Summary
Pleiotropic genetic factors (e.g., DNA polymorphisms) may be involved in the initiation of neuroblastoma (NB) and coronary artery disease (CAD) given their common origin from defects in neural crest development. To discover novel NB susceptibility genes, we conducted a three-stage survey including a meta-analysis of NB and CAD genome-wide association data, prioritization of NB causal variants, and validation in an independent cohort of affected individuals–control subjects. The lead SNP, rs13337397 at the 16q23.1 locus, associated with both diseases in the meta-analysis and with NB in the validation study. All the SNPs in linkage disequilibrium with rs13337397 were annotated using the H3K27ac epigenetic marker of neural crest cells (NCC) and NB cell lines. Indeed, we identified the functional SNP rs13337017, mapping within an enhancer of NCCs and NB cell lines and showing long-range interactions with CFDP1 by Hi-C analysis. Luciferase assays indicated that the risk allele of rs13337017 increased CFDP1 expression in NB cell lines. Of note, CFDP1 high expression associated with unfavorable prognostic markers in an analysis including 498 NB transcriptomes. Moreover, depletion of CFDP1 markedly decreased viability and migration and increased apoptotic rates in NB cell lines. Finally, transcriptome and qPCR analyses revealed that the depletion of CFDP1 may affect noradrenergic neuron differentiation by downregulating master regulators of sympathetic noradrenergic identity, including PHOX2B, HAND2, and GATA3. Our data strongly suggest that CFDP1 acts as oncogene in NB. In addition, we provide evidence that genetic predisposition to NB can be mediated by the alteration of noradrenergic lineage-specific gene expression.
Keywords: GWAS, SNP, neuroblastoma, neural crest cells, coronary artery disease, pleiotropic variants, cancer predisposition
Pleiotropic genetic factors may promote both neuroblastoma and coronary artery disease, as they originate from neural crest development defects. To discover novel neuroblastoma susceptibility genes, we analyzed data of GWAS, chromatin conformation, and gene expression. Further biological investigations indicated oncogenic roles of CFDP1 in influencing the transcriptional programs in neuroblastoma.
Introduction
Neuroblastoma (NB), the most common extracranial tumor in childhood, is an aggressive metastasis-prone tumor of the sympathetic nervous system.1 Clinical and biological factors, including age at diagnosis, stage, tumor histopathology, ploidy, and genomic aberrations, define distinct risk strata and determine treatment plans for patients.1 In young children, MYCN amplification and other chromosomal arm-level alterations, such as the deletion of 1p and 11q, gain of 17q2, and TERT rearrangements, have been reported as poor prognostic features.3 Children older than 6 years present with unique structural variants, with 19p loss and 1q gain being among the most frequent.4 Somatic point mutations in ALK and ATRX5 and NB-specific active regulatory elements6,7 have been reported in primary tumors as cancer drivers with the potential to predict patient prognosis. In addition, several variants and SNPs involving genes of the base excision repair and of the mRNA post transcriptional modification pathways are known for their association with the occurrence of neuroblastoma.8,9
Nevertheless, despite progress in discovering clinically actionable alterations, NB still causes 15% of all deaths due to cancer in children. Furthermore, less than 40% of children survive for more than 5 years after being diagnosed with a high-risk disease.10
NB arises from the sympathetic ganglia and adrenal medulla, which originate from the trunk neural crest (NC). During early sympathetic neurogenesis, proper migration and specification of human NC cells (NCC) is regulated by a network of transcription factors, including three master regulators of sympathetic noradrenergic identity of NB: PHOX2B, GATA3, and HAND2.11 Notably, GATA3 and HAND2 are also required for coronary artery development and physiological formation of the outflow tract.12,13,14 In addition, Arima and colleagues15 reported the involvement of preotic NC in the development of smooth muscles in coronary arteries and adjacent cardiac tissues. Indeed, they observed that ablation of preotic NCs in chick and mouse embryos leads to coronary defects.
Recent genome-wide association studies (GWASs)16 have demonstrated that common single nucleotide polymorphisms (SNPs) are risk factors for NB and coronary artery disease (CAD). In NB, GWASs have identified several susceptibility loci, including CASC15, BARD1, LMO1, DUSP12, HSD17B12, DDX4/IL31RA, HACE1, LIN28B, TP53, MLF1, CPZ, CDKN1B, NEFL, and SLC16A1.17 Other loci, including PCSK9, SORT1, MIA3, PHACTR1, LPA, CDKN2A, CDKN2B, CXCL12, and LDLR have been associated with CAD.18,19 Some of these loci, such as LPA, CDKN2A, CDKN2B, CXCL12, and LDLR, also play a role in NB.20,21,22,23,24 For example, the genomic region of 9p21 confers susceptibility to CAD. In addition, deletion of 9p21 is a marker of poor prognosis for NB patients.25 Taken together, given the common origins of NB and CAD, we had strong suggestion that these two diseases might share causal pathways. Furthermore, our hypothesis was also supported by our previous work in which we found genetic risk loci, regulating the expression of relevant genes of NCC development, associated with both congenital heart disease and NB susceptibility.26
Based on the above observations, we reasoned that pleiotropic DNA polymorphisms could be modestly associated with both NB and CAD and that, in a single phenotype-based GWAS, these SNPs could remain undetected at the standard threshold for genome-wide significance, mainly because of sample size limitations and multiple testing corrections. Previous studies have shown that new risk variants can be identified by combining GWAS results of two or more disease phenotypes.27,28,29 Here, to identify novel susceptibility loci for NB, we performed a cross-phenotype meta-analysis of two large GWASs of NB and CAD, including a total of 36,785 individuals and a replication study of Italian NB affected individuals and control subjects (N = 1,716).
This strategy, coupled with biological investigations, allowed us to identify CFPD1 as a novel NB susceptibility gene that acts as an oncogene by influencing the transcriptional regulatory circuitry of noradrenergic identity in NB.
Materials and methods
Meta-analysis
To identify genetic loci sharing the GWAS signal between NB and CAD, we performed a cross-phenotype meta-analysis using two large GWAS datasets.
In Stage 1 we analyzed the SNP genotyping data of a large CAD GWAS dataset (n = 15,420 affected individuals and n = 15,062 control subjects) obtained from the CARDIoGRAMplusC4D Consortium30 and the SNP genotyping data obtained from a recent NB GWAS (n = 2,101 affected individuals and n = 4,202 control subjects) published by McDaniel and colleagues.31 See details in the supplemental information.
First, for each dataset, we performed whole-genome imputation on a multipopulation reference panel to increase the number of detected SNPs. We then combined the significant SNPs (p < 0.05) of each dataset to test for shared genetic variants between these two diseases (cross-phenotype meta-analysis) following a 2-fold approach in which we searched for SNPs with the same direction of association (same effect) and for SNPs with inverse direction of association in CAD (inverse effect).
In Stage 2, we annotated SNPs emerging from these two meta-analysis studies (n = 434) with epigenomic profiling of NC using H3K27Ac chromatin immunoprecipitation sequencing (ChIP-seq) data (GEO: GSM2664365, GSM2664367, GSE90683) to prioritize SNPs mapping in promoters and/or enhancers (active in NCs) with a significance of association of p < 1.0 × 10−4.
In Stage 3 we selected four SNPs to perform direct genotyping by TaqMan assay (TaqMan Universal PCR Master Mix; Applied Biosystems, Thermo Fisher Scientific) in an independent Italian cohort of 636 NB affected individuals and 1,080 control subjects (details are described in the supplemental information). This study was approved by the Ethics Committee of the University of Naples Federico II.
Hardy-Weinberg equilibrium was evaluated using the goodness-of-fit chi-square test in control subjects. Two-sided chi-square tests were used to evaluate differences in the distributions of allele frequencies between patients and control subjects. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to assess the relative disease risk conferred by a specific allele.
The statistical power was calculated by using the Genetic Association Study (GAS) Power Calculator (http://csg.sph.umich.edu/abecasis/gas_power_calculator/index.html). The false positive report probability (FPRP) for the significant SNP (rs13337397) was calculated according to the method reported in the published paper by Wacholder et al.32 We had a power of 82.3% to identify disease-associated SNPs with p = 0.05, effect size of 1.35, and a disease allele frequency of 0.10. If we set the disease allele frequency at 0.20 we were able to identify disease-associated SNPs with statistical power of 95.8%.
Analysis of public ChIP-seq data
ChIP-seq reads from the GEO: GSE90683 public dataset were reanalyzed, as reported by Avitabile et al.33 Briefly, alignment to the human reference genome (assembly GRCh37/hg19) was performed using Bowtie2 (v2.3.4.3). Duplicate and low mapping quality (MQ < 20) reads were removed. Enriched regions (peaks) were detected with HMCan (v1.30) using the data from the input DNA to subtract the background noise from the ChIP signals. To identify enhancers and super-enhancers, we used the LILY package.11
DNase-I hypersensitivity level (DHS) signal intensity files of the SK-N-SH NB cell line (GEO: GSE96240) and fetal adrenal gland (FAD) tissues (GEO: GSM530653, GSM817165, GSM1027310, GSM1027311, and GSM817167) were downloaded from the GEO database as processed files. The details are described in the supplemental information.
Identification of causal variants at 16q23.1
To identify potential functional SNPs, we first selected variants in linkage disequilibrium (LD) with the lead SNP rs13337397 (r2 ≥ 0.8) (n = 57). LD calculations (r2 and D′) were performed using the LDlink web tool and allele frequencies of Europeans from the 1000 Genomes Project.
Next, we mapped the selected SNPs onto the predicted regulatory elements (super-enhancers [SEs], enhancers, and promoters) obtained in the previous step (see above). From this analysis, we retained only the SNPs (n = 12) that achieved the highest number of SE annotations (SE = 13). Finally, we selected, for further consideration, the rs13337017 SNP showing the highest levels of both H3K27ac (in NB cell lines) and DHS signals (in FAD tissues).
To obtain additional functional annotations on the prioritized loci and SNPs, we used the GeneHancer database.34 GeneHancer assigns confidence scores to gene-regulatory element associations based on a combination of evidence annotations. From the list of CFDP1 associated regulatory elements, we selected those supported by two or more evidence sources.
HiC data analysis
In-house-generated HiC data of the SK-N-BE NB cell line were processed as reported previously.35,36 Briefly, sequencing was performed using an Illumina HiSeq platform. Paired-end reads of 150 base pairs (bp) were mapped to the reference genome (build hg19/GRCH37) using Bowtie2. HiCExplorer (v3.7) was used to (1) build the interaction matrix at a resolution of 10 Kb (bin size = 10 Kb), (2) normalize the observed read counts, (3) determine topologically associating domains (TADs, self-interacting genome regions) and their boundaries, and (4) plot the results. Subsequently, starting from the selected SNP coordinates, we extended our region of interest of 0.5 Mb up- and downstream and calculated the statistical significance of the interactions between bins with FitHiC (v2.0.8). p-values were corrected for multiple tests using the Benjamini-Hochberg method (false discovery rate, FDR), and the cutoff was set at 5%. Finally, we annotated these bins using the R-Bioconductor package ChIPseeker (v 1.32.0) to map genomic bins to gene coordinates.
Cell cultures
Human HEK293T, SK-N-BE, SK-N-AS, and SH-EP cell lines were obtained from the American Type Culture Collection (respectively ATCC #CRL-3216, #CRL-2268, #CRL-2137, #CRL-2269). The cell lines used for all experiments were re-authenticated and tested as mycoplasma-free and grown in commercial media, as reported in the supplemental information. Early passage cells were used, and the cumulative culture length was less than 3 months after resuscitation.
Treatment of cells with siRNA CFDP1
SK-N-BE, SK-N-AS, and SH-EP cells were plated into a six-well at 70% confluence. Cells were transfected with 10 nM of three unique 27-mer small interfering RNA (siRNA) duplexes for CFDP1 (human) (Origene, Locus ID 10428) and 10 nM of Trilencer-27 Universal Scrambled Negative Control siRNA Duplex (Origene) using X-tremeGENE (Roche). After 48 h of transfection, the cells were used to assess the silencing of protein and mRNA of CFDP1. The experiments were performed in triplicate, and three experimental points were analyzed for each experiment.
In vitro functional assays
We used a luciferase reporter assay to assess the effects of the rs13337017 alleles on gene expression. The functional implications of CFDP1 in regulating cell behavior and properties were assessed on siRNA silenced (siCFDP1) and control (siScrambled) NB cell lines using viability, wound-healing, and Caspase-3 activity assays. Moreover, we used NB cells to quantify the gene expression of CFDP1 and regulated TFs by quantitative real-time PCR and to assess CFDP1 protein concentrations by western blotting. Detailed descriptions of the experimental conditions for each experiment, quantitative real-time PCR primers, and antibodies are provided in the supplemental information. In each case, the experiments were replicated, and the statistical significance of the results was assessed using a t test.
Analysis of RNA-sequencing data and differential expression
Total RNA was isolated from two biological replicates of the SK-N-AS NB cell line under treated (siCFDP1) and control (siScrambled) experimental conditions and processed as reported in the supplemental information. The RNA-sequencing (RNA-seq) data (obtained on the Illumina platform) were first checked for quality using FastQC and then analyzed using the Tuxedo suite: TopHat (v2.0.14) and Cufflinks v2.1.0. TopHat was run using the default options by providing the reference genome and its related RefSeq reference transcriptome (assembly GRCh37/hg19). Next, Cufflinks was used to assemble the mapped reads into possible transcripts and generate a final transcriptome assembly. Gene- and transcript-level expression is reported as normalized fragments per kilobase of exon per million fragments mapped (FPKM) counts. Finally, Cuffdiff was used to detect differentially expressed genes and transcripts between the two experimental conditions (siCFDP1 versus siScrambled). The results were analyzed using the R-Bioconductor package CummeRbund (v2.16.0). We deemed differentially expressed genes, those showing FDR below 0.05, and the Log2 transformed fold change greater than +0.5 (up-regulated) or lower than −0.5 (down-regulated). Next, we used the expression data of these genes to perform k-means clustering (using k = 4) to identify groups of genes showing similar expression patterns. The four lists of differentially expressed genes (DEGs) were then used to query the Gene Ontology database of Biological Processes (GO BP) with the R-Bioconductor package ClusterProfileR (v3.18.1). BPs were considered enriched if FDR was less than 0.05.
Results
NB and CAD share risk polymorphisms
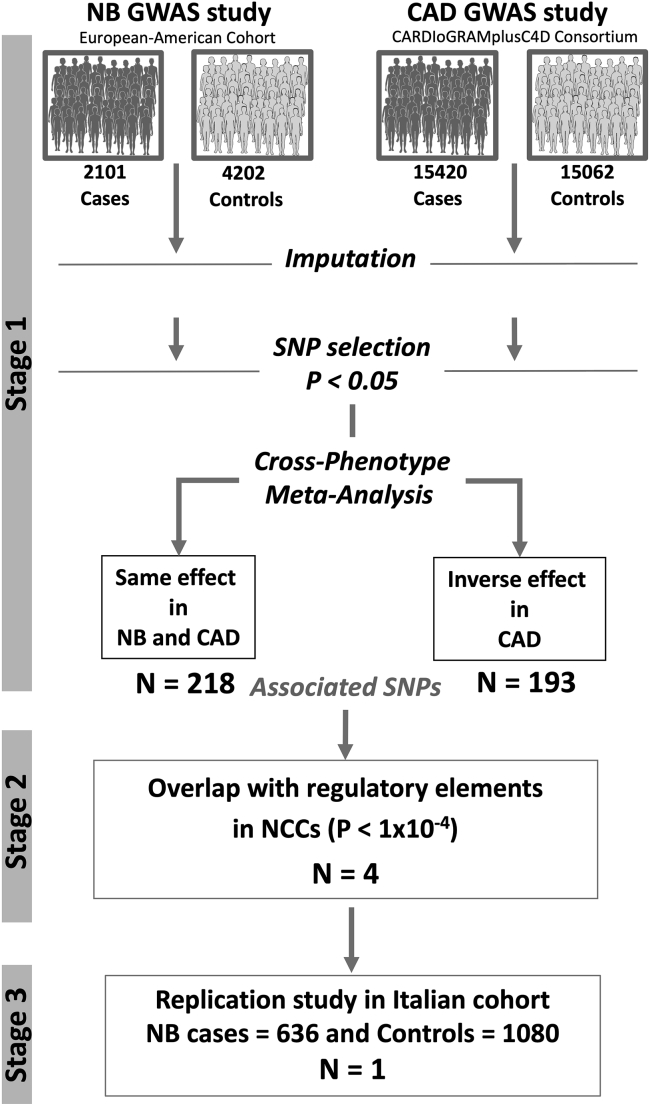
Candidate susceptibility loci were selected through a cross-phenotype meta-analysis using two GWAS datasets (Figure 1). The results of this analysis are presented below.
Figure 1.
Workflow of the cross-phenotype meta-analysis
Stage 1 | Combination of the summary statistics of two large NB and CAD GWAS data using two approaches: (1) meta-analysis study across NB and CAD and (2) meta-analysis study across NB and CAD with inverted effect in CAD.
Stage 2 | Identification and prioritization of candidate causal SNPs using epigenomic data.
Stage 3 | Replication study in an Italian cohort of 636 NB affected individuals and 1,080 control subjects by direct, PCR-based, genotyping.
Stage 1: meta-analysis of NB and CAD GWAS data | We conducted a fixed-effects meta-analysis of two large GWAS studies, 2,101 NB affected individuals and 4,202 control subjects31 and 15,420 CAD affected individuals and 15,062 control subjects.30 Whole-genome imputation of the two GWASs was performed using a multipopulation reference panel,36 which yielded up to 9 million SNPs. As described in the materials and methods section and detailed in the supplemental information, we compared the GWAS data (p value and odds ratio for 9,671,310 SNPs) of NB and CAD using two approaches. First, meta-analysis across NB and CAD returned 218 SNPs with the same direction of association (same effect) (Table S1). With the second approach, the meta-analysis across NB and CAD with inverted effects in CAD, we obtained 193 SNPs with an inverse direction of association (opposite effect) (Table S2).
Stage 2: Identification of causal variants | To prioritize the top potential functional SNPs, we integrated NB and CAD meta-analysis data with H3K27ac epigenetic markers using three publicly available ChIP-seq data of human NCCs 11,37 to select SNPs mapping in human promoters and/or enhancers (active in NCs) with a p value of association below 1.0 × 10−4 (Figure 1). We identified two SNPs with the same direction of association (green in Table S1) and two SNPs with opposite directions of association (green in Table S2).
Stage 3: Replication in an Italian cohort of NB | We next sought to replicate the results of the association for the four prioritized risk loci in an independent cohort of Italian subjects (n = 636 NB affected individuals and n = 1,080 control subjects) (Figure 1). We identified an association of the minor allele A for rs13337397 SNP at 16q23.1 locus with the NB risk (p = 0.033; OR = 1.339, Table 1).
Table 1.
Replication study in Italian cohort
| dbSNP identifier | Position (hg19) | Major allele | Minor allele | MAF controls (n = 1,080) | MAF cases (n = 636) | p value | Odds ratio | CI 95% (low-high) |
|---|---|---|---|---|---|---|---|---|
| rs11879191 | chr19:10,512,911 | G | A | 0.18 | 0.18 | 0.90 | 1.02 | 0.803–1.284 |
| rs4387287 | chr10:105,677,897 | C | A | 0.23 | 0.24 | 0.63 | 1.04 | 0.881–1.233 |
| rs2838330 | chr21:45,023,876 | A | T | 0.44 | 0.42 | 0.33 | 0.93 | 0.807–1.074 |
| rs13337397∗ | chr16:75,295,639 | C | A | 0.08 | 0.10 | 0.03 | 1.339 | 1.024–1.751 |
Significant SNPs obtained in Stage 2 (see Figure 1) were tested by direct genotyping in a cohort of Italian subjects (N = 1,080 healthy control subjects and N = 636 NB patients). MAF, minor allele frequency; CI, confidence interval. Genomic coordinates have been reported for the hg19 assembly. This significant association is in bold. ∗False Positive Report Probability (FPRP) values equal to 0.11, 0.271, 0.804, 0.976, 0.998, and 1.000 according to the prior probability of 0.25, 0.1, 0.01, 0.001, 0.0001, and 0.00001, respectively.
Functional characterization of NB-CAD cross-associated locus 16q23.1
In order to better understand the functional role of the 16q23.1 locus in NB biology, we further investigated a subset of 57 SNPs in LD (0.8 < R2 ≤ 1) with the lead SNP rs13337397 (Table S3). We mapped the selected 57 SNPs on predicted regulatory elements (SEs, enhancers, and promoters) obtained by re-analysis of H3K27ac ChIP-seq peaks of 25 NB cell lines and two human NCCs published in Boeva et al.11 (GEO: GSE90683). Furthermore, the SNPs were annotated using H3K27ac signal intensity levels from the cell lines listed above. We have also added information regarding the DHS of the SK-N-SH NB cell line (from GEO: GSE96240) and FAD (N = 5) DHS data from GEO: GSE18927 (Figures 2 and S1 and Table S3).
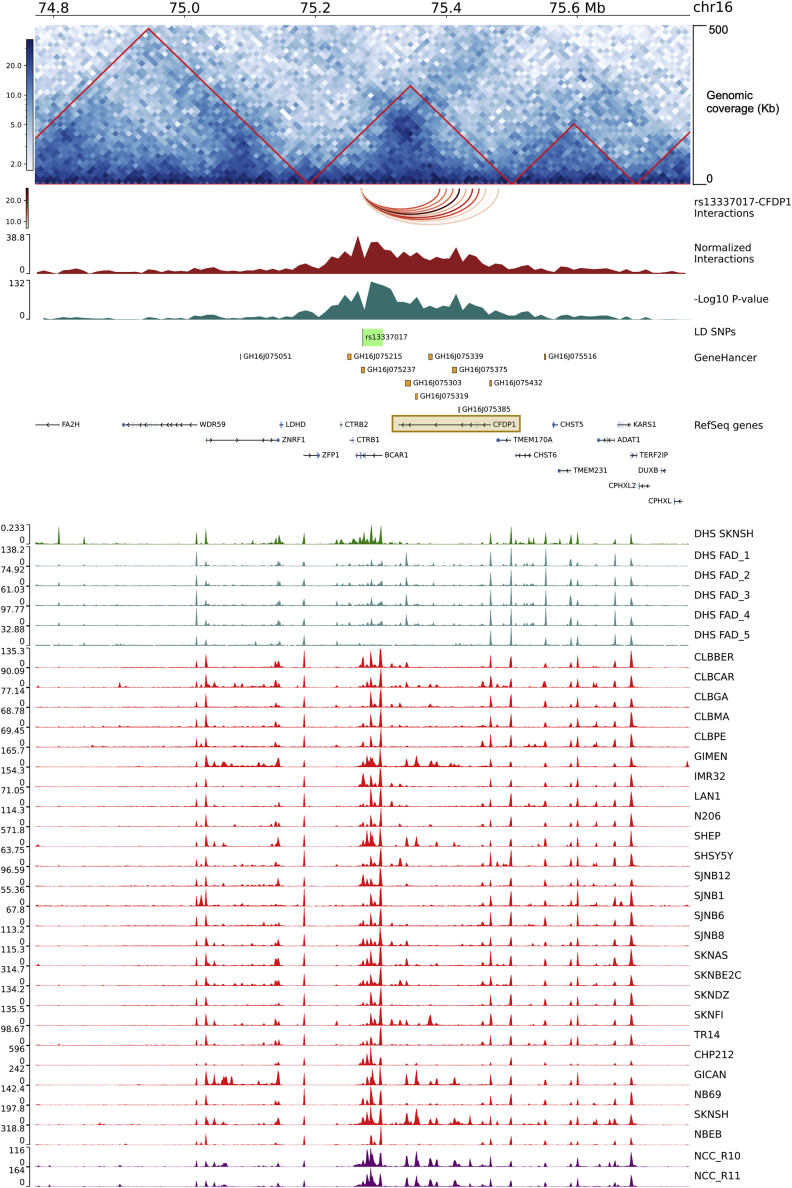
Figure 2.
rs13337017 showed long-range significant interactions with CFPD1 in SK-N-BE NB cell line
The figure reports the genomic interactions from the rs13337017 point of view. The genomic tracks are named from top to bottom and described below. The genomic coordinates are from human genome hg19. The interaction matrix is centered on rs13337017 (chr16:75,272,366-75272367) and extended of 0.5 Mb up- and downstream. Genomic coverage is of 500 Kb and the matrix resolution is of 10 Kb (the interactions are calculated between bins of 10 Kb). Red-bordered triangles represent the Topologically Associated Domains (TADs). The arcs track shows the interactions between rs13337017 and CFPD1 annotated bins. The normalized number of interactions (a measure of the strength of interactions). The minus Log10 of the FDR adjusted p value. The region of 30,631 bp containing the list of 58 in Linkage Disequilibrium SNPs (LD SNPs) with rs13337017 is in green. The GeneHancer track reports the regulatory elements, from the GeneHancer database, showing strong associations with CFDP1 (see materials and methods). The NCBI RefSeq genes (we plotted the longest transcript for each gene). A brown-bordered rectangle highlights the CFDP1 locus. The ChIP peak-based tracks show, from top to bottom, the DNase-I hypersensitivity levels (DHS) sites of the SK-N-SH NB cell line in dark green (from ENCODE v3); the DHS signal of fetal adrenal gland (n = 5) tissues from Roadmap Epigenomics Consortium (GEO: GSM530653, GSM817165, GSM1027310, GSM1027311, GSM817167) in dark cyan; the H3K27ac data of NB cell lines (from GEO: GSE90683 and GSE65664) in red; and H3K27ac peaks from two human neural crest cells (NCCs) (from GEO: GSE90683) in purple. Data ranges and heatmap color keys are shown on the left of each track, when needed.
To further filter the list of SNPs, we first selected those SNPs (n = 12) with the highest number of super-enhancer annotations (n = 13) (Table S3). Among the 12 SNPs, we focused on rs13337017, which showed the highest levels of both H3K27ac and DHS (Figures 2 and S1 and Table S3).
Subsequently, we used in-house Hi-C data from the SK-N-BE NB cell line to assess the strength and significance of long-range interactions between rs13337017 SNP and its surrounding genomic regions. We found that rs13337017 and the CFDP1 locus were mapped within the same topologically associated domain. Furthermore, rs13337017 significantly interacted with the CFDP1 locus (FDR ≤1.45 × 10−16) (Figure 2 and Table S4). We also queried the GeneHancer database to retrieve the known regulatory elements associated with CFDP1 and found that rs13337017 fell within a regulatory element (GeneHancer ID: GH16J075237), with multiple supporting evidence (including Ensembl: ENSR00000542243 and dbSUPER: SE_01027) for its association with CFDP1 (Figure 2). Our analyses of H3K27ac ChIP-seq data strongly indicated the super-enhancer functions of our prioritized locus at 16q23.1 in NB and NCCs. Nevertheless, we wanted to assess its regulatory activity in the adrenal gland tissues from which NB commonly arises.38 For this purpose, we used the public database 3DIV (http://3div.kr/). The analysis showed that long-range interactions between the rs13337017 SNP and the promoter of CFDP1 were maintained in the adrenal gland tissue (Figure S2).
Taken together, these findings provide strong evidence for the regulatory functions of our prioritized locus on chromosome 16q23.1 (including rs13337017) in NB, and are consistent with recent evidence that disease-associated SNPs frequently affect regulatory regions that control tissue-specific and developmental stage-specific gene expression.39
The expression profile of CFDP1 in cells of origin of NB and CAD is comparable
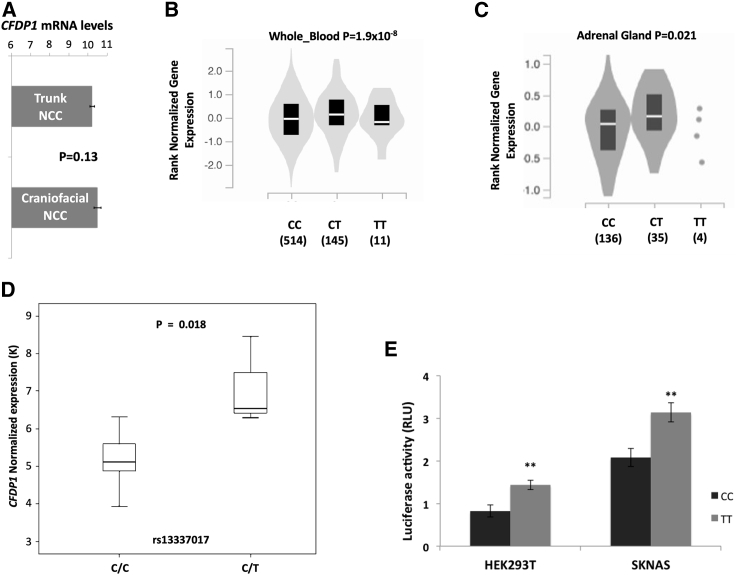
This study was based on the assumption that NB and CAD share the same embryonic origin and regulatory pathways in the early phases of development. NB arises from trunk-NCC,40 whereas CAD originates from craniofacial-NCC.15 To exclude the differential regulation of CFDP1 in these two branches of NC, we compared the expression profiles of trunk-NCC and craniofacial-NCC in murine embryos (GEO: GSE39191). As expected, we found no statistically significant difference in CFDP1 expression between the two NC derivatives (Figure 3A).
Figure 3.
rs13337017 genotypes and CFDP1 expression association
(A) Comparison of CFDP1 expression across trunk-NCC and craniofacial-NCC of murine embryos (GEO: GSE39191).
(B) Violin plot reporting the eQTL analysis of rs13337017 in whole blood.
(C) Violin plot showing eQTL analysis of rs13337017 in adrenal gland tissue.
(D) Boxplots reporting CFDP1 expression grouped by rs13337017 genotypes of NB cell lines in GEO: GSE78061.
(E) Barplots of luciferase reporter gene assays carried out in HEK293T and SK-N-AS. Data shown are the mean ± standard deviation from nine independent transfection experiments, each done in triplicate and compared with promoter-less control. ∗∗p < 0.001, t test. eQTL analysis performed with GTEx Release v7.
The rs13337017 acts as an eQTL for CFDP1
Expression quantitative trait loci (eQTL) analysis using whole blood tissue from the GTEx database revealed that rs13337017 acts as an eQTL for CFDP1 (p = 1.9 × 10−8, GTEx Release v7, Figure 3B). Moreover, we observed a significant association of the eQTL in adrenal gland tissues (p = 0.021, Figure 3C). To assess the eQTL function of rs13337017 genotypes in NB cell lines, we first obtained CFDP1 expression profiles in a set of 13 NB cell lines using expression arrays (GEO: GSE78061). We directly genotyped rs13337017 in this set of cell lines (C/C = 11, C/T = 2, and T/T = 0). Finally, we compared CFDP1 expression according to the rs13337017 genotype and found that the T risk allele was significantly correlated with increased mRNA expression of CFDP1 (p = 0.018, Figure 3D). This association was further confirmed by luciferase reporter gene assays, which showed a higher enhancer activity for the T allele than for the C allele (p < 0.01, Figure 3E) in HEK293T and SK-N-AS cell lines.
High CFDP1 expression associates with worst patient prognosis
The R2 Genomics Analysis and Visualization Platform (http://r2.amc.nl) was used to query the transcriptomic data of 498 NB samples (GEO: GSE62564). Our analysis showed that high CFDP1 expression was associated with poor overall and event-free survival probabilities (p ≤ 5.2 × 10−7) (Figure S3A). Moreover, the expression of CFDP1 was significantly higher in stage 4 tumors than in lower tumor stages (p ≤ 3.7 × 10−3) (Figure S3B). In addition, CFDP1 expression was significantly higher in four independent NB datasets (including 281 samples) than in normal adrenal gland tissues (p < 1.0 × 10−4, Figure S3C). Finally, by querying gene expression data from the Cancer Cell Line Encyclopedia (GEO: GSE36133) (including 917 samples from 36 cancer types), we found that NB cell lines showed higher expression of CFDP1 than other cell lines (p = 4.82 × 10−7; ANOVA) (Figure S3D).
In vitro functional analysis of CFDP1
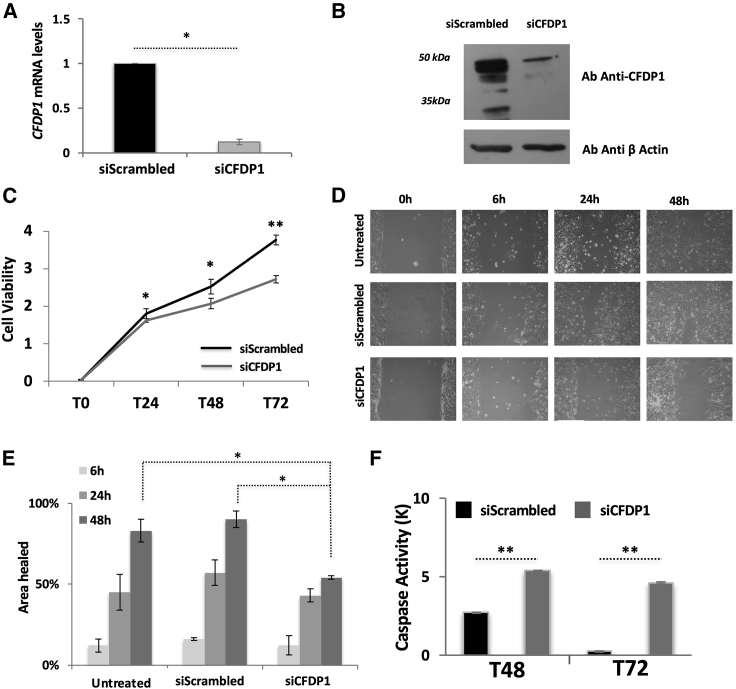
To investigate the biological role of CFDP1 in NB, we transiently knocked down endogenous CFDP1 using specific siRNA transfection. To conduct this experiment, we first assessed the expression level of CFPD1 protein in NB cell lines by western blotting (Figure S4). From this analysis, we selected the SK-N-BE cell line given its high levels of CFDP1 and the SK-N-AS cell line, given their intermediate noradrenergic identity between NB and NCCs, as reported in Boeva et al. 11 Compared with the control siRNA (siScrambled), three different siRNAs targeting CFDP1 (siCFPD1) significantly reduced its mRNA (Figure 4A) and protein levels (Figure 4B) in SK-N-AS cells (p < 0.05). Moreover, depletion of CFDP1 markedly decreased cell viability (p < 0.05) (Figure 4C), and migration capabilities (p < 0.05) (Figures 4D and 4E) and increased apoptotic rates (p < 0.001) (Figure 4F) at different time points. The same assays performed on SK-N-BE cells confirmed these results (Figures S5A–S5F). These findings support the involvement of CFDP1 in the initiation and progression of NB.
Figure 4.
The depletion of CFDP1 correlates with decreased tumorigenicity in SK-N-AS NB cell line
(A) Barplots showing the results of expression quantification by quantitative real-time PCR of CFDP1 Control (siScrambled) and CFDP1 silenced cells (siCFDP1).
(B) CFDP1 protein levels assessed using western blot analysis in Scrambled and siCFDP1 cells.
(C) Line plot reporting cell viability and proliferation measured by MTT assay in Scrambled and siCFDP1 cells. In (A) to (C), the data (mean of three experiments ± standard deviation) are represented as the fold change of siCFDP1 compared with control (siScrambled) cells.
(D) Bright-light microscopy images reporting the results of wound-healing assay observed at different time points (6, 24, and 48 h). The wound distance was measured with ImageJ software.
(E) Barplots reporting the quantifications of distances obtained in (D). The experiments in (D) and (E) were made in triplicate and conducted on untreated, siScrambled, and siCFDP1 SK-N-AS cells.
(F) Barplot of the Caspase-3 activity assay on SK-N-AS cells. The data in siCFDP1 were normalized on the negative (promoter-less) control and reported in thousands (K). Data are shown as the mean ± standard deviation from three independent transfection experiments, each done in triplicate. In Panel (A), (C), (E), and (F), statistical significance was assessed by t test. ∗p < 0.05; ∗∗p < 0.001; ∗∗∗p < 0.0001.
The depletion of CFDP1 affects noradrenergic neuron differentiation
To elucidate the molecular mechanisms involved in CFDP1, we sequenced the whole transcriptome (RNA-seq) of the SK-N-AS NB cell lines under siCFDP1 and siScrambled experimental conditions (silenced and control, respectively; see above) and performed differential gene expression analysis. Details of the quality control of RNA-seq analysis are reported in Figures S6A–S6C.
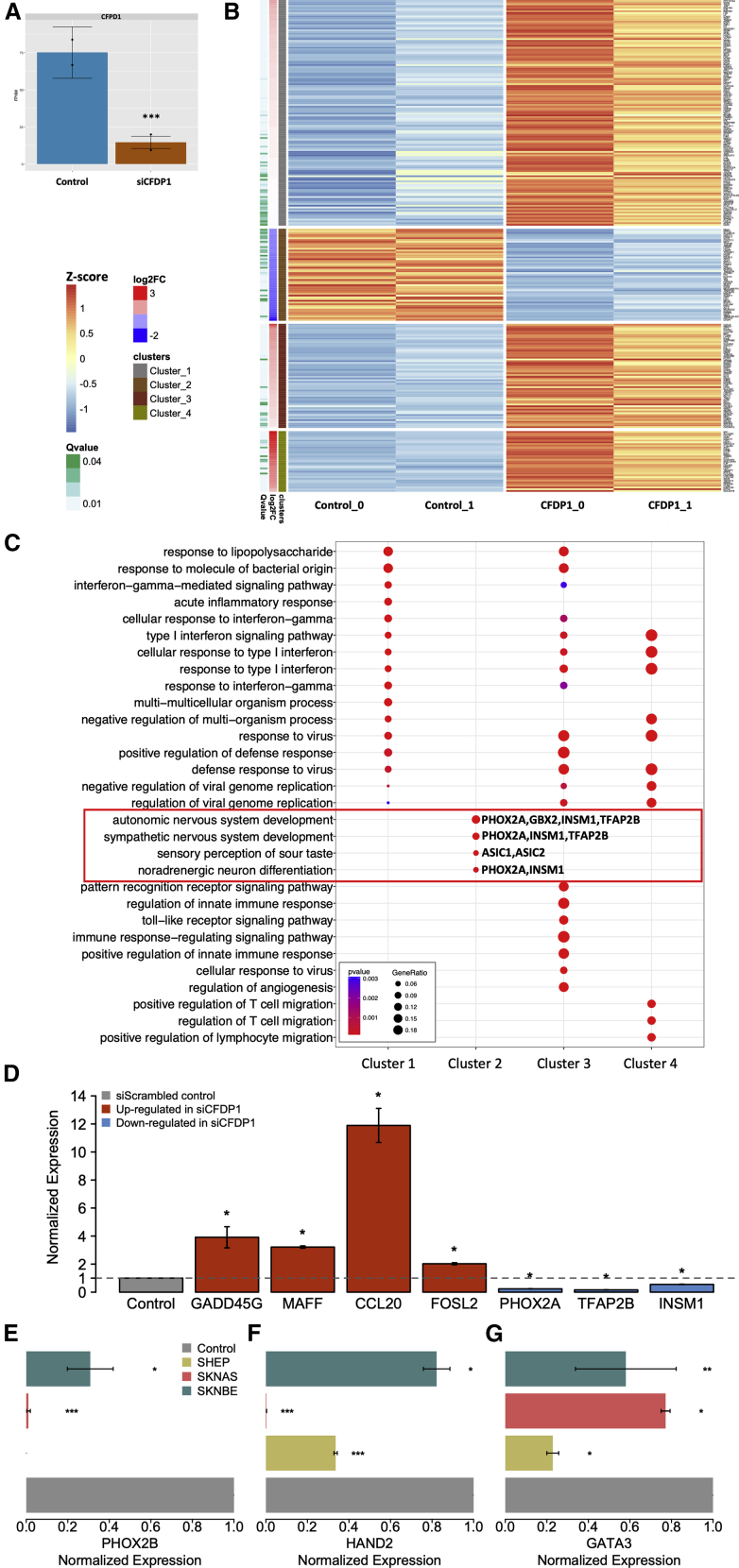
RNA-seq analysis confirmed that CFDP1 expression was significantly decreased in siCFPD1 compared with that in the control (Log2 FC = −2.36; FDR = 3.6 × 10−3) (Figure 5A and Table S5). From the differential expression analysis, we identified 278 significantly regulated genes (FDR ≤0.05, Table S5). Subsequently, we performed k-means clustering (k = 4) to group the list of genes based on their expression patterns. In siCFPD1 compared with the control, the Clusters 1, 3, and 4 showed increased gene expression, whereas Cluster 2, including CFDP1, exhibited decreased expression (Figure 5B). Next, we performed the gene ontology (GO) enrichment analysis of biological processes for the genes included in the four clusters. The results clearly showed that Clusters 1, 3, and 4 (up-regulated genes) contained genes mainly involved in processes related to inflammation, the interferon pathway, and the immune system (Figure 5C and Table S6). The genes in Cluster 2 (down-regulated genes) were mainly involved in processes related to autonomic and sympathetic nervous system development and differentiation of noradrenergic neurons (Figure 5C and Table S6). Notably, the Cluster 2 contained genes known to be involved in conferring noradrenergic cell identities in NB (PHOX2A, 41 INSM1, 42 TFAP2B 43).
Figure 5.
The depletion of CFDP1 affects the expression of gene regulators of NB cell identity
(A) Barplots showing the expression levels of CFDP1 in control (siScrambled) and CFDP1 silenced cells (siCFDP1) as measured by RNA-seq in SK-N-AS.
(B) Heatmap of the differentially expressed genes grouped according to the k-means clustering algorithm. Color key on the left.
(C) Dotplots reporting the Gene Ontology biological process enrichment results of clustered gene lists.
(D) Barplot showing the results of expression quantification by quantitative real-time PCR for four up-regulated genes (red) and three down-regulated genes (blue) in SK-N-AS.
(E) Expression quantification by quantitative real-time PCR of PHOX2B in SK-N-AS and SK-N-BE NB cell lines.
(F) Expression quantification by quantitative real-time PCR of HAND2 in SK-N-AS, SK-N-BE, and SH-EP NB cell lines.
(G) Expression quantification by quantitative real-time PCR of GATA3 in SK-N-AS, SK-N-BE, and SH-EP NB cell lines. The data in (D) to (G) are reported as fold-changes of induction in siCFDP1 compared with control cells. In Panel (A), (D), (E), (F), and (G) statistical significance was assessed using a t test. ∗p < 0.05; ∗∗p < 0.001; ∗∗∗p < 0.0001.
To assess the reliability of RNA-seq data, we measured gene expression by quantitative real-time PCR in our two experimental conditions using seven DEGs that play key roles in conferring noradrenergic and NCC-like identity to NB cells. We confirmed the up-regulation of GADD45G, MAFF, FOSL2, and CCL20 (p ≤ 0.05) (Figure 5D) and down-regulation of PHOX2A, TFAP2B, and INSM1 (p ≤ 0.05) (Figure 5D) in siCFDP1 compared with the siScrambled control. Based on these results, we also assessed the expression levels of the transcription factors (TFs) HAND2, GATA3, and PHOX2B, which together participate in a complex network regulating noradrenergic neuron differentiation.11 For this purpose, we silenced CFPD1 in three different NB cell lines representative of the three NB cell types: SK-N-BE (noradrenergic cell type), SK-N-AS (intermediate cell type), and SH-EP (NCC type). Quantitative real-time PCR showed down-regulation of PHOX2B in SK-N-BE and SK-N-AS cells (p ≤ 0.05), but not in SH-EP cells (Figure 5E), whereas HAND2 and GATA3 were down-regulated in all three cell lines (p ≤ 0.05) (Figures 5F and 5G). In the RNA-seq data, we observed the same decrease in the expression of these TFs without reaching the statistical threshold of significance, probably because of the low sensitivity of the method (Table S5).
CFDP1 and master regulators of noradrenergic cell identity are co-expressed in NB tumors
Consequently, we assessed whether the observed positive correlation between CFDP1 and noradrenergic-related TFs (PHOX2B, HAND2, GATA3, PHOX2A, and INSM1) also subsisted in NB tumors. By querying a public dataset of 498 NB (GEO: GSE62564), we confirmed the direct correlation between CFDP1 and PHOX2B, GATA3, and PHOX2A (R > 0.180; p ≤ 5.24 × 10−5), but not with HAND2 and INSM1 (Figures S7A–S7E). We can comment on the latter result by speculating that other and more complex regulatory circuitries could involve HAND2 and INSM1. In an additional expression analysis, we observed that CFDP1 and the noradrenergic-related TFs showed similar expression trends from the undifferentiated NCs to the differentiated adrenal glands (p ≤ 8.23 × 10−3) (Figures S8A–S8F). Together, these findings provide strong evidence supporting a direct correlation between CFDP1 and master regulators of NB, and add a piece to the complex mosaic of the regulatory circuitries of NB.
Discussion
Based on the hypothesis that NB and CAD are NCC-derived diseases that may share the same genetic factors, we conducted a cross-phenotype meta-analysis of GWAS data and replication-based studies to identify novel pleiotropic variants that contribute to NB risk. This strategy exploits the pleiotropic effect of two distinct phenotypes to increase the statistical power necessary for discovering novel disease-predisposing variants discarded by stringent multiple-testing correction in GWAS. We are aware that the different sample size of our two cohorts in the Stage 1 analysis might represent a limitation of this work. However, the reliability of our data was assessed in the replication-based genotyping of an independent cohort of NB affected individuals and control subjects that confirmed the association of the SNPs selected in Stages 1 and 2.
The meta-analysis of NB and CAD GWASs identified the rs13337397 SNP at 16q23.1, in an active enhancer of NCCs, cross-associated with CAD and NB. The same SNP replicated the genetic association with NB risk in an Italian cohort of patients and control subjects. This genetic association supports our hypothesis that the early phases of embryonic development can be altered by pleiotropic risk SNPs. We then focused on functional investigation of the 16q231 locus to unravel its involvement in NB initiation. Fine mapping analyses indicated that the SNP rs13337017 is a potential causative variant; indeed, it resides in a regulatory region of NCCs, the fetal adrenal gland, and NB cell lines. Notably, this genomic region functions as a super-enhancer and strongly interacts with CFDP1 in NB cells. Moreover, the minor allele of the rs13337017 SNP was associated with increased NB risk and higher CFDP1 expression in whole blood, adrenal glands, and NB cell lines. Together, these data suggest that NB and CAD are NC-derived diseases that may share the same molecular mechanisms and that our strategy is useful for the identification of novel disease susceptibility genes. Interestingly, CFDP1 has also been reported as a new CAD susceptibility gene44; however, its functional role in CAD development remains unknown.
CFDP1 belongs to the evolutionarily conserved Bucentaur (BCNT) protein superfamily and is expressed during early embryogenesis. In mice, CFDP1 is highly expressed during neural tube development45 and its ablation causes early embryonic lethality.45 Recent studies have proposed CFDP1 involvement in craniofacial development and osteogenesis in vertebrates and linked this gene with Williams-Beuren syndrome, which is characterized by developmental disorders affecting many body sites, including the heart, blood vessels, bone, and eye.46 Craniofacial birth defects involving bones can be caused by abnormal development of NCC.47,48 Based on these observations, we propose that risk variants can induce CFDP1 overexpression and contribute to cellular malignant transformation in the early phases of embryogenesis by altering the normal program of NCC differentiation into sympathetic neurons. Our hypothesis was supported by literature reporting predisposing variants with cis-effects on LIN28B in NB. 49 LIN28B is involved in reprogramming of human and mouse fibroblasts into pluripotent stem cells during the embryogenesis.50,51
Our work demonstrated that the minor allele of rs13337017 was correlated with increased CFDP1 mRNA levels and conferred a risk of NB development, suggesting that CFPD1 acts as an oncogene. Accordingly, CFDP1 expression was significantly higher in NB than in other types of tumors and normal tissues, and was correlated with poor clinical outcomes in patients with NB. Transient knockdown of CFDP1 results in significant growth inhibition and of apoptosis induction in NB cells. This result is consistent with previous reports indicating that CFDP1 stimulates cell proliferation and prevents apoptosis in embryonic fibroblasts. 52
Evidence of high expression levels of CFDP1 has been reported in a wide range of human tissues, including cancer tissues.16,53 Furthermore, recent studies have associated the 16q23.1 locus, which contains CFDP1, with different cancer types, such as multiple myeloma,54 acute lymphoblastic leukemia,55 pancreatic cancer,56,57 and breast cancer.58 Taken together, these results provide evidence that CFDP1 may play an oncogenic role in NB and potentially in other cancer diseases. Nevertheless, further studies are necessary to assess the implications of CFDP1 in NB.
In addition, using RNA-seq, we investigated the possible molecular mechanisms involving CFDP1 in NB cell lines. We compared the transcriptome of CFDP1 depleted cells with that of untreated control cells. Analysis of the DEGs revealed that sympathetic nervous system development and noradrenergic neuron differentiation were the most significantly enriched GO terms among the down-regulated genes. Interestingly, depletion of CFDP1 also reduced the expression of three master regulators (PHOX2B, HAND2, and GATA3) of noradrenergic cell identity in NB.11 Since CFPD1 seems to play a role in chromatin organization59 and in promoting neural differentiation,48 we speculated that its alteration could affect normal differentiation programs controlled by the master regulators PHOX2B, HAND2, and GATA3 and thus promote NB initiation and/or progression.
In light of our results, additional studies, involving mouse models, become necessary to better understand the oncogenic role of CFPD1. Furthermore, they could help in deepening the biochemical and physiological processes behind the occurrence and development of NB. In the future, such levels of information could also be useful for alternative and personalized therapy strategies.
In conclusion, our results indicate that a polymorphism within a super-enhancer element influences NB susceptibility by modulating CFDP1 expression leading to oncogenic dependency in tumor cells, likely by altering the control of noradrenergic differentiation. Furthermore, this study demonstrated that using GWAS data through cross-phenotype meta-analysis and integrating functional genomics data could be a useful strategy to identify novel disease susceptibility loci and genes.
Acknowledgments
This study was supported by grants from Associazione Italiana per la Ricerca sul Cancro (Grant no. 25796 to M.C. and Grant no. 20757 to A.I.); Fondazione Italiana per la Lotta al Neuroblastoma (to M.C.); Associazione Oncologia Pediatrica e Neuroblastoma (to M.C.); Regione Campania “SATIN” grant 2018-2020 (to M.C.); and Associazione Giulio Adelfio onlus (to M.C. and A.I.).
Author contributions
M.C., D.F., and V.A.L. drafted the manuscript. D.F., A.C., and M.A. performed in vitro functional assays. V.A.L. performed bioinformatic analyses and curated data collection, analysis, and visualization. A.T. performed bioinformatic analyses of the GWAS data. S.C. supported in DNA sample collecting and genotyping. S.D. provided GWAS data for neuroblastoma. A.C., S.C., and M.A. critically reviewed the manuscript. S.D. and A.I. critically reviewed the study design and the manuscript. M.C. designed and supervised the study. All the authors have read and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xhgg.2022.100158.
Web resources
GEO DataSets: https://www.ncbi.nlm.nih.gov/gds
GAS Power Calculator: http://csg.sph.umich.edu/abecasis/gas_power_calculator/index.html
GTEx: https://gtexportal.org/home/
R2 Genomics Analysis and Visualization Platform: http://r2.amc.nl
3DIV: http://3div.kr/
Supplemental information
Data and code availability
The RNA-sequencing data generated and analyzed in this study are available at the NCBI GEO database under the accession number: GEO: GSE216674. Public data and data repositories are referenced within the manuscript. This study did not generate code.
References
- 1.Matthay K.K., Maris J.M., Schleiermacher G., Nakagawara A., Mackall C.L., Diller L., Weiss W.A. Neuroblastoma. Nat. Rev. Dis. Primers. 2016;2:16078. doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 2.Capasso M., Diskin S.J. Genetics and genomics of neuroblastoma. Cancer Treat Res. 2010;155:65–84. doi: 10.1007/978-1-4419-6033-7_4. [DOI] [PubMed] [Google Scholar]
- 3.Peifer M., Hertwig F., Roels F., Dreidax D., Gartlgruber M., Menon R., Kramer A., Roncaioli J.L., Sand F., Heuckmann J.M., et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature. 2015;526:700–704. doi: 10.1038/nature14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lasorsa V.A., Cimmino F., Ognibene M., Mazzocco K., Erminio G., Morini M., Conte M., Iolascon A., Pezzolo A., Capasso M. 19p loss is significantly enriched in older age neuroblastoma patients and correlates with poor prognosis. NPJ Genom Med. 2020;5:18. doi: 10.1038/s41525-020-0125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pugh T.J., Morozova O., Attiyeh E.F., Asgharzadeh S., Wei J.S., Auclair D., Carter S.L., Cibulskis K., Hanna M., Kiezun A., et al. The genetic landscape of high-risk neuroblastoma. Nat. Genet. 2013;45:279–284. doi: 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasorsa V.A., Montella A., Cantalupo S., Tirelli M., de Torres C., Aveic S., Tonini G.P., Iolascon A., Capasso M. Somatic mutations enriched in cis-regulatory elements affect genes involved in embryonic development and immune system response in neuroblastoma. Cancer Res. 2022 doi: 10.1158/0008-5472. CAN-20-3788. [DOI] [PubMed] [Google Scholar]
- 7.Capasso M., Lasorsa V.A., Cimmino F., Avitabile M., Cantalupo S., Montella A., De Angelis B., Morini M., de Torres C., Castellano A., et al. Transcription factors involved in tumorigenesis are over-represented in mutated active DNA-binding sites in neuroblastoma. Cancer Res. 2020;80:382–393. doi: 10.1158/0008-5472.CAN-19-2883. [DOI] [PubMed] [Google Scholar]
- 8.Zhuo Z., Lu H., Zhu J., Hua R.X., Li Y., Yang Z., Zhang J., Cheng J., Zhou H., Li S., et al. METTL14 gene polymorphisms confer neuroblastoma susceptibility: an eight-center case-control study. Mol. Ther. Nucleic Acids. 2020;22:17–26. doi: 10.1016/j.omtn.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuo Z., Zhou C., Fang Y., Zhu J., Lu H., Zhou H., Wu H., Wang Y., He J. Correlation between the genetic variants of base excision repair (BER) pathway genes and neuroblastoma susceptibility in eastern Chinese children. Cancer Commun. 2020;40:641–646. doi: 10.1002/cac2.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J.R., Bagatell R., Cohn S.L., Pearson A.D., Villablanca J.G., Berthold F., Burchill S., Boubaker A., McHugh K., Nuchtern J.G., et al. Revisions to the international neuroblastoma response criteria: a consensus statement from the national cancer institute clinical trials planning meeting. J. Clin. Oncol. 2017;35:2580–2587. doi: 10.1200/JCO.2016.72.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boeva V., Louis-Brennetot C., Peltier A., Durand S., Pierre-Eugene C., Raynal V., Etchevers H.C., Thomas S., Lermine A., Daudigeos-Dubus E., et al. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat. Genet. 2017;49:1408–1413. doi: 10.1038/ng.3921. [DOI] [PubMed] [Google Scholar]
- 12.Raid R., Krinka D., Bakhoff L., Abdelwahid E., Jokinen E., Karner M., Malva M., Meier R., Pelliniemi L.J., Ploom M., et al. Lack of Gata3 results in conotruncal heart anomalies in mouse. Mech. Dev. 2009;126:80–89. doi: 10.1016/j.mod.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Song H., Suehiro J., Kanki Y., Kawai Y., Inoue K., Daida H., Yano K., Ohhashi T., Oettgen P., Aird W.C., et al. Critical role for GATA3 in mediating Tie2 expression and function in large vessel endothelial cells. J. Biol. Chem. 2009;284:29109–29124. doi: 10.1074/jbc.M109.041145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes R.M., Firulli B.A., VanDusen N.J., Morikawa Y., Conway S.J., Cserjesi P., Vincentz J.W., Firulli A.B. Hand2 loss-of-function in Hand1-expressing cells reveals distinct roles in epicardial and coronary vessel development. Circ. Res. 2011;108:940–949. doi: 10.1161/CIRCRESAHA.110.233171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arima Y., Miyagawa-Tomita S., Maeda K., Asai R., Seya D., Minoux M., Rijli F.M., Nishiyama K., Kim K.S., Uchijima Y., et al. Preotic neural crest cells contribute to coronary artery smooth muscle involving endothelin signalling. Nat. Commun. 2012;3:1267. doi: 10.1038/ncomms2258. [DOI] [PubMed] [Google Scholar]
- 16.Orian-Rousseau V., Mink S., Mengwasser J., HogenEsch H., Guo F., Thies W.G., Hofmann M., Herrlich P., Ponta H. Genes upregulated in a metastasizing human colon carcinoma cell line. Int. J. Cancer. 2005;113:699–705. doi: 10.1002/ijc.20644. [DOI] [PubMed] [Google Scholar]
- 17.Tonini G.P., Capasso M. Genetic predisposition and chromosome instability in neuroblastoma. Cancer Metastasis Rev. 2020;39:275–285. doi: 10.1007/s10555-020-09843-4. [DOI] [PubMed] [Google Scholar]
- 18.Mehta N.N. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Circulation. Cardiovascular Genet. 2011;4:327–329. doi: 10.1161/CIRCGENETICS.111.960443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z., Schunkert H. Genetics of coronary artery disease in the post-GWAS era. J. Intern. Med. 2021;290:980–992. doi: 10.1111/joim.13362. [DOI] [PubMed] [Google Scholar]
- 20.Tan S.L., Nishi M., Ohtsuka T., Matsui T., Takemoto K., Kamio-Miura A., Aburatani H., Shinkai Y., Kageyama R. Essential roles of the histone methyltransferase ESET in the epigenetic control of neural progenitor cells during development. Development. 2012;139:3806–3816. doi: 10.1242/dev.082198. [DOI] [PubMed] [Google Scholar]
- 21.Pan L., Ma X., Wen B., Su Z., Zheng X., Liu Y., Li H., Chen Y., Wang J., Lu F., et al. Microphthalmia-associated transcription factor/T-box factor-2 axis acts through Cyclin D1 to regulate melanocyte proliferation. Cell Prolif. 2015;48:631–642. doi: 10.1111/cpr.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams A.L., Bohnsack B.L. Neural crest derivatives in ocular development: discerning the eye of the storm. Birth defects research. Part C, Embryo today : reviews. 2015;105:87–95. doi: 10.1002/bdrc.21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh C.W., Kao S.H., Cheng Y.C., Hsu L.S. Knockdown of cyclin-dependent kinase 10 (cdk10) gene impairs neural progenitor survival via modulation of raf1a gene expression. J. Biol. Chem. 2013;288:27927–27939. doi: 10.1074/jbc.M112.420265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong D., Jiang M., Xu X., Guan M., Wu J., Chen Q., Xiang L. The effects of NB-UVB on the hair follicle-derived neural crest stem cells differentiating into melanocyte lineage in vitro. J. Dermatol. Sci. 2012;66:20–28. doi: 10.1016/j.jdermsci.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Takita J., Hayashi Y., Nakajima T., Adachi J., Tanaka T., Yamaguchi N., Ogawa Y., Hanada R., Yamamoto K., Yokota J. The p16 (CDKN2A) gene is involved in the growth of neuroblastoma cells and its expression is associated with prognosis of neuroblastoma patients. Oncogene. 1998;17:3137–3143. doi: 10.1038/sj.onc.1202232. [DOI] [PubMed] [Google Scholar]
- 26.Testori A., Lasorsa V.A., Cimmino F., Cantalupo S., Cardinale A., Avitabile M., Limongelli G., Russo M.G., Diskin S., Maris J., et al. Exploring shared susceptibility between two neural crest cells originating conditions: neuroblastoma and congenital heart disease. Genes. 2019;10 doi: 10.3390/genes10090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kar S.P., Beesley J., Amin Al Olama A., Michailidou K., Tyrer J., Kote-Jarai Z., Lawrenson K., Lindstrom S., Ramus S.J., Thompson D.J., et al. Genome-wide meta-analyses of breast, ovarian, and prostate cancer association studies identify multiple new susceptibility loci shared by at least two cancer types. Cancer Discov. 2016;6:1052–1067. doi: 10.1158/2159-8290.CD-15-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fehringer G., Kraft P., Pharoah P.D., Eeles R.A., Chatterjee N., Schumacher F.R., Schildkraut J.M., Lindstrom S., Brennan P., Bickeboller H., et al. Cross-cancer genome-wide analysis of lung, ovary, breast, prostate, and colorectal cancer reveals novel pleiotropic associations. Cancer Res. 2016;76:5103–5114. doi: 10.1158/0008-5472.CAN-15-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung R.J., Ulrich C.M., Goode E.L., Brhane Y., Muir K., Chan A.T., Marchand L.L., Schildkraut J., Witte J.S., Eeles R., et al. Cross cancer genomic investigation of inflammation pathway for five common cancers: lung, ovary, prostate, breast, and colorectal cancer. J. of the Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coronary Artery Disease Genetics C. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nat. Genet. 2011;43:339–344. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 31.McDaniel L.D., Conkrite K.L., Chang X., Capasso M., Vaksman Z., Oldridge D.A., Zachariou A., Horn M., Diamond M., Hou C., et al. Common variants upstream of MLF1 at 3q25 and within CPZ at 4p16 associated with neuroblastoma. PLoS Genet. 2017;13:e1006787. doi: 10.1371/journal.pgen.1006787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wacholder S., Chanock S., Garcia-Closas M., El Ghormli L., Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. Journal of the National Cancer Institute. 2004;96:434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avitabile M., Lasorsa V.A., Cantalupo S., Cardinale A., Cimmino F., Montella A., Capasso D., Haupt R., Amoroso L., Garaventa A., et al. Association of PARP1 polymorphisms with response to chemotherapy in patients with high-risk neuroblastoma. J. Cell Mol. Med. 2020;24:4072–4081. doi: 10.1111/jcmm.15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fishilevich S., Nudel R., Rappaport N., Hadar R., Plaschkes I., Iny Stein T., Rosen N., Kohn A., Twik M., Safran M., et al. Database; 2017. GeneHancer: Genome-wide Integration of Enhancers and Target Genes in GeneCards. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardinale A., Cantalupo S., Lasorsa V.A., Montella A., Cimmino F., Succoio M., Vermeulen M., Baltissen M.P., Esposito M., Avitabile M., et al. Functional annotation and investigation of the 10q24.33 melanoma risk locus identifies a common variant that influences transcriptional regulation of OBFC1. Hum. Mol. Genet. 2022;31:863–874. doi: 10.1093/hmg/ddab293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howie B.N., Donnelly P., Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rada-Iglesias A., Prescott S.L., Wysocka J. Human genetic variation within neural crest enhancers: molecular and phenotypic implications. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2013;368:20120360. doi: 10.1098/rstb.2012.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolbert V.P., Coggins G.E., Maris J.M. Genetic susceptibility to neuroblastoma. Curr. Opin. Genet. Dev. 2017;42:81–90. doi: 10.1016/j.gde.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker S.C., Stitzel M.L., Taylor D.L., Orozco J.M., Erdos M.R., Akiyama J.A., van Bueren K.L., Chines P.S., Narisu N., Program N.C.S., et al. Chromatin stretch enhancer states drive cell-specific gene regulation and harbor human disease risk variants. Prod. of the Natl. Acad. of Sci. of the USA. 2013;110:17921–17926. doi: 10.1073/pnas.1317023110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brodeur G.M. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 41.Seo H., Hong S.J., Guo S., Kim H.S., Kim C.H., Hwang D.Y., Isacson O., Rosenthal A., Kim K.S. A direct role of the homeodomain proteins Phox2a/2b in noradrenaline neurotransmitter identity determination. J. Neurochem. 2002;80:905–916. doi: 10.1046/j.0022-3042.2002.00782.x. [DOI] [PubMed] [Google Scholar]
- 42.Wildner H., Gierl M.S., Strehle M., Pla P., Birchmeier C. Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development. 2008;135:473–481. doi: 10.1242/dev.011783. [DOI] [PubMed] [Google Scholar]
- 43.Ikram F., Ackermann S., Kahlert Y., Volland R., Roels F., Engesser A., Hertwig F., Kocak H., Hero B., Dreidax D., et al. Transcription factor activating protein 2 beta (TFAP2B) mediates noradrenergic neuronal differentiation in neuroblastoma. Mol. Oncol. 2016;10:344–359. doi: 10.1016/j.molonc.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klarin D., Zhu Q.M., Emdin C.A., Chaffin M., Horner S., McMillan B.J., Leed A., Weale M.E., Spencer C.C.A., Aguet F., et al. Genetic analysis in UK Biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat. Genet. 2017;49:1392–1397. doi: 10.1038/ng.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diekwisch T.G., Marches F., Williams A., Luan X. Cloning, gene expression, and characterization of CP27, a novel gene in mouse embryogenesis. Gene. 1999;235:19–30. doi: 10.1016/s0378-1119(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 46.Makeyev A.V., Bayarsaihan D. Molecular basis of Williams-Beuren syndrome: TFII-I regulated targets involved in craniofacial development. Cleft Palate Craniofac J. 2011;48:109–116. doi: 10.1597/09-093. [DOI] [PubMed] [Google Scholar]
- 47.Trainor P.A. Craniofacial birth defects: the role of neural crest cells in the etiology and pathogenesis of Treacher Collins syndrome and the potential for prevention. Am. J. Med. Genet. 2010;152A:2984–2994. doi: 10.1002/ajmg.a.33454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snider T.N., Mishina Y. Cranial neural crest cell contribution to craniofacial formation, pathology, and future directions in tissue engineering. Birth defects research. Part C, Embryo today : reviews. 2014;102:324–332. doi: 10.1002/bdrc.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diskin S.J., Capasso M., Schnepp R.W., Cole K.A., Attiyeh E.F., Hou C., Diamond M., Carpenter E.L., Winter C., Lee H., et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat. Genet. 2012;44:1126–1130. doi: 10.1038/ng.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 51.Melton C., Judson R.L., Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–626. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luan X., Diekwisch T.G. CP27 affects viability, proliferation, attachment and gene expression in embryonic fibroblasts. Cell Prolif. 2002;35:207–219. doi: 10.1046/j.1365-2184.2002.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Messina G., Celauro E., Atterrato M.T., Giordano E., Iwashita S., Dimitri P. The Bucentaur (BCNT) protein family: a long-neglected class of essential proteins required for chromatin/chromosome organization and function. Chromosoma. 2015;124:153–162. doi: 10.1007/s00412-014-0503-8. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell J.S., Li N., Weinhold N., Forsti A., Ali M., van Duin M., Thorleifsson G., Johnson D.C., Chen B., Halvarsson B.M., et al. Genome-wide association study identifies multiple susceptibility loci for multiple myeloma. Nat. Commun. 2016;7:12050. doi: 10.1038/ncomms12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi Y., Du M., Fang Y., Tong N., Zhai X., Sheng X., Li Z., Xue Y., Li J., Chu H., et al. Identification of a novel susceptibility locus at 16q23.1 associated with childhood acute lymphoblastic leukemia in Han Chinese. Hum. Mol. Genet. 2016;25:2873–2880. doi: 10.1093/hmg/ddw112. [DOI] [PubMed] [Google Scholar]
- 56.Childs E.J., Mocci E., Campa D., Bracci P.M., Gallinger S., Goggins M., Li D., Neale R.E., Olson S.H., Scelo G., et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat. Genet. 2015;47:911–916. doi: 10.1038/ng.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolpin B.M., Rizzato C., Kraft P., Kooperberg C., Petersen G.M., Wang Z., Arslan A.A., Beane-Freeman L., Bracci P.M., Buring J., et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat. Genet. 2014;46:994–1000. doi: 10.1038/ng.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higginbotham K.S., Breyer J.P., McReynolds K.M., Bradley K.M., Schuyler P.A., Plummer W.D., Freudenthal M.E., Trentham-Dietz A., Newcomb P.A., Parl F.F., et al. A multistage genetic association study identifies breast cancer risk loci at 10q25 and 16q24. Cancer Epidemiol. Biomarkers Prev. 2012;21:1565–1573. doi: 10.1158/1055-9965.EPI-12-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Messina G., Atterrato M.T., Prozzillo Y., Piacentini L., Losada A., Dimitri P. The human Cranio Facial Development Protein 1 (Cfdp1) gene encodes a protein required for the maintenance of higher-order chromatin organization. Sci. Rep. 2017;7:45022. doi: 10.1038/srep45022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-sequencing data generated and analyzed in this study are available at the NCBI GEO database under the accession number: GEO: GSE216674. Public data and data repositories are referenced within the manuscript. This study did not generate code.