Abstract
Background
Forkhead Box Protein C2 (FOXC2) belongs to the Forkhead/Wing-helix family. The regulatory role of this transcription factor in physiological function and carcinogenic activity has been proven in subsequent investigations. However, there is still scarcity of evidence on the relationship between FOXC2 expression and prognosis in human solid tumors. We conducted this meta-analysis to evaluate the role of FOXC2 as a prognosis factor and a possible target marker in human solid tumors.
Methods
PubMed, Web of Science, Embase, and the Cochrane library database were all searched methodically. Eligible publications on FOXC2 in human solid tumors were gathered and reviewed. The effect sizes were calculated using pooled hazard ratios (HRs) or odds ratios (ORs) with the corresponding 95% confidence interval (CI). Statistical analysis was conducted with Stata SE12.0.
Results
This meta-analysis comprised 3,267 patients from 20 studies covering a variety of solid tumors. Increased FOXC2 expression was related to shorter overall survival (OS) (HR = 2.05, 95% CI: 1.73–2.42). High expression of FOXC2 is associated with lymph node metastases (OR = 3.33, 95% CI: 2.65–4.19), TNM stage (OR = 3.09, 95% CI: 2.00–4.78), and age (OR = 1.26, 95% CI: 1.06–1.50), according to the pooled ORs. However, no significant association was observed between the high expression of FOXC2 and sex, tumor size or tumor differentiation.
Conclusion
Increased expression of FOXC2 is associated with unfavored OS, lymph node metastases, TNM stage, and age. FOXC2 is a promising prognostic marker and a novel target marker in human solid tumors.
Keywords: FOXC2, solid tumor, prognosis, survival, tumor biomarker
Introduction
The transcription factor forkhead box (FOX) is a family with a highly conserved winged-helix DNA-binding domain (1, 2). FOX family members are involved in cell growth, differentiation, aging and carcinogenesis, and various regulatory and functional activities (3, 4). From FOXA to FOXR, there are 17 gene subfamilies of FOX and more than 14 have been identified in humans (5). FOXC2, also known as the mesenchyme forkhead-1 (MHF1), consists of a single exon located on the chromosomal band 16p24.1 (6). FOXC2 is necessary for the development of the lungs (7), bone (8), cardiovascular system (9), adipose tissue in adults (10), and various other organs or tissues. In addition to physiologic functions as cellular metabolism, angiogenesis and wound healing, dysregulated FOXC2 contributes to tumorigenesis and malignancy progression in cell proliferation, metabolic reprogramming, lymph-angiogenesis, epithelial-mesenchymal transition (EMT), and drug resistance (11–16). Recent studies have reported that FOXC2 is dysregulated in malignancies, including breast cancer (13), gastric cancer (17), esophageal carcinoma (18).
Currently, there is an increasing interest on the oncogenic role of FOXC2 both in vivo and in vitro. FOXC2 has also merged as a potential molecular target in preclinical/clinical studies due to the dysregulated expression level and nuclear localization. Previous studies have associated expression levels of FOXC2 with clinical and pathological characteristics including tumor size, differentiation, metastasis, and stage (19, 20). However, there is still lack of proof that FOXC2 expression in human solid tumors has significant predict value. This analysis was carried out in order to systematically assess the potential prognostic significancy of FOXC2.
Methods
Literature search
A systematic literature search was undertaken in PubMed, Web of Science, Embase, and Cochrane library databases before April, 2022. The following keywords and search terms were used to find potentially eligible studies: (“Forkhead box protein C2” OR “FOXC2” OR “MHF1” OR “mesenchyme forkhead1”) AND (“cancer” OR “carcinoma” OR “neoplasm” OR “tumor” OR “malignancy”) AND (“prognosis” OR “survival”). Additional research was found by looking through the references of the selected articles. The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement was used in this analysis (21).
Study selection
The following were the selection criteria of this analysis: (1) patients with solid tumors diagnosed pathologically; (2) the expression FOXC2 in tissue were determined by immunohistochemistry (IHC) or quantitative real-time polymerase chain reaction (q-PCR); (3) available data for calculating odds ratio (OR) with 95% CIs were depicted; (4) only the study with the most extensive or recent data was considered, if multiple publications used employed overlapping samples from the same institution; (5) patients were categorized into groups based on high and low FOXC2 expression levels. Exclusion criteria in this meta-analysis were as follows: (1) duplicate publications; (2) research with no data or data from animal or cellular experiments; (3) only serum levels of FOXC2 expression were detected; (4) studies only provided Kaplan-Meier curves but no multivariate data; (5) reviews, letters, case reports, or expert opinions.
Date extraction and quality assessment
The two independent investigators screened the all publications, classified and sorted out the titles and abstracts of the literature retrieved from reading, excluded duplicate literatures and literature failed inclusion criteria, contacted the original author for relevant information for the literature with incomplete information report, and determined whether it could be included in the final study after obtaining the full text. The research team shall assist in solving any dispute. The following information was retrieved from eligible articles: name of first author; publication year; sample size; cancer kind; criteria for increased expression of FOXC2; detecting methodology; outcome measuring; patient follow-up; HRs with corresponding 95% CI; and clinical characteristics (age, sex, tumor size, lymph node metastases, distant metastases, TNM stage). We preferred multivariate analysis in research with both univariate and multivariate analyses because it is better at explaining confounding factors. If there was a disagreement, a compromise was sought through debate until everyone agreed. The quality evaluation for eligible studies was undertaken by 2 independent investigators (CW and LZ), and any discrepancies were handled by consensus among all authors. The Newcastle-Ottawa Scale (NOS) tool was used to evaluate the quality of all eligible studies (22). The NOS scores ranged from 0 to 9. High quality was assigned to studies with NOS score ≥ 6.
Statistical analysis
Stata SE12.0 was applied to conduct this meta-analysis (Stata Corp., College Station, USA). The heterogeneity of the included studies used Chi-square-based Q test and I2 statistic (23). P < 0.05 for the Q test and an I2 > 50% indicates significant heterogeneity. For studies with no obvious heterogeneity (Ph > 0.05, I2 < 50%), the fixed-effects model was adopted, and the random-effects model was used for others (Ph ≤ 0.05, I2 ≥ 50%). The sensitivity analysis was conducted to check the stability of results. Begg's and Egger's tests were conducted to investigate potential publication bias (24). Differences with P < 0.05 were considered as statistically significant.
Results
Study characteristics
The procedure of literature retrieval was depicted in Figure 1. A total of 3,267 patients with solid tumors were included in eligible articles published between 2011 and 2021 (17–19, 25–40). These studies were conducted in China (n = 12), Japan (n = 5), Singapore (n = 1), Spain (n = 1), and Norway (n = 1). Mean of patient sample size was 163 (from 61 to 338). In this meta-analysis, 15 varying solid tumor kinds were summarized, including 2 non–small-cell lung cancer, 2 breast cancers, 2 esophageal squamous cell carcinoma, 2 colorectal cancer, 2 cervical cancer, and 1 each of glioma, oral squamous cell carcinoma, pulmonary neuroendocrine tumors, extrahepatic cholangiocarcinoma, hepatocellular carcinoma, gastric cancer, oral tongue squamous cell carcinoma, ovarian cancer, prostate cancer, and phyllodes tumor of the breast. All of the specimens were well preserved, and diagnosis was made based on pathological findings. The main characteristics of enrolled studies are summarized in Supplementary Table S1. Eligible studies included in this meta-analysis had NOS scores ranging from 5 to 9, with a mean of 6.5.
Figure 1.
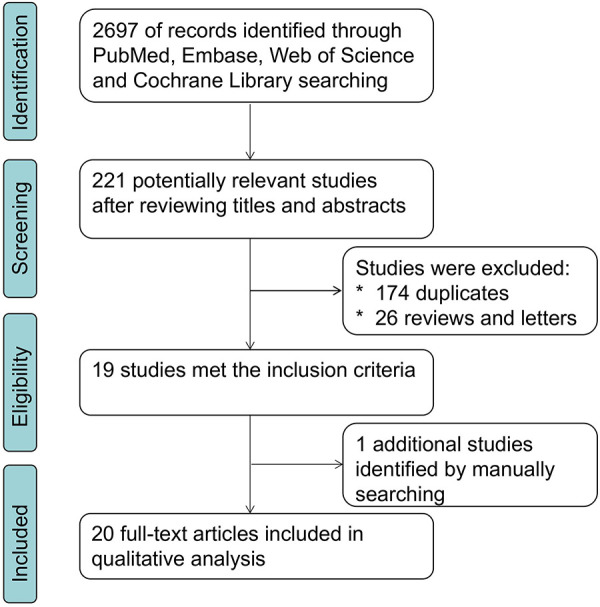
Literature search and selection flowchart.
Prognostic value of FOXC2 in patients with solid tumors
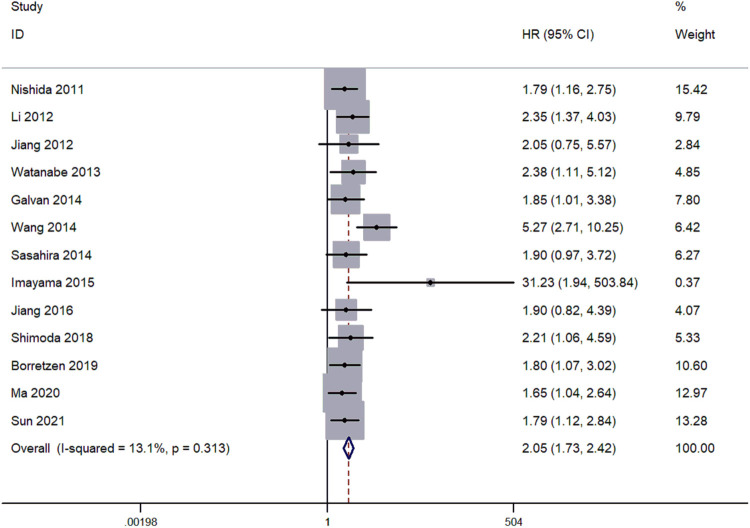
In 13 articles, the overall survival (OS) was reported. The pooled hazard ratios (HRs) and corresponding 95% CI were estimated by the fixed-effects model. The results indicated a mild heterogeneity in studies (I2 = 13.1%, Ph = 0.313). HRs for the increased FOXC2 expression against the low FOXC2 expression were 2.31 (95% CI: 1.73–2.42) (Figure 2). Patients with increased expression of FOXC2 presented significantly shorter OS, indicating that increased FOXC2 expression was associated with unfavored OS.
Figure 2.
Forest plots for the relationship between high FOXC2 expression and OS.
Clinical and pathological characteristics associated with FOXC2 expression
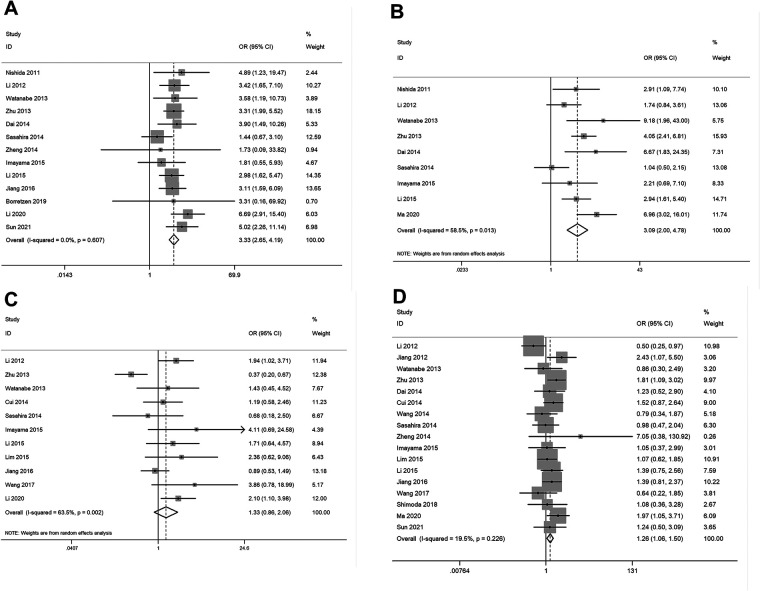
The pooled results (Supplementary Table S2) showed that elevated expression of FOXC2 was significantly related with lymph node metastases (OR = 3.33, 95% CI: 2.65–4.19, P < 0.05) (Figure 3A), TNM stage (OR = 3.09, 95% CI: 2.00–4.78, P < 0.05) (Figure 3B), and age (OR = 1.26, 95% CI: 1.06–1.50, P < 0.05) (Figure 3D). However, no significant correlation was observed between increased expression of FOXC2 and tumor differentiation (Figure 3C), sex, or tumor size (data not shown). Due to a lack of data, we were unable to detect the relationship between FOXC2 overexpression and other clinical and pathological characteristics.
Figure 3.
Forest plots for the relationship between FOXC2 overexpression and clinical and pathological characteristics. (A) Lymph node metastases, (B) TNM stage, (C) tumor differentiation, (D) patient age.
Sensitivity analysis
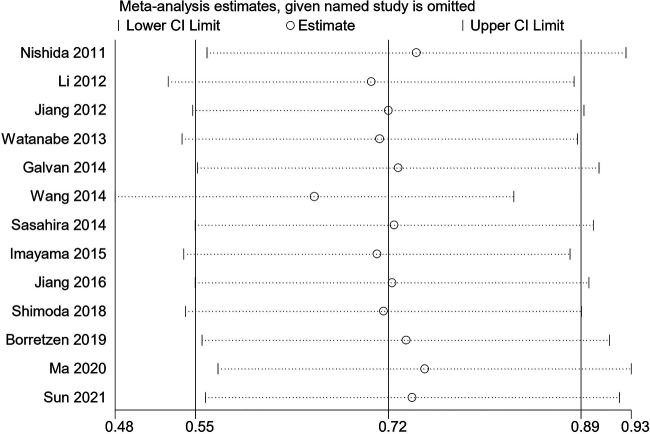
Sensitivity analysis was conducted to assess the FOXC2 expression and OS by gradually deleting each individual research from the pooled analysis. The purpose of this approach is to evaluate the impact of the deleted data set on the overall HRs. The fingdings were reliable, and the exclusion of any study had no effect on the results (Figure 4).
Figure 4.
Sensitivity analysis regarding overall survival.
Publication bias
Begg's Test and Egger's tests were conducted to evaluate the publication bias. Findings revealed that there was no publication bias between FOXC2 expression and OS in the included studies (Figure 5).
Figure 5.
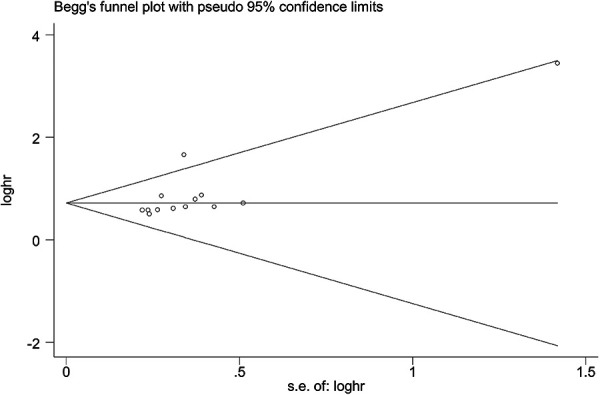
Publication bias in this meta-analysis.
Discussion
As a kind of genomic disease, lots of somatic mutations, structural mutations and gene recombination occur during the carcinogenesis process (41). There were 19.3 million new cancer cases and 10.0 million cancer deaths estimated in 2020 worldwide, with cancer burden anticipated to rise to 28.4 million by 2040 (42). Despite advancements in cancer surveillance enrollment, surgical techniques, systematic therapy and palliative care, the survival of individuals with solid tumors still remains unsatisfactory. Finding novel tumor markers is critical in order to provide accurate diagnosis and prospective therapeutic targets.
FOXC2 acts as a key mediator of tumor initiation and progression, involving tumor proliferation, migration, invasion, metastasis, and EMT (3). FOXC2 overexpression has been reported in a range of tumor kinds, including lung cancer (19), colorectal cancer (15), gastric cancer (16), ovarian cancer (40) and glioma (32). Furthermore, FOXC2 overexpression was associated with clinical characteristics and a poor prognosis (43, 44). FOXC2 is a novel independent biological marker for predicting tumor progression and survival because of its prognostic significancy and association with clinicopathological features. The prognosis effect of elevated FOXC2 expression was assessed in patients with solid tumors. The findings indicated that elevated FOXC2 expression was related with shorter OS in solid tumors. Additionally, increased FOXC2 was closely associated with age, TNM stage and lymph node metastasis, suggesting that FOXC2 could be a useful biomarker for predicting prognosis in human solid tumors based on clinical pathology. Targeting FOXC2 might be a viable approach for these patients.
The limitations of this analysis were as follows: first, in this meta-analysis, the majority of the studies were conducted among Asian population. Other ethnic groups, such as Europeans, Africans and Americans, are relatively under-studied, which may limit the global applicability of the results discussed. Further high-quality studies from diverse ethnical origins are necessary to investigate the therapeutic importance of FOXC2. Second, despite the fact that FOXC2 overexpression was associated with patient age, tumor differentiation, lymph node metastasis and TNM stage, we were unable to evaluate the association between FOXC2 overexpression and other clinical and pathological characteristics due to insufficient data. Third, the number of studies included in this analysis could restrict its statistical power. Although no publication bias was found, potential publication bias may still exist due to the insufficient studies available for assessments. Then, inconsistencies in detecting platforms, methodologies, and criteria for IHC or RT-PCR, and distinct tumor kinds with varying prognostic differences may lead to skewed results. Furthermore, the mean of NOS scores is 6.5, implying that the quality of studies in this analysis is acceptable but not supreme, which might be inevitable in meta-analysis. Finally, the combined predictive significance of FOXC2 and other tumor markers was not assessed. As a result, higher quality multicenter studies with larger population, as well as consistent criteria for assessing the expression of FOXC2, are necessary for validation of the findings.
Conclusion
In this analysis, increased expression level of FOXC2 is associated with poor prognosis, as well as TNM stage, lymph node metastases, and age. FOXC2 could serve as a novel prognostic marker in solid tumors. For these patients, targeting FOXC2 could be a feasible treatment option. To corroborate the findings, further well-designed pre-clinical/clinical studies with high-quality data are needed.
Funding
The research was supported by the Natural Science Foundation of Jiangxi Province (NO. 20202BABL206076) and the Doctoral Research Fund of Ganzhou People's Hospital (NO. BSQD2020008) to LZ, and Chen Xiao-ping Foundation for the Development of Science and Technology of Hubei Province (NO. CXPJJH12000002-2020058) and Scientific Research Funding of Tongji Hospital (NO. 2021A11) to CW.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
LZ, YH, XHT, and CW participated in the date collection and manuscript drafting. LZ, YH, XJD, RQY, YCX, and JYS performed the data analysis. LZ, YFJ, and XHD conceived the study and designed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.960698/full#supplementary-material.
References
- 1.Hargadon KM, Győrffy B, Strong EW. The prognostic significance of FOXC2 gene expression in cancer: a comprehensive analysis of RNA-seq data from the cancer genome atlas. Cancer Genet. (2021) 254–255:58–64. 10.1016/j.cancergen.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 2.Herman L, Todeschini AL, Veitia RA. Forkhead transcription factors in health and disease. Trends Genet. (2021) 37:460–75. 10.1016/j.tig.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 3.Xu J, Wang K, Zhang Z, Xue D, Li W, Pan Z. The role of forkhead box family in bone metabolism and diseases. Front Pharmacol. (2021) 12:772237. 10.3389/fphar.2021.772237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch S. Regulation of wnt signaling by FOX transcription factors in cancer. Cancers. (2021) 13. 10.3390/cancers13143446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myatt SS, Lam EW. The emerging roles of forkhead box (fox) proteins in cancer. Nat Rev Cancer. (2007) 7:847–59. 10.1038/nrc2223 [DOI] [PubMed] [Google Scholar]
- 6.Kume T. Foxc2 transcription factor: a newly described regulator of angiogenesis. Trends Cardiovasc Med. (2008) 18:224–8. 10.1016/j.tcm.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji M, Morishima M, Shimizu K, Tsuji M, Morishima M, Shimizu K, Morikawa S, Heglind M, Enerbäck S, et al. FOXC2 influences alveolar epithelial cell differentiation during lung development. Dev Growth Differ. (2017) 59:501–14. 10.1111/dgd.12368 [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Cho KW, Choi HS, Kim SH, Cho KW, Choi HS, Park SJ, Rhee Y, Jung HS, et al. The forkhead transcription factor FOXC2 stimulates osteoblast differentiation. Biochem Biophys Res Commun. (2009) 386:532–6. 10.1016/j.bbrc.2009.06.071 [DOI] [PubMed] [Google Scholar]
- 9.Seo S, Kume T. Forkhead transcription factors, FOXC1 and FOXC2, are required for the morphogenesis of the cardiac outflow tract. Dev Biol. (2006) 296:421–36. 10.1016/j.ydbio.2006.06.012 [DOI] [PubMed] [Google Scholar]
- 10.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 Is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. (2001) 106:563–73. 10.1016/S0092-8674(01)00474-3 [DOI] [PubMed] [Google Scholar]
- 11.Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, FOXC1 and FOXC2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. (2006) 294:458–70. 10.1016/j.ydbio.2006.03.035 [DOI] [PubMed] [Google Scholar]
- 12.Hader C, Marlier A, Cantley L. Mesenchymal-epithelial transition in epithelial response to injury: the role of FOXC2. Oncogene. (2010) 29:1031–40. 10.1038/onc.2009.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Fu X, Liu R, Li W, Fu X, Liu R, Wu C, Bai J, Xu Y, et al. FOXC2 often overexpressed in glioblastoma enhances proliferation and invasion in glioblastoma cells. Oncol Res. (2013) 21:111–20. 10.3727/096504013X13814233062171 [DOI] [PubMed] [Google Scholar]
- 14.Lin F, Li X, Wang X, Sun H, Wang Z, Wang X. Stanniocalcin 1 promotes metastasis, lipid metabolism and cisplatin chemoresistance via the FOXC2/ITGB6 signaling axis in ovarian cancer. J Exp Clin Cancer Res. (2022) 41:129. 10.1186/s13046-022-02315-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui YM, Jiao HL, Ye YP, Cui YM, Jiao HL, Ye YP, Chen CM, Wang JX, Tang N, et al. Retraction note to: FOXC2 promotes colorectal cancer metastasis by directly targeting MET. Oncogene. (2022) 41:2529. 10.1038/s41388-022-02287-w [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Zhang Z, Han Y, Liu H, Zhang Z, Han Y, Fan A, Liu H, Zhang X, et al. The FENDRR/FOXC2 axis contributes to multidrug resistance in gastric cancer and correlates with poor prognosis. Front Oncol. (2021) 11:634579. 10.3389/fonc.2021.634579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu JL, Song YX, Wang ZN, Zhu JL, Song YX, Wang ZN, Gao P, Wang MX, Dong YL, et al. The clinical significance of mesenchyme forkhead 1 (FOXC2) in gastric carcinoma. Histopathology. (2013) 62:1038–48. 10.1111/his.12132 [DOI] [PubMed] [Google Scholar]
- 18.Nishida N, Mimori K, Yokobori T, Nishida N, Mimori K, Yokobori T, Sudo T, Tanaka F, Shibata K, et al. FOXC2 Is a novel prognostic factor in human esophageal squamous cell carcinoma. Ann Surg Oncol. (2011) 18:535–42. 10.1245/s10434-010-1274-y [DOI] [PubMed] [Google Scholar]
- 19.Jiang W, Fan H, Qian C, Ding J, Wang Q, Pang X. Prognostic value of high FOXC2 expression in resectable non-small cell lung cancer, alone or in combination with E-cadherin expression. BMC Cancer. (2016) 16:16. 10.1186/s12885-016-2056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Rong X, Liu X, Chen J, Rong X, Liu X, Zheng D, Rong X, Chen F, et al. FOXC2 is a prognostic biomarker and contributes to the growth and invasion of human hepatocellular carcinoma. Cancer Cell Int. (2020) 20:196. 10.1186/s12935-020-01265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J. (2009) 339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 25.Dai J, Wang JY, Yang LL, Dai J, Wang JY, Yang LL, Xiao Y, Qu ZL, Qin SH, et al. Correlation of forkhead box C2 with subtypes and invasive ability of invasive breast cancer. J Huazhong Univ Sci Technolog Med Sci. (2014) 34:896–901. 10.1007/s11596-014-1370-5 [DOI] [PubMed] [Google Scholar]
- 26.Imayama N, Yamada S, Yanamoto S, Imayama N, Yamada S, Yanamoto S, Naruse T, Matsushita Y, Takahashi H, et al. FOXC2 Expression is associated with tumor proliferation and invasion potential in oral tongue squamous cell carcinoma. Pathol Oncol Res. (2015) 21:783–91. 10.1007/s12253-014-9891-6 [DOI] [PubMed] [Google Scholar]
- 27.Jiang W, Pang XG, Wang Q, Shen YX, Chen XK, Xi JJ. Prognostic role of twist, slug, and FOXC2 expression in stage I non-small-cell lung cancer after curative resection. Clin Lung Cancer. (2012) 13:280–7. 10.1016/j.cllc.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Yang W, Yang Q, Zhou S. Nuclear localization of GLI1 and elevated expression of FOXC2 in breast cancer is associated with the basal-like phenotype. Histol Histopathol. (2012) 27:475–84. 10.14670/HH-27.475 [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Wu J, Wei P, Li Q, Wu J, Wei P, Xu Y, Zhuo C, Wang Y, et al. Overexpression of forkhead box C2 promotes tumor metastasis and indicates poor prognosis in colon cancer via regulating epithelial-mesenchymal transition. Am J Cancer Res. (2015) 5:2022–34. PMID: [PMC free article] [PubMed] [Google Scholar]
- 30.Lim JC, Koh VC, Tan JS, Tan WJ, Thike AA, Tan PH. Prognostic significance of epithelial-mesenchymal transition proteins twist and FOXC2 in phyllodes tumours of the breast. Breast Cancer Res Treat. (2015) 150:19–29. 10.1007/s10549-015-3296-4 [DOI] [PubMed] [Google Scholar]
- 31.Sasahira T, Ueda N, Yamamoto K, Sasahira T, Ueda N, Yamamoto K, Kurihara M, Matsushima S, Bhawal UK, et al. PROX1 and FOXC2 act as regulators of lymphangiogenesis and angiogenesis in oral squamous cell carcinoma. PLoS One. (2014) 9:e92534. 10.1371/journal.pone.0092534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YW, Yin CL, Zhang HY, Wang YW, Yin CL, Zhang HY, Hao J, Yang YY, Liao H, et al. High expression of forkhead box protein C2 is related to poor prognosis in human gliomas. Asian Pac J Cancer Prev. (2014) 15:10621–5. 10.7314/APJCP.2014.15.24.10621 [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Yue X. Role and importance of the expression of transcription factor FOXC2 in cervical cancer. Oncol Lett. (2017) 14:6627–31. 10.3892/ol.2017.7004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe A, Suzuki H, Yokobori T, Watanabe A, Suzuki H, Yokobori T, Altan B, Kubo N, Araki K, et al. Forkhead box protein C2 contributes to invasion and metastasis of extrahepatic cholangiocarcinoma, resulting in a poor prognosis. Cancer Sci. (2013) 104:1427–32. 10.1111/cas.12249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng CH, Quan Y, Li YY, Deng WG, Shao WJ, Fu Y. Expression of transcription factor FOXC2 in cervical cancer and effects of silencing on cervical cancer cell proliferation. Asian Pac J Cancer Prev. (2014) 15:1589–95. 10.7314/APJCP.2014.15.4.1589 [DOI] [PubMed] [Google Scholar]
- 36.Shimoda Y, Ubukata Y, Handa T, Shimoda Y, Ubukata Y, Handa T, Yokobori T, Watanabe T, Gantumur D, et al. High expression of forkhead box protein C2 is associated with aggressive phenotypes and poor prognosis in clinical hepatocellular carcinoma. BMC Cancer. (2018) 18:597. 10.1186/s12885-018-4503-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Børretzen A, Gravdal K, Haukaas SA, Beisland C, Akslen LA, Halvorsen OJ. FOXC2 expression and epithelial-mesenchymal phenotypes are associated with castration resistance, metastasis and survival in prostate cancer. J Pathol Clin Res. (2019) 5:272–86. 10.1002/cjp2.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galván JA, Astudillo A, Vallina A, Crespo G, Folgueras MV, González MV. Prognostic and diagnostic value of epithelial to mesenchymal transition markers in pulmonary neuroendocrine tumors. BMC Cancer. (2014) 14:855. 10.1186/1471-2407-14-855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma L, Yang R, Gu J, Jiang H, Li H. The expression of AGGF1, FOXC2, and E-cadherin in esophageal carcinoma and their clinical significance. Medicine. (2020) 99:e22173. 10.1097/MD.0000000000022173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y, Wang X, Wen H, Zhu B, Yu L. Expression and clinical significance of the NCAPH, AGGF1, and FOXC2 proteins in serous ovarian cancer. Cancer Manag Res. (2021) 13:7253–62. 10.2147/CMAR.S329688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bird A. Genetic determinants of the epigenome in development and cancer. Swiss Med Wkly. (2017) 147:w14523. 10.4414/smw.2017.14523 [DOI] [PubMed] [Google Scholar]
- 42.Sung H, Ferlay J, Siegel RL, Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 43.Cui YM, Jiang D, Zhang SH, Cui YM, Jiang D, Zhang SH, Wu P, Ye YP, Chen CM, et al. FOXC2 promotes colorectal cancer proliferation through inhibition of FOXO3a and activation of MAPK and AKT signaling pathways. Cancer Lett. (2014) 353:87–94. 10.1016/j.canlet.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 44.Wang T, Zheng L, Wang Q, Hu YW. Emerging roles and mechanisms of FOXC2 in cancer. Clin Chim Acta. (2018) 479:84–93. 10.1016/j.cca.2018.01.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.