Abstract
Background
The Mycobacterium tuberculosis complex causes tuberculosis, a severe public health problem. Close contacts of someone who has active pulmonary tuberculosis were at a greater risk of contracting the disease. Despite the large number of primary research available in Sub-Saharan African nations, there are no systematic reviews or meta-analyses that estimate the pooled prevalence of tuberculosis among tuberculosis patients' household contacts (HHC). Thus, this study aimed to estimate the pooled prevalence of tuberculosis in a household contact of tuberculosis patients in the sub-Saharan African region.
Methods
Potential papers were systematically searched from electronic databases (PubMed, Google scholar and web of science). To analyze the quality of the papers featured, we used the Joanna Briggs Institute Critical Appraisal methods. Data were analyzed using STATA Version 16.
Result
After screening 373 studies, the final analysis includes 20 articles from twelve countries. The overall prevalence of tuberculosis among household contacts was 3.29 % (95 % CI; 2.35 %-4.23 %). The overall prevalence rate of active tuberculosis in children aged less than five years was 2.60 % (95 % CI; 1.81 %-3.39 %). When the index patient age was less than 18 years old, the pooled prevalence of active TB in HHC was 2.64 % (95 % CI; 1.46 %-3.81 %). The pooled proportion of HIV in index TB patients was 53.12 % (95 % CI, 39.73 %-66.51 %). The overall pooled prevalence of HIV in household contacts was 7.75 % (95 % CI, 4.21 %-11.29 %).
Conclusion
Our systematic review showed that, in Sub-Saharan African nations, household contacts are at a high risk of contracting tuberculosis from their index patient. According to this study, one out of every thirty household contacts will develop active tuberculosis. This demonstrated the significance of doing thorough active tuberculosis case tracing in household contacts to locate missing tuberculosis patients.
Keywords: Household contact, Tuberculosis, Sub-Saharan Africa
1. Introduction
Globally, Tuberculosis (TB) is one of the top 13 causes of death, and it is the second leading cause of death caused by a single infectious agent after COVID 19. Tuberculosis is a disease of poverty and it affects all age groups [1]. It is caused by the Mycobacterium tuberculosis bacteria. Pulmonary tuberculosis (PTB) affects the lung whereas extrapulmonary tuberculosis (EPTB) was the disseminated type of TB other than the lung [2]. Pulmonary tuberculosis is the most common type of tuberculosis, which is spread by inhaling aerosolized bacilli from coughing, sneezing, or talking. Close contacts of someone who has active infectious PTB are at a high risk of contracting the disease. The predominant source of TB infection was untreated smear-positive PTB patients [3].
In 2019, an estimated 10.0 million persons become infected with tuberculosis (TB). Africa accounted for one-fourth of all people diagnosed with tuberculosis in this year. [1]. Household contacts (HHC) who live with infectious TB patients are one of the groups at high risk of developing TB [4]. Preventive treatment for HHC of people with TB falls far short of what is planned in 2020 [1].
The chance of contracting tuberculosis is determined by the person's immunity, the infectiousness of the TB patient, the proximity of the contact, and the amount of time spent in contact with the source of infection. One of the pillars of the End TB approach is a systematic screening of high-risk groups, such as close contacts with infectious TB [5]. According to a global TB report, almost 30 % of new TB cases are missed each year because they are not diagnosed or underreported to the competent national authority. [1].
To fill undiagnosed cases the World Health Organization (WHO) recommends active case finding and contact investigation among household and close contacts of infectious TB cases [6]. Contact investigation had an importance on early diagnosis and treatment of contacts with active TB, so it gives opportunity to interrupt transmission and reduce morbidity and mortality due to TB [7]. Contact investigation involves the systematic evaluation of the contacts to identify, find, and assess active or latent TB. It is a key for active case detection to increase the case detection rate [7]. Contact investigation involves clinical screening, chest X-ray, or microbiological evaluation of sputum for active TB and testing for latent TB.
In Africa, individuals who had diseases symptom goes to health facility and the health professionals screen for the presence of TB, which is a passive case finding [8]. Although WHO promotes contact tracing as a TB control strategy, it was not widely used in underdeveloped and African nations [9]. In these countries prevalence of bacteriologically confirmed active TB among contacts was 1.2 %, while latent TB was 51.5 % and Multi drug resistance (MDR) or extensive drug resistance (XDR) TB was 3.4 %. Additionally, 3.1 % of household contacts of TB patients were diagnosed with TB [7].
To our knowledge, there was no systematic review and meta-analysis done in sub-Saharan African countries on the prevalence of TB in household contacts of TB patients. So, this systematic review and meta-analysis was conducted to review the pooled prevalence of tuberculosis among household contacts of TB patients in the sub-Saharan African countries.
2. Materials and methods
2.1. Search strategy and selection criteria
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and meta-Analyses (PRISMA) follow digram (S. Table 1) [10]. Since this study was based on previously published primary studies no ethical clearance was taken from anywhere. The study was conducted to estimate the pooled prevalence of TB in household contacts of TB patients in sub-Saharan African countries.
Two investigators (GS and AA) searched major electronic databases; PubMed/Medline, and Web of Science for articles published after 2000 in English language. We also sought for papers in gray literature sources such as unpublished studies, Google Scholar and Google. The inconsistency between the two authors was solved by the third author (BG).All studies were searched electronically. Our search included the terms: ‘‘tuberculosis’’, ‘‘Mycobacterium tuberculosis’’, ‘‘pulmonary tuberculosis’’, and ‘‘contact tracing’’, ‘‘contact’’, ‘‘contact screen’’, ‘‘household’’, ‘‘household contact’’, Sub-Saharan Africa, ‘case finding“ or ‘‘case detection’’. The Boolean operators AND and OR were used accordingly. The Boolean operator AND term was used when we are looking for articles that contain our entire search terms combination. While OR is used when we are looking for articles that contain at least one of our search terms. Key term combination was similar in both Web of science and PubMed data bases. Any duplicated studies from the data bases were removed after screening. The full search strategy for PubMed was available in the supplementary material (S. Table 2).
Following the search, full-text publications were evaluated by two reviewers (BD and WS) based on the study's inclusion criteria; any disagreement between the two reviewers was discussed, and the agreed one gets acceptance.
2.2. Inclusion and exclusion criteria
All published studies that included a measure of prevalence or incidence of TB among household contacts of patients with new or previously treated TB. Studies that reported the prevalence of any type of tuberculosis diagnosed with any WHO recommended laboratory diagnosis for active tuberculosis were eligible.
All titles and abstracts were assessed for inclusion according to the following inclusion criteria; published in English language, cross-sectional and retrospective, that reported a quantitative measure of the TB or LTBI diagnosed among HHC of patients with TB. Additionally studies where the number of cases tested were reported. We excluded studies where the diagnosis of TB was not made according to microbiological criteria. Editorials papers, conference abstracts, studies with inaccurate data (incomplete data), and systematic reviews (meta-analyses) studies were excluded from the final analysis.
2.3. Study definition
Index patients were patients of any age who were positive for any pulmonary tuberculosis. Household contacts were any person at any age that has resided in the same household or dwelling with the index tuberculosis patients. Tuberculosis of index patients was defined as bacteriologically confirmed Pulmonary TB. The dependent variable for the systematic review and meta-analysis was prevalence of active tuberculosis among house hold contacts. Household contacts of TB patients were the independent variable of the study since it was prevalence studies none of the included article used control group.
2.4. Data extraction
The following data were extracted from the included studies; Author year, a country study conducted, study design, data source, study period, index type, index age, number of an index, household contact age, number of HHC, the prevalence of tuberculosis among HHC, the prevalence of HIV in index and prevalence of HIV in HHC in pre-formatted excel sheet.
2.5. Quality assessment
Quality of included articles was assed using the Joanna Briggs Institute Critical Appraisal (JBI) tools designed for prevalence studies [11]. The JBI prevalence study checklist consists of 9 quality indicators. These quality indicators were turned into 100 % and the quality score was graded as high if >80 %, a medium between 60 and 80 %, and low. Publication bias was assessed by funnel plot and Egger’s regression tests.The quality assessment was carried out by two authors (GS and WS), and the difference between the two assessors was managed by the third author (AA) (S. Table 3).
2.6. Data synthesis and statistical analysis
The extracted data was summarized and saved in Microsoft Excel 2016 before being exported to STATA 16.0 for analysis. We estimated the pooled proportions of TB prevalence among HHC with their 95 % Confidence Interval (CI) using a random-effect meta-analysis model. Heterogeneity among studies was examined using forest plots and I2 heterogeneity tests. In the current review, I2 >50 % a random effect model was used for analysis. We also used a funnel plot and an Egger's test (p-value 0.1 as a significant level) to see if there was any potential for publication bias.
2.7. Role of the funding source
No fund was obtained to carry out this systematic review and meta-analysis.
3. Results
3.1. Study selection
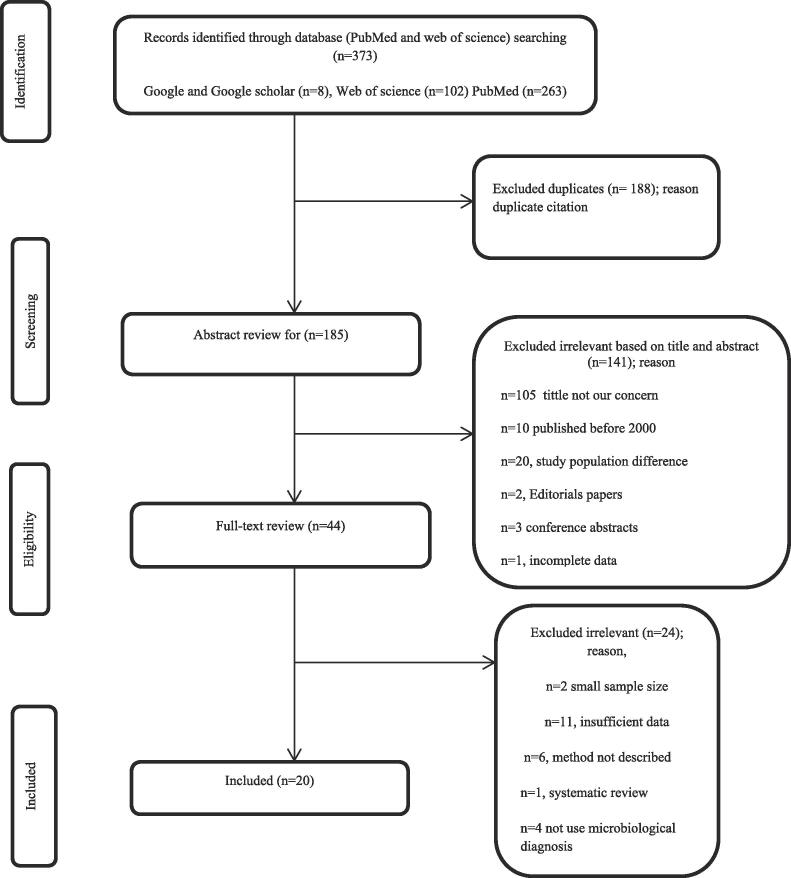
A total of 373 potentially relevant articles were initially searched in the systematic literature search of the different databases), of which 188 were duplicates, 185 were screened based on the review of their titles and abstracts, of which 141 were excluded based on title and abstract review. Forty-one studies undergo full text review. But 24 articles were excluded after full-text review based on the exclusion criteria of the study. The final analysis of this systematic review and meta-analysis includes 20 publications. The study selection process and a flow diagram were presented in Fig. 1. These included papers were done in sub-Saharan African countries including Ethiopia, Uganda, South Africa, Kenya, Gambia, Tanzania, Botswana, Ghana, Rwanda, Benin, Malawi, Central Republic of Africa, Burkina Faso, and Cameroon (Table 1).
Fig. 1.
Flowchart diagram describing a selection of studies for the systematic review and meta-analysis.
Table 1.
Characteristics of individual included studies in this systematic review and meta-analysis.
| Author_year | Country | Study period | Study design | Index age | Index type | No of _index | Data source | HHC_Age | NO of _HHC | Lab method used |
|---|---|---|---|---|---|---|---|---|---|---|
| Abinet et al.,2020[16] | Ethiopia(Haramaya) | February to March 2019 | Cross-sectional | ≥18 | SSP | 240 | Health facility | >18 | 451 | flst mcscp |
| Adriene et al,2012[17] | South Africa (Matlosana) | February and September 2009 | _ | ≥18 | SSP | 723 | Community-based | All age | 2843 | culture,mcscp |
| Bonnet et al., 2017[18] | Uganda | April 2012 to April 2013 | prospective | ≥18 | SSP | 204 | hospital based | less than5 | 281 | TST,GX,Culture |
| Fana et al.,2019[12] | Ethiopia(Northern) | February 04, 2015 to July 28, 2015 | Cross-sectional | ≥18 | All form | 363 | Health facility | All age | 1509 | mcscp |
| Irene et al.,2016[19] | Cameroon(Buea) | January 2014 to May 2015 | Cross-sectional | all age | SSP | 103 | Health facility | All age | 570 | mcscp |
| Jerene et al., 2015[20] | Ethiopia (Amhara and Oromia) | April 2013 and March 2014 | Retrospective | all age | SSP | 6015 | Routine data | All age | 15,527 | mcscp |
| Ken et al.,2020[21] | Kenya | March 2014 to June 2016 | prospective cohort | all age | SSP | 330 | Health facility | All age | 1155 | TST,mcscp |
| Kristen et al., 2018[22] | South Africa | December 1, 2013 – September 30, 2014 | Cross-sectional | ≥18 | SSP | 130 | Health facility | All age | 282 | mcscp,culture |
| Sanjay et al., 2015[14] | South Africa | May 2010 to August 2012. | Observational | less than7 | SSP,EPTB | 576 | Health facility | All age | 1342 | mcscp, culture |
| Medard et al.,018[23] | Tanzania | August to December 2016 | Retrospective | all age | SSP | 93 | Health facility | All age | 456 | gx, culture,mcscp |
| Puryear et al., 2013[15] | Botswana | January 2009 to December 2011 | Operational research | less than13 | SSP | 163 | Health facility | All age | 548 | mcscp |
| Ohene et al.,2018[13] | Ghana | June 2010 to December 2014 | Retrospective | all age | SSP,EPTB | 3267 | Health facility | All age | 8166 | mcscp |
| Velen et al.,2021[24] | S.Africa | August 2013 to July 2015 | Cross-sectional | ≥18 | SSP,MDR | 216 | Health facility | All age | 619 | gx,culture,mcscp |
| Christopher et al., 2011[25] | Uganda | 1995–2004 | Cross-sectional | _ | SSP | 497 | Community based | All age | 1918 | cuture,mcscp,TST |
| Zacharia et al., 2003[26] | Malawi | October 2001 to May 2002 | Cross-sectional | ≥18 | SSP | 87 | Community based | All age | 461 | mcscp |
| Hill et al.,2008[27] | Gambia | June 2002 – October 2002 | prospective cohort | ≥15 | SSP | 317 | Health facility | All age group | 2348 | ELISPOT,TST,mcsco |
| Mubarek et al., 2020[28] | Ethiopia | March – June 2015 | Retrospective | all age | SSP | 344 | Health facility | All age group | 1517 | flst mcscp |
| Michael et al., 2020[29] | Uganda | April -September 2017 | Retrospective | ≥18 | SSP | 454 | Health facility | All age group | 2707 | gx |
| Schwoebel et al., 2020[30] | Benin, Burkina Faso, Cameroon and Central African Republic | 1 April 2016 to 30 September 201 | Multi center | _ | SSP | 1095 | Health facility | less than5 child | 1965 | TST,mcscp,gx |
| Egere et al.,2017[31] | Gambia | February2012 to December 2013 | Prospective | ≥18 | SSP | 551 | Health facility | less than15 | 4042 | TST,mcscp |
Ssp = Sputum smear positive; EPTB = Extra pulmonary tuberculosis; mcscp = microscope;flst mcscp = fluorescence microscope; gx = gene xpert Mtb RIF assay,TST = Tuberculin skin test; IGRA = Interferon-gamma release assay.
3.2. Study characteristics
This systematic review and meta-analysis comprised 20 papers from 14 Sub-Saharan African nations. Four studies each from Ethiopia, South Africa, and the others from other countries mentioned above. There was heterogeneity across the studies. The majority of the study's data source for index patients was collected from health facilities. The study period for the included studies was from 2001 to 2019. Based on JBI checklist for prevalence Studies [11] fourteen studies(12,14–18,21,22,24–28 and 30) score high quality and six studies(13,19,20,23,26 and 29) score medium quality (Table 1).
The majority of the studies used smear-positive pulmonary tuberculosis (SSP) as index cases whereas one study [12] used all types of tuberculosis, two studies [13], [14] use SSP and extrapulmonary tuberculosis patients among included studies. Two [14], [15] articles used less than 7 and less than 13 years old children and eight studies used patients at all ages as index patients.More than half of the studies used index patients at any age (Table 1).
The number of HHC investigated in the studies ranged from 191 to 15,572 HHC of 87 to 6015 index cases. In the twenty studies included in this review, a total of 15,768 index patients, and a total of 48,707 household contacts were screened for tuberculosis (Table 1).
3.3. Prevalence of active tuberculosis among household contacts
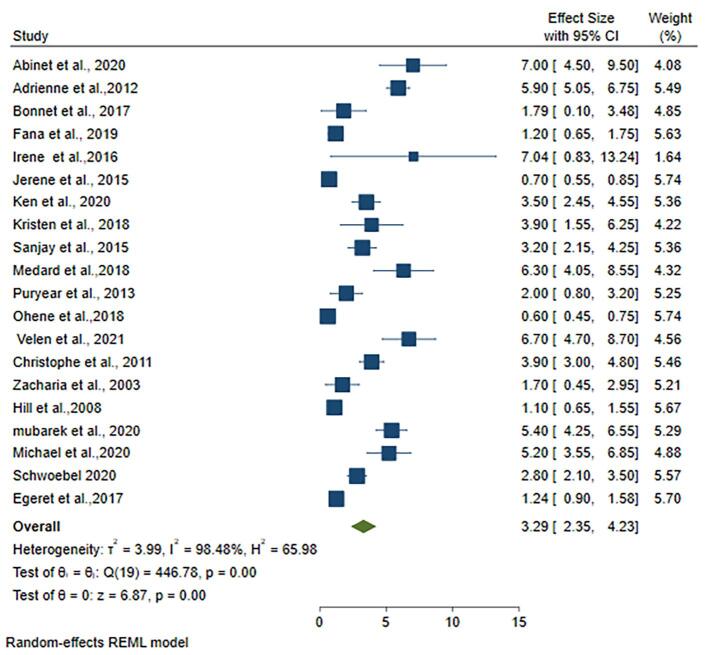
The smallest sample size of household contact was 281 [18], while the largest sample size was 15,527 [20]. The highest proportion of tuberculosis among household contact was, 7.04 % [19], while the lowest was 0.60 % [13]. In Sub-Saharan Africa, the pooled prevalence of active tuberculosis among household contacts was 3.29 % (95 %CI; 2.35 %-4.23 %, I2; 98.48 %) (Fig. 2).To see the presence of publication bias funnel plot was analyzed for the twenty studies and two studies over lapped which have high weight. The funnel plot of the analyzed studies showed the presence of publication bias (S. Fig. 1).
Fig. 2.
Forest plots for pooled prevalence of active tuberculosis among household contacts of tuberculosis patients in Sub-Saharan countries.
3.4. Prevalence of HIV in index tuberculosis patients and their household contacts
Only eleven of the articles included in this systematic review report the prevalence of HIV in index patients. The highest proportion of HIV prevalence in index patients was 87.0 % (95 % CI, 84.5 % – 89.5 %) [17]. The lowest proportion of HIV in index patients was 8.0 % (95 % CI, 5.5 % – 10.5 %) [12]. The pooled proportion of HIV in index tuberculosis patients was 53.12 % (95 % CI, 39.73 % – 66.51 %) (S. Fig. 2).
Thirteen of the papers included in the review reports the prevalence of HIV among household contacts of tuberculosis patients. A study conducted in South Africa [14] has the largest sample size (n = 221) of household contacts diagnosed with HIV. The overall pooled prevalence of HIV in household contacts was 7.34 % (95 % CI, 3.62 % – 11.07 %) (S. Fig. 3).
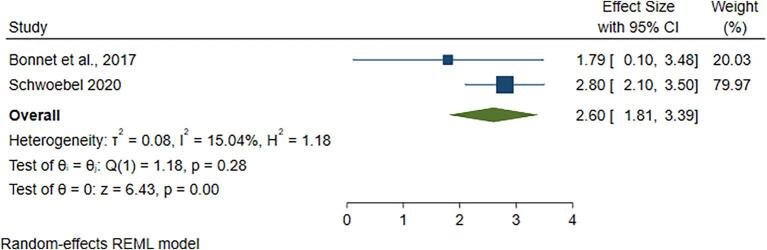
In this systematic review, two studies (18 and 30) use a total of 2246 under five years, children, as household contact. The pooled prevalence rate of active tuberculosis in children aged less than five years revealed a prevalence of 2.60 % (95 % CI; 1.81 %-3.39 %) (Fig. 3).
Fig. 3.
The pooled prevalence of active tuberculosis among household contacts aged less than 5 years.
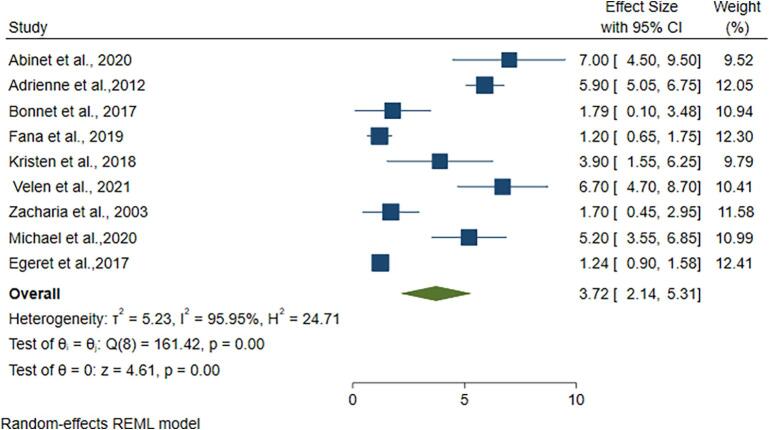
Nine of the included studies screen index patients' household contacts who are over the age of 18. When the index patient age was greater than or equal to 18 years, the prevalence of active tuberculosis in HHC was 3.72 % (95 %CI; 2.14–5.31) (Fig. 4). The pooled prevalence of active TB in HHC of index patients aged less than 18 was 2.64 % (95 % CI; 1.46–3.81) (S. Fig. 4).
Fig. 4.
Pooled prevalence of active tuberculosis in HHC age > 18 years.
3.5. The pooled prevalence of active tuberculosis among household contacts based on study country
For sub group analysis,More than one studies were included from four countries.Subgroup analysis by country-level showed that pooled prevalence of tuberculosis infection in household contacts of a tuberculosis patient in South Africa, Uganda, Ethiopia, and Gambia was 4.91 %, 3.65 %, 3.42 %, and 1.19 % respectively (S. Fig. 5).
4. Discussion
This is the first systematic and meta-analysis study describing the prevalence of tuberculosis infection in a household contact of tuberculosis patients in sub-Saharan African countries. This review estimated the pooled prevalence of tuberculosis in HHC of tuberculosis patients and pooled prevalence of HIV in index patients in sub-Saharan Africa countries. To prevent the high heterogeneity of studies we used the random-effect model analysis method. In this first systematic review and meta-analysis, we collected and analyzed data from 20 studies from--countries to estimate the pooled prevalence of active tuberculosis in HHC of TB patients in sub-Saharan Africa countries. Using a random effect model the pooled prevalence of tuberculosis among HHC was 3.29 % (95 %CI; 2.35 % – 4.23 %). The prevalence was lower in children HHC aged less than 5 years than the other age group HHC.
This study revealed that the pooled prevalence of tuberculosis was 3.29 % among household contacts of TB patients. Comparable results were reported from different review studies done in low-and middle-income countries [7], [32] that reported 3.1 % and 2.87 % prevalence respectively. The explanation for this is that most sub- Saharan African countries had a similar burden of TB and living conditions with lower and middle-income countries. This research suggests that governments should improve their active tracing of TB patients to accomplish an End-TB plan. Countries that fully trace tuberculosis in HHC will increase their case detection rate by >3 % per year [7], [32].
However, the results of this investigation were lower than those of a recent systematic review conducted in high-burden tuberculosis settings [33], which found 8.70 % active TB in HHC of MDR-TB source cases. The difference between the two studies might be due to the index case drug susceptibility difference, that the previous study used drug resistance (DR) index patients. Contacts of drug-resistant tuberculosis were clearly at a higher risk of Mycobacterium tuberculosis infection than drug-susceptible tuberculosis contacts [34].
The prevalence of active TB was found to be greater in HHC of index patients over the age of 18 than in HHC of index patients under the age of 18. Even though it needs further research, this data suggests that the age of the index patient had an impact on tuberculosis transmission. Of course, younger people in the household had lower responsibility to care for others than the older people which lower exposure time between household members. It also suggests that younger index patients were a smaller source of TB Transmission than older index patients. This finding was in support of the previous study finding which stated that the transmission of tuberculosis is associated with the age of source cases as well as the age of secondary cases [39].
We found that the overall prevalence of HIV among household contacts was 7.34 %; however, a study conducted in eight countries indicated a lower incidence of HIV [35]. Two previous meta-analyses reported 31.8 % [36] and 31.25 % [37] prevalence of HIV in TB patients in sub-Saharan African countries, which was lower than the present review estimate of 53.12 %. This is because our analysis included only studies that had HHC and conducts HIV screening for the index patients which minimizes the number of included studies for the analysis. due to other included continents and countries, it is clear that this review estimate was higher than the global HIV prevalence among tuberculosis patients (8.2 %) [1]. Furthermore, the high prevalence of HIV/AIDS in this study is related to a higher prevalence of TB-HIV co-infection in sub-Saharan Africa countries. This result should be interpreted carefully, as the characteristics of HIV-positive and -negative TB patients differed in many ways that are or could be related to the risk of Mycobacterium tuberculosis transmission.
In this review, the prevalence of active tuberculosis among children HHC under the age of five was less than the pooled prevalence of TB in the general age group HHC. This finding was comparable with different research studies finding that TB age-specific risk is higher in older contacts than under 5 children. According to a previous study, when compared to children the risk of infection with Mycobacterium tuberculosis is highest during adolescence and young adulthood and that between the ages of 12 and 24 years [38].
The pooled prevalence of tuberculosis among children household contacts under the age of five in the present review (2.60 %) was relatively lower than the previous systematic review [7] report which reported the prevalence of active TB was 10.0 %. This substantial difference could be due to the previous study done on low and middle-income countries all over the world. Additionally, the living habit of the community and screening of children for tuberculosis varies between sub-Saharan African countries and other middle-income countries.
We also conducted a sub-group analysis to determine the pooled prevalence of tuberculosis among HHC by country (Ethiopia, S/Africa, Malawi, and Gambia). South Africa had the highest pooled prevalence of Tuberculosis than other countries (4.91 %). of course, the studies included from South Africa report a higher prevalence of HIV among HHC than other countries' studies. This could also be because South Africa has one of the highest tuberculosis and HIV burdens on the continent.
4.1. Limitation of the study
First, some include papers that use smear microscopic only for diagnosis of active tuberculosis which had less sensitivity than other tests like gene Xpert MTB/RIF assay and culture. Secondly, this review did not do a subgroup analysis of TB prevalence among HHC based on HIV status. Due to the high heterogeneity of studies, this result should be interpreted carefully.
5. Conclusion
In conclusion, our systematic review showed that household contacts are at high risk of getting tuberculosis infection from their index patient in sub-Saharan African countries. This review estimate around one household contact will develop active tuberculosis among thirty household contacts. Additionally, they are a potential reservoir of reactivated tuberculosis. This was indicative of the importance of intensive active tracing of tuberculosis cases in household contacts to reach out to missing TB patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Our great acknowledge goes to the author of primary studies included in this systematic review and meta-analyses.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jctube.2022.100337.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Global tuberculosis report 2020. Geneva: World Health Organization; 2020. License: CC BY-NC-SA 3.0 IGO.
- 2.FMOH . 6th edition, Adis Ababa; Ethiopia: 2016. Guidelines for Clinical and Programmatic Management TB, TB/HIV and Leprosy in Ethiopia, Federal Ministry of Health. [Google Scholar]
- 3.Jilani T.N., Avula A., Zafar Gondal A., et al. Stat Pearls Publishing; Treasure Island (FL): 2021. Active Tuberculosis. [PubMed] [Google Scholar]
- 4.Padmanesan Narasimhan, James Wood, Chandini Raina MacIntyre, and Dilip Mathai. Risk Factors for Tuberculosis.Pulmonary Medicine Volume 2013. [DOI] [PMC free article] [PubMed]
- 5.WHO. The End TB Strategy Global strategy and targets for tuberculosis prevention, care and control after 2015. May, 2014.
- 6.World Health Organization . WHO; Geneva, Switzerland: 2016. The End TB Strategy: global strategy and targets for tuberculosis prevention, care and control after 2015. [Google Scholar]
- 7.Fox G.J., Barry S.E., Britton W.J., Marks G.B. Contact investigation in households of patients with tuberculosis in Hanoi, Vietnam: a prospective cohort study. PLoS ONE. 2012;7(11):e49880. doi: 10.1371/journal.pone.0049880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ereso B.M., Yimer S.A., Gradmann C., Sagbakken M. Barriers for tuberculosis case finding in Southwest Ethiopia: A qualitative study. PLoS ONE. 2020;15(1):e0226307. doi: 10.1371/journal.pone.0226307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begun M., Newall A.T., Marks G.B., Wood J.G. Contact tracing of tuberculosis: a systematic review of transmission modeling studies. PLoS ONE. 2013;8(9):e72470. doi: 10.1371/journal.pone.0072470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 12.Tefera F., Barnabee G., Sharma A., Feleke B., Atnafu D., Haymanot N., et al. Evaluation of facility and community-based active household tuberculosis contact investigation in Ethiopia: a cross-sectional study. BMC Health Serv Res. 2019;19(1):234. doi: 10.1186/s12913-019-4074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohene S.A., Bonsu F., Hanson-Nortey N.N., et al. Yield of tuberculosis among household contacts of tuberculosis patients in Accra. Ghana Infect Dis Poverty. 2018;7:14. doi: 10.1186/s40249-018-0396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lala SG, Little KM, Tshabangu N, Moore DP, Msandiwa R, van der Watt M, et al. Integrated Source Case Investigation for Tuberculosis (TB) and HIV in the Caregivers and Household Contacts of Hospitalised Young Children Diagnosed with TB in South Africa: An Observational Study. plos one 2015;10(9): e0137518. [DOI] [PMC free article] [PubMed]
- 15.Puryear S., Seropola G., Ho-Foster A., et al. Yield of contact tracing from pediatric tuberculosis index cases in Gaborone. Botswana Int J Tuberc Lung Dis. 2013;17(8):1049–1055. doi: 10.5588/ijtld.12.0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abinet Adane, Melake Damena, Fitsum Weldegebreal, Hussein Mohammed. “Prevalence and Associated Factors of Tuberculosis among Adult Household Contacts of Smear Positive Pulmonary Tuberculosis Patients Treated in Public Health Facilities of Haramaya District, Oromia Region, Eastern Ethiopia”, Tuberculosis Research and Treatment, vol. 2020. [DOI] [PMC free article] [PubMed]
- 17.Shapiro A.E., Variava E., Rakgokong M.H., Moodley N., Luke B., Salimi S., et al. Community-based targeted case finding for tuberculosis and HIV in household contacts of patients with tuberculosis in South Africa. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201111-1941OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnet M., Kyakwera C., Kyomugasho N., Atwine D., Mugabe F., Nansumba M., et al. Prospective cohort study of the feasibility and yield of household child tuberculosis contact screening in Uganda. Int J Tuberc Lung Dis. 2017;21(8):862–868. doi: 10.5588/ijtld.16.0889. [DOI] [PubMed] [Google Scholar]
- 19.Anyangwe I.A., Meriki H.D., Anong D.N., Shu C.J., Tufon K.A., Nsonghomanyi F.R., et al. Prevalence and Risk of Active Tuberculosis among Symptomatic Household Contacts of Bacteriologically Confirmed Pulmonary Tuberculosis Subjects Treated at the Buea Regional Hospital of the Southwest Region of Cameroon”. American Journal of Epidemiology and Infectious Disease. 2016;4(2):34–41. [Google Scholar]
- 20.Jerene D., Melese M., Kassie Y., Alem G., Daba S.H., Hiruye N., et al. The yield of a tuberculosis household contact investigation in two regions of Ethiopia. Int J Tuberc Lung Dis. 2015;19(8):898–903. doi: 10.5588/ijtld.14.0978. [DOI] [PubMed] [Google Scholar]
- 21.Warria K., Nyamthimba P., Chweya A., Agaya J., Achola M., Reichler M., et al. Tuberculosis disease and infection among household contacts of bacteriologically confirmed and non-confirmed tuberculosis patients. Trop Med Int Health. 2020;25(6):695–701. doi: 10.1111/tmi.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little K.M., Msandiwa R., Martinson N., Golub J., Chaisson R., Dowdy D. Yield of household contact tracing for tuberculosis in rural South Africa. BMC Infect Dis. 2018;18(1):299. doi: 10.1186/s12879-018-3193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beyanga M., Kidenya B.R., Gerwing-Adima L., et al. Investigation of household contacts of pulmonary tuberculosis patients increases case detection in Mwanza City. Tanzania BMC Infect Dis. 2018;18:110. doi: 10.1186/s12879-018-3036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velen K. Laura J Podewils, N Sarita Shah, James J Lewis, Tiro Dinake, Gavin J Churchyard, Mary Reichler, Salome Charalambous, Performance of GeneXpert MTB/RIF for Diagnosing Tuberculosis Among Symptomatic Household Contacts of Index Patients in South Africa. Open Forum Infectious Diseases. 2021;8(4) doi: 10.1093/ofid/ofab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whalen C.C., Zalwango S., Chiunda A., Malone L., Eisenach K., et al. Secondary Attack Rate of Tuberculosis in Urban Households in Kampala. Uganda plos one. 2011;6(2):e16137. doi: 10.1371/journal.pone.0016137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zachariah R., Spielmann M.P., Harries A.D., Gomani P., Graham S.M., Bakali E., et al. Passive versus active tuberculosis case finding and isoniazid preventive therapy among household contacts in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7(11):1033–1039. PMID: 14598961. [PubMed] [Google Scholar]
- 27.Hill P.C., Jackson-Sillah D.J., Fox A., Brookes R.H., de Jong B.C., et al. Incidence of Tuberculosis and the Predictive Value of ELISPOT and Mantoux Tests in Gambian Case Contacts. PLoS ONE. 2008;3(1):e1379. doi: 10.1371/journal.pone.0001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yassin M.A., Yirdaw K.D., Datiko D.G., et al. Yield of household contact investigation of patients with pulmonary tuberculosis in southern Ethiopia. BMC Public Health. 2020;20:737. doi: 10.1186/s12889-020-08879-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakinda M. Matovu JKB.A yield and cost comparison of tuberculosis contact investigation and intensified case finding in Uganda. PLoS ONE. 2020;15(6):e0234418. doi: 10.1371/journal.pone.0234418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwoebel V., Koura K.G., Adjobimey M., Gnanou S., Wandji A.G., Gody J.C., et al. Tuberculosis contact investigation and short-course preventive therapy among young children in Africa. Int J Tuberc Lung Dis. 2020;24(4):452–460. doi: 10.5588/ijtld.19.0712. [DOI] [PubMed] [Google Scholar]
- 31.Egere U., Togun T., Sillah A., Mendy F., Otu J., Hoelscher M., et al. Identifying children with tuberculosis among household contacts in The Gambia. Int J Tuberc Lung Dis. 2017;21(1):46–52. doi: 10.5588/ijtld.16.0289. [DOI] [PubMed] [Google Scholar]
- 32.Velleca M., Malekinejad M., Miller C., Abascal Miguel L., Reeves H., Hopewell P., et al. The yield of tuberculosis contact investigation in low- and middle-income settings: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):1011. doi: 10.1186/s12879-021-06609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah N.S., Yuen C.M., Heo M., Tolman A.W., Becerra M.C. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):381–391. doi: 10.1093/cid/cit643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suggaravetsiri,H. Yanai,V. Chongsuvivatwong,O. Naimpasan, P. Akarasewi.Integrated counseling and screening for tuberculosis and HIV among household contacts of tuberculosis patients in an endemic area of HIV infection: Chiang Rai, Thailand P. 2003 int j tuberc lung dis 7(12):S424–S431. [PubMed]
- 35.Opollo V.S., Wu X., Hughes M.D., et al. HIV testing uptake among the household contacts of multidrug-resistant tuberculosis index cases in eight countries. Int J Tuberc Lung Dis. 2018;22(12):1443–1449. doi: 10.5588/ijtld.18.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelaw Y.A., Williams G., Soares Magalhães R.J., Gilks C.F., Assefa Y. HIV Prevalence Among Tuberculosis Patients in Sub-Saharan Africa: A Systematic Review and Meta-analysis. AIDS Behav. 2019;23(6):1561–1575. doi: 10.1007/s10461-018-02386-4. [DOI] [PubMed] [Google Scholar]
- 37.Gao J., Zheng P., Fu H. Prevalence of TB/HIV Co-Infection in Countries Except China: A Systematic Review and Meta-Analysis. PLoS ONE. 2013;8(5):e64915. doi: 10.1371/journal.pone.0064915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snow KathrynJ., Sismanidis Charalambos, Denholm Justin, Sawyer Susan M., Graham Stephen M. The incidence of tuberculosis among adolescents and young adults: a global estimate. Eur Respir J. 2018;51:1702352. doi: 10.1183/13993003.02352-2017. [DOI] [PubMed] [Google Scholar]
- 39.Borgdorff M.W., Nagelkerke N.J.D., de Haas P.E.W., van Soolingen D. Transmission of Mycobacterium tuberculosis Depending on the Age and Sex of Source Cases. Am J Epidemiol. November 2001;154(10):934–943. doi: 10.1093/aje/154.10.934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.