Highlights
-
•
Efficacy of immunotherapy and RT for advanced ESCC is not well-described.
-
•
Immunotherapy with RT improved the survival of locoregional recurrent ESCC.
-
•
RT led to better resolution of dysphagia compared to immunotherapy alone.
Keywords: Immunotherapy, Radiotherapy, Esophageal squamous cell carcinoma, Progression-free survival, Overall survival
Abstract
Background and purpose
To evaluate the efficiency and safety of immunotherapy combined with or without radiotherapy (RT) for metastatic or recurrent esophageal squamous cell carcinoma (ESCC).
Methods
We retrospectively reviewed data of 127 patients with metastatic or recurrent ESCC, who received immunotherapy with or without RT at Tianjin Medical University Cancer Institute between 2017 and 2021.
Results
The median follow-up time was 15.7 months (95 % confidence interval (CI): 12.42–18.99). The median PFS of the RT and NRT groups was 5.45 months (95 % CI: 2.89–8.28) and 4.60 months (95 % CI: 3.75–7.06), respectively (P = 0.660). The median OS was 11.9 (95 % CI: 8.61–19.2) and 10.3 (95 % CI: 7.56–15.8) months, respectively (P = 0.890). The median PFS of locoregional recurrence patients in the RT and NRT groups was 11.27 months (95 % CI: 2.45–20.09) and 4.17 months (95 % CI: 2.64–5.71), respectively (P = 0.081). The median OS of locoregional recurrent patients in the RT and NRT groups was 19.48 months (95 % CI: 8.37–30.60) and 7.69 months (95 % CI: 3.45–11.93), respectively (P = 0.026). 64 % of patients in the RT group and 30 % of patients in the NRT group experienced an improvement in dysphagia (P = 0.033). No significant increase in treatment-related toxicity was observed in the RT group compared with the NRT group, except for some hematological complications.
Conclusions
Locoregional recurrent patients gained survival benefits from immunotherapy combined with RT. The combination of immunotherapy and RT was safe in metastatic/recurrent ESCC patients. RT for the esophagus leads to the improvement of dysphagia compared to immunotherapy alone.
Introduction
Esophageal cancer is an aggressive disease. Almost half of the patients had metastatic disease at the time of diagnosis, and the majority of patients treated with curative intent developed locoregional or distant metastases [1]. For those patients, pembrolizumab was recommended for the first-line treatment in combination with fluoropyrimidine plus platinum-based chemotherapy, the second-line treatment as a single agent, and maintenance treatment after the first-line treatment [2].
In metastatic or recurrent ESCC, RT is frequently used for the relief of symptoms, such as pain, dysphagia, dyspnea, and tumor bleeding [3]. Results of several preclinical and clinical studies have suggested that RT can enhance the systemic antitumor immune response through multiple mechanisms. For example, RT leads to cell death and subsequent release of antigens and pro-inflammatory mediators called damage-associated molecular patterns (DAMPs), which can trigger the recruitment and activation of antigen-presenting cells (APC) and initiate tumor antigen-specific T cell responses [4]. Combined radiation and immunotherapy have gained survival benefits in various cancers including locally advanced ESCC [5], [6], [7]. However, the efficacy of this strategy in metastatic or recurrent esophageal cancer patients remains unclear.
In advanced ESCC patients, dysphagia is the most common complaint, which may lead to nutritional compromise and deterioration of quality of life [8]. EBRT has been an effective method of palliation of dysphagia and carries the advantage of being a non-invasive procedure. Chemotherapy alone takes several weeks to achieve symptom relief, and patients in poor general condition are not fit enough to undergo effective chemotherapy [9]. Nowadays, with immunotherapy being widely used in advanced ESCC patients, we’d like to know the effect of immunotherapy combined with or without RT on dysphagia relief.
We therefore did a real-world study to investigate whether adding RT to immunotherapy could improve the prognoses and palliation of dysphagia, and the safety in patients with metastatic or recurrent ESCC.
Materials and methods
Patients
The medical records of metastatic/recurrent ESCC patients treated with immunotherapy with or without RT at Tianjin Medical University Caner Institute between January 2017 and July 2021 were screened. The inclusion criteria for this study were as follows: (1) histological diagnosis of ESCC, (2) diagnosis with metastatic or recurrent disease, (3) treatment with immunotherapy with or without RT, and (4) Karnofsky performance status ≥ 70. Patients with other malignancy histories, and/or serious heart, liver, lung, kidney, or blood system diseases were excluded.
Metastatic disease was defined as non-regional lymph node metastases and any distant organ metastases. The recurrent disease after definitive chemoradiotherapy, surgery-based comprehensive therapy, or chemotherapy includes both locoregional and distant recurrence. Tumor staging was performed according to the 8th edition of the International Union against Cancer (UICC) TNM classification. The study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital.
Treatments
Radiotherapy
A total of 48 RT courses were delivered to 40 patients: 21 palliatives (dose 30–48 Gy in 10–24 fractions), 4 ablatives (36–50 Gy in 4–6 fractions), and 23 conventional radical doses (dose: 50–60 Gy in 25–33 fractions; mostly as initial treatment in locally advanced setting). The median equivalent dose in 2 Gy fractions (EQD2) was 56 Gy (range: 32.5–93.75 Gy) using the linear-quadratic model with a/b = 10 Gy. And all patients received involved field radiation rather than extended field radiation.
Immunotherapy
The anti-PD-1 antibodies used as systemic immunotherapy included pembrolizumab, nivolumab, camrelizumab, sintilimab, and tislelizumab. A median (range) of 4 (1–21) and 4(1–35) cycles of anti-PD-1 antibodies were administered in the RT and NRT groups, respectively (P = 0.830).
As for the sequence of immunotherapy and radiotherapy, both synchronous and sequential treatment were included in our study. In fact, 8 patients received synchronous treatment without interruption of immunotherapy during radiotherapy, and the other 32 patients received sequential treatment with radiotherapy before or after immunotherapy.
Chemotherapy
77.0 % of patients in the NRT group and 87.5 % of patients in the RT group received systemic chemotherapy consisted of docetaxel, taxane, platinum, fluoropyrimidine or irinotecan.
Follow up
Patients attended regularly scheduled follow-up physical examinations, blood tests, chest and abdominal CT scans, barium esophagography, and ultrasonography at 6-weeks intervals for the first two years, every 6 months for the next three years, then annually. All patients were followed up by telephone or clinical visits.
Statistical methods
The primary endpoints of this study were OS and PFS. OS was defined as the time from the start of immunotherapy to the date of death or last follow-up. PFS was defined as the time from the start of immunotherapy to the date of the first documented disease progression, death, or last contact (whichever occurred first). The second endpoint was the improvement of dysphagia and the safety of immunotherapy with or without radiotherapy in the metastatic/recurrent ESCC. Dysphagia relief rate in the metastatic or recurrent patients was defined as the number of patients with complete or partial dysphagia relief as a percentage of the total number of patients with dysphagia after the initiation of immunotherapy.
The Immune-related Response Criteria (irRC) was used to determine the response and progression after the initiation of immunotherapy [10]. Adervse effects (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03 (NCI CTCAE v4.03).
χ2 or Fisher’s exact test was used to analyze differences in categorical variables and the Mann-Whitney U test for differences in continuous variables. Survival analysis was performed using the Kaplan-Meier approach with a log-rank test and the median follow-up was calculated using the reverse Kaplan-Meier approach. The univariate and multivariate analyses were performed using the Cox proportional hazard regression model. Variables with a P ≤ 0.25 in the univariate analyses were subjected to multivariate analyses. All statistical analyses were conducted using GraphPad Prism V.9.3.0 (GraphPad Software, San Diego, California, USA, https://www.graphpad.com) or R V.4.1.2 (The R Foundation for Statistical Computing, https://www.R-project.org). And P value < 0.05 was statistically significant.
Results
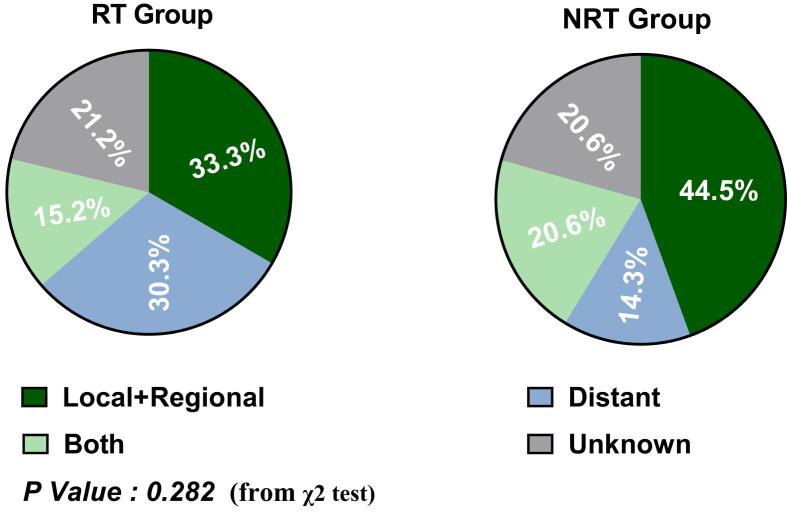
A total of 127 patients with metastatic/recurrent ESCC who met the inclusion criteria were included in the study. As of March 13, 2022, the median follow-up time was 15.7 months (95 % CI:12.42–18.99). The patient’s characteristics are detailed in Table 1. 35.4 % (45/127) patients presented with metastatic disease at diagnosis, and others showed recurrent disease. There were 6.7 % (3/45) patients with distant lymph nodes metastasis in the chest wall or axillary in the primary metastatic patients, and 93.3 % (42/45) patients had metastatic lesions involving one or more organs. We grouped the total patients into two groups according to whether they received RT or not (RT and NRT group). The RT group included 31.5 % (40/127) patients who received immunotherapy combined with RT. The NRT group included 68.5 % (87/127) patients who received immunotherapy but not RT. The clinical characteristics were well balanced between the two groups except for the lines of immunotherapy (P = 0.011) (Supplement Table 1).
Table 1.
Clinical characteristics of all patients.
| Characteristics | No. of patients (%) |
|---|---|
| n | 127 |
| Age (median [IQR]) | 59.0 [55.0, 65.0] |
| Sex | |
| Female | 9.4 % (12/127) |
| Male | 90.6 % (115/127) |
| Drinking history | |
| No | 20.5 % (26/127) |
| Yes | 70.9 % (90/127) |
| Unknown | 8.6 % (11/127) |
| Clinical T Stage | |
| T1-T3 | 79.5 % (101/127) |
| T4 | 20.5 % (26/127) |
| Clinical N Stage | |
| N0-N2 | 80.3 % (102/127) |
| N3 | 19.7 % (25/127) |
| Cancer type | |
| Primary metastatic disease | 35.4 % (45/127) |
| Recurrent disease | 64.6 % (82/127) |
| Treatment type | |
| RT | 31.5 % (40/127) |
| NRT | 68.5 % (87/127) |
| Lines of immunotherapy | |
| 1 | 70.9 % (90/127) |
| ≥2 | 29.1 % (37/127) |
| Site of metastases or recurrence | |
| Distant | 69.3 % (88/127) |
| Locoregional | 30.7 % (39/127) |
| No. of involved organs | |
| 0 | 33.1 % (42/127) |
| 1 | 51.2 % (65/127) |
| ≥2 | 15.7 % (20/127) |
| Site of metastases(PMD) | |
|---|---|
| Lung only | 17.8% (8/45) |
| Liver only | 44.4% (20/45) |
| Bone only | 4.4% (2/45) |
| Other single organ | 4.4% (2/45) |
| Two or more organs | 22.2% (10/45) |
| Distant lymph node | 6.7% (3/45) |
| Site of progression(RD) | |
| Locoregional | 47.6% (39/82) |
| Distant | 9.8% (8/82) |
| Both | 42.7% (35/82) |
| Irradiated tumour site | |
| Locoregional | 17 |
| Lymph node | 17 |
| Bone | 9 |
| Chest wall | 3 |
| Adrenal gland | 3 |
IQR- Interquartile range, RT- Radiotherapy, NRT- Non-radiotherapy, PMD- Primary metastatic disease, RD- Recurrent disease.
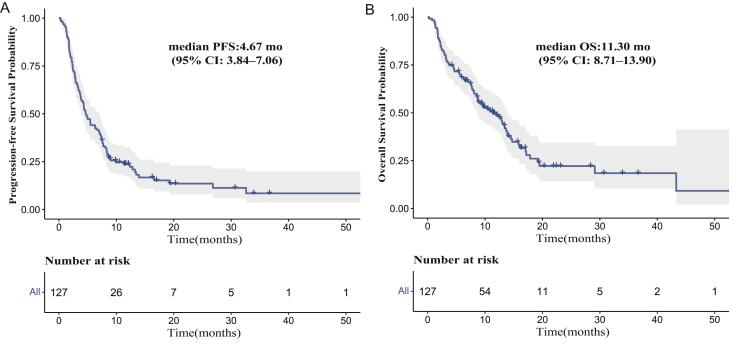
For the entire cohort, the median PFS was 4.67 months (95 % CI: 3.84–7.06) (Supplement Fig. 1A) and the median OS was 11.30 months (95 % CI: 8.71–13.90) (Supplement Fig. 1B). Univariate analysis showed using immunotherapy as first-line therapy was positively associated with PFS and OS. Consistantly, multivariate analysis revealed that using immunotherapy as first-line therapy independently predicted better PFS and OS (Table 2).
Table 2.
Univariable and multivariable analysis of covariables associated with progression-free survival and overall survival in all patients.
| Progression-free survival (n = 127) | Overall survival (n = 127) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| Characteristics | HR (CI95) | P | HR (CI95) | P | HR (CI95) | HR (CI95) | HR (CI95) | P |
| Age years | ||||||||
| ≥60 vs < 60 | 0.89 (0.6–1.3) |
0.538 | 0.88 (0.57–1.37) |
0.574 | ||||
| Sex | ||||||||
| Female vs male | 1.13 (0.59–2.18) |
0.714 | 0.92 (0.4–2.11) |
0.836 | ||||
| Drinking history | ||||||||
| No vs yes | 1.00 (0.61–1.64) |
0.992 | 0.95 (0.53–1.69) |
0.866 | ||||
| Clinical T Stage | ||||||||
| T1-T3 vs T4 | 0.69 (0.43–1.09) |
0.112 | 0.74 (0.46–1.18) |
0.205 | 0.51 (0.3–0.84) |
0.009 | 0.53 (0.32–0.90) |
0.018 |
| Clinical N Stage | ||||||||
| N0-N2 vs N3 | 0.72 (0.45–1.14) |
0.163 | 0.76 (0.48–1.21) |
0.243 | 0.62 (0.37–1.04) |
0.072 | 0.66 (0.39–1.11) |
0.114 |
| Cancer type | ||||||||
| PMD vs RD | 0.87 (0.58–1.31) |
0.510 | 0.81 (0.51–1.3) |
0.382 | ||||
| Lines of immunotherapy | ||||||||
| 1 vs ≥ 2 | 0.55 (0.37–0.83) |
0.005 | 0.58 (0.38–0.88) |
0.010 | 0.56 (0.36–0.89) |
0.014 | 0.61 (0.38–0.96) |
0.034 |
| Site of metastases or recurrence | ||||||||
| Locoregional vs distant | 0.99 (0.66–1.5) |
0.977 | 1.15 (0.72–1.83) |
0.569 | ||||
| No. of involved organs | ||||||||
| 0 vs ≥ 2 | 1.20 (0.66–2.18) |
0.546 | 1.56 (0.78–3.12) |
0.213 | 1.38 (0.68 – 2.79) |
0.372 | ||
| 1 vs ≥ 2 | 1.17 (0.66–2.08) |
0.581 | 1.36 (0.69–2.68) |
0.367 | 1.15 (0.58–2.29) |
0.690 | ||
| Treatment type | ||||||||
| RT vs NRT | 1.10 (0.73–1.64) |
0.656 | 0.97 (0.61–1.54) |
0.894 | ||||
PMD- Primary metastatic disease, RD- Recurrent disease, RT-Radiotherapy.
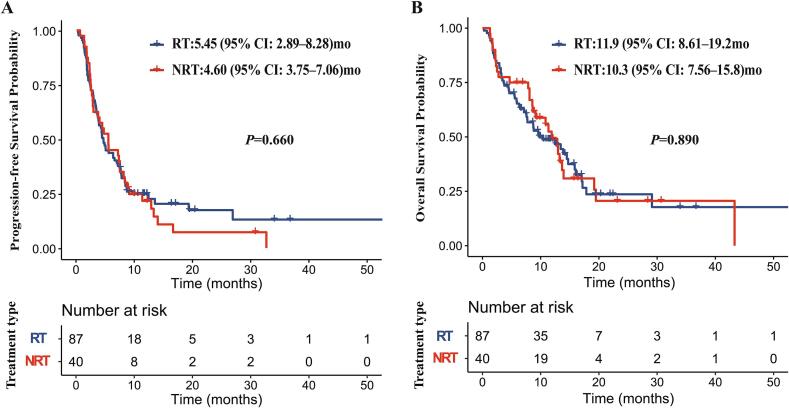
Progression events occurred in 90.0 % (36/40) patients in the RT group and 79.3 % (69/87) patients in the NRT group. The sites of progression in the RT and NRT groups were shown in Supplement Fig. 2. The proportion of progression in locoregional sites was slightly lower in the RT group compared with that in the NRT group (33.3 % vs 44.5 %), despite without significant difference (P = 0.282). Median PFS was 5.45 months (95 % CI: 2.89–8.28) in the RT group versus 4.60 months (95 % CI: 3.75–7.06) in the NRT group (P = 0.660) (Fig. 1A). The 12- and 24-month PFS rate was 21.7 % (95 %CI: 22.5 %-53.1 %), 7.2 % (95 % CI: 5.7 %-31.6 %) in the RT group, respectively, and 24.8 % (95 % CI: 17.1 %-35.9 %) and17.4 % (95 % CI: 10.0 %-30.2 %) in the NRT group, respectively.
Fig. 1.
Progression-free survival (A) and overall survival (B) for all patients in RT and NRT groups.
Death events occurred in 67.5 % (27/40) patients in the RT group and 62.1 % (54/87) patients in the NRT group. Median OS was 11.9 months (95 % CI: 8.61–19.2) in the RT group versus 10.3 months (95 % CI: 7.56–15.8) in the NRT group (P = 0.890) (Fig. 1B). The 12- and 24-month OS rate was 49.2 % (95 % CI: 50.0 %-80.9 %), 20.6 % (95 % CI: 11.0 %-47.7 %) in the RT group, respectively, and 48.7 % (95 % CI: 39.0 %%-60.9 %), 23.6 % (95 % CI: 14.0 %-39.9 %) in the NRT group, respectively.
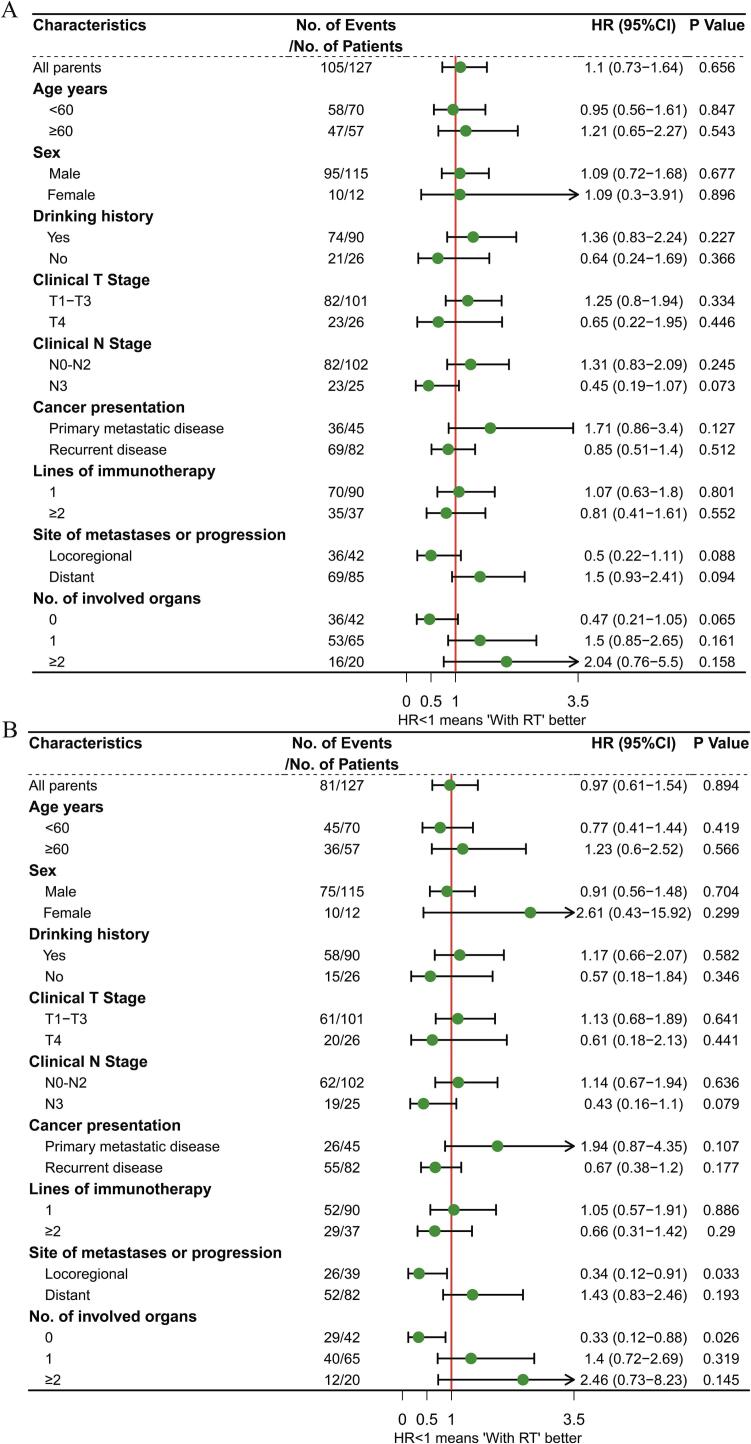
We then performed exploratory subgroup analysis between the RT and NRT group. In the subgroup analysis of PFS, no subgroup patients benefitted from the combination of immunotherapy and RT (Fig. 2A). In the subgroup analyses of OS, patients with locoregional recurrence (HR: 0.34, P = 0·033), and those without distant organ metastasis (HR: 0.33, P = 0.026) had significantly prolonged OS in the RT group (Fig. 2B).
Fig. 2.
Forest plots show factors associated with progression-free survival (A) and overall survival (B) in all patients.
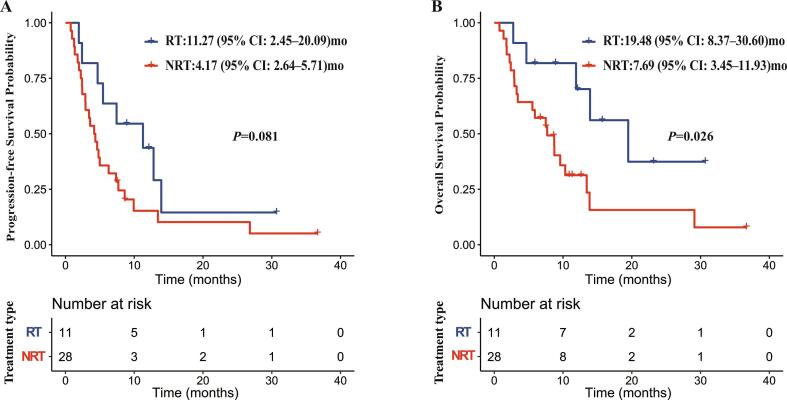
We did a survival analysis for patients with locoregional recurrence. The median PFS were 11.27 months (95 % CI: 2.45–20.09) and 4.17 months (95 % CI: 2.64–5.71) in the RT and NRT group (Fig. 3A). The median OS were 19.48 months (95 % CI: 8.37–30.60) and 7.69 months (95 % CI: 3.45–11.93) in the RT and NRT group (Fig. 3B).
Fig. 3.
Progression-free survival (A) and overall survival (B) for patients with locoregional recurrence in RT and NRT groups.
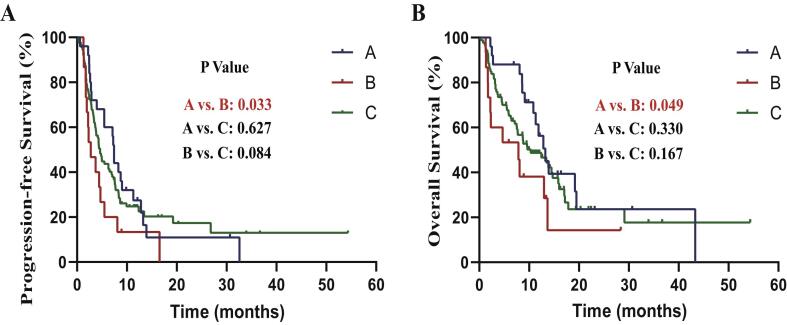
To investigate the impact of different RT timing on patients’ prognosis, we subdivided patients into three groups: Group A-patients with RT within 90 days prior to or after ICI use, Group B-patients with RT outside 90 days prior to or after ICI use, and Group C-patients without RT. Results showed that patients in Group A had a significant longer PFS (median PFS: 7.43 months vs 2.76 months; P (Group A vs Group B) = 0.033) (Supplement Fig. 3A) and OS (median OS: 13.24 months vs 7.85 months; P (Group A vs Group B) = 0.049) (Supplement Fig. 3B) than patients in Group B. The median PFS and OS in Group C was 4.60 months and 10.32 months, respectively. And there was no significant difference in PFS and OS between Group C and other groups.
87 % (39/45) of patients with primary metastatic disease at diagnosis and 52 % (14/27) of local recurrence patients complained of dysphagia. The grade of dysphagia was determined by the dysphagia score shown in Supplement Table 2. Of these 53 patients who had dysphagia, 12 patients were not assessable for dysphagia relief due to missing data, nasogastric tube insertion or esophagectomy after immunotherapy, leaving 41 patients assessable to analysis. The clinical characteristics in patients with dysphagia were well balanced between the RT and NRT groups (Supplement Table 3). In the RT group, 78.6 % of patients (11/14) received radical RT (dose 50.4–60 Gy in 28–30 fractions), and 21.4 % of patients (3/14) received palliative radiation (dose 39.6–46 Gy in 22–23 fractions). The median (range) cycle of anti-PD-1 antibodies administered in the RT and NRT groups was 4 (2–14) and 4 (1–21), respectively (P = 0.758). The median (range) cycle of chemotherapy administered in the RT and NRT groups was 4 (1–10) and 4 (1–16), respectively (P = 0.986).
64 % (9/14) patients in the RT group and 30 % (8/27) patients in the NRT group achieved dysphagia relief (P = 0.033). 36 % (5/14) patients in the RT group and 55 % (15/27) patients in the NRT group had no change in the dysphagia score. 15 % (4/27) patients in the NRT group developed worse dysphagia, but no one did in the RT group. There were 3 patients who had a relapse of dysphagia in the NRT group and no one in the RT group by the end of the last follow-up time.
In terms of treatment-related toxicities of patients with dysphagia, the incidence of thrombocytopenia (71 % vs 30 %; P = 0.026) and lymphopenia (93 % vs 52 %; P = 0.023) were higher in the RT group than that in the NRT group (Supplement Table 4). As for AEs associated with RT, there was 1 patient having an interruption of treatment after receiving 46 Gy radiation due to the risk of esophageal fistula, 3 patients experienced 2-grade radiation esophagitis, and 1 patient had late esophagus stricture in the RT group.
The most common AEs in the entire corhort reported were hematological complications (anemia, leukopenia, thrombocytopenia, and lymphopenia) and nausea. The incidence of anemia (80 % vs 48 %; P = 0.002), leukopenia (65 % vs 36 %; P = 0.004), thrombocytopenia (60 % vs 33 %; P = 0.008), and lymphopenia (95 % vs 52 %; P < 0.001) was higher in the RT group than that in the NRT group. Rates of grade 3 or higher neutrophilic granulopenia (28 % vs 11 %; P = 0.046) and lymphopenia (60 % vs 25 %; P < 0.001) were higher in the RT group versus the NRT group. 8 % and 5 % of patients had pneumonitis in the RT group and NRT group, the difference was not statistically significant (P = 0.805) (Table 3), and there was only one patient who developed grade 3 pneumonitis in the RT group. The majority of toxicities were grade 1 to 2. No grade 5 toxicity was recorded.
Table 3.
AEs of all patients in the NRT and RT group.
| All No. (%) | Grade ≥ 3 No. (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| RT (n = 40) |
NRT (n = 87) |
P-Value | All patients (n = 127) | RT (n = 40) |
NRT (n = 87) |
P-Value | All patients (n = 127) | ||
| Fatigue | 5(13) | 9(10) | 0.956 | 14(11) | 0(0) | 2(2) | 0.842 | 2(2) | |
| Anorexia | 8(20) | 15(17) | 0.899 | 23(18) | 0(0) | 1(1) | 1.000 | 1(1) | |
| Hypoalbuminemia | 12(30) | 22(25) | 0.733 | 34(27) | 0(0) | 0(0) | NA | 0(0) | |
| Nausea | 17(43) | 34(39) | 0.865 | 51(40) | 0(0) | 2(2) | 0.842 | 2(2) | |
| Vomiting | 11(28) | 23(26) | 1.000 | 34(27) | 0(0) | 1(1) | 1.000 | 1(1) | |
| Diarrhea | 3(8) | 3(3) | 0.583 | 6(5) | 1(3) | 0(0) | 0.689 | 1(1) | |
| Abdominal Pain | 0(0) | 2(2) | 0.842 | 2(2) | 0(0) | 0(0) | NA | 0(0) | |
| Radiation esophagitis | 9(53) | 9(7) | 0(0) | 0(0) | |||||
| Hypothyroidism | 2(5) | 1(1) | 0.485 | 3(2) | 0(0) | 0(0) | NA | 0(0) | |
| Adrenal insufficiency | 1(3) | 2(2) | 1.000 | 3(2) | 0(0) | 0(0) | NA | 0(0) | |
| Hyperthyroidism | 0(0) | 1(1) | 1.000 | 1(1) | 0(0) | 0(0) | NA | 0(0) | |
| Anemia | 32(80) | 42(48) | 0.002 | 74(58) | 4(10) | 4(5) | 0.441 | 8(6) | |
| Leukopenia | 26(65) | 31(36) | 0.004 | 57(45) | 3(8) | 4(5) | 0.805 | 7(6) | |
| Neutrophilic granulopenia | 19(48) | 26(30) | 0.084 | 45(35) | 11(28) | 10(11) | 0.046 | 21(17) | |
| Thrombocytopenia | 24(60) | 29(33) | 0.008 | 53(42) | 3(8) | 1(1) | 0.175 | 4(3) | |
| Lymphopenia | 38(95) | 45(52) | <0.001 | 83(65) | 24(60) | 22(25) | <0.001 | 46(36) | |
| Elevated liver enzymes | 9(23) | 18(21) | 1.000 | 27(21) | 2(5) | 1(1) | 0.485 | 3(2) | |
| Blood creatinine increased | 1(3) | 3(3) | 1.000 | 4(3) | 0(0) | 0(0) | NA | 0(0) | |
| Hepatitis | 0(0) | 1(1) | 1.000 | 1(1) | 0(0) | 1(1) | 1.000 | 1(1) | |
| Dyspnea | 1(3) | 2(2) | 1.000 | 3(2) | 0(0) | 0(0) | NA | 0(0) | |
| Cough | 4(10) | 9(10) | 1.000 | 13(10) | 0(0) | 2(2) | 0.842 | 2(2) | |
| Hemoptysis | 3(8) | 3(3) | 0.583 | 6(5) | 0(0) | 0(0) | NA | 0(0) | |
| Pneumonitis | 3(8) | 4(5) | 0.805 | 7(6) | 1(3) | 0(0) | 0.689 | 1(1) | |
| Pruritis | 0(0) | 1(1) | 1.000 | 1(1) | 0(0) | 0(0) | NA | 0(0) | |
| Rash | 1(3) | 1(1) | 1.000 | 2(2) | 0(0) | 0(0) | NA | 0(0) | |
| Cutaneous capillary hemangioma | 0(0) | 4(5) | 0.406 | 4(3) | 0(0) | 0(0) | NA | 0(0) | |
| Radiation dermatitis | 1(3) | 1(1) | 0(0) | 0(0) | |||||
| Peripheral sensory neuropathy | 2(5) | 3(3) | 1.000 | 5(4) | 1(3) | 0(0) | 0.689 | 1(1) | |
We also investigated the AEs of patients with RT within 90 days and outside 90 days prior to or after ICI use in the RT group, the results showed that there were no differences between the two groups for all-grade and grade 3 or higher AEs (Supplement Table 5). Considering the effect of different radiation doses on safety, we explored the AEs between palliative doses group (14 patients received palliative radiotherapy only) and radical doses group (23 patients received radical radiotherapy with or without palliative or stereotactic ablative radiotherapy), 3 patients who received stereotactic ablative radiotherapy only were not analyzed. The results showed only vomiting and cough were higher in the palliative doses group (Supplement Table 6). We also analyzed the AEs of different irradiated sites, and patients were divided into three groups: Group 1-patients who only received locoregional radiation, Group 2-patients who received radiation only to lymph nodes, and Group 3-patients who received radiation to distant organs or multiple sites. It turned out that the AEs were evenly distributed in the three groups (Supplement Table 7).
Discussion and conclusions
To the best of our knowledge, this retrospective study is the first large-scale study to demonstrate the efficacy of the combination of immunotherapy and RT in patients with metastatic or recurrent ESCC. The results revealed that there was no survival benefit in the RT group for the entire cohort. However, patients with locoregional recurrence had significantly improved OS with immunochemotherapy combined with RT compared to immunochemotherapy. The combination of immunochemotherapy and RT was safe and tolerable, despite a relatively higher number of hematological AEs than the immunochemotherapy group.
Of the entire cohort in our study, the median PFS and OS were 4.67 and 11.30 months, respectively. The median PFS and OS of advanced ESCC patients treated with immunochemotherapy were 6.3 and 12.6 months in the KEYNOTE-590 study, and 6.9 and 15.3 months in the ESCORT-1 s study [11], [12]. The median PFS and OS of the patients who received immunotherapy as the first-line therapy (70.9 %) in our study were 6.90 (95 % CI: 5.23–8.57) and 13.24 (95 % CI: 10.83–15.65) months, which was similar to the results of the KEYNOTE-590 study.
In our study, immunochemotherapy combined with RT significantly prolonged OS in patients with locoregional recurrence compared with immunochemotherapy. The OS of these patients in the RT group was 19.43 months. Several studies have been reported of locoregional recurrent esophageal cancer treated with radiotherapy or chemoradiotherapy, with an OS of 7.0–24.3 months [13], [14], [15], [16], [17], [18], [19]. The OS in our study was estimated from the initiation of immunotherapy not the start of radiation or the date of relapse like the studies mentioned above, so we recalculated the OS of locoregional recurrence patients in the RT group from the first date of radiation, and the results was 22.60 months (95 % CI: 12.58–32.62), which was only inferior to the 24.3 months in Kobayashi’s study (whose OS was also defined from the start of radiotherapy) [15]. As Jeene et al. reported, patients with only lymphnode recurrence had significantly longer survival than patients with recurrence at anastomosis. And patients with only lymphnode recurrence treated with radiotherapy were 63.6 % in ours, which was relatively less than the 73.8 % in Kobayashi’s study, which may explain the slightly shorter OS in our study [19]. So it is imperative that large scale trials be conducted to investigate the efficiency of immunotherapy combined with local therapy in locoregional recurrent ESCC.
We invested the impact of different RT timing on the survival of all patients, and it turned out that without increased AEs, patients with RT within 90 days of ICI use had a significantly longer OS and PFS than patients with RT outside 90 days of ICI use. So it would be necessary to add RT within 3 months of ICI use to prolong the survival of metastatic/recurrent ESCC patients.
Our study revealed that immunochemotherapy combined with RT had greatly improved dysphagia compared to immunochemotherapy. 64 % of patients in the RT group had an improvement in dysphagia, which was similar to some existing studies, of which 69 %-75 % of patients had dysphagia relief treated with palliative RT [20], [21], [22]. The ESCORT study and the phase 3 ESCORT-1st study showed that camrelizumab combined with or without chemotherapy was associated with statistically significant improvements in some health-related quality-of-life metrics compared with chemotherapy [12], [23]. However, neither of these studies showed an advantage of immunotherapy in improving dysphagia. This highlights the important role of RT in improving dysphagia in advanced patients.
Natasja’s and Halla’s study revealed that the dysphagia relief rate showed no difference in patients treated with 30–39 Gy in 10–13 fractions and 20 Gy in 5 fractions [22], [24]. But there was a long time to second intervention and fewer re-interventions in the higher doses group. A few early studies revealed that doses above 45 Gy were shown to provide a longer duration of symptom relief [25], [26], [27]. With the survival being significantly prolonged in the era of immunotherapy, a more aggressive but convenient RT strategy may be preferred to obtain a more sustainable duration of response. While a definitive dose of radiation may be inappropriate for advanced ESCC due to the AEs such as radiation esophagitis, so the optimal radiation dose for palliation of dysphagia needs further investigation.
The safety profile observed in our study was consistent with previous published prospective ICI trials for patients with advanced ESCC [28], [29]. There was no increased toxicity with the addition of RT other than some hematological complications. The esophagitis rate in the RT group was similar to previous chemoradiotherapy combined with or without immunotherapy trials for locally advanced ESCC [6], [30], [31]. The pneumonitis rate was low and tolerable in our study, which was consistent with other studies for locally advanced ESCC patients treated with immunotherapy with RT.
Our study has some limitations that should be addressed. Firstly, this study was a retrospective single-center research, and more prospective multi-center studies are needed for external validation. Secondly, an inherent selection bias existed because of the retrospective design, although the only variable that differed significantly was the lines of immunotherapy between the RT and NRT groups. Thirdly, we didn’t analyze the impact of the level of PD-L1 expression on survival due to lacking detection of PD-L1 expression in most patients. Thus, further well designed randomized studies are needed to investigate the subsets of patients with metastatic or relapsed ESCC who would benefit from RT combined with immunotherapy.
In conclusion, our study shows that RT combined with immunotherapy is effective for locoregional recurrent ESCC patients. Immunotherapy combined with RT was safe in metastatic or recurrent ESCC patients. And adding local RT to immunotherapy was associated with better resolution of dysphagia for metastatic or recurrent ESCC.
Funding
This work was supported by the National Nature Science Foundation of China under Grant [number 81872462, 82073348].
Data sharing
Data is available upon reasonable request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Conception and design: PW, QP, WZ. Administrative support: PW, QP, WZ. Provision of study materials or patients: QP, WZ, PW. Collection and assembly of data: XW, YL. Data analysis and interpretation: XW, YL. Manuscript writing: All authors. Final approval of manuscript: All authors. All authors contributed to the article and approved the submitted version.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2022.10.011.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
Supplementary figure 3.
References
- 1.Krug S., Michl P. Esophageal Cancer: New Insights into a Heterogenous Disease. Digestion. 2017;95:253–261. doi: 10.1159/000464130. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers (Version 2,2022), <https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf> (2022). [DOI] [PubMed]
- 3.National Health Commission Of The People's Republic Of, C. Chinese guidelines for diagnosis and treatment of esophageal carcinoma 2018 (English version). Chin. J. Cancer Res. = Chung-kuo yen cheng yen chiu31, 223-258, doi:10.21147/j.issn.1000-9604.2019.02.01 (2019). [DOI] [PMC free article] [PubMed]
- 4.Menon H., Ramapriyan R., Cushman T.R., Verma V., Kim H.H., Schoenhals J.E., et al. Role of Radiation Therapy in Modulation of the Tumor Stroma and Microenvironment. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park S., Oh D., Choi Y.-L., Chi S.A., Kim K., Ahn M.-J., et al. Durvalumab and tremelimumab with definitive chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Cancer. 2022;128(11):2148–2158. doi: 10.1002/cncr.34176. [DOI] [PubMed] [Google Scholar]
- 6.Zhang, W. et al. Safety and Feasibility of Radiotherapy Plus Camrelizumab for Locally Advanced Esophageal Squamous Cell Carcinoma. The oncologist26, e1110-e1124, doi:10.1002/onco.13797 (2021). [DOI] [PMC free article] [PubMed]
- 7.Zhang W., Yan C., Zhang T., Chen X.i., Dong J., Zhao J., et al. Addition of camrelizumab to docetaxel, cisplatin, and radiation therapy in patients with locally advanced esophageal squamous cell carcinoma: a phase 1b study. Oncoimmunology. 2021;10(1) doi: 10.1080/2162402x.2021.1971418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watt E., Whyte F. The experience of dysphagia and its effect on the quality of life of patients with oesophageal cancer. Europ. J. Cancer care. 2003;12:183–193. doi: 10.1046/j.1365-2354.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- 9.Grünberger B., Raderer M., Schmidinger M., Hejna M. Palliative chemotherapy for recurrent and metastatic esophageal cancer. Anticancer Res. 2007;27:2705–2714. [PubMed] [Google Scholar]
- 10.Wolchok, J. D. et al. Guidelines for the Evaluation of Immune Therapy Activity in Solid Tumors: Immune-Related Response Criteria. Clinical Cancer Research15, 7412-7420, doi:10.1158/1078-0432.CCR-09-1624 %J Clin Cancer Res (2009). [DOI] [PubMed]
- 11.Kato, K. et al. KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. 15, 1057-1066, doi:10.2217/fon-2018-0609 (2019). [DOI] [PubMed]
- 12.Xu, R.-h. et al. ESCORT-1st: A randomized, double-blind, placebo-controlled, phase 3 trial of camrelizumab plus chemotherapy versus chemotherapy in patients with untreated advanced or metastatic esophageal squamous cell carcinoma (ESCC). 39, 4000-4000, doi:10.1200/JCO.2021.39.15_suppl.4000 (2021).
- 13.Jingu K., Matsushita H., Takeda K., Umezawa R., Takahashi C., Sugawara T., et al. Long-term bresults of radiotherapy combined with nedaplatin and 5-fluorouracil for postoperative loco-regional recurrent esophageal cancer: update on a phase II study. BMC Cancer. 2012;12(1) doi: 10.1186/1471-2407-12-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shioyama Y., Nakamura K., Ohga S., Nomoto S., Sasaki T., Yamaguchi T., et al. Radiation Therapy for Recurrent Esophageal Cancer after Surgery: Clinical Results and Prognostic Factors. Jpn J Clin Oncol. 2007;37(12):918–923. doi: 10.1093/jjco/hym138. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi R., Yamashita H., Okuma K., Shiraishi K., Ohtomo K., Nakagawa K. Salvage radiation therapy and chemoradiation therapy for postoperative locoregional recurrence of esophageal cancer. Dis Esophagus. 2014;27(1):72–78. doi: 10.1111/dote.12068. [DOI] [PubMed] [Google Scholar]
- 16.Zhao K., Si Y., Sun L., Meng X., Yu J. Efficacy and toxicity of re-irradiation for esophageal cancer patients with locoregional recurrence: a retrospective analysis. Radiat Oncol. 2020;15(1) doi: 10.1186/s13014-020-01685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita H., Jingu K., Niibe Y., Katsui K., Matsumoto T., Nishina T., et al. Definitive salvage radiation therapy and chemoradiation therapy for lymph node oligo-recurrence of esophageal cancer: a Japanese multi-institutional study of 237 patients. Radiat Oncol. 2017;12(1) doi: 10.1186/s13014-017-0780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J., Yin W., Yao H., Gu W. Salvage treatment for lymph node recurrence after radical resection of esophageal squamous cell carcinoma. Radiat Oncol. 2019;14(1) doi: 10.1186/s13014-019-1377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeene P.M., Versteijne E., van Berge Henegouwen M.I., Bergmann J.J.G.H.M., Geijsen E.D., Muller K., et al. Definitive chemoradiation for locoregional recurrences of esophageal cancer after primary curative treatment. Diseas Esoph Off J Int Soc Diseases of the Esophagus. 2016 doi: 10.1111/dote.12539. [DOI] [PubMed] [Google Scholar]
- 20.Murray L.J., Din O.S., Kumar V.S., Dixon L.M., Wadsley J.C. Palliative radiotherapy in patients with esophageal carcinoma: A retrospective review. Pract Radiat Oncol. 2012;2:257–264. doi: 10.1016/j.prro.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Kassam Z., Wong R.K.S., Ringash J., Ung Y., Kamra J., DeBoer G., et al. A phase I/II study to evaluate the toxicity and efficacy of accelerated fractionation radiotherapy for the palliation of dysphagia from carcinoma of the oesophagus. Clin Oncol (Roy Coll Radiol (Great Britain)) 2008;20(1):53–60. doi: 10.1016/j.clon.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Walterbos N.R., Fiocco M., Neelis K.J., van der Linden Y.M., Langers A.M.J., Slingerland M., et al. Effectiveness of several external beam radiotherapy schedules for palliation of esophageal cancer. Clin Transl Radiat Oncol. 2019;17:24–31. doi: 10.1016/j.ctro.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J., Xu J., Chen Y., Zhuang W.u., Zhang Y., Chen Z., et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 24.Ólafsdóttir H.S., Klevebro F., Ndegwa N., Alexandersson von Döbeln G. Short-course compared to long-course palliative radiotherapy for oesophageal cancer: a single centre observational cohort study. Radiat Oncol. 2021;16(1) doi: 10.1186/s13014-021-01880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caspers R.J., Welvaart K., Verkes R.J., Hermans J., Leer J.W. The effect of radiotherapy on dysphagia and survival in patients with esophageal cancer. Radiother Oncol J Europ Soc Therapeut Radiol Oncol. 1988;12:15–23. doi: 10.1016/0167-8140(88)90188-0. [DOI] [PubMed] [Google Scholar]
- 26.Albertsson M., Ewers S.-B., Widmark H., Hambraeus G., Lillo-Gil R., Ranstam J. Evaluation of the palliative effect of radiotherapy for esophageal carcinoma. Acta oncologica (Stockholm, Sweden) 1989;28(2):267–270. doi: 10.3109/02841868909111261. [DOI] [PubMed] [Google Scholar]
- 27.Kellokumpu-Lehtinen P., Huovinen R., Nikkanen V. Survival and esophageal passage after radiotherapy of inoperable esophageal carcinoma. A retrospective study of 106 cases. Acta oncologica (Stockholm Sweden) 1990;29:175–178. doi: 10.3109/02841869009126541. [DOI] [PubMed] [Google Scholar]
- 28.Sun J.-M., Shen L., Shah M.A., Enzinger P., Adenis A., Doi T., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet (London, England) 2021;398(10302):759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 29.Luo H., Lu J., Bai Y., Mao T., Wang J., Fan Q., et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA. 2021;326(10):916. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner B., Ilson D.H., Minsky B.D., Bains M.S., Tong W., Gonen M., et al. Phase I trial of combined-modality therapy for localized esophageal cancer: escalating doses of continuous-infusion paclitaxel with cisplatin and concurrent radiation therapy. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(1):45–52. doi: 10.1200/JCO.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y., Wu X., Bu S., He C., Wang W., Liu J., et al. Promising outcomes of definitive chemoradiation and cetuximab for patients with esophageal squamous cell carcinoma. Cancer Sci. 2012;103(11):1979–1984. doi: 10.1111/j.1349-7006.2012.02393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.