attending meetings and/or travel from the ARSEP Foundation (paid directly to herself, unrelated to this work).
Sandra Vukusic: received grants or contracts (paid to her university hospital) from Biogen, BMS-Celgene, Janssen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva; received consulting fees from Biogen, BMS-Celgene, Janssen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva (paid to her university hospital); received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva (paid to her university hospital); received support for attending meetings and/or travel from Biogen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva, participated on a Data Safety Monitoring Board or Advisory Board for Biogen (contracts with her university hospital), all of the above unrelated to this work.
Emmanuelle Le Page: received payment or honoraria for consulting or lectures from Biogen, Merck, Teva, Sanofi-Genzyme, Novartis Alexion; received research support from Teva and Biogen, and received academic research grants from PHRC and LFSEP, and a travel grant from the ARSEP Foundation; received payment for consulting from Biogen, Merck, Sanofi-Genzyme, and Novartis; received invitations for national and international congresses from Biogen, Merck, Sanofi-Genzyme, Novartis Alexion, all of the above unrelated to this work.
Jonathan Ciron: participated on a Data Safety Monitory Board of Advisory Board with Biogen, Novartis, Merck, Sanofi, Roche, Alexion and BMS-Celgene (all unrelated to this work).
Aurélie Ruet: Consultancy fees from Roche and Biogen, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Merck, Roche, Biogen, research grants (paid to the institution) from Roche, Biogen and Sanofi-Genzyme, and support for attending meetings and/or travel from Biogen, Novartis and Alexion, all of the above unrelated to this work.
Hélène Zephir: received research support for one PhD student from Roche, and research support for one MD student from FHU Imminent, consulting fees from Biogen IDEC (Symposium Biogen Idec in ISNI congress); received payment or honoraria for lectures from Merck, received payment or honoraria for lectures and boards from Novartis, all of the above unrelated to this work.
David-Axel Laplaud: received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Biogen, Merck, Alexion, BMS, Roche and Novartis, all of the above unrelated to this work.
Pierre Clavelou: received consulting fees from Biogen, Janssen, Medday, Merck, Novartis, Roche, Sanofi-Genzyme and Teva Pharma; and support for attending meetings and/or travel from Sanofi-Genzyme, and participated on a Data Safety Monitoring Board or Advisory Board for Medday, Merck and Novartis. All of the above unrelated to this work.
Eric Berger: received consulting fees from Novartis, Sanofi Aventis, Biogen, Genzyme, Roche and Teva Pharma; received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis, Sanofi Aventis, Biogen, Genyme, Roche and Teva Pharma (all of the above unrelated to this work).
Olivier Heinzlef: consulting fees from Bayer Schering, Merck, Teva, Genzyme, Novartis, Almirall and BiogenIdec, support for attending meetings and/or travel grants from Novartis, Teva, Genzyme, Merck Serono and Biogen Idec and other financial or non-financial interests from Novartis, Teva, Genzyme, Merck Serono and BiogenIdec (all of the above unrelated to this work).
Eric Thouvenot: received grants or contracts from Novartis and Biogen (paid to the institution), consulting fees from Merck, Novartis, Biogen and Celgene (paid directly to himself); received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Merck, Novartis, Biogen, Celgene (paid directly to himself). All of the above unrelated to this work.
Jean Philippe Camdessanché: received grants or contracts from CSL-Behring, Grifols, Laboratoire Français des Biotechnologies, consulting fees from Akcea, Alexion, Alnylam, Argenx, Bristol Myers Squibb, Laboratoire Français des Biotechnologies, Pfizer, UCB Pharma, SNF-Floeger, received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Akcea, Alexion, Alnylam, Argenx, Biogen, CSL-Behring, Genzyme, Grifols, Laboratoire Français des Biotechnologies, Merck-Serono, Natus, Novartis, Pfizer, UCB Pharma and Teva. Received support for attending meetings and/or travel from Akcea, Alexion, Alnylam, Argenx, Biogen, CSL-Behring, Genzyme, Grifols, Laboratoire Français des Biotechnologies, Merck-Serono, Natus, Novartis, Pfizer, Teva, SNF-Floeger, all of the above unrelated to this work.
Emmanuelle Leray: received consulting fees from Alexion, Merck, Novartis, Roche and Biogen, received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Sanofi Genzyme, and received support for attending meetings and/or travel from Sanofi Genzyme, all of the above unrelated to this work.
Gilles Defer Received research grants (paid to institution) from Biogen, Merck Serono, Novartis, Sanofi Genzyme; payment for speaker honoraria from Biogen, Merck Serono, Novartis, Sanofi Genzyme, Teva Pharmaceuticals, BMS; funding for travel from Biogen, Merck Serono, Novartis, Sanofi Genzyme, Teva Pharmaceuticals; and personal compensation for scientific advisory boards from Biogen, Merck Serono, Novartis, Sanofi Genzyme, Teva Pharmaceuticals, and BMS. All of the above unrelated to this work.
Acknowledgements
The authors thank the patients and neurologists for their participation in the OFSEP project by contributing and facilitating access to clinical data; the research assistants in all participating centers who contributed to data collection; and the geographical platform MAPinMED and “Cancers and Preventions” group for performing geolocation of our study population of MS patients, with a particular mention to Ludivine Launay for her large contribution in this task.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2022.100542.
Appendix A. Supplementary data
References
- 1.Atlas of MS 2020 - epidemiology report. https://www.msif.org/resource/atlas-of-ms-2020/
- 2.McGinley M.P., Goldschmidt C.H., Rae-Grant A.D. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325:765. doi: 10.1001/jama.2020.26858. [DOI] [PubMed] [Google Scholar]
- 3.Marrie R.A., Elliott L., Marriott J., et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85:240–247. doi: 10.1212/WNL.0000000000001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leray E., Vukusic S., Debouverie M., et al. Excess mortality in patients with multiple sclerosis starts at 20 years from clinical onset: data from a large-scale French observational study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rollot F., Fauvernier M., Uhry Z., et al. Effects of age and disease duration on excess mortality in patients with multiple sclerosis from a French nationwide cohort. Neurology. 2021;97:e403–e413. doi: 10.1212/WNL.0000000000012224. [DOI] [PubMed] [Google Scholar]
- 6.Kingwell E., van der Kop M., Zhao Y., et al. Relative mortality and survival in multiple sclerosis: findings from British Columbia, Canada. J Neurol Neurosurg Psychiatry. 2012;83:61–66. doi: 10.1136/jnnp-2011-300616. [DOI] [PubMed] [Google Scholar]
- 7.Koch-Henriksen N., Sørensen P.S. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 8.Magyari M., Koch-Henriksen N., Pfleger C.C., Sørensen P.S. Physical and social environment and the risk of multiple sclerosis. Mult Scler Relat Disord. 2014;3:600–606. doi: 10.1016/j.msard.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Landfeldt E., Castelo-Branco A., Svedbom A., Löfroth E., Kavaliunas A., Hillert J. Personal income before and after diagnosis of multiple sclerosis. Value Health. 2018;21:590–595. doi: 10.1016/j.jval.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Castelo-Branco A., Landfeldt E., Svedbom A., Löfroth E., Kavaliunas A., Hillert J. Clinical course of multiple sclerosis and labour-force absenteeism: a longitudinal population-based study. Eur J Neurol. 2019;26:603–609. doi: 10.1111/ene.13863. [DOI] [PubMed] [Google Scholar]
- 11.Tron L., Fauvernier M., Bouvier A.-M., et al. Socioeconomic environment and survival in patients with digestive cancers: a French population-based study. Cancers. 2021;13:5156. doi: 10.3390/cancers13205156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell R.a.S., Colhoun H.M., Kennon B., et al. Socio-economic status and mortality in people with type 1 diabetes in Scotland 2006–2015: a retrospective cohort study. Diabet Med. 2020;37:2081–2088. doi: 10.1111/dme.14239. [DOI] [PubMed] [Google Scholar]
- 13.Calocer F., Dejardin O., Kwiatkowski A., et al. Socioeconomic deprivation increases the risk of disability in multiple sclerosis patients. Mult Scler Relat Disord. 2020;40:101930. doi: 10.1016/j.msard.2020.101930. [DOI] [PubMed] [Google Scholar]
- 14.Harding K.E., Wardle M., Carruthers R., et al. Socioeconomic status and disability progression in multiple sclerosis: a multinational study. Neurology. 2019;92:e1497–e1506. doi: 10.1212/WNL.0000000000007190. [DOI] [PubMed] [Google Scholar]
- 15.Rotstein D., Maxwell C., Tu K., Schultz S.E., Fung K., Marrie R.A. Risk of mortality in immigrants with multiple sclerosis in Ontario, Canada. Neuroepidemiology. 2020;54:148–156. doi: 10.1159/000506161. [DOI] [PubMed] [Google Scholar]
- 16.Mackenbach J.P. The persistence of health inequalities in modern welfare states: the explanation of a paradox. Soc Sci Med. 2012;75:761–769. doi: 10.1016/j.socscimed.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Lunde H.M.B., Assmus J., Myhr K.-M., Bø L., Grytten N. Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J Neurol Neurosurg Psychiatry. 2017;88:621–625. doi: 10.1136/jnnp-2016-315238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scalfari A., Knappertz V., Cutter G., Goodin D.S., Ashton R., Ebers G.C. Mortality in patients with multiple sclerosis. Neurology. 2013;81:184–192. doi: 10.1212/WNL.0b013e31829a3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estève J., Benhamou E., Croasdale M., Raymond L. Relative survival and the estimation of net survival: elements for further discussion. Stat Med. 1990;9:529–538. doi: 10.1002/sim.4780090506. [DOI] [PubMed] [Google Scholar]
- 20.Perme M.P., Stare J., Estève J. On estimation in relative survival. Biometrics. 2012;68:113–120. doi: 10.1111/j.1541-0420.2011.01640.x. [DOI] [PubMed] [Google Scholar]
- 21.Vukusic S., Casey R., Rollot F., et al. Observatoire Français de la Sclérose en Plaques (OFSEP): a unique multimodal nationwide MS registry in France. Mult Scler. 2020;26:118–122. doi: 10.1177/1352458518815602. [DOI] [PubMed] [Google Scholar]
- 22.Confavreux C., Compston D.A., Hommes O.R., McDonald W.I., Thompson A.J. EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry. 1992;55:671–676. doi: 10.1136/jnnp.55.8.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pornet C., Delpierre C., Dejardin O., et al. Construction of an adaptable European transnational ecological deprivation index: the French version. J Epidemiol Community Health. 2012;66:982–989. doi: 10.1136/jech-2011-200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remontet L., Uhry Z., Bossard N., et al. Flexible and structured survival model for a simultaneous estimation of non-linear and non-proportional effects and complex interactions between continuous variables: performance of this multidimensional penalized spline approach in net survival trend analysis. Stat Methods Med Res. 2019;28:2368–2384. doi: 10.1177/0962280218779408. [DOI] [PubMed] [Google Scholar]
- 25.Fauvernier M., Roche L., Uhry Z., Tron L., Bossard N., Remontet L. Multi-dimensional penalized hazard model with continuous covariates: applications for studying trends and social inequalities in cancer survival. J R Stat Soc Ser C Appl Stat. 2019;68 doi: 10.1111/rssc.12368. [DOI] [Google Scholar]
- 26.Abrahamowicz M., Beauchamp M.-E., Sylvestre M.-P. Comparison of alternative models for linking drug exposure with adverse effects. Stat Med. 2012;31:1014–1030. doi: 10.1002/sim.4343. [DOI] [PubMed] [Google Scholar]
- 27.Wood S.N. 2nd ed. Chapman and Hall/CRC; New York: 2017. Generalized additive models: an introduction with R. [DOI] [Google Scholar]
- 28.Fauvernier M., Remontet L., Uhry Z., Bossard N., Roche L. survPen: an R package for hazard and excess hazard modelling with multidimensional penalized splines. JOSS. 2019;4:1434. [Google Scholar]
- 29.Harding K., Zhu F., Alotaibi M., Duggan T., Tremlett H., Kingwell E. Multiple cause of death analysis in multiple sclerosis: a population-based study. Neurology. 2020;94:e820–e829. doi: 10.1212/WNL.0000000000008907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nejatinamini S., Godley J., Minaker L.M., et al. Quantifying the contribution of modifiable risk factors to socio-economic inequities in cancer morbidity and mortality: a nationally representative population-based cohort study. Int J Epidemiol. 2021;50:1498–1511. doi: 10.1093/ije/dyab067. [DOI] [PubMed] [Google Scholar]
- 31.Albus C. Psychological and social factors in coronary heart disease. Ann Med. 2010;42:487–494. doi: 10.3109/07853890.2010.515605. [DOI] [PubMed] [Google Scholar]
- 32.Walker J.J., Livingstone S.J., Colhoun H.M., et al. Effect of socioeconomic status on mortality among people with type 2 diabetes: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetes Care. 2011;34:1127–1132. doi: 10.2337/dc10-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaffar R., Rachet B., Belot A., Woods L.M. Estimation of net survival for cancer patients: relative survival setting more robust to some assumption violations than cause-specific setting, a sensitivity analysis on empirical data. Eur J Cancer. 2017;72:78–83. doi: 10.1016/j.ejca.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 35.Calocer F., Dejardin O., Droulon K., Launoy G., Defer G. Socio-economic status influences access to second-line disease modifying treatment in relapsing remitting multiple sclerosis patients. PLoS One. 2018;13:e0191646. doi: 10.1371/journal.pone.0191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adler N.E., Boyce T., Chesney M.A., et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 37.Vitturi B.K., Rahmani A., Dini G., et al. Occupational outcomes of people with multiple sclerosis: a scoping review. BMJ Open. 2022;12:e058948. doi: 10.1136/bmjopen-2021-058948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tremlett H., Marrie R.A. The multiple sclerosis prodrome: emerging evidence, challenges, and opportunities. Mult Scler. 2021;27:6–12. doi: 10.1177/1352458520914844. [DOI] [PubMed] [Google Scholar]
- 39.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagedoorn P., Vandenheede H., Vanthomme K., Gadeyne S. Socioeconomic position, population density and site-specific cancer mortality: a multilevel analysis of Belgian adults, 2001-2011. Int J Cancer. 2018;142:23–35. doi: 10.1002/ijc.31031. [DOI] [PubMed] [Google Scholar]
- 41.Kavaliunas A., Danylaitė Karrenbauer V., Binzer S., Hillert J. Systematic review of the socioeconomic consequences in patients with multiple sclerosis with different levels of disability and cognitive function. Front Neurol. 2021;12:737211. doi: 10.3389/fneur.2021.737211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diez Roux A.V. A glossary for multilevel analysis. J Epidemiol Community Health. 2002;56:588–594. doi: 10.1136/jech.56.8.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woods L.M., Rachet B., Coleman M.P. Choice of geographic unit influences socioeconomic inequalities in breast cancer survival. Br J Cancer. 2005;92:1279–1282. doi: 10.1038/sj.bjc.6602506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merville O., Launay L., Dejardin O., et al. Can an ecological index of deprivation be used at the country level? the case of the French Version of the European Deprivation Index (F-EDI) IJERPH. 2022;19:2311. doi: 10.3390/ijerph19042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marrie R.A., Salter A., Tyry T., Fox R.J., Cutter G.R. Health literacy association with health behaviors and health care utilization in multiple sclerosis: a cross-sectional study. Interact J Med Res. 2014;3:e3. doi: 10.2196/ijmr.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanplain B. L’espérance de vie par niveau de vie - Méthode et principaux résultats - documents de travail - F1801 | Insee. https://www.insee.fr/fr/statistiques/3322051
- 47.Rijnhart J.J.M., Lamp S.J., Valente M.J., MacKinnon D.P., Twisk J.W.R., Heymans M.W. Mediation analysis methods used in observational research: a scoping review and recommendations. BMC Med Res Methodol. 2021;21:226. doi: 10.1186/s12874-021-01426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.