Abstract
The human bocavirus (HBoV) is an agent of upper and lower respiratory infections, affecting mainly children under 5 years of age. Community-acquired pneumonia (CAP) is an important public health problem in developing countries, representing one of the main causes of hospitalizations and deaths in children. The aim of this study was to describe the prevalence of HBoV and the clinical and epidemiological characteristics in children diagnosed with CAP. For this purpose, nasopharyngeal aspirates were collected from 545 children aged 0 to 60 months diagnosed with CAP between January 2013 and December 2014 in a reference pediatric hospital in Fortaleza, Ceará, Brazil. The samples were subjected to PCR for detection of HBoV and parainfluenza 4 (PIV4) and indirect immunofluorescence for detection of respiratory syncytial virus (RSV), adenovirus (AdV), influenza A and B (FLU A and FLU B), and parainfluenza 1, 2, and 3 (PIV1, PIV2, PIV3). Clinically, most CAP were non-complicated (487/545; 89.3%); however, 10.7% (58/545) of children were treated in the ICU/resuscitation sector. Among the total samples analyzed, 359 (65.8%) were positive for at least one virus surveyed and 105 (19.2%) samples had two or more viruses. HBoV was detected in 87 samples (15.9%), being the second most prevalent virus. RSV, AdV, FLU A, FLU B, and PIV 1–3 were detected in 150 (27.5%), 45 (8.2%), 30 (5.5%), 3 (0.5%), and 131 (24%) samples, respectively. The age average was 12.1 months in children infected with HBoV, and the most frequent symptoms were dyspnea and cough. In addition, 90.6% of HboV-positive children received antibiotics as empirical treatment. HBoV did not show any circulation pattern; however, it seemed to be more frequent in the first half of the year, totaling 68.9% of the cases. HBoV is a frequent agent of pneumonia in the child population studied.
Keywords: Community-acquired pneumonia, Human bocavirus, Respiratory viruses, Children, Viral pneumonia, Epidemiology
Introduction
Pneumonia is an important infection that affects children worldwide, and was responsible for 800,694 deaths among children under 5 years of age in 2017 — nearly 15% of all deaths in this population that year [1]. In Brazil, the estimated incidence of pneumonia among children under 5 years of age in 2015 was approximately 150/1000 inhabitants. It is believed that the country has the highest incidence of infantile pneumonia in Latin America [2]. Although community-acquired pneumonia is clinically managed without laboratorial identification of its etiology, the prevalence of viruses in these cases reaches up to 66% [3].
The human bocavirus (HBoV) belongs to the Parvoviridae family, and it is classified in the genus Bocaparvovirus. They are small viruses with a non-enveloped capsid ranging from about 23 nm to about 28 nm in diameter that contains a single-strand DNA genome [4]. HBoV was described in 2005 in respiratory secretions of children with lower respiratory infections [5], and, since its discovery, it has been detected worldwide in respiratory and fecal samples from children with respiratory and gastrointestinal infections [6].
The pathogenicity of HBoV is still questionable because this agent can be detected in asymptomatic individuals, and it is quite common in co-infections with other infectious agents during respiratory infections and/or gastroenteritis [7, 8]. However, Jiang et al. [6] reported that respiratory infections in which only HBoV was detected showed a higher viral load and this result was associated with a greater severity of the disease [9]. A study with primary human airway epithelial cells (HAE-ALI) showed that HBoV can replicate in cells without high replicative activity causing the loss of cellular integrity, loss of cilia, rupture of cell junctions, hypertrophy of epithelial cells, and persistent infection, as well as cell death induced by caspase-1 activation. These findings suggest that the pathogenesis of HBoV is related to damage to aerial epithelium cells [10].
The present study aimed to estimate the prevalence of HBoV in children under 5 years diagnosed with pneumonia in Fortaleza, Ceará (Northeast Brazil), and to describe the clinical-epidemiological profile of HBoV infections alone and in co-infection with other respiratory viruses.
Material and methods
Location
The study was conducted at the Albert Sabin Children Hospital (HIAS), located in the city of Fortaleza (3° 43′ S-38° 32′ W), State of Ceará, Brazil. The hospital is a referral institution for children and adolescent patients in the State of Ceará and offers public emergency service, pediatric intensive care unit, and specific services for diagnosis and therapy. Each month, on average, 17,000 outpatient consultations and 830 hospitalizations are performed at the HIAS.
The Ceará State has an estimated population of 9,240,580 in 2020; Fortaleza has the highest population density among all Brazilian capitals, 7786.44 inhabitants per kilometer. According to official data, the Ceará State has a Gini coefficient of 0.56 and nearly half of its inhabitants live with less than 50% minimum wage. Nearly 18% have daily earnings below US$ 1.9, and are regarded as people living in extreme poverty by the World Bank. Economy is roughly based on agriculture (5%), industry (18%), and services (78%). The tropical semiarid climate comprises 80% of the State area; the climate in Fortaleza city is classified as tropical sub-humid climate-coastal region [11].
Study design, ethical approval, and definitions
This is a single-center, retrospective study conducted at HIAS. Ethical approval was obtained by the institutional ethics committee (approval number 070/09), and written informed consent was obtained from the parents of the participating children. Patients aged between 0 and 60 months diagnosed with pneumonia at the HIAS from January 2013 to December 2014 were enrolled in the study. Only patients that met the inclusion criteria were included in the study. CAP diagnosis was based on clinical findings (cough, fever, tachypnea with or without dyspnea, tachydyspnea, hypoxemia) and radiological findings (condensation, interstitial infiltrate and pleural effusion) [12]. Children hospitalized in the previous 14 days of pneumonia onsetting, with a history of aspiration pneumonia or nosocomial pneumonia diagnosis, were excluded from the study. A single nasopharyngeal aspirate (NPA) sample was collected shortly after the diagnosis of pneumonia. NPA samples were aspirated through a catheter inserted into the nostril with a depth of 5 to 7 cm and connected to a syringe. All samples were collected in viral transport medium and transported at 4 to 8 °C to the laboratory, where they were processed [13].
Data collection
Clinical-epidemiological data were collected from the medical records using a structured questionnaire comprising closed-ended questions. The main variables studied were gender, age at admission, hospital care sector, risk factors (acute respiratory infection cases in the family, passive smoking, attendance to daycare or school, breastfeeding, prematurity, immunosuppression), and comorbidities (heart diseases, pneumopathies, neuropathies, asthma).
Virus detection
The detection of HBoV and parainfluenza 4 (PIV4) was carried out by conventional PCR. The viral nucleic acid was extracted using the AxyPrep™ Body Fluid Viral DNA/RNA Miniprep Kit (AxioGen Biotechnology, CA, USA), following the manufacturer’s recommendations. HBoV NS1 gene amplification was performed with the primers F-5′-TATGGCCAAGGCAATCGTCCAAG-3′, R-5′-GCCGCCTGAACATGAGAAACAGA-3′. Reactions were performed in a thermocycler (MultiGene™, Labnet, New Jersey, USA) with an initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C for 45 s, and extension at 72 °C for 45 s, and a final extension at 72 °C for 5 min [12]. For the detection of PIV 4, cDNA synthesis was performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems™, MA, EUA), following the manufacturer’s recommendations. Phosphoprotein P genes were targeted for PIV 4 detection by nested PCR reaction with primers F-5′-CTGAACGGTTGCATTCAGGT-3′ R-5′-AGGACTCATTCTTGATGCAA-3′ in the first reaction and F-5′-AAAGAATTAGGTGCAACCAG-3′ R-5′-GTGTCTGATCCCATAAGCAGC-3′ in the second PCR reaction following the protocol by Aguilar et al. [13]. PCR products were analyzed by 1.5% agarose gel electrophoresis. The expected product sizes of amplified fragments were 291 bp for the HBoV NS1 gene [12] and 246 bp for the PIV-4 phosphoprotein P gene[14] PCR reactions which were performed with strict protocols to avoid contamination, such as unidirectional workflow (pre-PCR and post-PCR in separate rooms), aseptic cleaning carried out periodically before and after reactions, and inclusion of negative and positive controls.
Respiratory syncytial virus (RSV), adenovirus (AdV), influenza A (FLU A) and B (FLU B), and parainfluenza 1 (PIV 1), 2 (PIV 2), and 3 (PIV 3) were detected by indirect immunofluorescence assay (IFA) using the Respiratory Panel I Viral Screening and Identification (Chemicon International, Temecula, USA), following the manufacturer’s recommendations.
Statistical analysis
Comparative statistical analyzes were performed in the following stages: (1) virus-positive and virus-negative patient groups were used; and (2) groups of patients positive for each of the nine virus species surveyed were used. For data analysis, Fisher’s exact test, Spearman’s correlation, and odds ratio were used. Significance was defined by a p value of less than 0.05. Data was analyzed by using SPSS Statistics for Windows, version 17.0.
Results
A total of 545 patients were enrolled in the study. The majority of children recruited in the study were 0–12 months old (357/545; 65.5%), 317 being male (58.2%) and 228 female (41.8%). Children positive for at least one viral species came mostly from emergency (170/359; 47.3%) and infirmary (150/359; 41.8%) wards. We found no significant differences regarding age, gender, and hospital sector. Table 1 shows the distribution of age, sex, and care sector for children with CAP enrolled in the present study.
Table 1.
Clinical, epidemiological, and demographic characteristics of the studied population. Number of patients for each variable and their respective percentual values are shown below
| Variables | General population (n = 545)* | Positive population (n = 359)* | Negative population (n = 186)* | HBoV (n = 43)* | RSV (n = 87)* | AdV (n = 22)* | PIV (n = 89)* | FLU (n = 13)* | Coinfections (n = 105)* |
| Gender | |||||||||
| Male | 317/58.2 | 206/57.4 | 111/59.7 | 24/55.8 | 52/59.8 | 11/50 | 49/55 | 9/69.2 | 61/58.1 |
| Female | 228/41.8 | 153/42.6 | 75/40.3 | 19/44.2 | 35/40.2 | 11/50 | 40/45 | 4/30.8 | 44/41.9 |
|
Age (months) Mean / median |
13.3/8 | 11.5/7 | 16.8/10 | 12.1/8 | 8.9/4 | 13.3/9 | 13.1/8 | 13.8/8 | 11.2/8 |
| Range | 1–60 | 1–58 | 1–60 | 1–58 | 1–56 | 1–34 | 1–55 | 1–41 | 1–55 |
| Age group (months) | |||||||||
| 0 to 12 | 357/65.6 | 248/69 | 109/58.6 | 28/65.2 | 68/78.1 | 13/59.1 | 59/66.2 | 9/69.2 | 71/67.7 |
| 13 to 24 | 95/17.4 | 66/18.3 | 29/15.6 | 11/25.6 | 11/12.6 | 6/27.2 | 15/16.9 | 1/7.7 | 22/21.9 |
| 25 to 36 | 38/7.0 | 24/6.9 | 14/7.5 | 2/4.6 | 3/3.5 | 3/13.7 | 7/7.9 | 1/7.7 | 8/7.6 |
| 37 to 48 | 36/6.6 | 16/4.4 | 20/10.8 | 1/2.3 | 3/3.5 | 0 | 7/7.9 | 2/15.3 | 3/2.9 |
| 49 to 60 | 19/3.4 | 5/1.4 | 14/7.5 | 1/2.3 | 2/2.3 | 0 | 1/1.1 | 0 | 1/0.9 |
| Hospital care sector | |||||||||
| Emergency | 279/51.2 | 170/47.3 | 109/58.6 | 26/60.5 | 39/44.8 | 14/63.6 | 49/55.1 | 1/7.7 | 41/39.1 |
| Wards | 208/38.2 | 150/41.8 | 58/31.2 | 16/37.2 | 37/42.6 | 8/36.4 | 28/31.4 | 11/84.6 | 50/47.6 |
| Resuscitation | 49/9 | 33/9.2 | 16/8.6 | 1/2.3 | 9/10.3 | 0 | 9/10.1 | 1/7.7 | 13/12.4 |
| ICU | 9/1.6 | 6/1.7 | 3/1.6 | 0 | 2/2.3 | 0 | 3/3.4 | 0 | 1/0.9 |
| Risk factor | |||||||||
| Not detected | 161/29.5 | 110/30.7 | 51/27.4 | 8/18.6 | 27/31 | 7/31.8 | 32/35.9 | 5/38.4 | 31/29.5 |
| ARI cases in the family | 233/42.7 | 148/41.2 | 85/45.7 | 21/48.8 | 36/41.3 | 9/40.9 | 31/34.8 | 5/38.4 | 46/43.8 |
| Passive smoking | 138/25.3 | 87/24.2 | 51/27.4 | 16/37.2 | 17/19.5 | 5/22.7 | 21/23.6 | 4/30.7 | 24/22.8 |
| Attendance to daycare or school | 31/5.6 | 15/4.2 | 16/8.6 | 2/4.6 | 2/2.3 | 2/9 | 5/5.6 | 0 | 4/3.8 |
| No breastfeeding | 96/17.6 | 70/19.5 | 26/14 | 9/2.9 | 19/21.8 | 3/13.6 | 15/16.8 | 2/15.3 | 22/20.9 |
| Immunosuppression | 4/0.7 | 4/1.1 | 0 | 1/2.3 | 0 | 0 | 1/1.1 | 0 | 1/0.9 |
| Prematurity | 49/9 | 38/10.6 | 11/5.9 | 5/11.6 | 10/11.4 | 1/4.5 | 11/12.3 | 1/7.7 | 10/9.5 |
| Comorbidities | |||||||||
| Not detected | 402/73.7 | 263/73.2 | 139/74.7 | 33/76.7 | 64/73.5 | 13/59.1 | 66/74.1 | 5/38.4 | 82/78 |
| Heart disease | 76/13.9 | 51/14.2 | 25/13.4 | 5/11.6 | 12/13.7 | 6/27.2 | 10/11.2 | 6/46.1 | 12/11.4 |
| Pneumopathies | 7/1.2 | 5/1.4 | 2/1 | 1/2.3 | 0 | 1/4.5 | 1/1.1 | 0 | 2/1.9 |
| Neuropathy | 44/8 | 31/8.6 | 13/7 | 2/4.6 | 8/9.1 | 4/18.1 | 5/5.6 | 1/7.7 | 9/8.5 |
| Asthma | 15/2.7 | 6/1.7 | 9/4.8 | 1/2.3 | 2/2.3 | 0 | 3/3.4 | 0 | 0 |
| Treatment | |||||||||
| Antipyretic | 253/46.2 | 171/47.6 | 82/44 | 20/46.5 | 30/34.4 | 12/54.5 | 49/55 | 5/38.4 | 55/52.3 |
| Aerosol | 369/67.7 | 250/69.6 | 119/63.9 | 37/86 | 52/59.7 | 12/54.5 | 62/69.6 | 6/46.1 | 81/77.1 |
| Bronchodilators | 11/2 | 5/1.4 | 6/3.2 | 0 | 2/2.3 | 1/4.5 | 1/1.1 | 0 | 1/0.9 |
| Corticoids | 221/40.5 | 152/42.3 | 69/37 | 23/53.4 | 36/41.3 | 6/27.2 | 39/43.8 | 4/30.7 | 44/41.9 |
| Antibiotic | 400/73.3 | 277/77.1 | 123/66.1 | 39/90.6 | 58/66.6 | 13/59.1 | 70/7.8 | 8/61.5 | 89/87.7 |
| Signs and symptoms | |||||||||
| Cough | 436/80.0 | 306/85.2 | 130/69.9 | 42/97.7 | 63/72.4 | 16/72.7 | 86/96.7 | 7/53.8 | 92/87.6 |
| Dyspnea | 421/77.2 | 286/79.7 | 135/72.6 | 41/95.3 | 59/67.9 | 15/68.2 | 76/85.4 | 8/61.5 | 87/82.8 |
| Coryza | 321/58.9 | 209/58.2 | 112/60.2 | 26/60.5 | 36/41.4 | 11/0.5 | 59/66.3 | 5/38.5 | 42/40.0 |
| Fever | 318/58.3 | 211/58.8 | 107/57.5 | 30/69.8 | 41/47.1 | 12/54.5 | 56/63.0 | 5/38.5 | 67/63.8 |
| Nasal obstruction | 255/46.8 | 171/47.6 | 84/45.2 | 20/46.5 | 38/43.7 | 9/41 | 41/46.1 | 4/30.8 | 59/56.2 |
| Sneezing | 201/36.9 | 140/39.0 | 61/32.8 | 21/48.4 | 36/41.4 | 5/22.7 | 28/31.5 | 3/23.1 | 47/44.8 |
| Anorexia | 125/23.0 | 84/23.4 | 41/22.0 | 11/25.6 | 18/2.7 | 7/31.8 | 23/25.9 | 1/7.7 | 24/22.8 |
| Vomit | 109/20.0 | 62/17.3 | 47/25.3 | 8/18.6 | 12/13.8 | 5/22.7 | 15/16.8 | 1/7.7 | 21/20.0 |
| Diarrhea | 57/10.4 | 41/11.4 | 16/8.6 | 7/16.3 | 12/13.8 | 2/9.0 | 6/6.7 | 1/7.7 | 13/12.4 |
| Cyanosis | 23/4.22 | 17/4.7 | 6/3.2 | 3/7.0 | 3/3.4 | 0 | 3/3.4 | 1/7.7 | 7/6.7 |
| Wheezing | 18/3.3 | 15/4.2 | 3/1.7 | 2/4.6 | 6/6.9 | 0 | 2/2.2 | 1/7.7 | 4/3.8 |
| Seizure | 17/3.1 | 11/3.1 | 6/3.2 | 0 | 4/4.6 | 0 | 1/1.1 | 0 | 6/5.7 |
| Rash | 4/0.7 | 4/1.1 | 0 | 0 | 0 | 0 | 3/3.4 | 1/7.7 | 0 |
| Conjunctivitis | 2/0.4 | 1/0.3 | 1/0.5 | 0 | 0 | 0 | 0 | 0 | 1/0.9 |
| Rattling | 1/0.2 | 1/0.3 | 0 | 0 | 0 | 0 | 1/1.1 | 0 | 0 |
HBoV human bocavirus, RSV respiratory syncytial virus, AdV adenovirus, PIV parainfluenza virus 1–4, FLU influenza virus, ARI acute respiratory infection, ICU intensive care unit
*Numbers in parenthesis correspond to the number of patients in each category
Most children with CAP had at least one risk factor (386/545; 70.8%), and 35.4% (137/386) were exposed to more than one risk factor. The most observed risk factor in children with CAP were the presence of acute respiratory infection in family members and secondhand smoking, detected in 42.7% (233/545) and 25.3% (138/545) children, respectively. The majority of the studied population did not present any underlying disease (402/545; 73.7%). The most common underlying diseases seen in this study were heart disease followed by neuropathy, observed in 11.9% (76/545) and 8% (44/545) of the children, respectively (Table 1). Respiratory diseases caused by FLU A were more frequent in children with heart disease (p < 0.003 [95% CI 1.58–16.1]); associations between other virus and comorbidities were not seen.
As for treatment, 77.1% (277/359) of the positive children and 61.1% (123/186) of the negative children for any of the viruses studied in their NPA received antibiotic therapy. Antibiotics were administered in 90.6% (39/43) of children who had only HBoV infection. In addition, of 105 cases of coinfections, 84.7% (89/105) of children received antibiotics as treatment. Aerosol and corticoids were also frequent treatments used in children positive for HBoV, 86% (37/43) and 53.4% (23/43), respectively. We found no significance differences regarding treatment of HBoV-positive and negative patients. Details are shown in Table 1. Children with CAP had cough (437/545; 80.1%), dyspnea (421/545; 77.2%), and rhinorrhea (322/545; 59%) as the most common manifestations. Gastrointestinal manifestations, such as anorexia, vomiting, and diarrhea, were also observed in children with pneumonia (Table 1). None of the clinical manifestations could be associated with HBoV infection; the onset of wheezing was associated in children with RSV (p < 0.03 [95% CI 1.22–4.72]). No significant differences among risk factors and underlying diseases were detected.
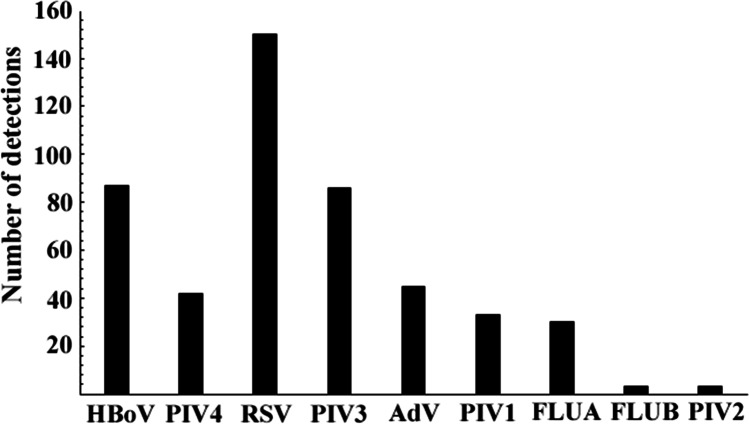
Of the 545 children studied, 359 (65.8%) were positive for at least one respiratory virus and 105 (19.2%) had coinfections, including 91 (86.7%) children with two viruses and 14 (13.3%) children with more than two viruses detected; therefore, using both techniques, 459 viruses could be detected. A total of 87 samples were positive for HBoV (15.9%; 87/545), 43 (49.5%; 43/87) being single detections. PIV4 was detected in 42 (7.6%; 42/545) samples, 17 (40.4%; 17/42) being in single detections. By way of IFA, a total of 294 samples were positive for at least one virus. With this technique, it was possible to detect the following respiratory viruses: RSV (n = 150; 27.4%), PIV3 (n = 86; 15.7%), AdV (n = 45; 8.2%), PIV1 (n = 33; 6%), FLUA (n = 30; 5.5%), FLU B (n = 3; 0.5%). and PIV2 (n = 3; 0.5%). A general picture of the prevalence of the detected viruses, considering single infections and coinfections using both techniques, in the study is shown in Fig. 1.
Fig. 1.
Number of virus detections in children diagnosed with pneumonia, considering single infections and coinfections, by conventional PCR and indirect immunofluorescence assay. HBoV = human bocavirus; RSV = respiratory syncytial virus; AdV = adenovirus; PIV1 = parainfluenza virus 1; PIV2 = parainfluenza virus 2; PIV3 = parainfluenza virus 3; PIV4 = parainfluenza virus 4; FLUA = influenza A; FLUB = influenza B
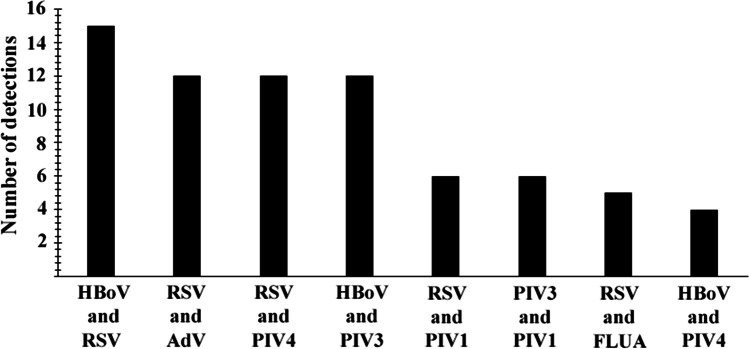
HBoV was detected in 44 (50.5%; 44/87) co-infections with other viral agents, 15 (34%; 15/44) with RSV, 12 (27.2%; 12/44) with PIV3, and five (11.3%; 5/44) with PIV4 as the most frequent agents. RSV was the most frequent virus detected in co-infections, corresponding to 63 (60%; 63/105) of the 105 positive samples with two or more viruses. The most frequent agents detected in co-infection with RSV were HBoV (23.8%; 15/63), AdV (11.4%; 12/63), and PIV4 (11.4%; 12/63). In addition, there was a case in which RSV, FLUA, PIV 1, and HBoV were detected in a single sample. Details regarding viral co-infections are shown in Fig. 2.
Fig. 2.
Number of virus coinfections detected in children diagnosed with pneumonia by conventional PCR and indirect immunofluorescence assay. HBoV = human bocavirus; RSV = respiratory syncytial virus; AdV = adenovirus; PIV1 = parainfluenza virus 1; PIV2 = parainfluenza virus 2; PIV3 = parainfluenza virus 3; PIV4 = parainfluenza virus 4; FLUA = influenza A; FLUB = influenza B
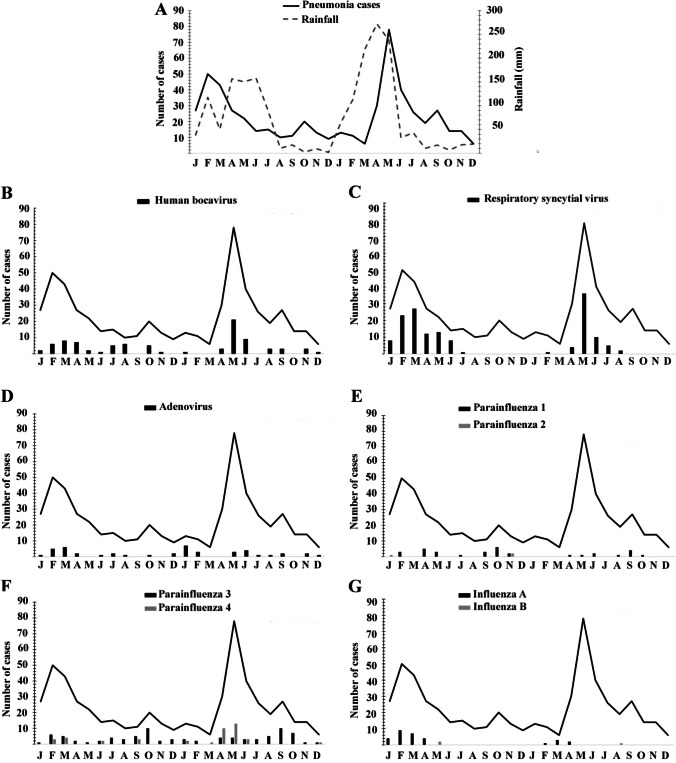
HBoV was detected throughout the year, but the majority of cases occurred in May 2014 (n = 21; 24.1%). RSV infections occurred only in the first half of the year, and the majority of cases occurred during February and May 2013 and May and June 2014. FLU A was also detected during the first half of the year. AdV circulated throughout the year, with a profile similar to HBoV. Most cases of PIV4 infections occurred from January to July, although the virus was also detected during September and December. The majority of cases of PIV3 infections occurred during the second half of the year. General circulation of the surveyed viruses is shown in Fig. 3.
Fig. 3.
Distribution of the studied viruses during the 24-month period. A Occurrence of pneumonia cases (bold line) and rainfall in millimeters (dashed line). Monthly occurrence of B human bocavirus; C respiratory syncytial virus; D adenovirus, E parainfluenza 1 (black bars) and parainfluenza 2 (gray bars); F parainfluenza 3 (black bars) and parainfluenza 4 (gray bars); G influenza A (black bars) and influenza B (gray bar). y-axis represents number of cases, x-axis represents months (2013–2014)
Discussion
The present study highlights the occurrence of HBoV infection among children under 5 years with PAC in a tropical semi-arid area. The detection rate of HBoV found in this study (15.9%) is similar to previous studies conducted in Brazil with children under 5 years of age presenting acute respiratory symptoms. By way of conventional or real-time PCR, the prevalence of HBoV in such studies varied from 2.44% to 23% [15–21].
More than 50% of the cases of HBoV in the present study were in co-infections with other viruses. High prevalence of HBoV co-infections has been detected, and according to previous studies they can reach up to 75% [22, 23]; recently, it was shown to 80% of co-infections with other respiratory viruses in infants from Saudi Arabia [24] and China [25], respectively. In addition, HBoV can be detected in asymptomatic children [15]. HBoV co-infections and casual detections may be related to persistent infection in tonsils and adenoids [26]. In a study carried out in patients with chronic tonsillar diseases, HBoV was detected in 31.1% of the samples, suggesting that these lymphoid tissues are important sites for replication of this agent, and can serve as a reservoir and thus remain detectable by molecular methods even weeks after the symptoms of the disease disappear [27]. A study conducted in Belgium, where children who had respiratory infections with HBoV were followed and samples taken at different times, showed that HBoV could be detected 97 days after the first detection, as it was with a child [28].
Although the pathogenic role of HBoV is not clear, clinical and experimental evidences have pointed that the agent is not an “innocent bystander” [16, 23]. In a prospective study performed between 2005 and 2013, HBoV was detected as single pathogen in 25% of positive population with virus respiratory diseases [23] Such infections were characterized by high fever, wheezing, pneumonia, hypoxia, moderate leukocytosis, and elevated C‐reactive protein [23]. In addition, high HBoV virus load is also associated with the severity of respiratory infections [9] (JIANG et al. 2016) and longer hospitalization [10]. The cytopathic effect in human airway epithelial cell cultures induced by HBoV confirms the pathogenic potential of this agent. However, one must keep in mind that non-tested viruses could not be excluded in the negative cases as well as in HBoV mono-infection.
Respiratory infections by HBoV are more common in children and are generally uncomplicated; however, there are cases of these infections in which intensive care is required [29]. In a cohort study in the UK [30], 9 of 29 (31%) of patients with HBoV mono-infection needed intensive care, including mechanical ventilation. A study conducted in Colombia with ninety-one adults with severe respiratory infections showed that 19.3% (5 of 26) of patients with HBoV infection died [31].
The majority of cases of pneumonia detected in the present study were non-complicated, but nearly one-third of children infected with of HBoV (single detection) required hospitalization. Previous studies have shown that HBoV seems to be associated with wheezing, during or after respiratory infection [28, 32], and even asthma [33]. In general, viral pneumonia is often associated with milder conditions, while bacterial pneumonia is associated with more severe conditions [34].
It was not possible to detect the presence of bacterial pathogens in the studied population, although it was seen that the majority of children positive for at least one virus agent was treated with antibiotics. The use of empirical antibiotics is common in cases of CAP worldwide mainly due to the difficulty in obtaining microbiological results, making it difficult to use specific drugs. Following this, the Brazilian Guidelines on Pulmonology and Tisiology and North and Latin American, European, and British health guidelines recommend the rational use of relatively narrow-spectrum antibiotics as empiric treatment [35, 36]. Although guided, the use of antibiotics not directed to specific targets favors the antimicrobial resistance [37, 38]. Hospitals should improve virus identification protocols in order to reduce the indiscriminate and excessive use of antibiotics in children with CAP.
Several respiratory viruses are able of participating in simultaneous infections [39], and children seems to be more likely to harbor viral co-infections. Investigators have found that children aged less than three years has higher propensity for simultaneous viral infections [40, 41]. Another DNA virus that causes respiratory infections has characteristics similar to HBoV; AdV is also frequently detected in co-infections [42] and can persist in adenoids and palatine tonsils [43]. Proenca-Modena and colleagues detected AdV in 52.8% (95/180) of tissue fragment samples, nasopharyngeal secretions and blood from children with chronic adenotonsillar diseases [44].
In this study, due to the limited number of patients studied, it was not possible to find any circulatory pattern for HBoV, although the majority of cases had occurred in the first half of the year. Although HBoV is more frequently detected during the rainy season, we do not find any statistical association, as RSV has already been demonstrated in the region [45]. The same seasonal circulation is observed with FLU A in the region [46]. Even in countries with temperate climates, the circulation of HBoV is diverse, being more frequent in winter [47], spring [48], and summer [49]. In a study conducted in a temperate area in the southeastern Brazil, HBoV was more prevalent during autumn and spring [50].
The present study shows, for the first time, molecular evidence for respiratory infection caused by HBoV in children under 5 years age in a tropical semiarid area in Brazil. Despite its relevance, this study has some limitations: the reduced number of investigated children did not allow us to define the clinical, epidemiological, and demographic variables statistically related to HBoV infection. Seasonal patterns of virus circulation were not possible to detect due to the limited period of time studied. In addition, although the IFA technique and PCR assays have been broadly performed for detection of respiratory viruses, more efficient multiplex qPCR kits are currently available [51] Although direct immunofluorescence assays are commercially available for detecting PIV4, their sensitivity is low [46] and therefore we chose to use PCR to detect this agent. Even though the prevalence of the investigated virus species is in agreement with data shown elsewhere, differences in methodologies should be considered. One of the approaches to determine HBoV as an etiological agent of pneumonia has been the use of serology that helps in the diagnosis of acute disease by the agent [16]. The persistence of the agent in the respiratory tract limits the use of the molecular diagnosis, which can present high sensitivity, but questionable specificity. Serology, on the other hand, has lower sensitivity [16]; however, when combined with PCR, it can be used for accurate diagnosis of HBoV infection [52].
Although the role of HBoV in the pathogenesis of CAP is unclear, the present study describes the occurrence of HBoV among symptomatic children in a tropical semi-arid area in northeastern Brazil. This study also reinforces that viral diagnosis may influence antimicrobial prescribing, as a high number of children with viral infections were treated with antibiotics.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pneumonia. World Health Organization. 2019. (available in: https://www.who.int/news-room/fact-sheets/detail/pneumonia). accessed in 01/18/2021.
- 2.McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health. 2019;7:47–57. doi: 10.1016/S2214-109X(18)30408-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain S. Epidemiology of viral pneumonia. Clin Chest Med. 2017;38:1–9. doi: 10.1016/j.ccm.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jianming Q, Maria SV, Neal SY. Human parvoviruses. Clin Microbiol Rev. 2017;30:43–113. doi: 10.1128/CMR.00040-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allander T, Tammi MT, Eriksson M, et al. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guido M, Tumolo MR, Verri T, et al. Human bocavirus: current knowledge and future challenges. World J Gastroenterol. 2016;22:8684–8697. doi: 10.3748/wjg.v22.i39.8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ljubin SS, Meštrović T, Ivković JI et al (2019) High detection rates of human bocavirus in infants and small children with lower respiratory tract infection from Croatia. Clin Lab 65(1) [DOI] [PubMed]
- 8.Campos GS, Silva ML, Menezes DL, et al. Human bocavirus in acute gastroenteritis in children in Brazil. J Med Virol. 2016;88:166–170. doi: 10.1002/jmv.24293. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, Yin F, Zhou W, Yan Y, Ji W. Clinical significance of different virus load of human bocavirus in patients with lower respiratory tract infection. Sci Rep. 2016;6:1–6. doi: 10.1038/srep20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng X, Yan Z, Cheng F, Engelhardt JF, Qiu J. Replication of an autonomous human parvovirus in non-dividing human airway epithelium is facilitated through the DNA damage and repair pathways. PLoS Pathog. 2016;12:e1005399. doi: 10.1371/journal.ppat.1005399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Instituto de Pesquisa e Estratégia Econômica do Ceará. Anuário Estatístico do Ceará. 2020. (available in: http://ipecedata.ipece.ce.gov.br/ipece-data-web/module/anuario.xhtml). Accessed in 07/13/2022.
- 12.Nascimento-Carvalho CM. Community-acquired pneumonia among children: the latest evidence for an updated management. Jornal de Pedriatria. 2020;96(S1):29–38. doi: 10.1016/j.jped.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung RY, Chan PK, Choi KC, et al. Comparative study of nasopharyngeal aspirate and nasal swab specimens for diagnosis of acute viral respiratory infection. J Clin Microbiol. 2018;46:3073–3076. doi: 10.1128/JCM.01209-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilar JC, Pérez-Breña MP, García ML, et al. Detection and identification of human parainfluenza viruses 1, 2, 3, and 4 in clinical samples of pediatric patients by multiplex reverse transcription-PCR. J Clin Microbiol. 2000;38(3):1191–1195. doi: 10.1128/jcm.38.3.1191-1195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro IA, Costa DC, Oliveira CR, et al. Circulation profile of respiratory viruses in symptomatic and asymptomatic children from Midwest Brazil. Braz J Microbiol. 2020;51:1729–1735. doi: 10.1007/s42770-020-00368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nascimento-Carvalho AC, Vilas-Boas AL, Fontoura MSH, et al. Serologically diagnosed acute human bocavirus 1 infection in childhood community-acquired pneumonia. Pediatr Pulmonol. 2018;53:88–94. doi: 10.1002/ppul.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caccia ERB, Watanabe ASA, Carraro E, et al. Frequency of human bocavirus respiratory infections among at-risk patients in São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2012;54:307–310. doi: 10.1590/s0036-46652012000600003. [DOI] [PubMed] [Google Scholar]
- 18.Nascimento-Carvalho CM, Cardoso RA, Meriluoto M, et al. Human bocavirus infection diagnosed serologically among children admitted to hospital with community-acquired pneumonia in a tropical region. J Med Virol. 2012;84:253–258. doi: 10.1002/jmv.22268. [DOI] [PubMed] [Google Scholar]
- 19.Pilger DA, Cantarelli VV, Amantea SL, Leistner-Segal S. Detection of human bocavirus and human metapneumovirus by real-time PCR from patients with respiratory symptoms in Southern Brazil. Mem Inst Oswaldo Cruz. 2011;106:56–60. doi: 10.1590/s0074-02762011000100009. [DOI] [PubMed] [Google Scholar]
- 20.Silva AK, Santos MC, Mello WA, Sousa RCM. Ocorrência de Bocavírus Humano associado às infecções respiratórias agudas em crianças de 0 a 2 anos de idade na Cidade de Belém, Pará. Brasil Revista Pan-Amazônica de Saúde. 2010;1:87–92. [Google Scholar]
- 21.Souza EL, Ramos JG, Proença-Módena JL, et al. Human bocavirus in very young infants hospitalized with acute respiratory infection in northeast Brazil. J Trop Pediatr. 2010;56:125–127. doi: 10.1093/tropej/fmp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esposito S, Daleno C, Prunotto G, et al. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses. Influenza Other Respir Viruses. 2013;7:18–26. doi: 10.1111/j.1750-2659.2012.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvo C, García ML, Pozo F, et al. Infections and coinfections by respiratory human bocavirus during eight seasons in hospitalized children. J Med Virol. 2016;88:2052–2058. doi: 10.1002/jmv.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alkhalf H, Almutairi AR, Almutairi A, et al. Prevalence and clinical characterization of bocavirus infection in a specialized children's hospital in Saudi Arabia. Cureus. 2022;14(2):e22127. doi: 10.7759/cureus.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji K, Sun J, Yan Y, Han L, Guo J, Ma A, Hao X, Li F, Sun Y. Epidemiologic and clinical characteristics of human bocavirus infection in infants and young children suffering with community acquired pneumonia in Ningxia. China Virol J. 2021;18:212. doi: 10.1186/s12985-021-01682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu M, Perdomo MF, Mattola S, et al. Persistence of human bocavirus 1 in tonsillar germinal centers and antibody-dependent enhancement of infection. MBio. 2021;12:1. doi: 10.1128/mBio.03132-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Proenca-Modena JL, Paula FE, Buzatto GP, et al. Hypertrophic adenoid is a major infection site of human bocavirus 1. J Clin Microbiol. 2014;52:3030–3037. doi: 10.1128/JCM.00870-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbeke V, Reynders M, Floré K, et al. Human bocavirus infection in Belgian children with respiratory tract disease. Adv Virol. 2019;164:2919–2930. doi: 10.1007/s00705-019-04396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Li Y, Liu J, et al. Genetic characterization of human bocavirus among children with severe acute respiratory infection in China. J Infect. 2016;73:155–163. doi: 10.1016/j.jinf.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bagasi AA, Howson HC, Clark G, et al. Human bocavirus infection and respiratory tract disease identified in a UK patient cohort. J Clin Virol. 2020;129:e104453. doi: 10.1016/j.jcv.2020.104453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remolina YA, Ulloa MM, Vargas H, et al. Viral infection in adults with severe acute respiratory infection in Colombia. PLoS ONE. 2011;10:e0143152. doi: 10.1371/journal.pone.0143152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobkowiak P, Mikoś M, Bręborowicz A, Szczepankiewicz A. Human bocavirus and metapneumovirus in acute wheezing in children Is there a link with atopy? Clin Respir J. 2020;14:1201–1207. doi: 10.1111/crj.13261. [DOI] [PubMed] [Google Scholar]
- 33.Del Rosal T, García ML, Calvo C, Gozalo F, Pozo F, Casas I. Recurrent wheezing and asthma after bocavirus bronchiolitis. Allergol Immunopathol. 2016;44:410–414. doi: 10.1016/j.aller.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Tramper-Stranders GA. Childhood community-acquired pneumonia: a review of etiology-and antimicrobial treatment studies Paediatric respiratory reviews. 2018;26:41–48. doi: 10.1016/j.prrv.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corrêa RDA, Costa NA, Lundgren F, et al. 2018 recommendations for the management of community acquired pneumonia. J Bras Pneumol. 2018;44:405–423. doi: 10.1590/S1806-37562018000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyd K. Back to the basics: community-acquired pneumonia in children. Pediatr Ann. 2017;46:e257–e261. doi: 10.3928/19382359-20170616-01. [DOI] [PubMed] [Google Scholar]
- 37.Peyrani P, Mandell L, Torres A, et al. The burden of community-acquired bacterial pneumonia in the era of antibiotic resistance. Expert Rev Respir Med. 2019;13:139–152. doi: 10.1080/17476348.2019.1562339. [DOI] [PubMed] [Google Scholar]
- 38.Wunderink RG, Yin Y. Antibiotic resistance in community-acquired pneumonia pathogens. Seminars in respiratory and critical care medicine. 2016;37:829–838. doi: 10.1055/s-0036-1593753. [DOI] [PubMed] [Google Scholar]
- 39.Pinky L, Dobrovolny HM. Coinfections of the respiratory tract: viral competition for resources. PLoS ONE. 2016;11(5):e0155589. doi: 10.1371/journal.pone.0155589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin ET, Fairchok MP, Stednick ZJ, et al. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis. 2013;207(6):982–989. doi: 10.1093/infdis/jis934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang G, Hu Y, Wang H, et al. High incidence of multiple viral infections identified in upper respiratory tract infected children under three years of age in Shanghai, China. PLoS ONE. 2012;7(9):e44568. doi: 10.1371/journal.pone.0044568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song E, Wang H, Kajon AE, et al. Diagnosis of pediatric acute adenovirus infections: is a positive PCR sufficient? Pediatr Infect Dis J. 2016;35:827–834. doi: 10.1097/INF.0000000000001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alkhalaf MA, Guiver M, Cooper RJ. Prevalence and quantitation of adenovirus DNA from human tonsil and adenoid tissues. J Med Virol. 2013;85:1947–1954. doi: 10.1002/jmv.23678. [DOI] [PubMed] [Google Scholar]
- 44.Proenca-Modena JL, Cardoso SR, Criado MF, et al. Human adenovirus replication and persistence in hypertrophic adenoids and palatine tonsils in children. J Med Virol. 2019;91:1250–1262. doi: 10.1002/jmv.25441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moura FE, Perdigão AC, Ribeiro JF, et al. Respiratory syncytial virus epidemic periods in an equatorial city of Brazil. Influenza and other respiratory viroses. 2013;7:1128–1135. doi: 10.1111/irv.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raboni SM, Moura FE, Caetano BC, et al. Global Influenza Hospital-based Surveillance Network (GIHSN): results of surveillance of influenza and other respiratory viruses in hospitalized patients in Brazil. BMJ Open. 2018;8:e017603. doi: 10.1136/bmjopen-2017-017603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrarca L, Nenna R, Frassanito A, et al. Human bocavirus in children hospitalized for acute respiratory tract infection in Rome. World Journal of Pediatrics. 2020;16:293–298. doi: 10.1007/s12519-019-00324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi E, Ha KS, Song DJ, et al. Clinical and laboratory profiles of hospitalized children with acute respiratory virus infection. Korean J Pediatr. 2018;61:180. doi: 10.3345/kjp.2018.61.6.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou JY, Peng Y, Peng XY, et al. Human bocavirus and human metapneumovirus in hospitalized children with lower respiratory tract illness in Changsha. China Influenza and other respiratory viroses. 2018;12:279–286. doi: 10.1111/irv.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva PE, Figueiredo CA, Luchs AP, et al. Human bocavirus in hospitalized children under 5 years with acute respiratory infection, Sao Paulo, Brazil, 2010. Adv Virol. 2018;163:1325–1330. doi: 10.1007/s00705-017-3694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yun SG, Kim MY, Choi JM, et al. Comparison of three multiplex PCR assays for detection of respiratory viruses: Anyplex II RV16, AdvanSure RV, and Real-Q RV. J Clin Lab Anal. 2017;32:e22230. doi: 10.1002/jcla.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang LL, Tang LY, Xie ZD, et al. Human bocavirus in children suffering from acute lower respiratory tract infection in Beijing Children’s Hospital. Chin Med J. 2008;121(17):1607–1610. [PubMed] [Google Scholar]