Abstract
The eubiotic lignocellulose has to be proven a useful source of dietary fiber. Dietary fiber is broken down into monosaccharides and then is fermented into short-chain fatty acids (SCFAs) by gut microorganisms of chickens. However, research on impacts of it on the gut microbiota of chickens was limited. Given this, three different levels (0%, 1%, and 2%) of eubiotic lignocellulose were added to the feed of ISA brown hens for 0–8 weeks, with the aim of observing the impacts of it on the performance, gut microbiota, and SCFAs. The results showed that there were no significant effects on the performance and the development of the cecum (P > 0.05); however, added eubiotic lignocellulose increased the relative abundance of the most excellent fiber-degradation species Bacteroides thetaiotaomicron and fiber digestibility (P < 0.05). The addition of 1% significantly increased Lactobacillus panis and Oscillospira (P < 0.05), resulting in increasing of the production of SCFAs. Adding 1% eubiotic lignocellulose is appropriate in this experiment, but one or more group less than 1% (e.g., 0.75%, 0.5%, or 0.25%) should be set to verify the impact and determine a more appropriate amount of it added in the feed of chickens between 0 and 8 weeks in the future.
Keywords: Eubiotic lignocellulose, Dietary fiber, Chicken, Gut microbiota, Short-chain fatty acids
Introduction
Dietary fiber is the substrate of microorganisms residing in the gut of chickens. Excellent fiber-degradation genera include Bacteroides, Prevotella, Fibrobacter, Roseburia [1], and so on. Excellent fiber-degradation species include Bacteroides thetaiotaomicron [2], Bacteroides ovatus [3], and so on. Short-chain fatty acids (SCFAs) including acetate, propionate, and butyrate are main fermentation products of dietary fibers by SCFA-producing bacteria. The cecum is the principal place for microbial fermentation in chickens. Bifidobacterium produces acetate using bifid-shunt [4]. Propionibacterium produces propionate via a succinate-propionate pathway [5]. Faecalibacterium and Roseburia can use acetate to generate butyrate [6]. SCFAs can serve as an important energy source [7] and also are in control of body weight [8]. Acetate is the main way for the host to obtain energy from dietary fiber. It can provide 1.2–10% of the total energy per day for human beings. Propionate synthesizes glycogen in the liver. Butyrate provides energy for normal colonic epithelial cells [9] and promotes their proliferation [10].
The eubiotic lignocellulose OptiCell is a new type of useful dietary fiber for chickens. It is a synergistic combination of soluble and insoluble fiber. Its proportion of total dietary fiber is as high as 85%. So, no great adjustment was required in terms of the composition of the feed, generally adding 1.0–1.5% can positively affect the growth performance and laying performance of poultry [11]. Results of experiments showed that the average daily gain and final weights of broilers increased by 9% and 7.8%, respectively, after adding 1% eubiotic lignocellulose for 32 days [12]. Chickens must rely on gut microorganisms to degrade dietary fiber due to a lack of endogenous fiber-degrading enzymes [13]. Therefore, we speculated that the effects of eubiotic lignocellulose on chickens should be partly related to the gut microbiota and microbial metabolites-SCFAs. However, there were few reports on impacts of adding it on the gut microbiota or SCFAs of chickens.
Given this, different levels of eubiotic lignocellulose were added to the feed of chickens for 0–8 weeks, with the aim of observing the effects of it on the growth performance, the gut microbiota, and microbial metabolite SCFAs of chickens. Increasing our understanding of this would be beneficial to provide a theoretical basis for the application of eubiotic lignocellulose and improve the growth performance and health of chickens.
Materials and methods
Animals and experimental design
This study was approved by the Shanxi Agricultural University Animal Experiment Ethics Committee and the license number was SXAU-EAW-2020-006Chi.001. A total of 108 1-day-old ISA brown hens (IBH) with a 40 g average weight were chosen. Chickens were randomly divided into three groups, each group had 6 cages with 6 chickens per cage. According to the actual production, different levels of eubiotic lignocellulose OptiCell (OC) were added to the basic feed (Jinzhong Shiyang Feed Ltd., Shanxi, China) (Table 1) for 0–8 weeks. Group one was given 1% eubiotic lignocellulose and was called the OC-low (OL) group. Group two was given 2% eubiotic lignocellulose and was called the OC-high (OH) group. The control group was not given it, it was OC-free (OF) group. Samples were harvested to measure the gut microbiota, the concentration of SCFAs, and so on of IBH at the end of 8 weeks.
Table 1.
Ingredients and nutrition level of feed during 0–8 weeks
| Ingredients (%) | Nutrition level | ||
|---|---|---|---|
| Corn | 61.95 | ME (MJ/kg) | 12.43 |
| Soybean meal | 23.7 | Crude protein (%) | 19.49 |
| Soybean oil | 1.1 | Crude fiber (%) | 3.21 |
| Corn gluten meal | 4 | Crude fat (%) | 4.27 |
| DDGS | 4 | Crude ash (%) | 5.83 |
| Stone power | 1.8 | Ca (%) | 1.05 |
| CaHPO4 | 1.3 | Total P (%) | 0.57 |
| NaCl | 0.3 | NaCl (%) | 0.3 |
| Met | 0.2 | ||
| Lys | 0.46 | ||
| Thr | 0.09 | ||
| Multivitamin1 | 0.4 | ||
| Minerals2 | 0.55 | ||
| Choline chloride | 0.1 | ||
| Complex enzyme | 0.05 | ||
| Total | 100 |
1Feed (per kg) contains: vitamin A 2100–2500 KIU, vitamin D3 800–1240 KIU, vitamin E ≥ 5900 IU, vitamin K3 ≥ 600 mg, vitamin B1 ≥ 620 mg, vitamin B2 ≥ 1600 mg, vitamin B6 ≥ 830 mg, niacinamide ≥ 7000 mg, vitamin B12 ≥ 4200 μg, pantothenic acid ≥ 2450 mg, folate ≥ 245 mg, biotin ≥ 35 mg
2Feed (per kg) contains: Cu 8 mg, Fe 80 mg, Mn 60 mg, Se 0.15 mg, Zn 40 mg, I 0.35 mg
The eubiotic lignocellulose (Beijing e-feed & e-vet cooperation, Beijing, China) was developed by Agromed Ltd. (Austria), and it is made from special fresh timber. It contains energy ~ 0%, moisture 8%, crude protein 0.9%, total dietary fiber (TDF) 88%, crude ash 1.0%, crude fat 0.8%, minerals and trace elements 1.3%, crude fiber 59%, soluble TDF 1.3%, NDF 78%, ADF 64%, and lignin 25–30%.
Management
Chickens were fed in brood cages for 0–8 weeks. Chickens were given free access to water and feed. The management of the temperature, light, and humidity was conducted according to the breeding manual of IBH. No conventional immunization schedule of chickens was performed to avoid impacts on gut microbiota. Body weights and feed intake of each group of chickens were recorded.
Sampling
A part of feed was collected and six chickens per group were chosen to collect the total feces for the determination of fiber digestibility. They were executed with humanitarian slaughter and the length and weight of the cecum were measured. Two pieces from the middle of the cecum were sampled and put them into 4% paraformaldehyde fixative solution for 24 h. The contents of the cecum were collected into multiple cryogenic tubes, and they were put into a liquid nitrogen tank and preserved at – 80 °C until the determination of the 16S rRNA gene sequence of the gut microbiota and SCFAs.
Determination
Growth performance
The average daily feed intake (ADFI) per chicken per group was calculated as the average daily consumption divided by the number of chickens. The average daily gain (ADG) of per chicken per group was calculated as follows: (current weight − previous weight) ÷ interval days ÷ number of chickens.
The development of the cecum
The length and weight of the cecum were measured. Moreover, post 4% paraformaldehyde fixation, the cecum samples were processed using the conventional methods for tissue section, including washing, dehydration, transparence, embedding, slicing, deparaffinization, and so on. The height of the cecum fold and the villous height on the cecum fold were measured.
16S rRNA gene sequencing
The 16S rRNA gene of the gut microbiota was sequenced by Genedenovo Biotechnology Ltd. (Guangzhou, China) using High-Throughput Sequencing Technology. First, DNA extraction was performed using the HiPure Stool DNA Kits (Magen, Guangzhou, China). V3-V4 regions of the 16S rRNA gene were amplified by PCR using primers 341F 5’-CCTACGGGNGGCWGCAG and 806R 3’-GGACTACHVGGGTATCTAAT. PCR reactions were as follows: 95 °C for 2 min, followed by 27 cycles at 98 °C for 10 s, 62 °C for 30 s, 68 °C for 30,s and a final extension at 68 °C for 10 min. Illumina Hiseq 2500 sequencing was then extracted. Amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA).
Bioinformatics analysis.
Operational taxonomic units (OTUs) were clustered using the UPARSE pipeline using the UPARSE pipeline [14]. Venn analysis was performed in R project (version 3.4.1) to identify unique and common OTUs.
Taxonomy classification. The representative sequences were classified into organisms by a Naive Bayesian Model using RDP classifier (version 2.2) [15] based on SILVA Database [16].
Alpha diversity analysis. Alpha diversity indexes including ACE, Chao1, Shannon and Simpson were calculated. ACE and Chao1 reflect the community richness, and Shannon and Simpson indexes reflect the community richness and diversity.
The pair-wise comparison of Metastats and LEfSe (linear discriminant analysis (LDA) effect size) between groups was performed. Metastats showed significantly different bacteria using P < 0.01 or 0.05. The value of LDA of certain microbes > 2 represents that the difference is significant.
Crude fiber digestibility
The contents of crude fiber in the feed and manure were determined by the conventional method. Crude fiber digestibility (%) was calculated.
The concentration of SCFAs
The concentration of SCFAs (mmol/100 g) in the cecum chyme was measured using the internal standard method with High Performance Gas Chromatography (HPGC) (Trace 1300, Thermo Fisher Scientific, America) [17].
Statistical analysis
In terms of the gut microbiota, abundance statistics of each taxonomy were visualized using Krona [18]. The comparison of alpha diversity indexes among groups was computed via Kruskal–Wallis H test using Vegan package in R project [19]. Bacteria biomarker features of each group were screened by Metastats [20] and LEfSe software [21]. Statistical analyses of other indexes were performed using the ANOVA with SPSS 22.0 software. The results were expressed as the means and SEM.
Results
The determination of gut microbial diversity and composition
OTUs and gut microbial diversity
IBHE refer to “ISA Brown Hens-eight weeks.” IBHE contained the OC-low (OL) group, OC-high (OH) group, and OC-free (OF) group, namely IBHE-OL, IBHE-OH, and IBHE-OF.
The total and unique numbers of OTUs in OL, OH, and OF group were similar (Fig. 1). This was consistent with the lack of a difference (P > 0.05) among groups in terms of α-diversity (Table 2). It indicated that eubiotic lignocellulose had little effect on the gut microbial diversity of chickens.
Fig. 1.

The Venn diagram of OTUs
Table 2.
Comparison of α-diversity of gut microbiota among groups
| OL group | OH group | OF group | SEM | P-value | |
|---|---|---|---|---|---|
| ACE | 1518.33 | 1572.97 | 1594.39 | 70.17 | 0.14 |
| Chao1 | 1522.07 | 1543.41 | 1547.78 | 71.80 | 0.79 |
| Shannon | 6.87 | 6.64 | 6.54 | 0.37 | 0.67 |
| Simpson | 0.97 | 0.96 | 0.95 | 0.018 | 0.95 |
Gut microbial composition
Dietary fiber including the eubiotic lignocellulose is broken down into monosaccharide by fiber-degradation bacteria. A part of monosaccharides is then fermented into SCFAs by SCFA-producing bacteria. Therefore, significantly different fiber-degradation bacteria and SCFAs-producing bacteria between groups were focused on.
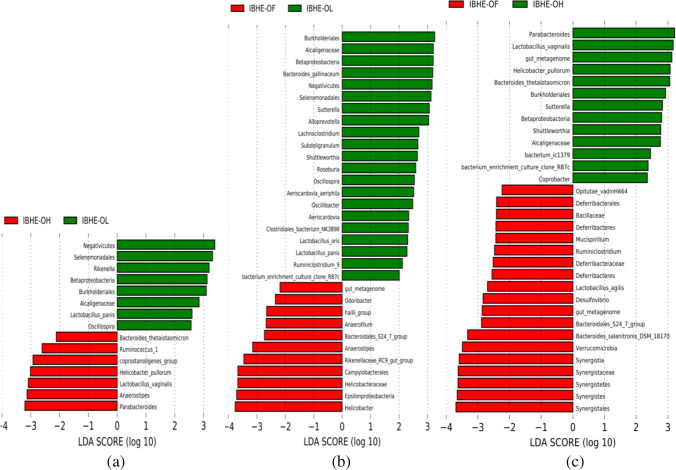
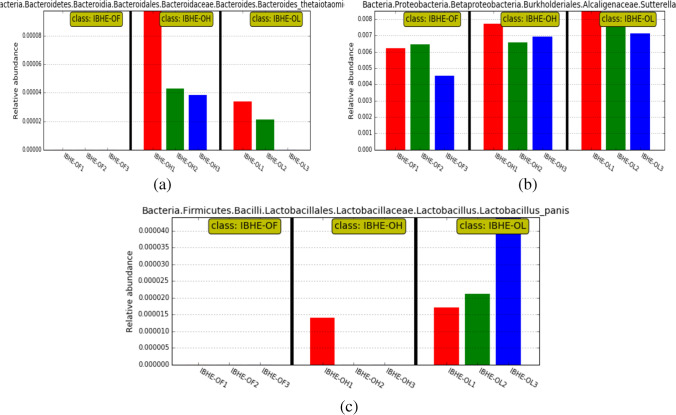
Compared with the OH and OF groups, the dominant bacteria of the OL group were the butyrate-producing genus Oscillospira (Fig. 2a) and the lactate-producing species Lactobacillus panis (Fig. 3b). Compared with the OF group, OL also had more of a low abundance of the fiber-degradation bacteria Prevotella, Roseburia, and Lactobacillus oris (Fig. 2b). Roseburia is also a butyrate producer. Compared with the OL and OF groups, the dominant bacteria of the OH group had the fiber-degradation bacterial species Bacteroides thetaiotaomicron. Bacteroides thetaiotaomicron is one of the most excellent fiber-degradation bacteria. Moreover, compared with the OF group, OL and OH groups both had B. thetaiotaomicron and the acetate-producing bacterium Sutterella (Figs. 2 and 3a). Notably, B. thetaiotaomicron was lacking in the OF group (Fig. 3a).
Fig. 2.
LDA (linear discriminant analysis) between the groups. a OH group vs. OL group, b OF group vs. OL group, c OF group vs. OH group
Fig. 3.
Abundance histogram of some dominant bacteria among three groups. a Abundance histograms of dominant fiber-degradation bacterium B. thetaiotaomicron and acetate-producing bacterium Sutterella in OL and OH groups. b Abundance histogram of dominant bacterium Lactobacillus panis in OL group
Crude fiber digestibility
The results showed that the crude fiber digestibility of OL and OH groups was higher (P < 0.05 and 0.01) than the OF group (Fig. 4). This was consistent with the fact that OL and OH groups had fiber-degrading bacterium B. thetaiotaomicron, while it was lack in the OF group (Fig. 3b).
Fig. 4.
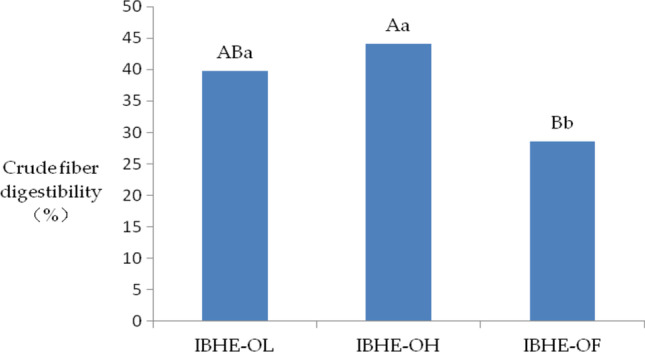
Histogram of crude fiber digestibility among three groups of chickens at 8 weeks. Note: means lacking a common uppercase superscript letter and lowercase superscript letter mean significant differences with a P < 0.01 and P < 0.05 respectively
The concentration of SCFAs
SCFAs are main fermentation products of dietary fibers by the gut microbiota. So the effects of the difference of the gut microbiota among groups on the concentration of SCFAs was next observed. The concentration of acetate, propionate and butyrate in the OL group was higher (P < 0.01 or 0.05) than the other two groups, indicating that the addition of 1% eubiotic lignocellulose was more conducive to the production of SCFAs at 8 weeks (Table 3).
Table 3.
Comparison of the concentration of SCFAs among groups
| SCFA | OL group | OH group | OF group | SEM | P-value |
|---|---|---|---|---|---|
| Acetate | 4.07Aa | 3.01Bb | 2.42Bb | 0.25 | 0.006 |
| Propionate | 0.96Aa | 0.72ABb | 0.62Bb | 0.077 | 0.010 |
| Butyrate | 0.28a | 0.20b | 0.19b | 0.026 | 0.027 |
Means within a line lacking a common lowercase superscript letter mean significant differences with a P-value < 0.05, and means within a row lacking a common uppercase superscript letter mean extremely significant differences with a P-value < 0.01
Growth performance
Given the concentration of SCFAs in the OL group was higher than the other groups (Table 3) and SCFAs provide energy for the host, the growth performance of chickens was determined. However, there was no difference (P > 0.05) among groups in terms of the growth performance including the body weight (BW), average daily feed intake (ADFI), and average daily gain (ADG) (Table 4).
Table 4.
Effects of eubiotic lignocellulose on the BW, ADFI, and ADG at 8 weeks
| OL group | OH group | OF group | SEM | P-value | |
|---|---|---|---|---|---|
| BW (g) | 775.50 | 764.38 | 766.25 | 15.44 | 0.25 |
| ADFI (g) | 52.39 | 51.62 | 50.59 | 1.04 | 0.91 |
| ADG (g) | 13.85 | 13.16 | 13.73 | 2.25 | 0.36 |
No superscript letters mean no significant difference (P > 0.05) in the same line
The development and histomorphology of the cecum
Because the concentration of butyrate in the OL group was the highest (Table 3) and butyrate can provide ~ 70% energy for normal intestinal epithelial cells and promotes their proliferation, therefore, the development and histomorphology of the cecum were next determined. However, there was no significant difference (P > 0.05) among groups in terms of the development (Table 5) and histomorphology of the cecum of chickens (Fig. 5).
Table 5.
Effects of eubiotic lignocellulose on the development of the cecum
| OL group | OH group | OF group | SEM | P-value | |
|---|---|---|---|---|---|
| Cecum length (cm) | 12.1 | 12.3 | 12.03 | 0.9 | 0.91 |
| Cecum weight (g) | 3.31 | 2.94 | 2.77 | 0.41 | 0.16 |
| Chyle weight (g) | 0.99 | 0.94 | 0.76 | 0.35 | 0.36 |
No superscript letters mean no significant difference (P > 0.05) in the same line
Fig. 5.
Histological section of cecum among groups at 8 weeks. a OL group, b OH group c OF group. Note: red arrows show the heights of cecum folds and yellow arrows show the heights of villi
Discussion
Effects of eubiotic lignocellulose on fiber-degradation bacteria and fiber digestibility
Dietary fiber is the substrate of fiber-degradation bacteria and it is broken down into monosaccharide by carbohydrate-active enZymes (CAZymes) released by fiber-degradation bacteria [22]. Bacteroidetes is “generalists” that degrade dietary fiber [23]. Excellent fiber-degradation Bacteroidetes bacteria include the Bacteroides and Prevotella genera. Bacteroides contains many excellent fiber-degrading species such as Bacteroides thetaiotaomicron. Firmicutes is regarded as “specialists” in fiber degradation. Among the members of Firmicutes, Fibrobacter [24], Ruminococcus, Butyrivibrio, and Roseburia genera are excellent fiber-degrading bacteria. Some members of Ruminococcus, such as Ruminococcus flavus, also can degrade cellulose and hemicellulose through the cellulosome pathway.
Other microorganisms that cannot degrade fiber can share the monosaccharides produced from the degradation of dietary fiber by these fiber-degradation bacteria. These monosaccharides can be used as a carbon source for gut microbial growth, thus increasing the microbial diversity. Report also showed that the consumption of a high-fiber diet helps to increase the richness and diversity of the gut microbiota [25].
In this experiment, the addition of eubiotic lignocellulose did not increase microbial diversity, but increased the relative abundance of B. thetaiotaomicron, one of the most excellent fiber-degradation species, resulting in an increasing of the crude fiber digestibility. Bacteroides thetaiotaomicron can degrade rhamnogalacturonan-II (RG-II) which is known to be the most complex glycan [26] by polysaccharide utilization locus (PUL) [27]. Of course, the impacts of adding eubiotic lignocellulose on the digestibility of cellulose and lignin of chickens are worth further discussing.
Moreover, the results suggested that the addition of 1% eubiotic lignocellulose is beneficial for increasing the abundance of certain fiber-degradation bacteria such as Prevotella and Roseburia. It was reported that Prevotella [1] and Roseburia [28] were excellent xylan-degrading genus, which could maximize the harvest of energy from plant polysaccharides. Prevotella can produce xylanase by PUL to decompose plant fiber [29]. Roseburia is a common genus of clostridium cluster XIVA. Roseburia intestinalis is a key xylan-degrading bacterium. The core apparatus that R. Intestinalis utilize xylan include extracellular xylanase RiXyn10A, ATP-binding cassette (ABC) transporter, and xylooligosaccharide-degrading enzyme in cytosol. RiXyn10A degrades xylan into xylooligosaccharides on the cell surface, and then substrate binding protein (SBP) binds xylooligosaccharides, which are transported to the cytosol and degraded into xylose by xylan-degrading enzyme [30].
Effects of eubiotic lignocellulose on SCFA-producing bacteria and SCFAs
Dietary fiber is cleaved into monosaccharides by fiber-degradation bacteria before it is fermented into SCFAs by SCFA-producing microorganisms. Acetate, propionate, and butyrate account for 90–95% of SCFAs. Bifidobacterium, Sutterella, and Blautia are acetate-producing bacteria. Propionate-producing bacteria include Propionibacterium, Phascolarctobacterium, and Veillonella [5]. Butyrate-producing bacteria include Faecalibacterium [31], Roseburia, Oscillospira, Anaerostipes, Coprococcus [32], and so on. SCFA-producing bacteria produce SCFAs through different pathways.
In this experiment, the relative abundances of the lactate-producing bacterium Lactobacillus panis and butyrate-producing bacterium Oscillospira in the OL group were higher than other two groups. Thus, the concentration of acetate and butyrate in this group was the highest among the three groups. SCFAs production of OH group was lower than OL group. It should be attributed to that microbial α-diversity indexes ACE and Chao1 of OH group were higher than OL group, this means that more bacteria compete for limited glucose as the carbon, and less glucose were fermented into SCFAs. It suggested that the addition of 1% eubiotic lignocellulose was more conducive to increasing the abundance of certain SCFA-producing bacteria and the concentration of SCFAs at 8 weeks in this study, but one or more group less than 1% (e.g., 0.75%, 0.5%, or 0.25%) should be set to determine a more appropriate amount of it in the next experiment.
Effects of eubiotic lignocellulose on growth performance
SCFAs, which are generated by the fermentation of dietary fiber by the gut microbiota, can constitute an important energy source for the host [5]. Acetate is the main way for the body to obtain energy from dietary fiber. The oxidation of acetate provides 0.876 MJ/mol of energy, which can provide 1.2–10% of the total energy per day for human beings [33]. In this study, though adding eubiotic lignocellulose have an significant effect on gut microbiota and the production of SCFAs, the effect of added eubiotic lignocellulose on the growth performance of chickens was not significant. One reason for this was that neither the difference values of the relative abundances of bacteria nor the concentration of acetate and butyrate among groups were great,the other reason was that eubiotic lignocellulose itself has little energy.
Effects of eubiotic lignocellulose on the development of the cecum
Studies have shown that about 95% of butyrate is absorbed into epithelial cells and is rapidly β-oxidized into ketones for ATP synthesis [9]. Butyrate is the first choice of intestinal epithelial cells [34]. Butyrate provides ~ 70% energy for normal intestinal epithelial cells [9] and promotes their proliferation [10]. Therefore, compared with mice fed a fiber-free diet, the colon length of mice fed a fiber-rich diet or normal diet increased [35]. However, in this experiment, although the butyrate content of the OL group was the highest, the difference among the three groups was not great, resulting in there was no significant difference in the development and histomorphology of the cecum.
Conclusions
The addition of eubiotic lignocellulose has little effects on the growth performance and the development and histomorphology of the cecum of chickens, but can increase the abundance of the fiber-degradation bacterium Bacteroides thetaiotaomicron and the fiber digestion. The addition of 1% eubiotic lignocellulose is beneficial to the production of SCFAs at 8 weeks in this study, but one or more group less than 1% should be set to verify the impact and determine a more appropriate amount of addition in the future.
Author contribution
All authors contributed to the study conception and design. Conceptualization, Yu Yang; methodology, Linyue Hou; formal analysis, Baosheng Sun and Linyue Hou; investigation, Baosheng Sun and Linyue Hou; resources, Yu Yang; data curation, Baosheng Sun and Linyue Hou; writing—original draft preparation, Baosheng Sun; writing—review and editing, Baosheng Sun; visualization, Linyue Hou; supervision, Yu Yang; project administration, Yu Yang and Baosheng Sun; funding acquisition, Yu Yang. All authors commented on previous versions of the manuscript and all authors read and approved the final manuscript.
Funding
This research was funded by Construction of Key Discipline in Animal Husbandry of “1331” Engineering in Shanxi Province (J202011315), the Construction of Double First-Class Key Disciplines of Animal Husbandry (J202111303), the Guizhou Science and Technology Planning Project (Qian Science Contract [2020] No. 1Z001), and the Zunyi Innovative Talent Team Training Project (Zunyi Science and Technology Talents [2021] No. 5).
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
Declarations
Ethics approval
This experiment was approved by the Shanxi Agricultural University Animal Experiment Ethics Committee, and the license number was SXAU-EAW-2020-006Chi.001.
Consent to participate
All authors concur with the submission.
Consent for publication
All authors concur with the publication.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Baosheng Sun and Linyue Hou contributed equally to this work.
References
- 1.Filippo CD, Cavalieri D, Paola MD, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. PNAS. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 3.Larsbrink J, Rogers TE, Hemsworth GR, McKee LS, Tauzin AS, Spadiut O, Klinter S, Pudlo K, Urs NA, Koropatkin NM, Creagh AL, Haynes CA, Kelly AG, Cederholm SN, Davies GJ, Martens EC, Brumer H. A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature. 2014;506:498–502. doi: 10.1038/nature12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derrien M, Van HylckamaVlieg JE. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23:354–366. doi: 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Hilpert W, Dimroth P. Conversion of the chemical energy of methylmalonyl-CoA decarboxylation into a Na+ gradient. Nature. 1982;296:584–585. doi: 10.1038/296584a0. [DOI] [PubMed] [Google Scholar]
- 6.Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA: acetate CoA-transferase gene. Environ Microbiol. 2010;12:304–314. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 7.Reese AT, Dunn RR. Drivers of microbiome biodiversity: a review of general rules feces and ignorance. mBio. 2018;9:01294–18. doi: 10.1128/mBio.01294-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, Carling D, Swann JR, Gibson G, Viardot A, Morrison D, Thomas EL, Bell JD. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:1–11. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroismayr A, Roberts SA. Eubiotic lignocellulose–a new tool for swine nutritionists. Int Pig Top. 2009;24:23–25. [Google Scholar]
- 12.Kroismayr A (2014) Choosing the right fibre for poultry-eubiotic lignocellulose. International poultry Production 22:17–19
- 13.Kaoutari AE, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 2013;11:1–9. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 14.Edgar RC. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pruesse E, Quast C, Katrin Fuchs BM, Ludwig W, Peplies J, Glöckner FO. SILVA, a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou L, Sun B, Yang Y. Effects of added dietary fiber and rearing system on the gut microbial diversity and gut health of chicken. Animals. 2020;10:1–22. doi: 10.3390/ani10010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ondov BD, Bergman NH, Phillippy AM. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011;12:385. doi: 10.1186/1471-2105-12-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oksanen J, Blanchet FG, Kindt R, Legendre P, O’Hara RB, Simpson GL, Solymos P, Stevens H (2010) Vegan, community ecology package. R package version 1.17–4. Accessed 23:1-287
- 20.White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol. 2009;5:e1000352. doi: 10.1371/journal.pcbi.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria, potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 24.Suen G, Weimer PJ, Stevenson DM, Stevenson DM, Aylward FO, Boyum J, Deneke J, Drinkwater C, Ivanova NN, Mikhailova N, Chertkov O, Goodwin LA, Currie CR, Mead D, Brumm PJ. The complete genome sequence of Fibrobacter succinogenes s85 reveals a cellulolytic and metabolic specialist. PLoS One. 2011;6:e18814. doi: 10.1371/journal.pone.0018814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, Turroni S, Biagi E, Peano C, Fiori SM, J, Gotti R, Bellis GD, Luiselli D, Brigidi P, Mabulla A, Marlowe F, Henry AG, Crittenden AN Gut microbiome of the Hadza hunter-gatherers. Nat Commun. 2014;5:1–13. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luis AS, Briggs J, Zhang X, Farnell B, Ndeh D, Labourel A, Baslé A, Cartmell A, Terrapon N, Stott K, Lowe EC, McLean R, Shearer K, Schückel J, Venditto I, Ralet MC, Henrissat B, Martens EC, Mosimann SC, Abbott DW, Gilbert HJ. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat Microbiol. 2017;3:210–219. doi: 10.1038/s41564-017-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terrapon N, Lombard V, Gilbert HJ, Henrissat B. Automatic prediction of polysaccharide utilization loci in Bacteroidetes species. Bioinformatics. 2015;31:647–655. doi: 10.1093/bioinformatics/btu716. [DOI] [PubMed] [Google Scholar]
- 28.Leth ML, Ejby M, Workman C, Ewald DA, Pedersen SS, Sternberg C, Bahl MI, Licht TR, LAachmann FL, Westereng B, Hachem MA Differential bacterial capture and transport preferences facilitate co-growth on dietary xylan in the human gut. Nat Microbiol. 2018;3:570–580. doi: 10.1038/s41564-018-0132-8. [DOI] [PubMed] [Google Scholar]
- 29.Gorvitovskaia A, Holmes SP, Huse SM. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome. 2016;4:15. doi: 10.1186/s40168-016-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92:521–526. doi: 10.1079/BJN20041225. [DOI] [PubMed] [Google Scholar]
- 31.Duncan SH, Hold GL, Harmsen HJM, Stewart CS, Flint HJ. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a pro-posal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int J Syst Evol Microbiol. 2002;52:2141–2146. doi: 10.1099/00207713-52-6-2141. [DOI] [PubMed] [Google Scholar]
- 32.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 33.Frost GS, Walton GE, Swann JR, Psichas A, Costabil A, Johnson LP, Sponheimer M, Gibson GR, Barraclough TG. Impacts of plant-based foods in ancestral hominin diets on the metabolism and function of gut microbiota in vitro. MBio. 2014;5:e00853–e914. doi: 10.1128/mBio.00853-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–429. doi: 10.1016/S0016-5085(82)80339-9. [DOI] [PubMed] [Google Scholar]
- 35.Macia T, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, McKenzie CI, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR (2015) Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 6:1–15 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Not applicable.