Abstract
Propolis has exhibited effective antibacterial activities in preventing the growth of multiple pathogenic bacteria. However, the antibacterial activity of Chinese propolis against methicillin-resistant Staphylococcus aureus (MRSA) is almost unknown. The present study aimed to explore the antibacterial activity and action mechanism of Chinese propolis ethanol extract (CPEE) against MRSA. Thirteen compounds of CPEE were identified using HPLC–DAD/Q-TOF–MS, and none of them showed better anti-MRSA activity than CPEE. The diameter of inhibition zone (DIZ) of CPEE was 20.1 mm. The minimal inhibitory concentration (MIC) of CPEE was 32 mg/L, while the minimal bactericidal concentration (MBC) against MRSA was 64 mg/L. Moreover, CPEE showed significant synergistic effects with β-lactam antibiotics (ampicillin and oxacillin). Nucleic acid and protein leakage assays showed that CPEE can stimulate the release of intracellular macromolecules by damaging the cell membrane integrity of MRSA. Live/dead-staining and SDS-PAGE assays further confirmed that CPEE could inhibit bacterial activities by disrupting the membrane. The reduction in PBP2a expression and β-lactamase activity, as shown by western blot and β-lactamase detection assays, suggested that CPEE was able to reverse the drug resistance of MRSA. These results demonstrated the anti-MRSA activity of CPEE was mainly due to changing the cell membrane and reversing resistance, which indicates that CPEE could be an attractive candidate for use in future food and medical applications.
Keywords: Chinese propolis, MRSA, Antibacterial activity, Synergy, Mechanism
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a common clinically virulent bacterium that causes diseases that seriously threaten the health of clinical patients worldwide, such as pneumonia, soft tissue infection, endocarditis and sepsis [1, 2]. As data from the China Antimicrobial Surveillance Network (CHINET) revealed in 2020, the detectable rate of MRSA accounts for 33.6% of total clinical infectious diseases. Furthermore, the abuse of antibiotics has greatly enhanced the range of MRSA resistance. At present, MRSA shows significant resistance to multiple broad-spectrum antibacterial agents, including fluoroquinolones, sulfonamides, tetracyclines, β-lactams, macrolides and aminoglycosides [3]. In addition, as it has expanded to an epidemic scope around the world, the detection and isolation rates of MRSA have also increased among the infections found in hospitals and communities [4]. High fatality rates, high detection rates and strong drug resistance have led to threat posed by MRSA becoming more serious. Thus, the development of novel anti-MRSA drugs is more urgently needed than ever before [5].
Propolis is a natural product collected by honeybees from exudates and the buds of various plants, which is then mixed with wax and bee enzymes [6]. As one of the major byproducts created during honey production, propolis has been used as a kind of natural medicine since ancient times in China. In fact, at the present, some types of propolis are included in the “Pharmacopoeia of the People’s Republic of China”. The chemical compositions of propolis are highly complex and variable, and so far, more than 600 kinds of natural components belonging to 20 categories have been identified, including flavonoids, phenolic acids, terpene acids, steroids, amino acids, etc. [7, 8]. Previous studies have shown that propolis possesses rich and unique biological activities due to its complex chemical composition, including antibacterial, antifungal, antiviral, anti-inflammatory, antioxidation, antitumor and immune regulation effects [9, 10]. Of these, the superior antibacterial activities of propolis are especially remarkable [11, 12]. However, the antibacterial activity of Chinese propolis has not yet been adequately investigated. Moreover, there has been almost no research focused on the anti-MRSA activities and action mechanisms of Chinese propolis specifically. Thus, the antibacterial activities and mechanisms of Chinese propolis against MRSA should be studied in depth in order to provide scientific support for its applications in the food and medical fields.
As such, our investigation concentrated on the antibacterial activities of Chinese propolis ethanol extract (CPEE) in regard to MRSA. Firstly, the antibacterial activity of CPEE was evaluated. Secondly, the major chemical components of CPEE were analyzed using HPLC–DAD/Q–TOF–MS; the anti-MRSA activities of these components were evaluated and are discussed below. Thirdly, we elucidated the mechanisms of CPEE against MRSA, with particular focus on its interactions with the integrity of cell membranes. Finally, we analyzed the effects of CPEE on PBP2a and β-lactamase, MRSA’s core resistance mechanisms, which endow it with much of its resistance to antibacterial agents. Until now, no literature has reported about the synergy of Chinese propolis and β-lactams, so the investigations into antibacterial activity of CPEE and its synergistic effect with β-lactams against MRSA are inadequate and need to be further studied.
Materials and methods
Propolis material and bacterial strain
Propolis was collected from Liaocheng (36°26′ N, 115°57′ E) in Shandong Province of China in 2018. The main plant sources were poplars (Populus sp.). A methicillin-resistant Staphylococcus aureus (MRSA, ATCC 43,300) strain was provided by the Guangdong Microbial Culture Collection Center (GDMCC) of China. After activation, the strain was stored at − 80 °C in 20% glycerin in order to achieve longer preservation.
Preparation of Chinese propolis ethanol extract
The extraction of Chinese propolis was based on the method previously published in [13]. In brief, propolis was extracted via 95% (v/v) ethanol overnight with constant stirring. The supernatants were filtered and concentrated until they reached a constant weight. The samples obtained were redissolved in anhydrous ethanol and filtered through filter papers followed by a bacteria filter with a 0.22-μm membrane. After being concentrated again, the Chinese propolis ethanol extract (CPEE) was preserved at − 20 °C.
HPLC–DAD/Q-TOF–MS analysis
The chemical composition of CPEE was analyzed by HPLC–DAD/Q-TOF–MS using the conditions noted in [14].
Determination of inhibition zone diameter
The diameter of the inhibition zone (DIZ) was detected according to the report published in [15]. Briefly, 100 μL of MRSA cell suspension (105 CFU/mL) was spread over trypticase soy agar (TSA) plates. The filter paper discs (6 mm) were sterilized and impregnated with 0.1 mg/disc CPEE. After drying, discs were moved to the plates and incubated overnight. CPEE was dissolved in trypticase soy broth (TSB) containing 2% tween 80. TSB medium was used as a negative control (control), and vancomycin hydrochloride (0.01 mg/disc) was used as the positive control. In addition, the DIZs of 2% tween 80 against MRSA were also measured.
Antibacterial activity assessments
Determination of minimal inhibitory concentration
The minimal inhibitory concentration (MIC) was assessed by the microdilution method, as reported in the literature, with several modifications [16, 17]. Briefly, a precultured bacterial suspension with TSB medium was mixed with the samples (CPEE, oxacillin and ampicillin) in 96-well plates; the final levels of MRSA cells and samples were 105 CFU/mL and 0.5–512 mg/L, respectively. After mixing evenly, the plates were incubated at 37 ℃ overnight. An amount equal to 20 µL of resazurin sodium (1 mg/mL) was added to the wells and incubated for 3 h at 37 °C in the dark. Non-treated with CPEE bacteria and TSB medium containing 2% tween 80 were used as a negative control, and vancomycin hydrochloride (10 mg/L) was used as the positive control. The MIC was at the lowest level of the samples, preventing the solution from changing from purple to orange.
Determination of minimal bactericidal concentration
The evaluation method of minimal bactericidal concentration (MBC) was measured using a subculture of 50 μL derived from the wells, with no visible bacterial growth on TSA plates. The MBC was the lowest concentration, and no MRSA growth could be observed on the plates after sub-culturing overnight.
Determination of Fractional Inhibitory Concentration Indices
Fractional Inhibitory Concentration (FIC) indices were measured according to the checkerboard method [12, 18]. In brief, the β-lactam antibiotics were serially diluted with TSB medium starting from a concentration of 2 MIC along the ordinate within the 96-well plate. CPEE was serially diluted with TSB medium (containing 2% tween 80) along the abscissa using 2 MIC as the highest level. An amount equal to 100 μL of MRSA suspension was added to every well, and its final level was 105 CFU/mL. After overnight incubation, the MICs of the CPEE and antibiotics were determined. After this, interactions between the CPEE and antibiotics were analyzed using FIC indices, calculated as follows: FICs = FIC A + FIC B. Here, FIC A is MICantibiotics in the combination/MICantibiotics alone and FIC B is MICCPEE in combination/MICCPEE alone. The FIC indices were interpreted as follows: synergistic for FIC ≤ 0.5, additive for 0.5 < FIC ≤ 1.0, indifferent for 1.0 < FIC ≤ 4.0 and antagonistic for FICI > 4.0 [19, 20].
Antibacterial mechanism assessments
Leakage of nucleic acids and proteins assays
The leaking proteins and nucleic acids were detected based on the report in [21, 22]. In brief, logarithmic MRSA cells (107 CFU/mL) were collected and resuspended in phosphate-buffered saline (PBS, pH 7.4). Different concentrations of CPEE (control, MIC and 2 MIC) were added to the above bacterial suspension for incubation. After the mixed suspensions were filtered through 0.22-μm filter membranes, the optical density (OD) values were determined at 260 and 280 nm by a microplate reader (Epoch2, Biotek, USA). Samples containing 2% tween 80, but without CPEE, were used as a control.
Live/dead bacteria staining assay
The live/dead bacteria-staining assay method was used for estimation according to the relevant study, albeit with some changes [23]. Briefly, the logarithmic MRSA cells were pre-collected, washed and resuspended in PBS. The suspension was mixed with different concentrations of CPEE (control, MIC, 2 MIC and 4 MIC) and incubated for 24 h. After this, the cultures were gently removed, washed with 0.85% NaCl solution and stained with a Live/Dead™ BacLight™ Bacterial Viability Kit (L13152, Invitrogen, Carlsbad, CA, USA) for 15 min in the dark. Finally, 50 μL of stained cell suspension was moved to special dishes for observation and photographed using a CLSM (FV1200, Olympus, Japan).
SDS-PAGE assay
The SDS-PAGE assay method was used for evaluation with reference to the previous literature, again with several changes [24]. In brief, the pre-collected logarithmic cells of MRSA were washed and resuspended in PBS. Then, the suspension was exposed to different concentrations of CPEE (control, ½ MIC and MIC) at 37 ℃ for 24 h. The treated MRSA cells were collected via centrifugation at 3000 rpm/min for 10 min. After that, an appropriate amount of RIPA lysate (containing 1% protease inhibitor and 1% phosphatase inhibitor) was used to extract bacterial proteins. The bacterial protein concentration was determined using a BCA protein detection kit. SDS-PAGE electrophoresis was performed with 12% separating and 5% stacking gel, which followed the approach of an SDS-PAGE kit. Protein bands were stained with blue fast-staining solution and de-stained in distilled water.
Western blotting analysis
Western blotting assay was executed as previously depicted in [13]. Briefly, the logarithmic MRSA cells were collected, washed and resuspended in PBS (pH 7.4). Subsequently, the MRSA suspensions were incubated with different concentrations of CPEE (control, ¼ MIC, ½ MIC and MIC) for 3 h. Then, lysozymes and protease inhibitors were added to the bacterial suspension. The proteins, including extracellular and intracellular, were extracted, and their concentrations were measured. An amount equal to 20 μL of the protein samples was separated by 12% SDS-PAGE and electro-blotted on a PVDF membrane using a semidry blotting apparatus.
Determination of β-lactamase activity
The activities of β-lactamase as expressed by MRSA were detected by referring to our previous study [14].
Statistical analysis
At minimum, all experiments were performed in triplicate. The statistical analysis was performed using a one-way analysis of variance (ANOVA), and p < 0.05 was considered statistically significant.
Results
Major chemical composition of CPEE
The major chemical contents of CPEE identified by HPLC–DAD/Q-TOF–MS are listed in Table 1. Thirteen compounds are quantified in the table, representing the main compounds in CPEE. These compounds are caffeic acid, p-coumaric acid, trans-cinnamic acid, ferulic acid, 3, 4-dimethoxycinnamic acid, quercetin, naringenin, kaempferol, apigenin, pinocembrin, chrysin, cape and galangin.
Table 1.
Major compounds identifed from CPEE by HPLC–DAD/Q-TOF–MS
| Compounds | Molecular formula | Retention time (min) | Content (mg/g) |
|---|---|---|---|
| Caffeic acid | C9H8O4 | 17.17 | 8.92 |
| p-Coumaric acid | C9H8O3 | 20.23 | 4.19 |
| trans-Cinnamic acid | C9H8O2 | 21.29 | 5.01 |
| Ferulic acid | C10H10O4 | 21.39 | 1.24 |
| 3,4-Dimethoxycinnamic acid | C11H12O4 | 24.68 | 7.75 |
| Quercetin | C15H10O7 | 26.81 | 1.14 |
| Naringenin | C15H12O5 | 27.11 | 5.93 |
| Kaempferol | C15H10O6 | 28.42 | 3.52 |
| Apigenin | C15H10O5 | 28.78 | 5.07 |
| Pinocembrin | C15H12O4 | 30.43 | 15.43 |
| Chrysin | C15H10O4 | 31.26 | 17.85 |
| CAPE | C17H16O4 | 31.27 | 4.66 |
| Galangin | C15H10O5 | 31.52 | 18.60 |
CAPE cafeic acid phenethyl ester
Antibacterial activity
The anti-MRSA activity of CPEE was assessed via the three main indicators: DIZ, MIC and MBC. DIZs were used to evaluate the antibacterial activity of MRSA qualitatively, while the MIC and MBC values were used for quantitative assessments. The DIZ values of CPEE against MRSA are shown in Table 2. As shown in the table, the obviously different DIZ values between the control group and the treated group indicate that CPEE showed significant antibacterial activity against MRSA. Meanwhile, the obvious DIZ value showed the MRSA strain was sensitive to vancomycin hydrochloride.
Table 2.
The DIZs of CPEE against MRSA
| Strain | DIZ (mm)a | |||
|---|---|---|---|---|
| Control | 2% tween 80 | CPEE | Vancomycin hydrochloride | |
| MRSA | 0 | 0 | 20.1 ± 0.5 | 20.6 ± 0.3 |
aDIZ, diameter of inhibition zone, the value indicated as an average of six replicates ± standard error
The values of the MIC, MBC and FIC indices are exhibited in Table 3. The MIC values of CPEE, ampicillin and oxacillin were 32, 128 and 128 mg/L, respectively, while the MBC values were 64, 128 and 256 mg/mL, respectively. These results were consistent with the conclusion of the DIZ assay, which indicated that CPEE displays a perfect antibacterial performance against MRSA. Furthermore, the values of the FIC indices were both ≤ 0.5, suggesting that ampicillin, oxacillin and CPEE have significant synergistic effects.
Table 3.
The individual and combinatorial effects of CPEE and β-lactams (ampicillin and oxacillin)
| Bacteria | Combinations | MIC (mg/L)a | MBC (mg/L)b | MIC (mg/L)c | FICs | Result |
|---|---|---|---|---|---|---|
| MRSA | CPEE | 32 | 64 | 8 | 0.38 | Synergy |
| Ampicillin | 128 | 128 | 16 | |||
| CPEE | 32 | 64 | 8 | 0.5 | Synergy | |
| Oxacillin | 128 | 256 | 32 |
aMIC values of individual samples
bMBC values of individual samples
cMIC values of the samples in combination
Antibacterial mechanism
Cell membrane damage studies
Information can be gleaned from the release of macromolecules in bacterial cells, thus revealing the structural integrity of the cell membrane. It was obvious in our work that nucleic acids (Fig. 1A) and proteins (Fig. 1B) were released into the bacterial suspension after their exposure to CPEE. In the first 4 h, the UV absorption of nucleic acids and proteins increased to 0.09, 0.14 and 0.10, and 0.18 for the MIC of CPEE. Afterward, the absorbance of nucleic acids and proteins continued to increase significantly within 24 h. Meanwhile, the 2 MIC groups displayed higher absorbance values, indicating that the degree of damage to the integrity of the bacterial cell membrane is concentration dependent. These results clearly suggest that CPEE is able to destroy the integrity of the cytomembrane and increase the cellular permeability of MRSA.
Fig. 1.
Effect of different concentrations of CPEE on (A) release of nucleic acids from MRSA and (B) release of proteins from MRSA
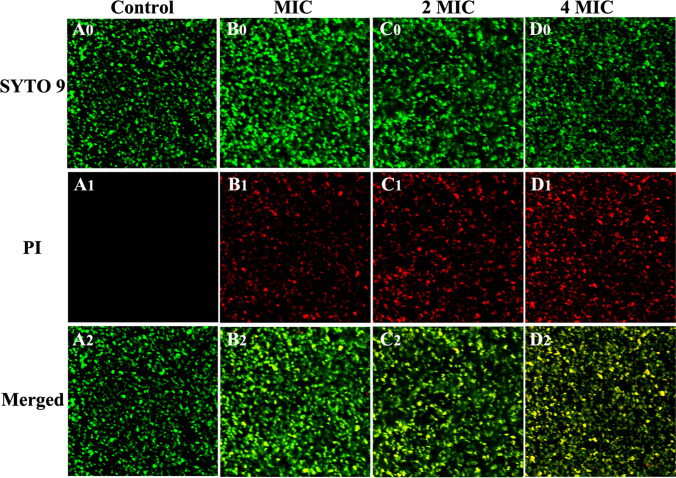
The Live/Dead™ BacLight™ Bacterial Viability Kit was used to detect the viability of bacterial cells. The kit included two types of stains: SYTO 9 and PI. SYTO 9 pass through the cell membrane of living or dead bacteria and stains bacterial cells with green fluorescence. PI cannot penetrate the living cell membrane and only stains cells with impaired cytomembrane with red fluorescence. Meanwhile, a yellow color indicates overlapping images of live and dead bacteria. In this work, staining was carried out on MRSA bacterial cells using different treatments. As shown in Fig. 2, red fluorescence from bacterial cells treated with CPEE is more apparent when compared to the control. These results prove that CPEE can disrupt bacterial cell membranes and enhance the permeability of the cytoplasmic membrane, which is in accordance with the results of the nucleic acid leakage test.
Fig. 2.
CLSM photos of MRSA treated with different concentrations of CPEE. Live cells exhibited green fluorescence (SYTO 9), whereas bacteria with damaged membranes exhibited red fluorescence (PI). (A) control; (B) MIC; (C) 2 MIC; (D) 4 MIC; (0) stained with SYTO9; (1) stained with PI; (2) merged
In order to display the leakage of proteins intuitively, the MRSA cells treated with CPEE were analyzed by an SDS-PAGE assay. As shown in Fig. 3, protein bands of MRSA treated with CPEE were significantly different from the control. The bands appeared clear and strong in the control group but slightly shallow after being treated with ½ MIC CPEE. The protein bands decreased more evidently with an increase in CPEE concentration (MIC) compared to the control. These results indicate that CPEE can apparently increase the permeability of the MRSA cytomembrane, which is consistent with the results of the protein leakage test. In addition, the disappearance of a large number of protein bands may be related to the inhibition of protein expression aroused by CPEE.
Fig. 3.
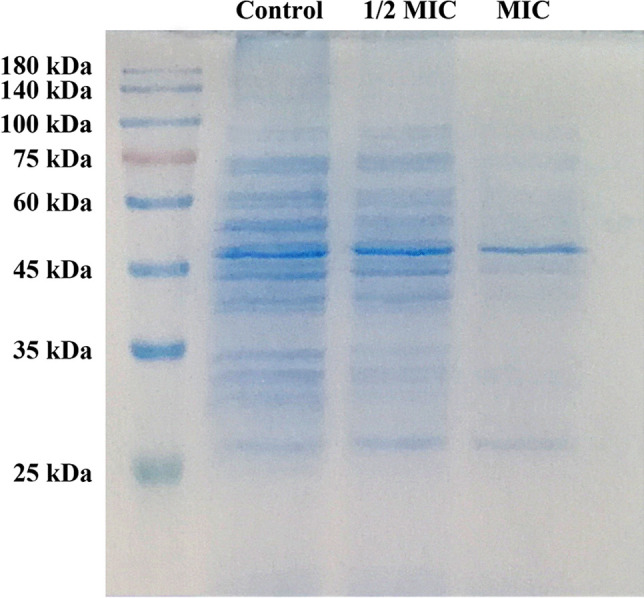
SDS-PAGE patterns of proteins from MRSA treated with different concentrations of CPEE
Resistance reversal studies
The major drug-resistant mechanism of MRSA is the expression of PBP2a and the production of β-lactamase. As shown in Fig. 4, the brightness of the PBP2a protein band decreased significantly after being treated with CPEE (1/4 MIC) compared to the control. The protein bands almost disappeared in the groups treated with ½ MIC and MIC CPEE. The above results indicate that the expression of PBP2a was regulated by CPEE.
Fig. 4.
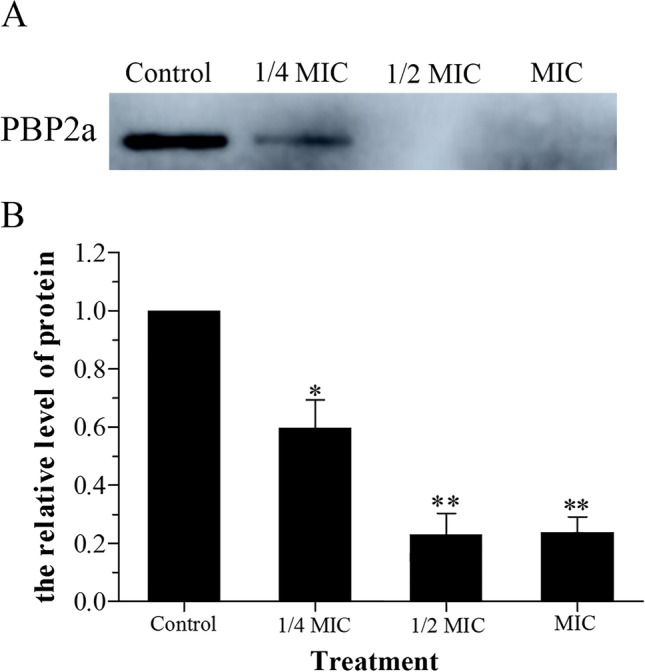
Effect of different concentrations of CPEE on the expression of PBP2a from MRSA. A, the expression of PBP2a; B, quantification of relative quantity of PBP2a. Values shown are means ± standard errors (n = 3). (*p < 0.05 and **p < 0.01 vs. control)
The effect of CPEE on β-lactamase expressed by MRSA is shown in Fig. 5; the OD values of the β-lactamase extract treated with CPEE (¼ MIC, ½ MIC and MIC) decreased markedly, which was similar to the behavior of sulbactam sodium (a competition inhibition). The OD value of β-lactamase treated with CPEE (1/8 MIC) did not apparently decrease compared to the control. These results also suggest CPEE is able to decrease the activities of the β-lactamase produced by MRSA.
Fig. 5.
Effect of different concentrations of CPEE on β-lactamase from MRSA. Values shown are mean ± standard error (n = 3). (*p < 0.05, **p < 0.01 vs. control)
Discussion
This work focused on the inhibitory activity of Chinese propolis against MRSA. We tested the inhibitory activity of propolis against MRSA using propolis sources obtained from various regions of China. In our study, thirteen major polyphenolic constituents, including caffeic acid, were identified by HPLC–DAD/Q-TOF–MS. However, none of these compounds showed better antibacterial activities against MRSA than CPEE, which indicates that the excellent anti-MRSA activity of CPEE is the comprehensive effect of multi-component synergistic action. This conclusion is similar to that of our recent publication on the anti‑MRSA effects of Australian propolis [14].
The values of DIZ, MIC, MBC and FIC indices were employed to assess the anti-MRSA activity of CPEE. Few entries in the literature have reported the antibacterial properties of Chinese propolis against MRSA. Up until now, only Taiwanese green propolis and Chinese red propolis have been found to have anti-MRSA effects [11, 25]. These results support the remarkable anti-MRSA effects found in our work, which suggest that Chinese propolis possesses great potential in the antibacterial field. In addition, similar to Australian propolis, we also found that CPEE shows strong antibacterial activity against methicillin-sensitive S. aureus (MSSA), but almost no inhibition against Escherichia coli and Salmonella enteritidis (data not shown) [14]. These results indicate that the antibacterial activity of Chinese propolis against Gram-positive bacteria is obviously different from that of Gram-negative bacteria. This phenomenon might be aroused by the different structures of the cell walls in Gram-positive and Gram-negative bacteria. Gram-negative bacteria (E. coli and S. typhimurium) have a thick layer of lipopolysaccharide on the outer membrane of the cell wall, while Gram-positive bacteria (MRSA and MSSA) do not possess this structure, which has been shown to be more resistant to multiple types of antibacterial agents [26–28]. Thus, different cell wall structures may be the major reason for the different antibacterial activities of CPEE against Gram-positive and Gram-negative bacteria. In brief, the significant inhibiting properties of Chinese propolis against Gram-positive bacteria will contribute to its great potential for use in a variety of medical fields. FIC indices were also determined, and high synergistic effects of CPEE and β-lactam antibiotics were found. The unique resistance mechanisms of MRSA are significant due to their resistance to β-lactam antibiotics [18]. Combinations that included both CPEE and β-lactam antibiotics reduced the MICs noticeably, suggesting that CPEE can impact the major resistance mechanisms of MRSA.
The integrity of the cell membrane is the basis for the survival of bacteria. Once a cell membrane is damaged, the cellular contents will be released into the bacterial suspension in sequence [29]. Proteins and nucleic acids are the typical macromolecular contents of bacterial cells, existing in the cytomembrane and cytoplasm, and they play indispensable roles in the normal operation of bacterial cells. Nucleic acids carry genetic information, and proteins provide the major structural information. Moreover, nucleic acids and proteins are both involved in the transmission of genetic information, including DNA replication, transcription and translation [30]. The leaking of macromolecules means that a large number of small, inorganic molecules, including H+, K+ and Na+, have leaked out of bacterial cells. These inorganic ions play an enormously important role in maintaining enzyme activities and the normal operation of metabolism in bacterial cells [31]. Therefore, the loss of these cell contents directly leads to the death of bacterial cells in multiple ways [32].
Our study also discussed the influence of CPEE on the drug-resistant mechanisms of MRSA. Controlling its drug resistance is the key to inhibiting the activity of MRSA. The main resistance mechanisms of MRSA are: (1) the expression of resistant proteins such as penicillin-binding protein 2a (PBP2a), regulated by mec A, which can function as a transpeptidase to promote the formation of cell walls [17]; (2) the secretion of large amounts of β-lactamase to hydrolyze antibacterial agents [33]. In addition, several other factors can also improve MRSA drug resistance, such as the promotion of drug efflux by enhancing the expression of efflux pump-related genes [34]. In this work, CPEE was able to inhibit the expression of PBP2a and reduce β-lactamase activity, indicating that CPEE is able to reverse the resistance of MRSA. These results suggest that a combination of CPEE and antibiotics may have better antibacterial effects than the use of antibiotics alone.
This study suggests that, individually, CPEE possesses significant antibacterial activity against MRSA. Meanwhile, CPEE paired with β-lactam antibiotics also has significant systemic effects. Thus, these action mechanisms were clarified by evaluating bacterial cell membrane integrity and the reversion of MRSA’s drug resistance aroused by CPEE. The results of the intracellular macromolecule leak detection assay revealed that the CPEE damaged the cytoplasmic membrane of the tested strain, causing proteins and nucleic acids to leak due to a loss of cell membrane integrity. The results of the live/dead-staining and SDS-PAGE assays confirmed these conclusions. Furthermore, the obvious decrease in the PBP2a expression levels and β-lactamase activity proved that CPEE has a strong ability to reverse the drug resistance of MRSA. These results indicate that CPEE, a byproduct of the honey industry, might be an efficient, functional ingredient for use in food preservation and clinical treatments.
Abbreviations
- CPEE
Chinese propolis ethanol extract
- DIZ
Diameter of inhibition zone
- FIC
Fractional inhibitory concentration
- MBC
Minimal bactericidal concentration
- MIC
Minimal inhibitory concentration
- MRSA
Methicillin-resistant Staphylococcus aureus
- PBS
Phosphate-buffered saline
- TSA
Trypticase soy agar
- TSB
Trypticase soy broth
Funding
This work was supported by grants from Shandong Province Modern Agricultural Technology System (SDAIT-24–05), Shandong Provincial Natural Science Foundation of China (ZR2021MC110) and the Doctoral Research Foundation of Liaocheng University (No. 318051826).
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fei Wang and Jie Yuan contributed equally to this work.
Contributor Information
Fuyao Wei, Email: weifuyao@lcu.edu.cn.
Hongzhuan Xuan, Email: xuanhongzhuan@126.com.
References
- 1.Flora M, Perrotta F, Nicolai A, Maffucci R, Pratillo A, Mollica M, Bianco A, Calabrese C. Staphylococcus aureus in chronic airway diseases: an overview. Respir Med. 2019;155:66–71. doi: 10.1016/j.rmed.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Mera RM, Suaya JA, Amrine-Madsen H, Hogea CS, Miller LA, Lu EP, Sahm DF, O’Hara P, Acosta CJ. Increasing role of Staphylococcus aureus and community-acquired methicillin-resistant Staphylococcus aureus infections in the United States: a 10-year trend of replacement and expansion. Microb Drug Resist. 2011;17:321–328. doi: 10.1089/mdr.2010.0193. [DOI] [PubMed] [Google Scholar]
- 3.Huang C, Wang XL, Zhang L, Shen W. Distribution and drug resistance of pathogenic bacteria in children with lower respiratory tract infection from Chengdu Children’s Hospital between 2001 and 2006. Chin J Contemp Pediatr. 2008;10:17–20. [PubMed] [Google Scholar]
- 4.Baek KT, Grundling A, Mogensen RG, Thogersen L, Petersen A, Paulander W, Frees D. beta-lactam resistance in methicillin-resistant Staphylococcus aureus USA300 is increased by inactivation of the ClpXP protease. Antimicrob Agents Ch 58, 4593–4603. c solvents applied in extraction and separation. J Sep Sci. 2014;39:3505–3520. doi: 10.1128/AAC.02802-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin N, Tan X, Jiao Y, Liu L, Zhao W, Yang S, Jia A. RNA-Seq-based transcriptome analysis of methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci Rep. 2014;4:5467. doi: 10.1038/srep05467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan WW, Chang HS, Liu XY, Wang SQ, Liu H, Xuan HZ. Brazilian green propolis inhibits Ox-LDL-stimulated oxidative stress in human umbilical vein endothelial cells partly through PI3K/Akt/mTOR-mediated Nrf2/HO-1 pathway. Evid-Based Compl Alt. 2019;2019:5789574. doi: 10.1155/2019/5789574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bankova V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol. 2005;100:114–117. doi: 10.1016/j.jep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Huang S, Zhang CP, Wang K, Li GQ, Hu FL. Recent advances in the chemical composition of propolis. Molecules. 2014;19:19610–19632. doi: 10.3390/molecules191219610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuliang HU, Hepburn HR, Xuan H, Chen M, Daya S, Radloff SE. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol Res. 2005;51:147–152. doi: 10.1016/j.phrs.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Braakhuis A. Evidence on the health benefits of supple mental propolis. Nutrients. 2019;11(11):2705. doi: 10.3390/nu11112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YW, Ye SR, Ting C, Yu YH. Antibacterial activity of propolins from Taiwanese green propolis. J Food Drug Anal. 2018;26:761–768. doi: 10.1016/j.jfda.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gezgin Y, Kazan A, Ulucana F, Yesil-Celiktas O. Antimicrobial activity of propolis and gentamycin against methicillin-resistant Staphylococcus aureus in a 3D thermo-sensitive hydrogel. Ind Crop Prod. 2019;139:111588. doi: 10.1016/j.indcrop.2019.111588. [DOI] [Google Scholar]
- 13.Xuan H, Yuan W, Chang H, Liu M, Hu F. Anti-inflammatory effects of Chinese propolis in lipopolysaccharide-stimulated human umbilical vein endothelial cells by suppressing autophagy and MAPK/NF-κB signaling pathway. Inflammopharmacology. 2019;27:561–571. doi: 10.1007/s10787-018-0533-6. [DOI] [PubMed] [Google Scholar]
- 14.Wang F, Liu H, Li J, Zhang W, Jiang B, Xuan H. Australian propolis ethanol extract exerts antibacterial activity against methicillin-resistant Staphylococcus aureus by mechanisms of disrupting cell structure, reversing resistance, and resisting biofilm. Braz J Microbiol. 2021;52:1651–1664. doi: 10.1007/s42770-021-00547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Said ZBS, Guemghar HH, Makhlouf LB, Rigou P, Remini H, Adjaoud A, Khoudja NK, Madani K. Essential oils composition, antibacterial and antioxidant activities of hydrodistillated extract of Eucalyptus globulus fruits. Ind Crop Prod. 2016;89:167–175. doi: 10.1016/j.indcrop.2016.05.018. [DOI] [Google Scholar]
- 16.Chen ZF, He B, Zhou J, He DH, Deng JD, Zeng RH. Chemical compositions and antibacterial activities of essential oils extracted from Alpinia guilinensis against selected foodborne pathogens. Ind Crop Prod. 2016;83:607–613. doi: 10.1016/j.indcrop.2015.12.063. [DOI] [Google Scholar]
- 17.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children (vol 52, pg e18, 2011) Clin Infect Dis. 2011;53:319–319. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 18.Catteau L, Reichmann NT, Olson J, Pinho MG, Nizet V, Van Bambeke F, Quetin-Leclercq J. Synergy between ursolic and oleanolic acids from Vitellaria paradoxa leaf extract and β-lactams against methicillin-resistant Staphylococcus aureus: in vitro and in vivo activity and underlying mechanisms. Molecules. 2017;22:2245. doi: 10.3390/molecules22122245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung PY, Navaratnam P, Chung LY. Synergistic antimicrobial activity between pentacyclic triterpenoids and antibiotics against Staphylococcus aureus strains. Ann Clin Microb Anti. 2011;10:25. doi: 10.1186/1476-0711-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YS, Kang OH, Choi JG, Oh YC, Chae HS, Kim JH, Park H, Sohn DH, Wang ZT, Kwon DY. Synergistic effects of the combination of galangin with gentamicin against methicillin-resistant Staphylococcus aureus. J Microbiol. 2008;46:283–288. doi: 10.1007/s12275-008-0012-7. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, Wei FY, Song CX, Jiang B, Tian SY, Yi JW, Yu CL, Song ZB, Sun LG, Bao YL, Wu Y, Huang YX, Li YX. Dodartia orientalis L. essential oil exerts antibacterial activity by mechanisms of disrupting cell structure and resisting biofilm. Ind Crop Prod. 2017;109:358–366. doi: 10.1016/j.indcrop.2017.08.058. [DOI] [Google Scholar]
- 22.Yang Y, Tian S, Wang F, Li Z, Liu L, Yang X, Bao Y, Wu Y, Huang Y, Sun L, Yu C, Li Y. Chemical composition and antibacterial activity of Kaempferia galanga essential oil. Int J Agric Biol. 2018;20:457–462. doi: 10.17957/IJAB/15.0560. [DOI] [Google Scholar]
- 23.Liu YC, Xu YJ, Song QH, Wang F, Sun LG, Liu L, Yang XG, Yi JW, Bao YL, Ma HF, Huang HL, Yu CL, Huang YX, Wu Y, Li YX. Anti-biofilm activities from Bergenia crassifolia leaves against Streptococcus mutans. Front Microbiol. 2017;8:1738. doi: 10.3389/fmicb.2017.01738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang B, Wang F, Liu L, Tian SY, Li WL, Yang XG, Wu Y, Huang YX, Yi JW, Yu CL, Sun LG, Zhang YW, Li YX. Antibacterial activity and action mechanism of the echinops ritro l. Essential oil against foodborne pathogenic bacteria. J Essent Oil Bear Pl. 2017;20:1172–1183. doi: 10.1080/0972060X.2017.1399090. [DOI] [Google Scholar]
- 25.Zhang WW, Margarita GE, Wu D, Yuan WQ, Yan S, Qi SZ, Xue XF, Wang K, Wu LM. Antibacterial activity of Chinese red propolis against Staphylococcus aureus and MRSA. Molecules. 2022;27:1693. doi: 10.3390/molecules27051693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsouna AB, Trigui M, Mansour RB, Jarraya RM, Damak M, Jaoua S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int J Food Microbiol. 2011;148:66–72. doi: 10.1016/j.ijfoodmicro.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Sikkema J, de Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YB, Liu XY, Wang YF, Jiang PP, Quek S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. doi: 10.1016/j.foodcont.2015.05.032. [DOI] [Google Scholar]
- 29.Chen CZ, Cooper SL. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23:3359–3368. doi: 10.1016/S0142-9612(02)00036-4. [DOI] [PubMed] [Google Scholar]
- 30.Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol. 2010;8(6):423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diao WR, Hu QP, Zhang H, Xu JG. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.) Food Control. 2014;35:109–116. doi: 10.1016/j.foodcont.2013.06.056. [DOI] [Google Scholar]
- 32.Sharma A, Bajpai VK, Baek KH. Determination of antibacterial mode of action of Allium sativum essential oil against foodborne pathogens using membrane permeability and surface characteristic parameters. J Food Safety. 2013;33:197–208. doi: 10.1111/jfs.12040. [DOI] [Google Scholar]
- 33.Tazi A, Chapron J, Touak G, Longo M, Hubert D, Collobert G, Dusser D, Poyart C, Morand PC. Rapid emergence of resistance to linezolid and mutator phenotypes in Staphylococcus aureus isolates from an adult cystic fibrosis patient. Antimicrob Agents Chemother. 2013;57:5186–5188. doi: 10.1128/AAC.01392-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nannini E, Murray BE, Arias CA. Resistance or decreased susceptibility to glycopeptides, daptomycin, and linezolid in methicillin-resistant Staphylococcus aureus. Curr Opin Pharmacol. 2010;10:516–521. doi: 10.1016/j.coph.2010.06.006. [DOI] [PubMed] [Google Scholar]