Abstract
Background:
Spinal cord injury (SCI) results in permanent impairment of motor and sensory functions at and below the lesion site. There is no therapeutic option to the functional recovery of SCI involving diverse injury responses of different cell types in the lesion that limit endogenous nerve regeneration. In this regard, cell replacement therapy utilizing stem cells or their derivatives has become a highly promising approach to promote locomotor recovery. For this reason, the demand for a safe and efficient multipotent cell source that can differentiate into various neural cells is increasing. In this study, we evaluated the efficacy and safety of human polysialylated-neural cell adhesion molecule (PSA-NCAM)-positive neural precursor cells (hNPCsPSA-NCAM+) as a treatment for SCI.
Methods:
One hundred thousand hNPCsPSA-NCAM+ isolated from human embryonic stem cell-derived NPCs were transplanted into the lesion site by microinjection 7 days after contusive SCI at the thoracic level. We examined the histological characteristics of the graft and behavioral improvement in the SCI rats 10 weeks after transplantation.
Results:
Locomotor activity improvement was estimated by the Basso–Beattie–Bresnahan locomotor rating scale. Behavioral tests revealed that the transplantation of the hNPCsPSA-NCAM+ into the injured spinal cords of rats significantly improved locomotor function. Histological examination showed that hNPCsPSA-NCAM+ had differentiated into neural cells and successfully integrated into the host tissue with no evidence of tumor formation. We investigated cytokine expressions, which led to the early therapeutic effect of hNPCsPSA-NCAM+, and found that some undifferentiated NPCs still expressed midkine, a well-known neurotrophic factor involved in neural development and inflammatory responses, 10 weeks after transplantation.
Conclusion:
Our results demonstrate that hNPCsPSA-NCAM+ serve as a safe and efficient cell source which has the potential to improve impaired motor function following SCI.
Keywords: Human pluripotent stem cell, Human embryonic stem cell, PSA-NCAM-positive neural precursor, Spinal cord injury, Transplantation
Introduction
Spinal cord injury (SCI) causes extensive neuronal and glial cell death. It leads to axon demyelination and the loss of axonal connections, resulting in permanent impairment in motor and sensory functions below the lesion site [1]. Spontaneous recovery from SCI is limited owing to the unfavorable environment for axon regrowth in the damaged area [2]. Thus far, there is no therapeutic option for improving functional recovery after SCI other than cell transplantation. Cell replacement therapy may be a potential therapeutic strategy to restore neuronal connectivity and remyelinate damaged axons. Several studies have shown that the transplantation of stem cells or their derivatives could promote locomotor recovery [3–8]. In particular, transplantation of neural precursor cells (NPCs) derived from human embryonic stem cells (hESCs) could lead to partial recovery of impaired neuronal circuits or remyelination of damaged axons and thus, the restoration of locomotor function in injured animals [9].
ESC-derived NPCs can be continuously cultured in the presence of some growth factors, such as basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) [10, 11], and are capable of differentiating into all neural lineages, including neurons, astrocytes, and oligodendrocytes [12]. Because of these properties, NPCs are considered a useful source of cell replacement therapy for neurodegenerative diseases, including SCI. Despite the therapeutic potential for cell therapy to treat SCI, NPCs are known to have tumorigenic potential. There have been several reports of tumorigenesis after NPC transplantation, although undifferentiated pluripotent stem cells (PSCs) were not detected [11, 13–15]. To overcome this issue in previous studies, we obtained highly pure populations of NPCs from hESCs [12] by sorting with a monoclonal antibody recognizing polysialylated-neural cell adhesion molecule (PSA-NCAM) and reported the sorted NPCs had no tumorigenicity [16, 17].
In this study, we describe the potential of highly pure, non-tumorigenic PSA-NCAM + -hNPCs (hNPCsPSA-NCAM+) as a therapeutic cell source for SCI treatment. In a contusive SCI rat model transplanted with hNPCsPSA-NCAM+, the behavioral recovery of the hindlimbs was significantly increased. Immunohistochemical analysis showed that transplanted hNPCsPSA-NCAM+ differentiated into neural lineage cells and did not form non-neural tissues or teratomas. Based on these results, hNPCsPSA-NCAM+ may be an effective and clinically applicable source to treat motor dysfunctions induced by SCI.
Materials and methods
Cell culture and differentiation of hNPCsPSA-NCAM+
hESCs (WA09, WiCell, Madison, WI, USA) were cultured and differentiated into NPCs following as previously described protocols [12, 18]. Briefly, undifferentiated hESCs were maintained with mitotically inactivated feeder cells (STO; ATCC, Manassas, VA, USA) in ES medium (DMEM/F12 medium supplemented with 20% Knock-out Serum Replacement (KSR; Invitrogen, Carlsbad, CA, USA), 1 × non-essential amino acids (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma, St Louis, MO, USA) with 4 ng/ml bFGF (Peprotech, Rocky Hill, NJ, USA). To induce neural differentiation, hESC colonies were cultured as embryoid bodies (EBs) in the presence of 5 μM dorsomorphin (Sigma) and 5 μM SB431542 (Calbiochem, San Diego, CA, USA) for 4 days and then placed on a matrigel-coated (Corning, Corning, NY, USA) culture dish in 1 × N2-containing (Invitrogen) medium supplemented with 20 ng/ml bFGF and 100 μg/ml insulin for an additional 5 days. Once neural rosettes appeared, they were mechanically isolated and passed onto a matrigel-coated culture dish after gentle trituration. The cells were expanded in N2B27 medium (composed of DMEM/F12 medium, 1 × N2 and 1 × B27 (Invitrogen)) supplemented with 20 ng/ml bFGF for another week. PSA-NCAM-positive cells were isolated using Anti-PSA-NCAM-MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) from the expanded neural rosette cells according to the manufacturer’s instructions. The isolated hNPCsPSA-NCAM+ were seeded on a matrigel-coated (Corning) culture dish at a density of ~ 2–3 × 105 cells per cm2.
Spinal cord injury and cell transplantation
Generation of contusive SCI model and cell transplantation were performed as previously described [8]. Briefly, adult male rats (Sprague–Dawley, 200–225 g, Orient Bio, Gyeonggi-do, Korea) were anesthetized under masked-inhalation anesthesia (enflurane: induction 3%; maintenance 2%), and laminectomy was performed at the T9 vertebral level. The exposed dorsal surface of the spinal cord was subjected to a weight-drop impact by dropping a 10 g weight rod from a 25 mm height onto the cord surface using the NYU weight-drop impactor. Manual bladder compression was performed until the animals were urinating independently. Animals that did not recover bladder function were excluded from further analysis.
One week after SCI, the rats received hNPCsPSA-NCAM+ transplantation or vehicle injection. With the rats under masked-inhalation anesthesia, the spinal cord was re-exposed at the SCI level, and a tiny hole was opened in the dura mater over the epicenter of the injury site. The fine tip of a glass capillary (850 μm in O.D. with 100 μm tip diameter) filled with hNPCsPSA-NCAM+ (1 × 105 cells per 5 μl) or PBS vehicle. The hNPCsPSA-NCAM+ or PBS vehicle were administered into the spinal cord for 30 s. The capillary was left in place for 1 min and then slowly withdrawn to avoid any outflow of the cell suspension. For immune suppression, the animals received cyclosporine-A (10 mg/kg, i.p.) every day beginning on the first day prior to transplantation and continuing until the second week after transplantation. All surgeries and experiments were done with the approval of the Institutional Animal Care and Use Committee of Yonsei University College of Medicine, Seoul, Korea (4-2015-1097).
Behavioral assessment
The improvement of locomotor activity for contusive SCI rats who received either hNPCPSA-NCAM+ transplantation (n = 9) or PBS vehicle injection (n = 7) was examined. The rats were trained as described by Basso, Beattie and Bresnahan (BBB), which ranged from complete paralysis (score = 0) to normal locomotion (score = 21) [19]. The rats were gently adapted to the open field, a molded-plastic circular enclosure with a smooth, non-slip floor (90 cm diameter, 30 cm wall height). Once a rat walked continuously in the open field, three blinded examiners conducted a 5-min, preoperative testing session using the BBB locomotor rating scale. Postoperative testing was performed on days 1, 4, and 7 after SCI and then weekly for 10 weeks after transplantation. The BBB scores collected from both hindlimbs were averaged for analysis.
Immunocytochemistry and flow cytometry
Cells were fixed in 4% paraformaldehyde-PBS solution and permeabilized with 0.3% Triton X-100-PBS solution. After blocking with 2% BSA-PBS solution for 1 h at room temperature, the cell was incubated overnight at 4 °C with primary antibodies: SOX1 (1:200, Millipore, Billerica, MA, USA), Nestin (1:1000, Millipore), and Midkine (1:200, R&D Systems, McKinley, MN, USA). Appropriate fluorescence-tagged secondary antibodies from Molecular Probes (Eugene, OR, USA) and Vector Laboratories, Burlingame, CA, USA) were used for visualization. Cells were mounted in DAPI mounting medium (Vector Laboratories), and images were obtained using an Olympus IX71 microscope equipped with a DP71 digital camera or Olympus FSX100 system.
To evaluate the purity of the isolated hNPCsPSA-NCAM+ after 3 ~ 4 passages, the cells were dissociated into single cells and incubated in 1% BSA-PBS solution. The cells were incubated with Anti-PSA-NCAM antibody (1:300, Millipore) and Alexa-Flour 488-conjugated anti-mouse IgG/IgM (Molecular Probes) for secondary antibodies. Flow cytometry was performed using FACSCalibur (BD Biosciences, San Jose, CA, USA) and analyzed using FlowJo software (BD Biosciences).
Immunohistochemistry
In order to evaluate the characteristics of the transplanted cells in vivo, we performed histological analyses after 10-week behavioral test. The animals were anesthetized with urethane (1.5 g/kg, i.p.) and perfused with PBS and 4% paraformaldehyde in PBS. After dissection of spinal cord including lesion site, the spinal cord post-fixed for 4–6 h with 4% paraformaldehyde, immersed overnight in 30% sucrose in 0.1 M PBS, and embedded in an OCT compound (Tissue-Tek, Torrance, CA, USA). Fixed cord segments were cut into 10 μm coronal sections using the cryostat. Every tenth cord section was collected, mounted onto gelatin-coated slides, and stored at − 70 °C before performing immunostaining. Sections incubated overnight at 4 °C with primary antibodies: Tuj1(1:200, BioLegend, San Diego, CA, USA), NeuN (1:200, Millipore), NG2 (1:200, Millipore), Glial fibrillary acidic protein (GFAP, 1:500, Millipore), Nestin (1:400, Millipore), Ki67 (1:150, Leica Biosystems, Buffalo Grove, IL, USA), human nuclear antigen (HNA, 1:100, Millipore), midkine (1:200, R&D System). After visualization with appropriate fluorescence-tagged secondary antibodies, sections were mounted in DAPI mounting medium (Vector Laboratories), and images were obtained using an Olympus IX71 microscope equipped with a DP71 digital camera or Olympus FSX100 system.
Conditioned medium collection from hNPCsPSA-NCAM+ and antibody array
In order to analyze secretome and identify soluble factors responsible for the therapeutic effects of hNPCPSA-MCAM+, conditioned medium was collected from hNPCPSA-MCAM+ under serum-free DMEM with low glucose supplemented with 1 × ITS (insulin, transferrin, and selenium, Invitrogen) during last 24 h of culture, centrifuged at 1,000 × g for 10 min at 4 °C to remove cell debris, filtered through a membrane with a pore size of 0.22 μm in diameter (Millipore), and then frozen in aliquots at − 80 °C before antibody array. The cytokines of conditioned medium from 2 independent batches were profiled by Cytokine Profiling antibody array (SCK100, Full Moon BioSystems, Sunnyvale, CA, USA) following the manufacturer’s instruction, normalized against the blank (medium) and data were analyzed using ExDEGA v.3.2.1 software (eBiogen, Seoul, Korea).
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of Midkine, GDNF (both from Aviva, San Diego, CA, USA), VEGF and TGF-β (both from R&D Systems) in conditioned medium were measured by ELISA following the manufacturer’s instructions. Optical densities were measured at 450 nm using a microplate reader (model 680, Bio-Rad, Hercules, CA, USA). The experiment was repeated at least 4 times.
Statistical analysis
The results from the behavioral tests were analyzed with a two-way repeated measures analysis of variance (ANOVA). When the ANOVAs identified significant differences, pair-wise comparisons between mean values were performed using post hoc Tukey’s tests. Significance was set at p < 0.05. The data are presented as the mean ± SEM.
Results
Generation of hNPCsPSA-NCAM+ from hESCs
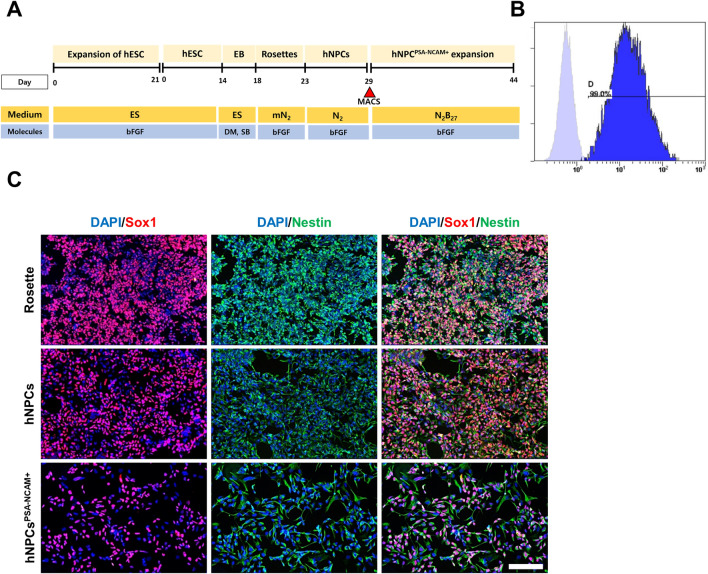
The overall scheme for hNPCPSA-NCAM+ differentiation is shown in Fig. 1A. NPCs were differentiated from hESCs using a previously reported protocol with slight modifications [16]. The hNPCsPSA-NCAM+ isolated with magnetic-activated cell sorting (MACS) were cultured through several passages. Subsequently, the high purity of the hNPCsPSA-NCAM+ was verified using FACS analysis (Fig. 1B, C). Immunostaining results indicated that the hNPCsPSA-NCAM+ expressed markers of human neural stem cells like their parental sources (neural rosettes and unsorted hNPCs).
Fig. 1.
Differentiation and characterization of hNPCsPSA-NCAM+ from human embryonic stem cells. A Schematic diagram of the differentiation conditions for hNPCsPSA-NCAM+. B Flow cytometry analysis for PSA-NCAM in hNPCsPSA-NCAM+. After several passages, the majority were still in the PSA-NCAM + form and had not differentiated into other cells. C Immunocytochemistry analysis of parental neural rosettes, rosette-derived NPCs, and hNPCsPSA-NCAM+. hNPCsPSA-NCAM+ expressed markers for NPCs like their parental cells and had the characteristics of NPCs. Scale bar: 50 μm
Improvement of locomotor activity in SCI rats after transplantation of hNPCsPSA-NCAM+
To test the therapeutic potential of hNPCsPSA-NCAM+ for treating SCI, we transplanted hNPCsPSA-NCAM+ into the lesion sites of SCI rats and measured locomotor activity according to the Basso–Beattie–Bresnahan (BBB) locomotor rating scale. After SCI, the rats had completely lost the function of both hindlimbs (BBB score = 0) (Fig. 2). However, SCI rats showed a behavioral improvement through spontaneous recovery with some movement of the hip, knee, and ankle joints (BBB score = 4–6). At 1 week post-SCI, the rats received hNPCsPSA-NCAM+ (n = 9) or PBS vehicle (n = 7) into the lesion site. The control group spontaneously recovered up to a BBB score of ~ 10 by 4 weeks after PBS treatment; they showed weight-supported stepping using the dorsum of the foot. However, the locomotor activity of the cell transplant group improved more rapidly than that of the control group. Although there was no statistical significance, hNPCsPSA-NCAM+-transplanted rats showed higher BBB scores than the control group during the first 3 weeks after transplantation.
Fig. 2.
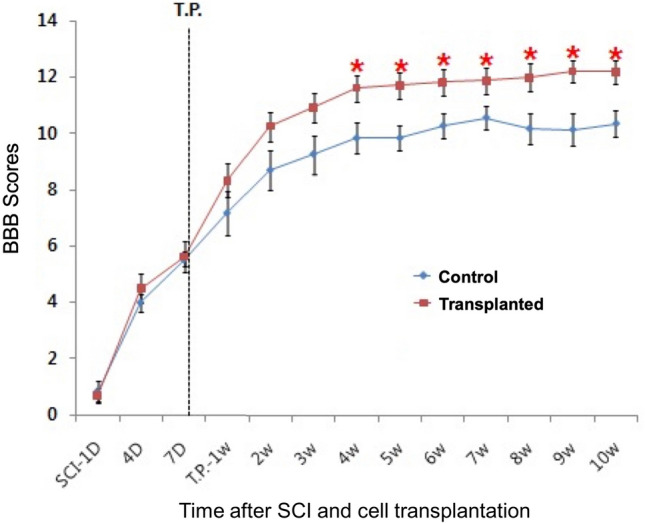
Recovery of hindlimb locomotor function 10 weeks after hNPCPSA-NCAM+ transplantation. The BBB score was assessed for each rat in the two groups: rats receiving cell transplants or PBS, 1 week after SCI. In the behavioral test, the results showed a significant improvement in the hNPCPSA-NCAM+-transplanted SCI rats compared with the control group from 4 weeks after cell transplantation. Error bars indicate s.e.m. *p < 0.05
Four weeks after transplantation, hNPCsPSA-NCAM+-transplanted rats showed significantly higher BBB scores than the control group with maintenance until the 10th week of the last behavioral evaluation (p < 0.05), indicating that the hNPCsPSA-NCAM+ transplantation significantly enhanced locomotor recovery in the SCI rats.
In vivo differentiation of hNPCsPSA-NCAM+ 10 weeks after transplantation
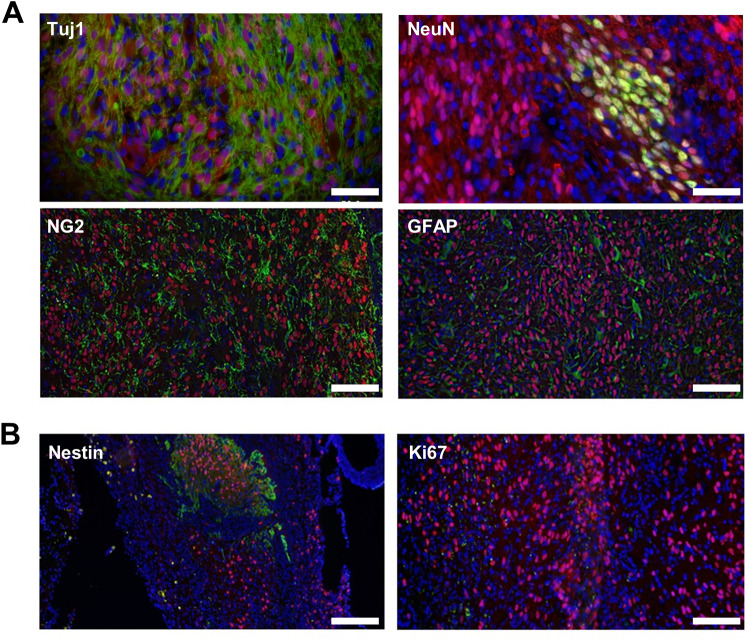
Next, we examined the differentiation of the hNPCsPSA-NCAM+ in the grafts within the injured spinal cords by immunostaining for the following neural lineage cell markers: neuron-specific class III β-tubulin (Tuj1) and neuronal nuclei (NeuN) for neurons, neural-glial antigen 2 (NG2) for oligodendrocytes, and glial fibrillary acidic protein (GFAP) for astrocytes. Ten weeks after transplantation, the grafts showed neural differentiation of hNPCsPSA-NCAM+ within the injured spinal cords. All lineage markers were co-labeled with human nuclear antigen (HNA) and observed throughout the injection area (Fig. 3A), suggesting that the hNPCsPSA-NCAM+ successfully differentiated into neural lineage cells in vivo and became engrafted into the host despite the harsh environment of the damaged tissue. We also identified undifferentiated and proliferating cells by the immunoreactivities of Nestin and Ki67. Nestin-positive cells were still detected in the graft, but Ki67-positive cells were not detected (Fig. 3B), indicating that some transplanted cells remained in undifferentiated, nonproliferating states even 10 weeks after transplantation. Collectively, our data show that the hNPCsPSA-NCAM+ could differentiate into neural lineage cells in the lesion site, which might help to improve recovery from contusion injuries.
Fig. 3.
Histological analysis for in vivo differentiation of transplanted hNPCPSA-NCAM+ in the injured spinal cord 10 weeks post-transplantation. A Coronal section image of a graft showing several types of surviving cells derived from the transplants. Scale bar: 50 μm (upper panel), 100 μm (lower panel). B Some cells remained undifferentiated (Nestin) but few cells were in a proliferating state (Ki67). DAPI for nuclear staining (blue), human nuclear antigen for hNPCPSA-NCAM+ (red). Scale bar: 100 μm
Expression of midkine in hNPCsPSA-NCAM+in vitro and in vivo
Although there was no statistical significance, behavioral recovery from the early stage after transplantation increased more in the transplanted rats than in the control group. Since this period was insufficient for the transplanted hNPCsPSA-NCAM+ to engraft into the host or differentiate into neural lineage cells, these effects might have resulted from factor(s) secreted by the transplanted cells rather than the transplanted cells themselves, and several reports have shown that NPCs secrete neurotrophic factors, which are involved in neuroprotection and neuroregeneration under pathogenic conditions [20–22]. To identify the high-quality candidate biomarkers and/or therapeutic factor(s) secreted by the hNPCsPSA-NCAM+, the protein profile of the hNPCsPSA-NCAM+-conditioned medium was analyzed by antibody array. Of the 310 antibodies used for profiling human cytokines and biomarkers, the top 10 proteins in the hNPCsPSA-NCAM+-conditioned medium compared to that of the blank (serum free medium) were midkine, TIMP metallopeptidase inhibitor 1 (TIMP-1), insulin-like growth factor-binding protein 7 (IGF-BP7), matrix metalloproteinase 2 (MMP-2), matrix metalloproteinase 10 (MMP-10), vascular endothelial growth factor (VEGF), endostatin, fibroblast growth factor 5 (FGF-5), follistatin, and Melanoma-derived growth regulatory protein (MIA) (Fig. 4A). Glial cell-derived neurotrophic factor (GDNF) and transforming growth factor-β (TGF-β) were also detected, but at low levels. Midkine was highly abundant in the antibody array, which was also validated by enzyme-linked immunosorbent assay (ELISA), while VEGF and TGF-β were at moderate levels and GDNF was in trace amounts (Fig. 4B).
Fig. 4.
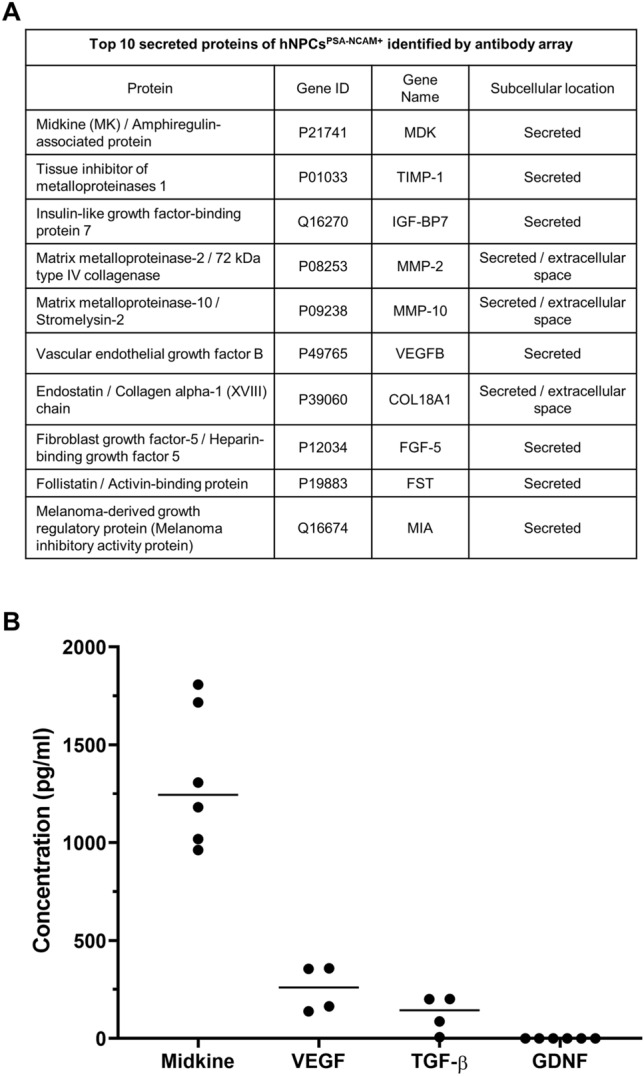
Major secreted proteins of the hNPCPSA-NCAM+ identified by antibody array and ELISA. A Top 10 secreted proteins identified in the hNPCPSA-NCAM+ conditioned medium by antibody array. B Secretion of key cytokines in the conditioned medium of hNPCPSA-NCAM+ by ELISA. Horizontal bars indicate the median values of 4 or 6 independent experiments
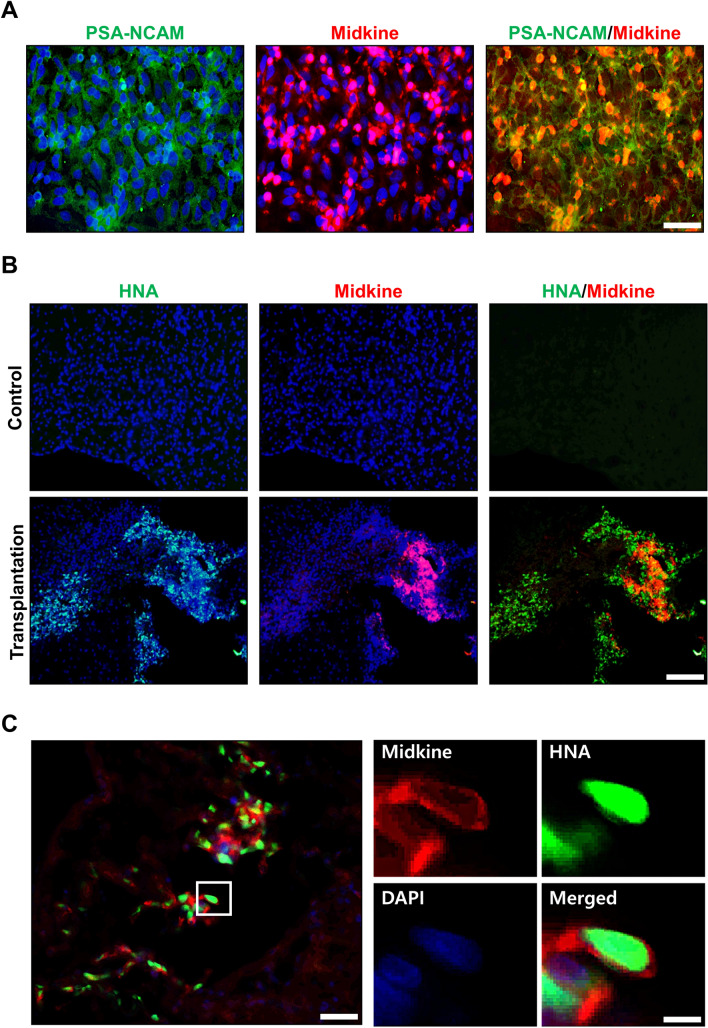
Midkine is a well-known neurotrophic factor, which is expressed in NPCs and plays an important role in regulating neural development and inflammatory responses [23–25]. Midkine is also expressed under pathogenic central nervous system (CNS) conditions, such as cerebral infarct or SCI [26–28], and known to be involved in repairing damaged nerve tissue [25, 27]. We performed immunostaining to confirm the expression of midkine in hNPCsPSA-NCAM+ or its derivatives in vitro and in vivo. Most of the hNPCsPSA-NCAM+ strongly expressed midkine in vitro (Fig. 5A), and some of the transplanted cells still strongly expressed midkine 10 weeks after transplantation in vivo (Fig. 5B, C). These results suggested that the behavioral improvement after cell transplantation might be due to neurotrophic factors secreted from hNPCsPSA-NCAM+ and cell replacement.
Fig. 5.
Expression of midkine in hNPCPSA-NCAM+ in vitro and in vivo. A hNPCsPSA-NCAM+ expressed midkine in vitro. Scale bar: 50 μm. B, C Some of the transplanted hNPCs.PSA-NCAM+ continued to express midkine in the injured spinal cord 10 weeks after transplantation in vivo. Scale bar: 100 μm (B), 50 μm (C, left panel) 10 μm (C, right panel)
Discussion
We showed that the hNPCsPSA-NCAM+ isolated from hESC-derived NPCs were well-integrated and successfully differentiated into three neural lineage cells after transplantation into traumatically injured rat spinal cords. After transplantation of hNPCsPSA-NCAM+, the impaired locomotor activity of SCI rats recovered more than that of the control group. Our in vivo results also showed that all neural lineage makers co-labeled with HNA were frequently observed in the cell injection site. In addition, there was no evidence of tumor formation induced by transplantation. These results demonstrate that hNPCsPSA-NCAM+ are effective and safe cell sources with the therapeutic potential to improve motor dysfunction caused by SCI.
SCI is a fatal event that leads to a permanent impairment of motor and sensory functions resulting from massive cell death in the lesion site, the loss of neural connections, and demyelination of spared axons [1]. NPCs could differentiate into neural cells, such as neurons, oligodendrocytes, and astrocytes, making them an excellent therapeutic candidate for regeneration and protection of neurons at SCI sites [29]. Ten weeks after transplantation, we confirmed that the transplanted hNPCsPSA-NCAM+ had differentiated into neurons, astrocytes, and oligodendrocytes (Fig. 3). Transplant-derived neural cells are thought to contribute to behavioral recovery by relaying damaged neural connections. These relays occur when the host axon extends beyond the injury and synapses with the graft-derived neurons [29–32]. Loss of myelin occurs early after SCI [33, 34], followed by apoptosis of oligodendrocytes [35]. Because demyelination and loss of oligodendrocytes with cell death lead to an impaired neural conduction, replacement of lost oligodendrocytes with cell transplantation and myelination may lead to the recovery of impaired locomotion after SCI [4, 8, 36].
The therapeutic benefits of NPC transplantation include not only the cell replacement effect but also the effect of substances secreted from the cells [37–40]. We identified some cells that remained undifferentiated within the transplanted site. These undifferentiated hNPCsPSA-NCAM+ might strongly secrete trophic factors at the transplanted site, thus reducing secondary damage following SCI. We confirmed the expression of midkine, a representative cytokine secreted from NPCs, in vitro and in vivo (Fig. 4). Midkine is strongly expressed in the nervous system during the embryonic stage [41] and observed at the beginning of neurogenesis [42]. Midkine promotes neurite extension, survival, and migration of embryonic neurons [43–45] and repair of the injured nervous system [23, 25]. This potential mechanism could explain the immediate and early effects after NPC transplantation before the integration of grafted cells into the host.
Despite the potential of using NPCs as a treatment for SCI, NPCs have the risk of tumor formation even in the absence of undifferentiated PSCs [11, 13–15]. We previously reported that hNPCsPSA-NCAM+ do not generate non-neural tissues or teratomas induced by NPCs [16, 17]. Similarly, in this study, no non-neural tissues or mesodermal tumors were found during the 10 weeks after hNPCsPSA-NCAM+ transplantation, confirming that hNPCsPSA-NCAM+ are safer than other cells derived from stem cells.
In conclusion, we suggest that hNPCsPSA-NCAM+ are a safe and effective therapeutic source for the treatment of SCI. hNPCsPSA-NCAM+ are non-tumorigenic cells that can differentiate into various neural lineage cells after transplantation and successfully induce functional recovery through direct or indirect mechanisms. We expect this knowledge to lead to the development of new treatment methods for SCI.
Acknowledgements
We thank Mrs. S.J. Jung for technical assistance. This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI20C0168).
Author’s contribution
D.-H. Kim Ph.D.: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing. H.-J. Cho MS: Collection and/or assembly of data, Data analysis and interpretation. C.-Y. Park Ph.D.: Collection and/or assembly of data, Data analysis and interpretation. M.S. Cho Ph.D.: Conception and design, Data analysis and interpretation, Manuscript writing and Final approval of manuscript. D.-W. Kim Ph.D.: Conception and design, Data analysis and interpretation, Manuscript writing and Final approval of manuscript.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical statement
All animal studies were done with the approval of the Institutional Animal Care and Use Committee of Yonsei University College of Medicine, Seoul, Korea (4-2015-1097).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/26/2022
A Correction to this paper has been published: 10.1007/s13770-022-00493-x
Contributor Information
Myung Soo Cho, Email: tpguy@sbiomedics.com.
Dong-Wook Kim, Email: dwkim2@yuhs.ac.
References
- 1.Noble M, Mayer-Pröschel M, Davies JE, Davies SJ, Pröschel C. Cell therapies for the central nervous system: How do we identify the best candidates? Curr Opin Neurol. 2011;24:570–576. doi: 10.1097/WCO.0b013e32834cd4c9. [DOI] [PubMed] [Google Scholar]
- 2.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 3.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 4.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno-Manzano V, Rodriguez-Jimenez FJ, Garcia-Rosello M, Lainez S, Erceg S, Calvo MT, et al. Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells. 2009;27:733–743. doi: 10.1002/stem.24. [DOI] [PubMed] [Google Scholar]
- 6.McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 7.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DS, Jung SJ, Lee JS, Lim BY, Kim HA, Yoo JE, et al. Rapid generation of OPC-like cells from human pluripotent stem cells for treating spinal cord injury. Exp Mol Med. 2017;49:e361. doi: 10.1038/emm.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest. 2012;122:3824–3834. doi: 10.1172/JCI64124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 11.Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim DS, Lee JS, Leem JW, Huh YJ, Kim JY, Kim HS, et al. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev Rep. 2010;6:270–281. doi: 10.1007/s12015-010-9138-1. [DOI] [PubMed] [Google Scholar]
- 13.Arnhold S, Klein H, Semkova I, Addicks K, Schraermeyer U. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 14.Doi D, Morizane A, Kikuchi T, Onoe H, Hayashi T, Kawasaki T, et al. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson's disease. Stem Cells. 2012;30:935–45. [DOI] [PubMed]
- 15.Seminatore C, Polentes J, Ellman D, Kozubenko N, Itier V, Tine S, et al. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke. 2010;41:153–159. doi: 10.1161/STROKEAHA.109.563015. [DOI] [PubMed] [Google Scholar]
- 16.Kim DS, Lee DR, Kim HS, Yoo JE, Jung SJ, Lim BY, et al. Highly pure and expandable PSA-NCAM-positive neural precursors from human ESC and iPSC-derived neural rosettes. PLoS ONE. 2012;7:e39715. doi: 10.1371/journal.pone.0039715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee DR, Yoo JE, Lee JS, Park S, Lee J, Park CY, et al. PSA-NCAM-negative neural crest cells emerging during neural induction of pluripotent stem cells cause mesodermal tumors and unwanted grafts. Stem Cell Reports. 2015;4:821–834. doi: 10.1016/j.stemcr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang J, Yoo JE, Lee JA, Lee DR, Kim JY, Huh YJ, et al. Disease-specific induced pluripotent stem cells: a platform for human disease modeling and drug discovery. Exp Mol Med. 2012;44:202–213. doi: 10.3858/emm.2012.44.3.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 20.Willis CM, Nicaise AM, Peruzzotti-Jametti L, Pluchino S. The neural stem cell secretome and its role in brain repair. Brain Res. 2020;1729:146615. doi: 10.1016/j.brainres.2019.146615. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Wang C, Chen H, Li L, Ma S, Wang H, et al. Neural stem cell-conditioned medium ameliorated cerebral ischemia-reperfusion injury in rats. Stem Cells Int. 2018;2018:4659159. doi: 10.1155/2018/4659159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Červenka J, Tylečková J, Kupcová Skalníková H, Vodičková Kepková K, Poliakh I, Valeková I, et al. Proteomic characterization of human neural stem cells and their secretome during in vitro differentiation. Front Cell Neurosci. 2021;14:612560. doi: 10.3389/fncel.2020.612560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weckbach LT, Muramatsu T, Walzog B. Midkine in inflammation. ScientificWorldJournal. 2011;11:2491–2505. doi: 10.1100/2011/517152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkler C, Yao S. The midkine family of growth factors: diverse roles in nervous system formation and maintenance. Br J Pharmacol. 2014;171:905–912. doi: 10.1111/bph.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida Y, Sakakima H, Matsuda F, Ikutomo M. Midkine in repair of the injured nervous system. Br J Pharmacol. 2014;171:924–930. doi: 10.1111/bph.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakakima H, Yoshida Y, Muramatsu T, Yone K, Goto M, Ijiri K, et al. Traumatic injury-induced midkine expression in the adult rat spinal cord during the early stage. J Neurotrauma. 2004;21:471–477. doi: 10.1089/089771504323004610. [DOI] [PubMed] [Google Scholar]
- 27.Muramoto A, Imagama S, Natori T, Wakao N, Ando K, Tauchi R, et al. Midkine overcomes neurite outgrowth inhibition of chondroitin sulfate proteoglycan without glial activation and promotes functional recovery after spinal cord injury. Neurosci Lett. 2013;550:150–155. doi: 10.1016/j.neulet.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida Y, Goto M, Tsutsui J, Ozawa M, Sato E, Osame M, et al. Midkine is present in the early stage of cerebral infarct. Brain Res Dev Brain Res. 1995;85:25–30. doi: 10.1016/0165-3806(94)00183-z. [DOI] [PubMed] [Google Scholar]
- 29.Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 30.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadoya K, Lu P, Nguyen K, Lee-Kubli C, Kumamaru H, Yao L, et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016;22:479–487. doi: 10.1038/nm.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu P, Woodruff G, Wang Y, Graham L, Hunt M, Wu D, et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron. 2014;83:789–796. doi: 10.1016/j.neuron.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Z, Jin Y. Intrinsic control of axon regeneration. Neuron. 2016;90:437–451. doi: 10.1016/j.neuron.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42:549–563. doi: 10.1038/sj.sc.3101670. [DOI] [PubMed] [Google Scholar]
- 35.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 36.Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, et al. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol. 2014;117:54–72. doi: 10.1016/j.pneurobio.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci USA. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnabé-Heider F, Frisén J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Kumamaru H, Ohkawa Y, Saiwai H, Yamada H, Kubota K, Kobayakawa K, et al. Direct isolation and RNA-seq reveal environment-dependent properties of engrafted neural stem/progenitor cells. Nat Commun. 2012;3:1140. doi: 10.1038/ncomms2132. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura S, Yasuda A, Iwai H, Takano M, Kobayashi Y, Nori S, et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. doi: 10.1186/1756-6606-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadomatsu K, Huang RP, Suganuma T, Murata F, Muramatsu T. A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J Cell Biol. 1990;110:607–616. doi: 10.1083/jcb.110.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan QW, Muramatsu T, Kadomatsu K. Distinct expression of midkine and pleiotrophin in the spinal cord and placental tissues during early mouse development. Dev Growth Differ. 2000;42:113–119. doi: 10.1046/j.1440-169x.2000.00497.x. [DOI] [PubMed] [Google Scholar]
- 43.Muramatsu H, Muramatsu T. Purification of recombinant midkine and examination of its biological activities: functional comparison of new heparin binding factors. Biochem Biophys Res Commun. 1991;177:652–658. doi: 10.1016/0006-291x(91)91838-4. [DOI] [PubMed] [Google Scholar]
- 44.Muramatsu H, Shirahama H, Yonezawa S, Maruta H, Muramatsu T. Midkine, a retinoic acid-inducible growth/differentiation factor: immunochemical evidence for the function and distribution. Dev Biol. 1993;159:392–402. doi: 10.1006/dbio.1993.1250. [DOI] [PubMed] [Google Scholar]
- 45.Michikawa M, Kikuchi S, Muramatsu H, Muramatsu T, Kim SU. Retinoic acid responsive gene product, midkine, has neurotrophic functions for mouse spinal cord and dorsal root ganglion neurons in culture. J Neurosci Res. 1993;35:530–539. doi: 10.1002/jnr.490350509. [DOI] [PubMed] [Google Scholar]