Abstract
Psychological stress in a chronic course is implicated in various diseases, such as coronary artery disease, diabetes, ulcerative colitis, and psychosomatic pain disorders. Commensal microbiota in the host tissues interact with each other and maintain overall health. Oral and gut microbiomes are considered as the most ecologically rich and taxonomically diverse microbiota communities in humans. The effects of psychological stress on the gut microbiome have been well documented, and the interaction is commonly referred as the microbiota-gut-brain axis. Like the gut microbiome, the oral microbiome contributes to maintaining both local and systemic health. Although the effects of psychological stress on the oral microbiome have been studied, comprehensive knowledge about the oral-brain axis is lacking. The oral cavity and gut can communicate with each other through the microbiota. Three-way interactions within the oral-gut-brain microbiota might exist in patients with psychological stress and disorders. The effect of psychological stress on the gut and oral microbiomes, and the potential interactions within the oral-gut-brain axis are discussed in this review.
Keywords: Psychology, Oral, Microbiome, Gut, Oral-gut-brain axis
1. Introduction
The term stress is derived from the Latin word “stringere”, meaning “tight” or “strained”. Stress refers to anything that threatens the homeostasis in the body. Psychological stress develops when the demands of the environmental factor exceed the adaptive capacity of the individual [1]. In chronic cases, this maladaptation can affect the emotional, physiological, and behavioral aspects and alter the etiology and course of many diseases. Psychological stress is implicated in various health conditions such as coronary heart disease, hypercholesterolemia, diabetes, peptic ulcer, ulcerative colitis, asthma, poor immunity, and psychosomatic pain disorders [2], [3].
Microorganisms live as a community within human tissues and maintain commensal, symbiotic, or pathogenic relationships with the host tissue [4]. These communities are referred to as “microbiomes”. Microbiomes are found in various tissues such as the oral cavity, gastrointestinal tract, skin, vagina, and uterus. They interact with each other and the host to maintain the overall health [5]. Several host factors, such as diet, drugs, and the systemic condition, affect the microbiome [6]. The Human Microbiome Project, the largest study on human microbes to date, suggests that the gastrointestinal or gut microbiome and the oral microbiome are the two most ecologically rich and taxonomically diverse microbiomes in the human body [7]. The effects of psychological stress and psychiatric conditions on gut microbiome are well documented [8]. Dysbiosis of the gut is often related to the mental status, and this association has been attributed to the microbiota-gut-brain axis [9]. The gut-brain axis refers to the bidirectional interaction between the brain and the gastrointestinal system, mostly mediated through the gut microbiome. This interaction involves the autonomic nervous system, which includes the sympathetic and parasympathetic limbs, central nervous system, hypothalamus-pituitary-adrenal axis, and the enteric nervous system of the gut [10]. Microbiome-mediated alterations of the gut-brain axis have been associated with many diseases, such as anxiety and depression, inflammatory bowel disease, poor cognitive function, Parkinson’s disease, and Alzheimer’s disease [8].
Additionally, the existence of an oral-gut-brain axis has been proposed recently [11], [12]. The oral-gut-brain axis was defined based on anatomical communications between the oral cavity and the intestines. Oral bacteria naturally translocate to the digestive tract, where they may form ectopic colonies and potentially cause gut dysbiosis [13]. Similar to the gut microbiome, the oral microbiome contributes to the maintenance of the health of the host. Moreover, alterations in the oral microbiome have been associated with various systemic diseases, such as Alzheimer’s disease, cardiovascular diseases, pancreatic cancer, colorectal cancer, and rheumatoid arthritis [14]. Although the effects of psychological stress on the oral microbiome have been studied, comprehensive knowledge with regard to the oral-brain axis remains lacking.
The aim of this review was to summarize the findings on the effect of psychological stress on the gut and oral microbiomes. Additionally, the potential interplay between the microbiota gut-oral-brain axis and psychological stress was discussed.
2. Psychological stress and general body response
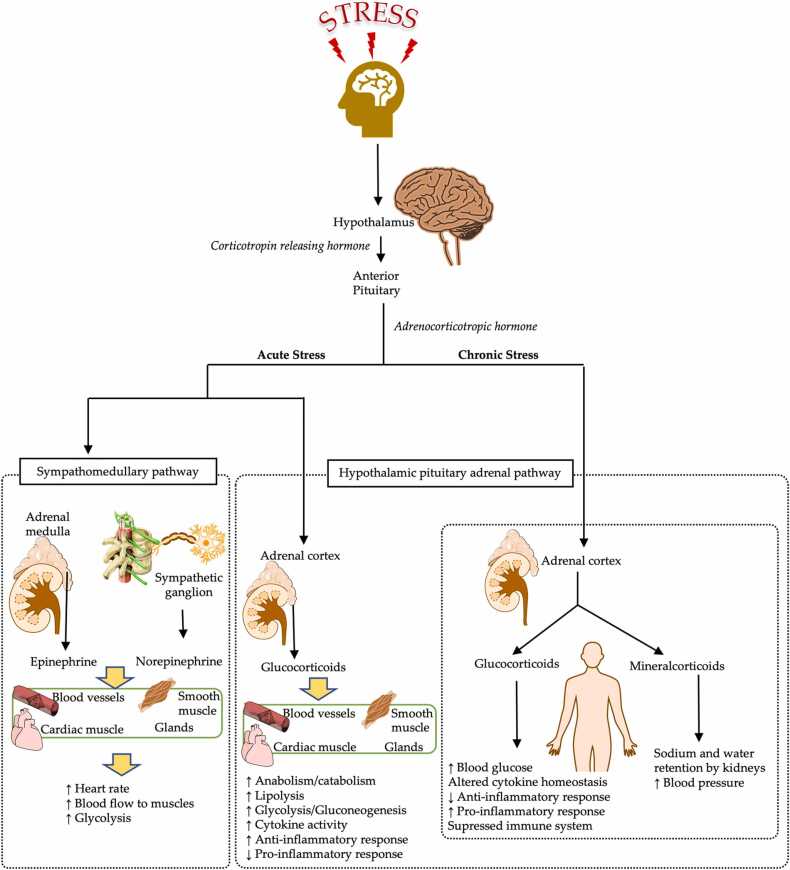
When exposed to stress, the body tries to maintain homeostasis by eliciting a “stress response”, a term introduced and defined by Selye in 1956 [1]. This physiological stress response was defined after taking the sympathetic adrenal-medullary (SAM) and hypothalamus-pituitary-adrenal (HPA) activities into consideration (Fig. 1). Acute stress activates the SAM and HPA pathways as an adaptive process and leads to the production of stress hormones and diverted distribution of the energy [1], [15], [16]. The SAM activity produces catecholamines through the adrenal medulla, and the HPA axis directs the hypothalamus to produce corticotrophin-releasing factor (CRF); CRF acts on the pituitary gland to produce adrenocorticotropin hormone (ACTH), which acts on the adrenal cortex to produce cortisol [15], [16]. The stress hormones, catecholamines and cortisol, increase lipolysis and glycogenolysis in the body to increase energy production. The increased energy is then distributed to various organs. This process is facilitated by the increase in the blood pressure and heart rate, and vasoconstriction [15], [16]. Activation of the innate immune system to produce more immune cells is another mechanism used to cope with acute stress; the immune cells act as a line of defense to protect the tissues from damage.[17]. Although these mechanisms aid in maintaining homeostasis in the body, they can become maladaptive under chronic stress conditions. Chronic activation of the sympathetic nervous system leads to a continuous increase in blood pressure, and chronic activation of the HPA axis leads to alterations in the expression levels of the inflammatory cytokines [1], [18]. These changes in the body eventually result in various stress-related diseases, such as coronary heart disease, hypercholesterolemia, diabetes, peptic ulcer, ulcerative colitis, asthma, poor immunity, and psychosomatic pain [19]. Furthermore, the stress-related hormones and proteins produced during the response may contribute to alterations in microbiomes, such as the gut and oral microbiomes directly.
Fig. 1.
The hypothalamus-pituitary-adrenal (HPA) axis. The HPA axis is activated differently during acute and chronic stress.
3. Psychological stress and the gut-brain axis
The gut-brain axis refers to the bidirectional intercommunication between the brain and the gastrointestinal system, which involves the autonomic nervous system [9], [10]. Several preclinical, clinical and epidemiological studies have shown that alterations in the gut can cause diseases related to the brain and vice-versa. Specifically, associations between psychological disorders (such as stress, anxiety, depression, and mood disorders) and gut diseases (such as irritable bowel syndrome and ulcerative colitis) have been reported [20].
Microbiota is one of the most important factors associated with gut-brain interactions [21]. Among several studies on the role of microbiota in the gut-brain axis, those involving germ-free rodents presented with the most convincing results; ablation of the gut microbiome in rodents from birth resulted in a wide range of physiological, immunological, and psychological problems [9]. Notably, the sensory-motor functions of the gut were affected. Moreover, a poorly-functioning blood-brain barrier and alterations in the regulation of neurotransmitters, neurogenesis, and myelination of the prefrontal cortex have been reported [22]. These changes resulted in poor cognition and the development of anxiety and depression-like symptoms. Interestingly, restoration of the gut microbiome in germ-free rodents improved the psychological symptoms [9]. Furthermore, individuals with Alzheimer’s disease, Parkinson’s disease, and depression experienced a shift in the gut microbiome profile when compared to healthy controls [23], [24], [25]. Transplantation of the gut microbiota from patients with these diseases to germ-free mice resulted in the development of symptoms specific to the disease in the animals [9], [26]. Taken together, these findings indicate the role of the microbiota-gut-brain axis in various psychological disorders.
Among the several possible pathways that act on the microbiota-gut-brain axis, the gut-vagus nerve-brain pathway, gut-immune system-brain pathways, and gut-microbiota derived metabolites-brain pathways are most notable. All these routes are mediated by several neuroactive compounds, such as microbiota-derived metabolites and products and gut hormones derived from the host [27].
The vagus nerve-mediated pathway is the most important route for gut-brain interactions [28], [29]. The vagus nerve is the tenth cranial nerve originating in the brain stem and extending to the neck, thorax, and abdomen. In the thorax, it innervates the heart for parasympathetic supply and regulates the heart rate, whereas in the abdomen, it innervates the intestines to regulate the contraction of the smooth muscle and the secretion from the gland [30]. Therefore, the vagus nerve connects the emotional areas of the brain with the functions of the gut. The efferent fibers of this nerve to the gut carry signals for the secretion of gastric acids and digestive enzymes. The afferent fibers control satiety and energy metabolism by sending signals to the brain through various afferents, such as chemoreceptors and mechanoreceptors [28]. The gut hormones and peptides act on these receptors to regulate gut function. The easiest way for the microbiota to alter the vagus-brain pathway is by altering the gut hormones and peptides produced by enteroendocrine cells. Moreover, some gut bacteria are known to produce serotonin, an important neurotransmitter, which acts on the vagus nerve to alter the gut-brain axis [28], [29].
Another important pathway through which gut bacteria can affect the gut-brain axis is by altering the microbiota-derived metabolites and products such as short-chain fatty acids (SCFA), tryptophan, branched-chain amino acids, and peptidoglycans [31]. SCFA is produced by certain gut bacteria such as Bifidobacteria, Lactobacillus, Akkermansia, Ruminococcus, Faecalibacterium, and Lachnospiraceae, among others. These bacteria are known to decompose carbohydrates to produce SCFA, which maintains glucose homeostasis, immune cell function, and the release of serotonin [31], [32]. Additionally, the role of SCFA in maintaining the integrity of the epithelial barrier is well demonstrated [33]. SCFAs contribute to preserving the integrity of the gut barrier and blood-brain barrier (BBB) [33], [34]. Other microbiota-derived metabolites and products such as tryptophan, amino acids, and lipopolysaccharides can alter the functions of immune cells, particularly cytokine production, and interfere with inflammatory pathways within the central nervous system [28]. Thus, psychological stress-mediated alterations of the gut microbiome can affect the gut-brain axis and contribute to various diseases.
4. Psychological stress and gut microbes
The gut harbors the most abundant microbiota within its habitat and is dominated by three main bacteria phyla: Firmicutes, Bacteroidetes, and Actinobacteria [35]. The balance among these bacteria is vital for the homeostasis of the gut; a slight shift in the relative abundance might lead to the development of various diseases. For example, a high Firmicutes/Bacteroidetes ratio has been associated with metabolic diseases such as obesity and diabetes, whereas a low Firmicutes/Bacteroidetes ratio has been associated with inflammatory bowel disease and colorectal cancer [36]. Psychological stress is one of the factors that can alter the diversity and composition of the microbiota in the gut.
Alterations in the gut microbiome during psychological stress have been reported previously [8], [37]. In brief, most animal studies using the chronic stress model showed a significant reduction in bacteria, such as Lactobacillus and Bifidobacterium, at the genus level under different stress conditions [8], [38], [39]. Lactobacillus and Bifidobacterium, used as probiotics, have been shown to be effective in improving mental health in humans [40]. Therefore, reductions in these bacterial strains within the gut might act as stress markers in animal models of psychological stress.
A recent systematic review focusing on the gut microbiota during anxiety and depression showed inconsistent results in the alpha and beta diversity. However, the differential abundance of few bacterial taxa could be associated with various psychological disorders. [41]. Decreases in the abundance of Bacteroidetes, Prevotellaceae, Fecalibacterium, and Coprococcus during depression, and Firmicutes, Ruminococcaceae, and Dialister during anxiety have been observed in some studies [41]. Alternatively, other studies reported higher levels of Actinobacteria and Eggerthella in depression, and Enterobacterales and Enterobacteriaceae in anxiety [41]. These findings are supported by a meta-analysis on the composition of the gut microbiota in several psychiatric disorders; a higher abundance of Eggerthella and lower abundance of Faecalibacterium and Coprococcus was commonly observed in adults with anxiety, depression, bipolar disorder, psychosis, and schizophrenia [42]. Faecalibacterium and Coprococcus are involved in the production of beneficial SCFAs, whereas Eggerthella is associated with the depletion of SCFAs [43]. These findings indicate that psychological stress can affect microbes and their metabolites, which could further affect the gut-brain axis.
Some studies have shown the effect of stress-related hormones such as CRF, ACTH, catecholamines, and cortisol produced through the SAM and HPA axis on the gut microbiota [44], [45], [46], [47]. Most of these hormones are released into the gut through circulation. Hormones such as CRF and cortisol is produced in small amount in the gut by enteric neurons, enterochromaffin cells, and epithelial cells of the gut [47]. The direct effect of catecholamines on gut bacteria such as Yersinia enterocolitica, Escherichia coli, Campylobacter jejuni, Helicobacter pylori, and Salmonella enetrica has been previously demonstrated [44]. The effect of cortisol on gut microbiome was shown in pigs where gut Ruminococcus negatively predicted the level of serum cortisol [45]. The serum cortisol fully mediated the relationship between fecal Ruminococcus and a brain metabolite N-acetyl aspartate which highlighted the role of the gut-brain axis [45]. Post-stressor salivary cortisol in humans was negatively associated with the alpha and beta diversity of gut microbiota [46]. This direct interaction of stress-related hormones with microbiota has been commonly termed microbial endocrinology [47]. Stress hormones may also affect the gut microbiota through the modulation of host epithelium. ACTH, catecholamines, and cortisol are known to increase the adherence of bacteria to gut mucosa as well contribute to the uptake of microbiota into Peyer’s patches [48], [49], [50], [51]. These evidences show the role of stress hormones derived from SAM and HPA axis in modulating gut microbiota.
5. Psychological stress and oral microbes
The oral cavity is the second-largest colonizer of the microbiome after the gut [52]. Additionally, it is the gateway to the respiratory and digestive tracts. Alterations in the oral microflora can cause or indicate various oral and systemic diseases. Oral bacteria such as Prevotella, Porphyromonas, Treponema, Aggregatibacter, and Fusobacterium are associated with periodontitis, whereas Streptococcus mutans, Lactobacillus, and Neisseria are associated with dental caries. Streptococcus salivarius, Capnocytophagia gingivalis, Streptococcus mitis, and Streptococcus gordonii are reported to be associated with oral cancer. Furthermore, oral bacteria such as Lactobacillus, Rothia, and Fusobacterium have been associated with colorectal cancer, whereas Leptotrichia, Porphyromonas gingivalis, and Aggregatibacter have been associated with pancreatic cancer. The roles of salivary Camplylobacter rectus, Porphyromonas gingivalis, and Prevotella intermedia in cardiovascular diseases have been reported; likewise, Porphyromonas gingivalis, Aggregatibacter, Neisseria, Actinomyces, and Streptococcus are known to be involved with diabetes [53]. Conversely, multiple local and systemic factors can affect the microbiome in the oral cavity. Systemic diseases such as diabetes, gastrointestinal disorders, and liver diseases alter the oral microflora [54]. Psychological stress is another systemic factor that can affect the oral microbiome.
Various in vitro, in vivo, and clinical studies suggest the role of psychological stress on the oral microbiome. As shown in Table 1 [55], [56], [57], [58], [59], several in vitro studies focused on the effect of stress-related hormones and proteins, such as cortisol, adrenaline, noradrenaline, and mucin, on the oral microbiome. Shifts in the gene expression profiles of the microbiome, similar to those observed in periodontitis, were observed in a metatranscriptomic analysis performed on samples derived from cortisol-treated dental plaque [55]. Members of the Fusobacteria phylum (mainly Leptotrichia goodfellowii) showed a significantly higher number of transcripts in the cortisol-treated plaque samples [55]. The response of Fusobacterium nucleatum toward stress-related proteins and hormones has been examined. F. nucleatum is an anaerobic periodontal pathogen and a producer of volatile sulfur compounds, which causes halitosis. Although mucin, a stress-related salivary protein, was reported to increase the viability of F. nucleatum, cortisol did not appear to have any effect on the bacteria [56]. On the other hand, adrenaline, noradrenaline, and cortisol significantly reduced the growth of F. nucleatum after 12 h of treatment in an in vitro study by Calil et al. [57]. Additional studies are required to clarify whether F.nucleatum is associated with psychological stress.
Table 1.
In vitro and in vivo studies focusing on the effect of psychological stress on the oral microbiome.
| Experiment | Results | Reference |
|---|---|---|
| In vitro | ||
| Samples of dental plaque treated with cortisol and performed metatranscriptomic analysis | Cortisol directly induced shifts in the gene expression profiles of the oral microbiome that reproduce results found in the expression profiles of periodontal disease and its progression. Members of the phylum Fusobacteria (class Fusobacteria and order Fusobacteriales) showed significantly increased activity (increased the number of transcripts significantly) after the addition of cortisol. |
[55] |
| Volatile sulfur-producing oral bacteria treated with mucin and cortisol | Mucin increased the viability of F. nucleatum. Cortisol did not affect S. moorei and F. nucleatum. |
[56] |
| Volatile sulfur-producing oral bacteria treated with adrenaline, noradrenaline, and cortisol | Adrenaline, noradrenaline, and cortisol significantly reduced F. nucleatum growth after 12 h and 24 while reduced Porphyromonas endodontalis growth after 24 h. Prevotella intermedia and Porphyromonas gingivalis showed no effects on bacterial growth. |
[57] |
| 43 microorganisms of subgingival microbial complexes treated with noradrenaline and adrenaline | Noradrenaline treatment showed positive growth in Actinomyces naeslundii, Actinomyces gerenscseriae, Eikenella corrodens, and Campylobacter gracilis. Noradrenaline treatment inhibited P. gingivalis and B. forsythus. Responses to adrenaline tended to mirror the responses seen with noradrenaline. |
[58] |
| Noradrenaline treated with P. gingivalis | Dose-dependent inhibition of P.gingivalis by noradrenaline was seen at 24 h and 36 h of treatment. | [59] |
| In vivo | ||
| Fecal glucocorticoid metabolites, a measure of hypothalamic–pituitary–adrenal axis correlated with the oral microbiome in wild red squirrel using 16 S rRNA gene sequencing | Alpha diversity of the oral microbiome was lower in individuals with elevated fecal glucocorticoid metabolites. Relative abundance of oral Pasteurellaceae increased with increasing fecal glucocorticoid metabolites. |
[60] |
| Evaluation of oral microbiome in chronic restraint stress using rat | Alpha diversity of the oral microbiome was significantly reduced in the stress group. Facklamia was increased while Corynebacterium was decreased in the stress group. |
[61] |
The growth of the periodontal pathogen Porphyromonas endodontalis was inhibited following treatment with adrenaline, noradrenaline, and cortisol for 24 h; however, no inhibitory effects were observed in the case of Porphyromonas gingivalis and Prevotella intermedia [57]. In contrast, stimulation with noradrenaline for 24 and 36 h inhibited the growth of P. gingivalis in a dose-dependent manner [59]. Roberts et al. reported that noradrenaline and adrenaline promoted the growth of Actinomyces, Eikenella, and Campylobacter, and inhibited the growth of P.gingivalis and Bacteroides forsythus within the subgingival microbial complexes [58]. These in vitro findings indicate that hormonal alterations during psychological stress could affect the oral microbiome.
Various preclinical (Table 1) [60], [61] and clinical studies (Table 2) [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72] have demonstrated the effect of psychological stress on the oral microbiome via alterations in the diversity and taxonomic abundance of the bacteria. The alpha diversity measures the richness or evenness of the species within a sample, which is commonly determined by 16 S rRNA sequencing. The alpha diversity and other phylogenetic diversities were reported to be higher in a group of high-distress patients compared to those in the low-distress patients [68]. In contrast, the study by Simpson et al. concluded that the composition of the oral microbiome, not the diversity, was associated with anxiety and depression in adolescents [66]. A significantly lower alpha diversity, measured by the Chao1 index, Shannon index, and phylogenetic diversity whole tree index, was observed in patients with Alzheimer’s disease compared to healthy controls [63]. The discrepancies in findings among these studies may be due to differences in the local and systemic factors among patients with similar psychological disorders.
Table 2.
Clinical studies focusing on the effect of psychological stress on the oral microbiota.
| Experiment | Findings | Reference |
|---|---|---|
| 16SrRNA sequencing of the oral microbiome in patients with depression and matched controls |
Genera Prevotella, Haemophilus, Rothia, Treponema, Neisseria, Solobacterium, Lepotrichia, Fusobacterium were less abundant in the depressed patients. Prevotella nigrescens and Neisseria genera were significantly more abundant in depressed patients.Clear separation of depressed and healthy control cohorts into distinct clusters. |
[62] |
| 16SrRNA sequencing of salivary flora in patients with Alzheimer’s disease and healthy controls |
Alpha diversity analysis showed significantly lower richness (Chao1 index) and diversity (Shannon index, phylogenetic diversity whole tree index) in Alzheimer’s disease patients than in healthy controls. Genera Moraxella increased significantly in AD patients. |
[63] |
| Terminal restriction fragment length polymorphism (T-RFLP) analysis of amplified 16SrRNA genes in individuals performing a challenging expedition for 7 days in the Himalayas with a destination 3000 m above the starting point | Twenty-eight individuals remained symptom-free (Group I), while 30 participants developed periodontal problems, mostly gingivitis (Group II). F. nucleatum was more prevalent in Group II, as shown by RTQ-PCR. T-RFLP analysis showed F. nucleatum was more prevalent in Group II, and the proportions were increased more frequently than in Group I corroborating the results obtained by RTQ-PCR. |
[64] |
| 16 S RNA sequencing of the oral microbiome in Israeli veterans with Post Traumatic Stress Disorder (PTSD) after participating in war |
A microbiota signature (reduced bacteria such as sp_HMT_914, 332, and 871, and Noxia) correlated with PTSD severity and additional psychopathological symptoms, including anxiety, hostility, memory difficulties, and idiopathic pain. | [65] |
| 16 S rRNA gene sequencing between patients of anxiety or depression with low and high symptoms |
No significant differences in alpha and beta diversity were seen between anxiety or depression groups (high versus low symptoms). Higher Spirochaetes and Spirochaetales in participants with high anxiety or depression symptoms. Depression and anxiety symptoms were associated with the differential abundance of Spirochaetaceae, Actinomyces, Treponema, Fusobacterium and Leptotrichia spp. |
[66] |
| 16 S rRNA gene sequencing of patients with schizophrenia, mania, major depressive disorder, and controls without a psychiatric disorder |
Neisseria subflava, Weeksellaceae, and Prevotella, were decreased, whereas Streptococci was increased in individuals with schizophrenia or mania as compared to controls. Neisseria subflava was also positively associated with cognitive functioning. There were no taxa significantly altered in individuals with major depression. |
[67] |
| 16 S rRNA gene sequencing in patients with low psychological distress and with high psychological distress |
The high-distress groups had higher alpha diversity compared to the low-distress groups. The top-ranked taxa positively associated with host distress included Leptotrichia, Bacteroidetes, Selenomonas, and Haemophilus, while the bottom-ranked negatively associated taxa predominantly consisted of the Prevotella genus. |
[68] |
| Investigating salivary-induced aggregation of Streptococcus gordonii 10 min before an academic examination and subsequently 2 and 6 weeks later, under non-stressed conditions | A significant reduction in the aggregation of Streptococcus gordonii was observed in saliva collected 10 min before an academic examination when compared to that observed in saliva collected 2 and 6 weeks after the examinations. | [69] |
| Examining the social, psychological, and behavioral predictors of salivary bacteria in children at risk for Early Childhood Caries | High baseline salivary cortisol was associated with increased salivary Streptococcus mutans. | [70] |
| Examining the role of stress and bacteria in childhood dental caries | Low socioeconomic status, high salivary cortisol, and high cariogenic bacteria (S. mutans and Lactobacillus) were each significantly associated with dental caries. | [71] |
| Investigating the associations among salivary bacteria, oral emanations of volatile sulfur compounds, and academic-related chronic stress | Academic-related chronic stress in students increased salivary Solobacterium moorei levels. S. moorei was correlated with F. nucleatum, which was further correlated with hydrogen sulfide in the stress group, resulting in halitosis. | [72] |
Thus, using an animal model might overcome the differences in local and systemic factors as seen in humans and provide reliable data on the alterations in the oral microbiome due to psychological stress. In our recent study comprising a rat model of chronic restraint stress, 16 S rRNA sequencing of oral microbiota revealed that the alpha diversity as measured by observed operational taxonomic unit and faith phylogenetic diversity was significantly lower in the stress group when compared to that in the control group [61]. Consistent with our findings, a previous experiment on wild red squirrels showed that the alpha diversity of the oral microbiome was lower in those with elevated fecal glucocorticoid metabolites, a measure of the hypothalamic-pituitary-adrenal axis [60]. Clinically, reductions in the alpha diversities of the oral microbiome have been observed in stress-related oral diseases, such as recurrent aphthous stomatitis, oral lichen planus, and Sjogren’s syndrome [73]. These findings indicate that a reduction in the alpha diversity of the oral microbiome might be associated with psychological stress. However, further studies are required to confirm this phenomenon.
Beta diversity measures the similarities or dissimilarities of the communities among various samples. The separation of healthy and stressed cohorts into different clusters using the principal coordinate analysis plot indicates that the communities in the two cohorts are different from each other. No significant differences in the beta diversities of the oral microbiome were observed between anxiety and depression patients with high and low symptoms [66]. A separate, but statistically non-significant, clustering was observed between the stress and control groups in an in vivo experiment using a rat model of chronic restraint stress [61]. However, a clinical study performing a constrained analysis of the oral microbiome reported significant clustering between the healthy and depressed cohorts [62]. Thus, additional preclinical and clinical studies are required to understand the alterations in the beta diversities of the oral microbiome due to psychological disorders.
A few preclinical and clinical studies have demonstrated the alterations in the taxonomic abundance of the oral microbiome due to psychological stress. Although evidence of a specific genera or species of oral bacteria as a potential biomarker is lacking, numerous bacteria, such as Prevotella, Neisseria, Haemophilus, and Fusobacterium are reported to be involved in this phenomenon. The abundance of oral Prevotella and Neisseria were reported to be decreased in psychological disorders, including distress, depression, schizophrenia, and mania [62], [67]. Moreover, oral Neisseria was found to be positively correlated with cognitive functions [67]. A reduction in the abundance of oral Haemophilus was observed in patients with depression [62]; conversely, the abundance of this bacteria was increased in those with host distress [68]. Similarly, reduced Fusobacterium was observed in patients with depression [62]. In contrast, F. nucleatum was found to be more prevalent in individuals who underwent a stressful event (reached the destination after a challenging expedition in the Himalayas) [64]. Significant increases in the abundance of the phylum Spirochaetes and order Spirochaetales were observed in oral cavity of participants with high depression symptoms when compared to those with low depression symptoms [66]. These bacteria were positively correlated with symptoms of anxiety and depression. In another study, Streptococci was increased in individuals with schizophrenia or mania [67]. A significant reduction in the aggregation of S. gordonii was observed in unstimulated whole saliva collected 10 min before an academic examination compared to that collected 2 and 6 weeks after the examinations [74]. Facklamia and Corynebacterium were reported to be significantly increased and decreased, respectively, in the oral cavities of rats with chronic resistant stress when compared to those in the control group [61].
The relationship between psychological stress and cariogenic bacteria has been demonstrated previously. A study comprising children with early childhood caries reported that high baseline salivary cortisol was associated with increased salivary Streptococcus mutans [70]. In another study, low socioeconomic status, high salivary cortisol, and high amounts of cariogenic bacteria (S. mutans and Lactobacillus) were found to be significantly associated with dental caries [71]. Furthermore, higher levels of salivary cortisol were associated with thinner and softer enamel surfaces in exfoliated teeth. Psychological stress might be involved in causing halitosis through alterations in the oral microbiome. In one study, increased levels of salivary Solobacterium moorei were observed in students with academic-related chronic stress. The levels of S. moorei were correlated with those of F. nucleatum, which were further correlated with that of hydrogen sulfide in the stress group, resulting in halitosis [72]. The diverse results of altered oral bacteria in psychological stress may be confusing for now but with more studies concrete findings could be expected in the future.
One important local factor in oral microbiome composition is the saliva and salivary glands. Saliva is a mixture of proteins, ions, and metabolites derived from the salivary glands and serum as well. The salivary glands and its secretion are under the control of ANS which is the most important pathway of the stress response. The salivary secretion is regulated by the parasympathetic branch of ANS while the salivary protein is regulated by both the sympathetic and parasympathetic branches [75]. Therefore, psychological stress can induce alteration of salivary glands and saliva including morphological and functional alterations, which may affect the oral microbiomes. The psychological stress has been shown to induce morphological changes in salivary glands in shape, size, and vacuolations of acinar cells, myoepithelial cells, and ductal cells in animal models [76], [77]. The administration of stress-related hormones such as cortisol in rats has been shown to induce disarranged and shrunk acinar cells, changes in the diameter of striated ducts, and height of ductal cells of striated ducts [78], [79]. These morphological changes may be involved in functional alterations manifested as xerostomia, sialorrhea, or altered salivary components, mainly proteins under stressed conditions.
Salivary proteins such as alpha-amylase, cortisol, dehydroepiandrosterone, IL-1β, IL-6, TNF-α, and IL-10 have been shown to alter during psychological stress conditions in humans [80], [81], [82], [83], [84]. Recently, we have demonstrated that chronic stress in mice models induces an increase in inflammatory cytokines such as IL-1β and IL-6 in the salivary gland and saliva [85]. The altered salivary proteins, mainly inflammatory cytokines may alter the oral microbiome. Oral microbiota such as Prevotella and Ruminococcaceae were positively associated with IL-1β while Prevotella and Granulicatella were negatively associated with IL-8 [86]. In another study, a negative correlation was observed between microbial alpha diversity and salivary interferon-γ, interleukin-17A, and interleukin-1β in patients with stress-related diseases such as oral lichen planus and recurrent aphthous stomatitis [73]. Salivary glands may affect the oral microbiome by acting as a route to transmit microbiota into saliva. Although studies on bacteria are limited, enteric viruses were shown to replicate in salivary glands and infect through saliva [87]. Further studies are required to prove the potential of bacteria to use the salivary gland route. Taken together, the findings suggest that the alteration in salivary glands and salivary proteins due to psychological stress may alter the oral microbiome.
It is still unclear whether stress hormones in saliva directly affect the oral microbiome. A study examining the effect of salivary cortisol at a concentration equivalent to that in patients with periodontitis found that the cortisol directly induced shifts in the gene expression profiles of the oral microbiome. The changes in gene expression profiles mimicked community-wide expression profiles seen in periodontitis [88]. A significant correlation between the level of salivary stress hormones including DHEA, cortisol, and β-endorphin, and parameters of periodontitis were observed. These findings indicate that salivary stress hormone may directly affect periodontal pathogens [89], [90].
6. Oral-gut axis
Oral-gut barrier, mainly the physical distance and difference in chemical environment of oral cavity and gut contributes to separate microbiota community in each of these habitats. The oral microbiome has been classified into 619 taxa in 13 phyla, with the Firmicutes and Bacteroidetes phyla accounting for nearly 50% of the bacteria [91]. This proportion is higher in the gut, where the Bacteroidetes and Firmicutes phyla account for more than 90% of the bacteria [92]. Large numbers of oral bacteria are swallowed in the saliva. The harsh chemical environment, owing to the low pH level of the gastric acid, makes it difficult for many of the oral bacteria to colonize in the gut. However, various circumstances, such as disease, drugs, and aging, aid the colonization of the oral bacteria in the gut [93]. Oral bacteria such as Rothia, Scardovia, Actinomyces, and Micrococcaceae were found to be over-represented in the fecal microbiome of patients taking protein pump inhibitor drugs, which lower the acidity of the gastric juice [94]. A comparative study showed high similarities in the beta diversities of the fecal and oral microbiota in the elderly (≥80 years old) when compared to those in the adult population (age, 35.9 ± 5.0 years); oral bacteria such as Eggerthella, Corynebacterium, Butyricimonas, Christensenellaceae, Dehalobacterium, Peptococcaceae, and Campylobacter were significantly increased in the fecal samples of the elderly [95]. Furthermore, oral bacteria such as Porphyromonas, Treponema, Fusobacterium, and Pseudoramibacter were found to be enriched in elderly-associated bacterial co-abundance groups compared to the adult- and infant-associated bacterial co-abundance groups [96]. Oral bacteria such as F. nucleatum and P. gingivalis have been detected in gastrointestinal diseases, such as inflammatory bowel disease and colorectal cancer [97], [98], [99]. There is convincing evidence of the transmission of microbiota from the oral cavity to the gut in healthy individuals. The translocation and colonization of oral microbiota to the large intestine were common and extensive among healthy individuals in a study comprising oral and fecal samples from 470 individuals belonging to five different countries [100]. These findings indicate that the oral bacteria can translocate to the gut and alter the microbiota of the gut.
The direct translocation of gut bacteria to the oral cavity may not be as common as that of oral bacteria to the gut. The human hand is a rich reservoir of microbiota; therefore, transmission through the fecal-oral route is possible. A study on the characterization of the fecal, oral, hand, and forehead microbiota showed that 48.9% had fecal signal and 67.2% had oral signal on palms [101]. Many bacteria such as Vibrio cholerae, Clostridium difficile, Shigella, Salmonella, Escherichia, and Campylobacter have been shown to transmit through this route and cause infectious disease [102]. The passage of these bacteria through the oral cavity may alter the composition of the oral microbiome and pass to the gastrointestinal tract. These findings indicate the presence of a bidirectional communication between the altered microbiota in the oral cavity and the gut (due to psychological stress), which affects the two habitats.
7. Oral-gut-brain axis
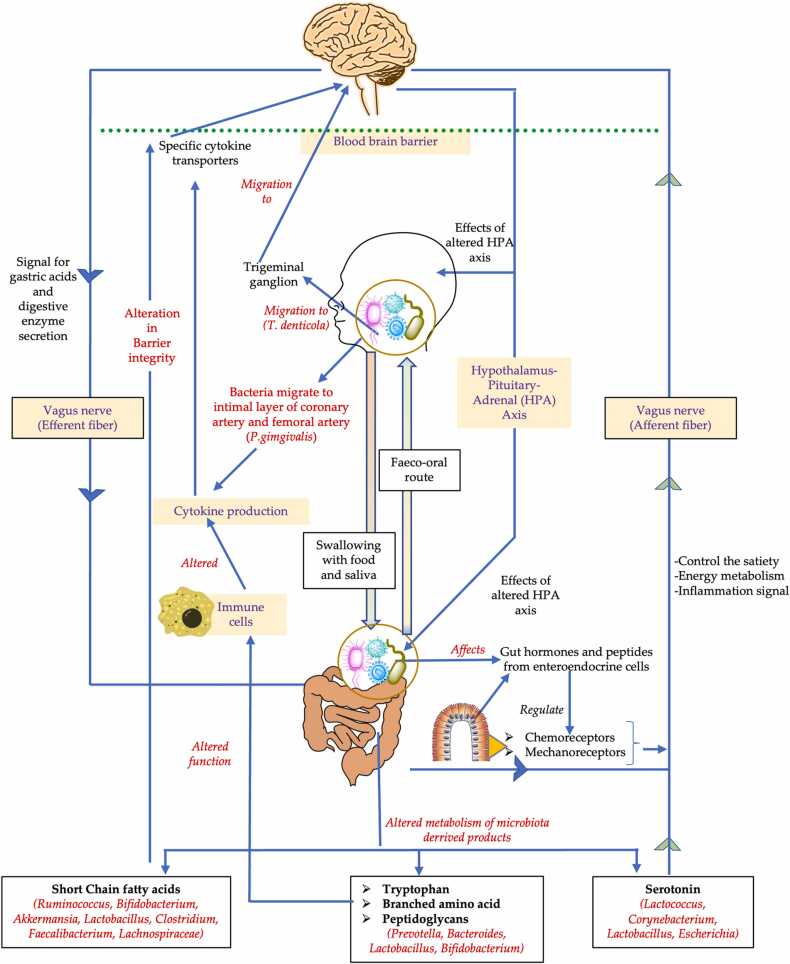
The oral-gut-brain axis is a theoretical concept rather than a materialized anatomical axis like the gut-brain axis. The idea of a microbiota oral-gut-brain axis is guided by evidence from each of the three components of the axis; oral-brain axis, gut-brain axis, and communication between the oral and gut microbiota (Fig. 2). These components are thought to interact with each other. The presence of an oral-brain axis has been demonstrated in some studies [11], [12]. The breakdown of the blood-brain barrier by bacteria or its products and the migration of bacteria to the brain through the trigeminal nerve has been identified as the two methods by which the oral microbiota reach or influence the function of the brain. P. gingivalis has been shown to migrate to the intimal layers of the coronary and femoral arteries through the bloodstream [103]. This migration may produce an acute inflammatory condition in the body resulting in the production of inflammatory cytokines, which can cross the blood-brain barrier through specific cytokine transporters to enter the brain [12]. Another pathway for the bacteria and their products to enter the brain could be via the trigeminal nerve. An oral bacterium, Treponema denticola, was found in the trigeminal ganglion and hippocampus of patients with Alzheimer’s disease [104]. This finding was supported in another preclinical study in mice, wherein oral T. denticola infection induced the production of amyloid-β in the hippocampus [105]. It remains unclear as to how the bacteria migrated to hippocampus; nonetheless, it is thought that the oral bacteria may have used the trigeminal route to reach the brain. Corynebacterium, a bacterium that produces serotonin in the gut, was found to be decreased in the oral cavities of rats exposed to chronic restraint stress [61]. The reduction of oral Corynebacterium that possibly produces serotonin in the chronic stress is of interest in oral-brain axis. Further investigations are needed to clarify the meaning of this phenomenon.
Fig. 2.
Basic pathways for the potential microbiota-oral-gut-brain pathway. The text marked in red shows the pathways that may be affected by the alteration in oral and gut microbiota. The bacteria name in the () shows some bacteria involved in the process.
Psychological stress may affect the oral and gut microbiomes independently thereby forming the oral-brain axis and oral-gut axis, respectively. The evidence of the oral-brain axis as discussed above has been gathered in recent years and may be established as a separate axis in the future. Additionally, due to psychological stress, the altered microbiota in the oral cavity and gut can communicate with and influence each other, thereby forming a complex of axes, including the potential oral-gut-brain axis. However, insufficient evidence warrants further studies to understand the microbiota-oral-gut-brain axis.
8. Future perspectives
Research on the effect of psychological stress on the gut and oral microbiota is challenging due to the factors such as stress response, coping, adaptation, and personality background, which are involved in the biological responses to psychological stress. Moreover, psychological stress is a feeling or state, and its quantification might not necessarily reflect the actual stress level. Therefore, individuals with the same levels of stress might not elicit the same responses [106].
Technological advancements and high throughput sequencing have enabled researchers to clarify the nature of the microbiota. However, it is difficult to determine the interactions between the microbiota (and their metabolites) and the host [107]. The gut and oral cavity consist of various tissues and fluids, which can be affected by multiple local and systemic factors. These factors may differ among individuals and affect the microbiota in different ways. Moreover, the oral health condition may vary among healthy individuals. These underlying confounding factors and ethical considerations make it difficult to accurately determine the effect of psychological stress on the gut and oral cavity microbiota in humans. Controlled studies, such as animal models of psychological stress, have been used to overcome this limitation. However, not all changes in animal models correlate with the stress-related effects in humans. Sample collection is another major challenge in oral microbiome studies. The microbiome of the oral cavity is diverse and site-dependent. Different microbial communities have been observed in oral sites, such as the tongue, palate, buccal mucosa, and gingiva [108]. Moreover, considerable diversity has been observed among various parts of the tongue [109]. The technique used for sample collection is sensitive in oral microbiome studies and could affect the results [52]. Thus, overcoming these difficulties in the future remains challenging. However, with the advancements in techniques and study designs, it might be possible to collect more evidence and clarify the interactions between the microbiota-oral-gut-brain axis.
9. Conclusion
Psychological stress has a profound effect on the gut and oral microbiomes. The impact of psychological stress on the gut microbiome has been extensively studied under the microbiota-gut-brain axis. However, the effect of psychological stress on oral microbiome is yet to be fully understood. This review summarized the findings of studies focusing on the effect of psychological stress on the oral microbiome. Bidirectional transmission of bacteria between the oral cavity and the gut and interactions within the microbiota-oral-brain axis were discussed. Evidence suggests the presence of a microbiota-oral-gut-brain axis that acts during psychological stress. Nonetheless, future studies are required to understand the complex interactions between psychological stress and the oral and gut microbiomes.
Conflict of interest
There is no conflict of interest associated with this study.
Acknowledgements
None.
References
- 1.Selye H. McGraw-Hill Book Company; New York: 1956. The Stress of Life. [Google Scholar]
- 2.Kim Y.-K., Maes M. The role of the cytokine network in psychological stress. Acta Neuropsychiatr. 2003;15:148–155. doi: 10.1034/j.1601-5215.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y.-Z., Wang Y.-X., Jiang C.-L. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017:11. doi: 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang D., Leung R.K.-K., Guan W., Au W.W. Involvement of gut microbiome in human health and disease: brief overview, knowledge gaps and research opportunities. Gut Pathog. 2018;10:3. doi: 10.1186/s13099-018-0230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y., Zhou J., Wang L. Role and mechanism of gut microbiota in human disease. Front Cell Infect Microbiol. 2021:11. doi: 10.3389/fcimb.2021.625913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulangé C.L., Neves A.L., Chilloux J., Nicholson J.K., Dumas M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karl J.P., Hatch A.M., Arcidiacono S.M., Pearce S.C., Pantoja-Feliciano I.G., Doherty L.A., et al. Effects of psychological, environmental and physical stressors on the gut microbiota. Front Microbiol. 2018:9. doi: 10.3389/fmicb.2018.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cryan J.F., O’Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 10.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol n.d.;28:203–209. [PMC free article] [PubMed]
- 11.Narengaowa Kong W., Lan F., Awan U.F., Qing H., Ni J. The oral-gut-brain AXIS: the influence of microbes in Alzheimer’s disease. Front Cell Neurosci. 2021:15. doi: 10.3389/fncel.2021.633735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sansores-España L.D., Melgar-Rodríguez S., Olivares-Sagredo K., Cafferata E.A., Martínez-Aguilar V.M., Vernal R., et al. Oral-gut-brain axis in experimental models of periodontitis: associating gut dysbiosis with neurodegenerative diseases. Front Aging. 2021:2. doi: 10.3389/fragi.2021.781582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitamoto S., Nagao-Kitamoto H., Hein R., Schmidt T.M., Kamada N. The bacterial connection between the oral cavity and the gut diseases. J Dent Res. 2020;99:1021–1029. doi: 10.1177/0022034520924633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willis J.R., Gabaldón T. The human oral microbiome in health and disease: from sequences to ecosystems. Microorganisms. 2020;8:308. doi: 10.3390/microorganisms8020308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadsworth M.E., Broderick A.V., Loughlin‐Presnal J.E., Bendezu J.J., Joos C.M., Ahlkvist J.A., et al. Co‐activation of SAM and HPA responses to acute stress: a review of the literature and test of differential associations with preadolescents’ internalizing and externalizing. Dev Psychobiol. 2019;61:1079–1093. doi: 10.1002/dev.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godoy L.D., Rossignoli M.T., Delfino-Pereira P., Garcia-Cairasco N., de Lima Umeoka E.H. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci. 2018:12. doi: 10.3389/fnbeh.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breen M.S., Beliakova-Bethell N., Mujica-Parodi L.R., Carlson J.M., Ensign W.Y., Woelk C.H., et al. Acute psychological stress induces short-term variable immune response. Brain Behav Immun. 2016;53:172–182. doi: 10.1016/j.bbi.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R., et al. Compr. Physiol. Wiley; 2016. Regulation of the hypothalamic‐pituitary‐adrenocortical stress response; pp. 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salleh M.R. Life event, stress and illness. Malays J Med Sci. 2008;15:9–18. [PMC free article] [PubMed] [Google Scholar]
- 20.Shah E., Rezaie A., Riddle M., Pimentel M. Psychological disorders in gastrointestinal disease: epiphenomenon, cause or consequence? Ann Gastroenterol. 2014;27:224–230. [PMC free article] [PubMed] [Google Scholar]
- 21.Morais L.H., Schreiber H.L., Mazmanian S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19:241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 22.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014:6. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuang Z.-Q., Shen L.-L., Li W.-W., Fu X., Zeng F., Gui L., et al. Gut microbiota is altered in patients with Alzheimer’s disease. J Alzheimer’s Dis. 2018;63:1337–1346. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 24.Li C., Cui L., Yang Y., Miao J., Zhao X., Zhang J., et al. Gut microbiota differs between parkinson’s disease patients and healthy controls in northeast China. Front Mol Neurosci. 2019:12. doi: 10.3389/fnmol.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barandouzi Z.A., Starkweather A.R., Henderson W.A., Gyamfi A., Cong X.S. Altered composition of gut microbiota in depression: a systematic review. Front Psychiatry. 2020:11. doi: 10.3389/fpsyt.2020.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastiaanssen T.F.S., Cowan C.S.M., Claesson M.J., Dinan T.G., Cryan J.F. Making sense of … the microbiome in psychiatry. Int J Neuropsychopharmacol. 2019;22:37–52. doi: 10.1093/ijnp/pyy067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L., Huh J.R., Shah K. Microbiota and the gut-brain-axis: implications for new therapeutic design in the CNS. EBioMedicine. 2022;77 doi: 10.1016/j.ebiom.2022.103908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fülling C., Dinan T.G., Cryan J.F. Gut microbe to brain signaling: what happens in vagus. Neuron. 2019;101:998–1002. doi: 10.1016/j.neuron.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Kaelberer M.M., Buchanan K.L., Klein M.E., Barth B.B., Montoya M.M., Shen X., et al. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;80-:361. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breit S., Kupferberg A., Rogler G., Hasler G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018:9. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agus A., Clément K., Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70:1174–1182. doi: 10.1136/gutjnl-2020-323071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne) 2020:11. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019:10. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker A., Fonseca S., Carding S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11:135–157. doi: 10.1080/19490976.2019.1638722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belizário J.E., Napolitano M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front Microbiol. 2015:6. doi: 10.3389/fmicb.2015.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stojanov S., Berlec A., Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8:1715. doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang S., Wu X., Jin F. Gut-brain psychology: rethinking psychology from the microbiota–gut–brain axis. Front Integr Neurosci. 2018:12. doi: 10.3389/fnint.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukhina A.Y., Medvedeva O.A., Svishcheva M.V., Shevchenko A.V., Efremova N.N., Bobyntsev I.I., et al. State of colon microbiota in rats during chronic restraint stress and selank treatment. Bull Exp Biol Med. 2019;167:226–228. doi: 10.1007/s10517-019-04496-y. [DOI] [PubMed] [Google Scholar]
- 39.Xu M., Wang C., Krolick K.N., Shi H., Zhu J. Difference in post-stress recovery of the gut microbiome and its altered metabolism after chronic adolescent stress in rats. Sci Rep. 2020;10:3950. doi: 10.1038/s41598-020-60862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong S.J., Tong T., Chew J., Lim W.L. Antidepressive mechanisms of probiotics and their therapeutic potential. Front Neurosci. 2020:13. doi: 10.3389/fnins.2019.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson C.A., Diaz-Arteche C., Eliby D., Schwartz O.S., Simmons J.G., Cowan C.S.M. The gut microbiota in anxiety and depression – a systematic review. Clin Psychol Rev. 2021;83 doi: 10.1016/j.cpr.2020.101943. [DOI] [PubMed] [Google Scholar]
- 42.Nikolova V.L., Hall M.R.B., Hall L.J., Cleare A.J., Stone J.M., Young A.H. Perturbations in gut microbiota composition in psychiatric disorders. JAMA Psychiatry. 2021;78:1343. doi: 10.1001/jamapsychiatry.2021.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ríos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., de los Reyes-Gavilán C.G., Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016:7. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyte M., Ernst S. Catecholamine induced growth of Gram negative bacteria. Life Sci. 1992;50:203–212. doi: 10.1016/0024-3205(92)90273-r. [DOI] [PubMed] [Google Scholar]
- 45.Mudd A.T., Berding K., Wang M., Donovan S.M., Dilger R.N. Serum cortisol mediates the relationship between fecal ruminococcus and brain n-acetylaspartate in the young pig. Gut Microbes. 2017;8:589–600. doi: 10.1080/19490976.2017.1353849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keskitalo A., Aatsinki A.-K., Kortesluoma S., Pelto J., Korhonen L., Lahti L., et al. Gut microbiota diversity but not composition is related to saliva cortisol stress response at the age of 2.5 months. Stress. 2021;24:551–560. doi: 10.1080/10253890.2021.1895110. [DOI] [PubMed] [Google Scholar]
- 47.Lyte M., Vulchanova L., Brown D.R. Stress at the intestinal surface: catecholamines and mucosa–bacteria interactions. Cell Tissue Res. 2010;343:23–32. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- 48.Schreiber K.L., Brown D.R. Adrenocorticotrophic hormone modulatesEscherichia coliO157:H7 adherence to porcine colonic mucosa. Stress. 2005;8:185–190. doi: 10.1080/10253890500188732. [DOI] [PubMed] [Google Scholar]
- 49.Ünsal H., Balkaya M., Ünsal C., Bıyık H., Başbülbül G., Poyrazoğlu E. The short-term effects of different doses of dexamethasone on the numbers of some bacteria in the ileum. Dig Dis Sci. 2007;53:1842–1845. doi: 10.1007/s10620-007-0089-6. [DOI] [PubMed] [Google Scholar]
- 50.Chen C., Brown D.R., Xie Y., Green B.T., Lyte M. Catecholamines modulate Escherichia coli O157:H7 adherence to murine cecal mucosa. Shock. 2003;20:183–188. doi: 10.1097/01.shk.0000073867.66587.e0. [DOI] [PubMed] [Google Scholar]
- 51.Green B.T., Lyte M., Chen C., Xie Y., Casey M.A., Kulkarni-Narla A., et al. Adrenergic modulation ofEscherichia coliO157:H7 adherence to the Colonic mucosa. Am J Physiol Gastrointest Liver Physiol. 2004:287. doi: 10.1152/ajpgi.00471.2003. [DOI] [PubMed] [Google Scholar]
- 52.Caselli E., Fabbri C., D’Accolti M., Soffritti I., Bassi C., Mazzacane S., et al. Defining the oral microbiome by whole-genome sequencing and resistome analysis: the complexity of the healthy picture. BMC Microbiol. 2020;20:120. doi: 10.1186/s12866-020-01801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He J., Li Y., Cao Y., Xue J., Zhou X. The oral microbiome diversity and its relation to human diseases. Folia Microbiol (Praha) 2015;60:69–80. doi: 10.1007/s12223-014-0342-2. [DOI] [PubMed] [Google Scholar]
- 54.Graves D.T., Corrêa J.D., Silva T.A. The oral microbiota is modified by systemic diseases. J Dent Res. 2019;98:148–156. doi: 10.1177/0022034518805739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duran-Pinedo A.E., Solbiati J., Frias-Lopez J. The effect of the stress hormone cortisol on the metatranscriptome of the oral microbiome. Npj Biofilms Micro. 2018;4:25. doi: 10.1038/s41522-018-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Lima P.O., Nani B.D., Almeida B., Marcondes F.K., Groppo F.C., de Moraes A.B.A., et al. Stress-related salivary proteins affect the production of volatile sulfur compounds by oral bacteria. Oral Dis. 2018;24:1358–1366. doi: 10.1111/odi.12890. [DOI] [PubMed] [Google Scholar]
- 57.Calil C.M., Oliveira G.M., Cogo K., Pereira A.C., Marcondes F.K., Groppo F.C. Effects of stress hormones on the production of volatile sulfur compounds by periodontopathogenic bacteria. Braz Oral Res. 2014;28:1–8. doi: 10.1590/1807-3107BOR-2014.vol28.0008. [DOI] [PubMed] [Google Scholar]
- 58.Roberts A., Matthews J.B., Socransky S.S., Freestone P.P.E., Williams P.H., Chapple I.L.C. Stress and the periodontal diseases: effects of catecholamines on the growth of periodontal bacteria in vitro. Oral Microbiol Immunol. 2002;17:296–303. doi: 10.1034/j.1399-302X.2002.170506.x. [DOI] [PubMed] [Google Scholar]
- 59.Saito T., Inagaki S., Sakurai K., Okuda K., Ishihara K. Exposure of P. gingivalis to noradrenaline reduces bacterial growth and elevates ArgX protease activity. Arch Oral Biol. 2011;56:244–250. doi: 10.1016/j.archoralbio.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 60.Stothart M.R., Bobbie C.B., Schulte-Hostedde A.I., Boonstra R., Palme R., Mykytczuk N.C.S., et al. stress and the microbiome: linking glucocorticoids to bacterial community dynamics in wild red squirrels. Biol Lett. 2016;12:20150875. doi: 10.1098/rsbl.2015.0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paudel D., Kuramitsu Y., Uehara O., Morikawa T., Yoshida K., Giri S., et al. Proteomic and microbiota analyses of the oral cavity during psychological stress. PLoS One. 2022;17 doi: 10.1371/journal.pone.0268155. e0268155–e0268155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wingfield B., Lapsley C., McDowell A., Miliotis G., McLafferty M., O’Neill S.M., et al. Variations in the oral microbiome are associated with depression in young adults. Sci Rep. 2021;11:15009. doi: 10.1038/s41598-021-94498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X.-X., Jiao B., Liao X.-X., Guo L.-N., Yuan Z.-H., Wang X., et al. Analysis of salivary microbiome in patients with Alzheimer’s disease. J Alzheimer’s Dis. 2019;72:633–640. doi: 10.3233/JAD-190587. [DOI] [PubMed] [Google Scholar]
- 64.Horz H.-P., Ten Haaf A., Kessler O., Said Yekta S., Seyfarth I., Hettlich M., et al. T-RFLP-based differences in oral microbial communities as risk factor for development of oral diseases under stress. Environ Microbiol Rep. 2012;4:390–397. doi: 10.1111/j.1758-2229.2012.00340.x. [DOI] [PubMed] [Google Scholar]
- 65.Levert-Levitt E., Shapira G., Sragovich S., Shomron N., Lam J.C.K., Li V.O.K., et al. Oral microbiota signatures in post-traumatic stress disorder (PTSD) veterans. Mol Psychiatry. 2022 doi: 10.1038/s41380-022-01704-6. [DOI] [PubMed] [Google Scholar]
- 66.Simpson C.A., Adler C., du Plessis M.R., Landau E.R., Dashper S.G., Reynolds E.C., et al. Oral microbiome composition, but not diversity, is associated with adolescent anxiety and depression symptoms. Physiol Behav. 2020;226 doi: 10.1016/j.physbeh.2020.113126. [DOI] [PubMed] [Google Scholar]
- 67.Yolken R., Prandovszky E., Severance E.G., Hatfield G., Dickerson F. The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr Res. 2021;234:51–57. doi: 10.1016/j.schres.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Kohn J.N., Kosciolek T., Marotz C., Aleti G., Guay-Ross R.N., Hong S.-H., et al. Differing salivary microbiome diversity, community and diurnal rhythmicity in association with affective state and peripheral inflammation in adults. Brain Behav Immun. 2020;87:591–602. doi: 10.1016/j.bbi.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bosch J.A., Brand H.S., Ligtenberg T.J., Bermond B., Hoogstraten J., Amerongen A.V. Psychological stress as a determinant of protein levels and salivary-induced aggregation of streptococcus gordonii in human whole saliva. Psychosom Med. 1996;58:374–382. doi: 10.1097/00006842-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 70.Kopycka-Kedzierawski D.T., Scott-Anne K., Ragusa P.G., Cvetanovska M., Flint K., Feng C., et al. Social, psychological, and behavioral predictors of salivary bacteria, yeast in caries-free children. JDR Clin Transl Res. 2022;7:163–173. doi: 10.1177/2380084421999365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boyce W.T., Den Besten P.K., Stamperdahl J., Zhan L., Jiang Y., Adler N.E., et al. Social inequalities in childhood dental caries: The convergent roles of stress, bacteria and disadvantage. Soc Sci Med. 2010;71:1644–1652. doi: 10.1016/j.socscimed.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nani B.D., Lima P.O., de, Marcondes F.K., Groppo F.C., Rolim G.S., Moraes A.B.A. de, et al. Changes in salivary microbiota increase volatile sulfur compounds production in healthy male subjects with academic-related chronic stress. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hijazi K., Morrison R.W., Mukhopadhya I., Martin B., Gemmell M., Shaw S., et al. Oral bacterial diversity is inversely correlated with mucosal inflammation. Oral Dis. 2020;26:1566–1575. doi: 10.1111/odi.13420. [DOI] [PubMed] [Google Scholar]
- 74.Bosch J.A., Brand H.S., Ligtenberg T.J.M., Bermond B., Hoogstraten J., Amerongen A.V.N. Psychological stress as a determinant of protein levels and salivary-induced aggregation of streptococcus gordonii in human whole saliva. Psychosom Med. 1996;58:374–382. doi: 10.1097/00006842-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Proctor G.B., Carpenter G.H. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 76.Pellegrini A., Grieco M., Materazzi G., Gesi M., Ricciardi M.P. Histochem J. 1998;30:695–701. doi: 10.1023/a:1003493921112. [DOI] [PubMed] [Google Scholar]
- 77.Ayuob N.N., Abdel-Tawab H.S., El-Mansy A.A., Ali S.S. The protective role of musk on salivary glands of mice exposed to chronic unpredictable mild stress. J Oral Sci. 2019;61:95–102. doi: 10.2334/josnusd.17-0440. [DOI] [PubMed] [Google Scholar]
- 78.Taher M.T. Effect of cortisol on submandibular salivary gland. Tikrit Med J. 2008;14:165–168. [Google Scholar]
- 79.Liu F.T.Y., Lin H.S. Effect of hydrocortisone acetate on dental caries and salivary glands in adrenalectomized female rats. J Dent Res. 1968;47:158–166. doi: 10.1177/00220345680470011101. [DOI] [PubMed] [Google Scholar]
- 80.Bauduin S.E.E.C., van Noorden M.S., van der Werff S.J.A., de Leeuw M., van Hemert A.M., van der Wee N.J.A., et al. Elevated salivary alpha-amylase levels at awakening in patients with depression. Psychoneuroendocrinology. 2018;97:69–77. doi: 10.1016/j.psyneuen.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Braithwaite E.C., Ramchandani P.G., Lane T.A., Murphy S.E. Symptoms of prenatal depression are associated with raised salivary alpha-amylase levels. Psychoneuroendocrinology. 2015;60:163–172. doi: 10.1016/j.psyneuen.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 82.Ali N., Pruessner J.C. The salivary alpha amylase over cortisol ratio as a marker to assess dysregulations of the stress systems. Physiol Behav. 2012;106:65–72. doi: 10.1016/j.physbeh.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Szabo Y.Z., Newton T.L., Miller J.J., Lyle K.B., Fernandez-Botran R. Acute stress induces increases in salivary IL-10 levels. Stress. 2016;19:499–505. doi: 10.1080/10253890.2016.1206885. [DOI] [PubMed] [Google Scholar]
- 84.Goodyer I.M., Herbert J., Altham P.M., Pearson J., Secher S.M., Shiers H.M. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol Med. 1996;26:245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- 85.Paudel D., Morikawa T., Yoshida K., Uehara O., Giri S., Neopane P., et al. Chronic stress-induced elevation of il-1β in the saliva and submandibular glands of mice. Med Mol Morphol. 2020;53:238–243. doi: 10.1007/s00795-020-00250-w. [DOI] [PubMed] [Google Scholar]
- 86.Sarkar A., Kuehl M.N., Alman A.C., Burkhardt B.R. Linking the oral microbiome and salivary cytokine abundance to circadian oscillations. Sci Rep. 2021:11. doi: 10.1038/s41598-021-81420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghosh S., Kumar M., Santiana M., Mishra A., Zhang M., Labayo H., et al. Enteric viruses replicate in salivary glands and infect through saliva. Nature. 2022;607:345–350. doi: 10.1038/s41586-022-04895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duran-Pinedo A.E., Solbiati J., Frias-Lopez J. The effect of the stress hormone cortisol on the metatranscriptome of the oral microbiome. npj Biofilms Micro. 2018:4. doi: 10.1038/s41522-018-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cakmak O., Tasdemir Z., Aral C.A., Dundar S., Koca H.B. Gingival crevicular fluid and saliva stress hormone levels in patients with chronic and aggressive periodontitis. J Clin Periodontol. 2016;43:1024–1031. doi: 10.1111/jcpe.12614. [DOI] [PubMed] [Google Scholar]
- 90.Rai B., Kaur J., Anand S.C., Jacobs R. Salivary stress markers, stress, and periodontitis: a pilot study. J Periodontol. 2011;82:287–292. doi: 10.1902/jop.2010.100319. [DOI] [PubMed] [Google Scholar]
- 91.Dewhirst F.E., Chen T., Izard J., Paster B.J., Tanner A.C.R., Yu W.-H., et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zeng M.Y., Inohara N., Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18–26. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park S.-Y., Hwang B.-O., Lim M., Ok S.-H., Lee S.-K., Chun K.-S., et al. Oral–gut microbiome axis in gastrointestinal disease and cancer. Cancers. 2021;13:2124. doi: 10.3390/cancers13092124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Imhann F., Bonder M.J., Vich Vila A., Fu J., Mujagic Z., Vork L., et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iwauchi M., Horigome A., Ishikawa K., Mikuni A., Nakano M., Xiao J., et al. Relationship between oral and gut microbiota in elderly people. Immun, Inflamm Dis. 2019;7:229–236. doi: 10.1002/iid3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Odamaki T., Kato K., Sugahara H., Hashikura N., Takahashi S., Xiao J., et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Strauss J., Kaplan G.G., Beck P.L., Rioux K., Panaccione R., DeVinney R., et al. Invasive potential of gut mucosa-derived fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011;17:1971–1978. doi: 10.1002/ibd.21606. [DOI] [PubMed] [Google Scholar]
- 98.Nakatsu G., Li X., Zhou H., Sheng J., Wong S.H., Wu W.K.K., et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727. doi: 10.1038/ncomms9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahn J., Segers S., Hayes R.B. Periodontal disease, porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33:1055–1058. doi: 10.1093/carcin/bgs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmidt T.S.B., Hayward M.R., Coelho L.P., Li S.S., Costea P.I., Voigt A.Y., et al. Extensive transmission of microbes along the gastrointestinal tract. Elife. 2019:8. doi: 10.7554/eLife.42693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shaffer M., Lozupone C. Prevalence and source of fecal and oral bacteria on infant, child, and adult hands. MSystems. 2018:3. doi: 10.1128/mSystems.00192-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meader E., Hunter P. Infect. Dis. Epidemiol. Oxford University Press; 2016. Faeco–oral infections; pp. 271–286. [DOI] [Google Scholar]
- 103.Marcelino S.L., Gaetti-Jardim E., Nakano V., Canônico L.A.D., Nunes F.D., Lotufo R.F.M., et al. Presence of periodontopathic bacteria in coronary arteries from patients with chronic periodontitis. Anaerobe. 2010;16:629–632. doi: 10.1016/j.anaerobe.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 104.Pisani F., Pisani V., Arcangeli F., Harding A., Singhrao S.K. The mechanistic pathways of periodontal pathogens entering the brain: the potential role of treponema denticola in tracing Alzheimer’s disease pathology. Int J Environ Res Public Health. 2022;19:9386. doi: 10.3390/ijerph19159386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su X., Tang Z., Lu Z., Liu Y., He W., Jiang J., et al. Oral treponema denticola infection induces Aβ1–40 and Aβ1–42 accumulation in the hippocampus of C57BL/6 Mice. J Mol Neurosci. 2021;71:1506–1514. doi: 10.1007/s12031-021-01827-5. [DOI] [PubMed] [Google Scholar]
- 106.Epel E.S., Crosswell A.D., Mayer S.E., Prather A.A., Slavich G.M., Puterman E., et al. More than a feeling: a unified view of stress measurement for population science. Front Neuroendocr. 2018;49:146–169. doi: 10.1016/j.yfrne.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mark Welch J.L., Rossetti B.J., Rieken C.W., Dewhirst F.E., Borisy G.G. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci. 2016:113. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilbert S.A., Mark Welch J.L., Borisy G.G. Spatial ecology of the human tongue dorsum microbiome. Cell Rep. 2020;30:4003–4015. doi: 10.1016/j.celrep.2020.02.097. [DOI] [PMC free article] [PubMed] [Google Scholar]