Abstract
Background:
Various methods based on gold nanoparticles (AuNPs) have been applied to enhance the photothermal effect. Among these methods, combining gold nanoparticles and stem cells has been suggested as a new technique for elevating the efficiency of photothermal therapy (PT) in terms of enhancing tumor targeting effect. However, to elicit the efficiency of PT using gold nanoparticles and stem cells, delivering large amounts of AuNPs into stem cells without loss should be considered.
Methods:
AuNPs, AuNPs-decorated silica nanoparticles, and silica-capped and AuNPs-decorated silica nanoparticles (SGSs) were synthesized and used to treat human mesenchymal stem cells (hMSCs). After evaluating physical properties of each nanoparticle, the concentration of each nanoparticle was estimated based on its cytotoxicity to hMSCs. The amount of AuNPs loss from each nanoparticle by exogenous physical stress was evaluated after exposing particles to a gentle shaking. After these experiments, in vitro and in vivo photothermal effects were then evaluated.
Results:
SGS showed no cytotoxicity when it was used to treat hMSCs at concentration up to 20 μg/mL. After intravenous injection to tumor-bearing mice, SGS-laden hMSCs group showed significantly higher heat generation than other groups following laser irradiation. Furthermore, in vivo photothermal effect in the hMSC-SGS group was significantly enhanced than those in other groups in terms of tumor volume decrement and histological outcome.
Conclusion:
Our results suggest that additional silica layer in SGSs could protect AuNPs from physical stress induced AuNPs loss. The strategy applied in SGS may offer a prospective method to improve PT.
Keywords: Gold nanoparticles, Silica capping, Photothermal therapy
Introduction
Photothermal therapy (PT) using various nanoparticles applies unique phenomenon related to temperature increment in tumor through exogenous laser irradiation to selectively remove abnormal tissues [1–3]. Unlike conventional therapies using chemicals or radiation, PT using tumor targeting techniques using PT can prevent unwanted damages to normal tissues. Moreover, tumor tissues are generally feebler to high temperature than normal tissues. Therefore, PT using various nanoparticles and exogenous laser irradiation can be useful for destroying tumor. Previously, many studies have applied gold nanoparticles (AuNPs) as key materials for PT [3–6]. Through localized surface plasmon resonance, light irradiation to AuNPs can convert light to heat energy [7]. Additionally, physiochemical properties of AuNPs can be easily modified by manipulating the dimension, shape, and combination with other atoms [8–10]. Generating heat energy in response to near-infrared (NIR) light irradiation can also be considered as an advantage of applying AuNPs for PT since NIR can penetrate deep inside the body where tumor tissue is located [11]. Lastly, AuNP is known to be bioinert. Thus, it can be applied as a promising material for PT [12].
However, insufficient temperature increment in tumor after exogenous laser irradiation remains as a critical problem to be solved when applying naked AuNPs without further modification. Among various methods used to solve this issue, human mesenchymal stem cells (hMSCs) have been suggested to as carriers for tumor targeting AuNPs [13, 14]. Previous studies have shown that stem cells such as hMSCs can migrate toward tumor tissue through chemotaxis. It is known that hMSCs can migrate to tumor tissues efficiently by recognizing various chemokines secreted from tumor tissues [15, 16]. However, loss of AuNPs delivered to hMSCs can diminish the effect of PT [14, 17]. Previous reports have shown that silica (Si) based particles can be utilized for destroying tumors. Si-based particles can deliver various materials including drug, gene, and other types of nanoparticles [18–20]. Therefore, a new strategy that can elicit the amount of intracellularly delivered AuNP in hMSCs could enhance the therapeutic PT effect. Here, we synthesized Si-capped and gold-decorated Si nanoparticles (SGSs) to augment cell viability and PT efficiency (Fig. 1). We hypothesized that treating SiNPs decorated by AuNPs to hMSCs enhance the effect of PT. Through Si capping on the surface of AuNPs decorated SiNPs, AuNP loss by physical stress might be prevented.
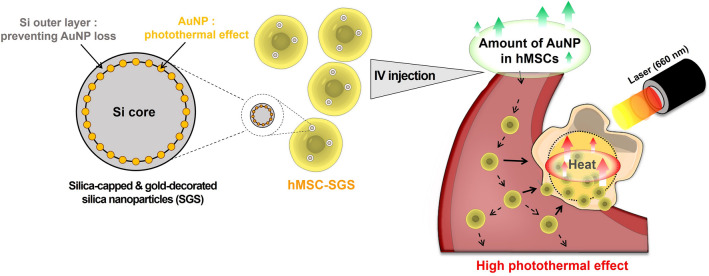
Fig. 1.
Schematic illustration of anti-tumor treatment using silica (Si)-capped gold (Au) decorated Si nanoparticles (SGSs). Si nanoparticles were applied to protect Au nanoparticles attached on the surface of Si nanoparticles from physical stress and Si degradation. Based on the improved long-term circulation and high photothermal effect induced by combining SGSs and human mesenchymal stem cells (hMSCs), therapeutic photothermal effect was significantly enhanced when compared to that of conventional photothermal therapy (PT) using Au nanoparticles (AuNPs)
In this study, we synthesized SGSs by applying SiNPs to AuNPs and incorporated SGSs to hMSCs. Treating hMSCs with optimized concentration of SGSs (hMSC-SGS) successfully induced prominent temperature increment required for PT without causing cytotoxicity. Intravenous injection of SGSs-laden hMSCs with exogenous laser irradiation dramatically decreased the size of the tumor compared to conventional methods. Thus, our SGSs may offer a new strategy for future PT based on AuNPs and stem cells combination.
Materials and methods
Synthesis of AuNPs, AuNPs-decorated Si nanoparticles (GSs), and SGSs
Gold (III) chloride trihydrate (HAuCl4), tetraethyl orthosilicate (TEOS), (3-aminopropyl) trimethoxysilane (APTMS), and trisodium citrate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methyl alcohol (MeOH, 99.9%), ethyl alcohol (EtOH, 99.9%), 1-propyl alcohol (PrOH, 99.8%), and ammonia solution (28–30%) were purchased from Samchun Chemicals (Seoul, Republic of Korea). Ultrapure water (18.2 MΩ cm) was used to prepare all aqueous solutions and samples unless otherwise indicated. A slightly modified Turkevich method was used to synthesize citrate stabilized AuNP [21]. Briefly, 300 mL of aqueous HAuCl4 (0.5 mM) was heated at 100 °C under gentle stirring. The color of the solution changed from yellow to deep red. After boiling, 30 mL of aqueous trisodium citrate (38.7 mM) was added to the solution and reacted for 7 min. The reaction was quenched by cooling in cold water. Finally, the resulting solution was collected by centrifugation and redispersed in 10 mL of water after removing the supernatant. GS and SGS were synthesized by seeded growth of Si and surface amino functionalization according to previously reported processes [22]. First, Si seeds were synthesized by reacting 4 mL of diluted TEOS solution (20 vol% in PrOH) and a mixture of 20 mL of PrOH, 2.7 mL of water, and 3.2 mL of ammonia solution for 1 h in a rotator at room temperature. Si seeds were washed using centrifugation with water and PrOH, respectively, then re-dispersed in 40 mL of water. For the synthesis of amino functionalization of Si, 10 mL of Si seed and 20 mL of MeOH were first mixed and heated to 55 °C. Then 0.6 mL of diluted APTMS (9.1 vol% in MeOH) was sequentially added to the suspension three times every 30 min (a total of 1.8 mL). It was then reacted overnight. Thereafter, the solution temperature was increased to 85 °C and kept for 30 min to finalize APTMS conjugation on the Si seed. The amino-functionalized Si was washed using centrifugation with acetic acid (10 vol% in EtOH) and water several times, respectively. It was finally re-dispersed in 10 mL of water. To electro-statically decorate AuNP on amino-functionalized Si surface, 8 mL of AuNP and 20 mL of EtOH were added to 10 mL of amino-functionalized Si suspension while applying ultrasonic in a sonicating bath for 30 min. AuNP-decorated Si suspension (GS) was washed with EtOH and water several times, respectively, by centrifugation and redispersed in 40 mL of water to prepare GS. To further prepare SGS, washed GS was dispersed in 30 mL of water/EtOH mixture (20 vol% in EtOH). Then, 1 mL of ammonia solution and 1 mL of diluted TEOS (20 vol% in PrOH) were added to this suspension. After 2 h of reaction at room temperature, the resulting product was washed with water and EtOH, respectively. It was finally dispersed in 40 mL of water for further characterization and experiments.
Characterization of AuNPs, AuNPs-decorated Si nanoparticles (GSs), and SGSs
Field-emission transmission electron microscopy (FE-TEM, TALOS F200X, Thermo Fisher Scientific, Waltham, MA, USA) and field-emission scanning electron microscopy (FE-SEM, Supra 40VP, Zeiss, Oberkochen, Germany) were employed to analyze morphologies of synthesized materials. The absorbance of each particle was measured using a spectrofluorometer (FS5, Edinburgh Instruments, Livingston, UK). Gold contents in GS and SGS were estimated using inductively coupled plasma optical emission spectroscopy (ICP-OES, Optima 8300, Perkin Elmer, Germany). The amount of gold detached from GS or SGS was measured with ICP-OES. Both particles were gently shaken (2 h, 37 °C and pH 7.0 water) and centrifuged to collect the gold in water for ICP-OES analysis.
Cells
hMSCs were purchased from Lonza (Allendale, NJ, USA). Human fibrosarcoma cell line HT-1080 was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Both cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (Gibco BRL) and 1% (v/v) penicillin/streptomycin (Gibco BRL). Both cells were incubated at 37 °C under 5% CO2. Cell culture medium was replenished every two days. hMSCs within five passages were used in the experiments.
In vitro photothermal effect
Various amounts of AuNPs, GSs, and SGSs (0, 10, 50, 100, 200 μg based on amounts of AuNP in each nanoparticle) were diluted with 100 μL distilled water and irradiated using a 660 nm continuous wave laser beam (UNIOTECH, Daejeon, Korea) with an output power of 2 W/cm2. Temperatures and photothermal images of AuNP, GS, and SGS suspensions were recorded with an infrared thermal imaging system every 30 s (FLIR E4, FLIR Systems Inc., Wilsonville, OR, USA).
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Relative p53 gene expression level was evaluated by qRT-PCR. Total ribonucleic acids (RNAs) were extracted using TRIzol reagent (1 mL, Life Technologies. Inc., Carlsbad, CA, USA) and chloroform (200 μL). Lysed samples were centrifuged and RNA pellets were washed with 35% (v/v) ethanol in water and dried. RNA samples were then dissolved in RNase-free water. qRT-PCR was then performed using the SsoAdvanced™ Universal SYBR Green Supermix kit (Bio-Rad, Hercules, CA, USA). Human specific glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization.
Cytotoxicity analysis
After hMSCs were seeded onto 6-well plates at density of 1 × 105 cells/well, they were cultured for 24 h and then incubated with various concentrations of AuNPs, GSs, and SGSs. A live and dead assay kit (FDA/EB, Sigma-Aldrich, St. Louis, MO, USA) was used to observe live and dead cells based on signals from fluorescein diacetate (FDA) and ethidium bromide (EB)-stained cells, respectively, according to the manufacturer’s protocol. FDA/EB results were evaluated by fluorescence microscopy (DMi8, Leica, Wetzlar, Germany).
Mouse tumor bearing modeling
Athymic nude mice (6 weeks old, BABL/c, female, Orient Bio, Seoul, Korea) were used to induce mouse tumor bearing model for in vivo experiments. After anesthetizing mice using a mixture of xylazine (10 mg/kg, Rompun; Bayer Korea, Ansan, Korea) and ketamine hydrochloride (100 mg/kg, ketamine, Yuhan, Seoul, Korea), HT-1080 cells (5 × 106 cells/100 μL PBS) were subcutaneously injected into both flanks of mice. Mice bearing tumors with diameters greater than 3.5 mm were selected for further experiments. The animal study was approved by the Institutional Animal Care and Use Committee of SungKyunKwan University (SKKUIACUC 2021-05-46-1).
In vivo photothermal tumor therapy and histology
PBS (100 μL), hMSCs (1 × 106 cells/100 μL PBS), hMSC-AuNPs (1 × 106 cells with AuNPs/100 μL PBS), hMSC-GSs (1 × 106 cells with GSs/100 μL PBS) or hMSC-SGSs (1 × 106 cells with SGSs/100 μL PBS) was intravenously injected into each tumor-induced mouse. At three days after injection, tumor tissues were irradiated with a 660 nm laser for 2 min under anesthesia. FLIR E4 was used to record temperature variations in tumor regions. The tumor volume was calculated using the formula of V = A × B2 × 0.5, where A and B indicated the largest and smallest diameters, respectively [13]. Tumor tissues were harvested at 7 days after laser irradiation and fixed in 4% (v/v) paraformaldehyde in PBS. For histology, samples were embedded in optimal cutting temperature compound (OCT compound, Sakura Finetek USA, Inc., Torrance, CA, USA) and cut into 10-μm thick sections at − 22 °C. These specimens were then stained with hematoxylin and eosin (H&E).
Statistical analysis
All statistical analyses were performed with one-way analysis of variance (ANOVA) and Tukey’s significant difference post hoc test using SPSS software (SPSS Inc., USA). Results are expressed as mean ± standard deviation. Statistical significance was set at p < 0.05.
Results
Synthesis and characterization of AuNP, GS, and SGS
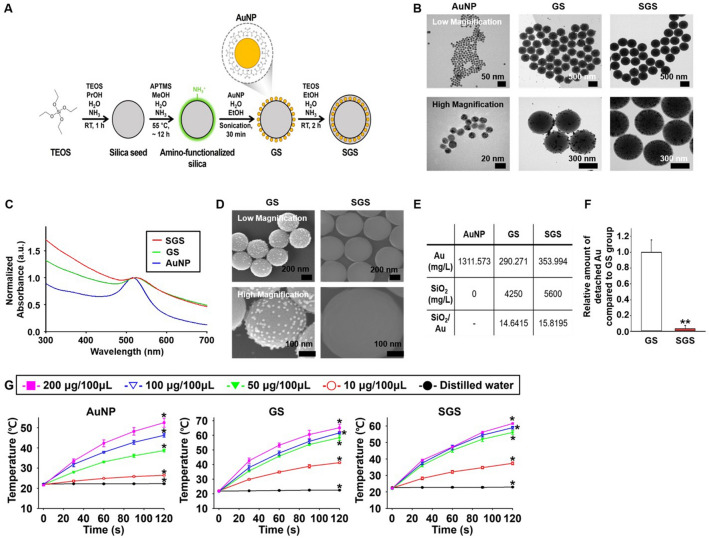
SGS was synthesized by stepwise Si seed synthesis, surface amino-functionalization, AuNP decoration, and additional Si coating as shown in Fig. 2A. At first, citrate-stabilized AuNP was synthesized following the Turkevich method with slight modification [21]. As shown in Fig. 2B, citrate-stabilized AuNP had a size of approximately 15 nm with a uniform size distribution. Owing to electrostatic attraction between the negative charge of citrate on AuNP and the positive charge of an amino group, AuNP was readily decorated on the surface of amino-functionalized Si. GS had a size of approximately 400–420 nm (Fig. 2B). SGS had a slightly larger size than GS of approximately 480–510 nm due to secondary Si capping as shown in Fig. 2B. It was noteworthy that AuNPs were uniformly embedded inside the Si without any morphological deformation. As shown in Fig. 2C, AuNP had a localized surface plasmon resonance (LSPR) peak at 516 nm with a high consistency, whereas GS and SGS had slightly red-shifted absorption peaks at 523 nm and 526 nm, respectively, probably due to agglomeration of AuNPs on Si surface. In the case of GS, although AuNP was electro-statically attached to the amino-functionalized Si, AuNP detached during the washing step. As a result, the gold content of GS was slightly lower than that of SGS (Fig. 2D, E). The amount of AuNP detached from the GS was significantly higher than that of SGS after physical stimulation (gentle shaking for 2 h, Fig. 2F). For PT, each nanoparticle was irradiated with a 660 nm laser to confirm the increment of temperature induced by photothermal effect. All three nanoparticles showed a photothermal effect upon irradiation for 2 min. The temperature increment in three nanoparticles became higher as the concentration of the three nanoparticles increased (Fig. 2G).
Fig. 2.
Preparation and physical property of AuNPs-decorated Si nanoparticle (GS) and SGS. A. Procedure used for preparing GS and SGS. B TEM images of AuNP, GS, and SGS at different magnifications. C Normalized absorbance of AuNP, GS, and SGS. D SEM images of GS and SGS at different magnifications. E Amount of gold and ratio between gold and Si in each particle. F Relative amount of gold detached from Si nanoparticles under physical stress (n = 5, **p < 0.001 versus GS group). G Temperature increment during 660 nm laser irradiation for 2 min according to concentration difference of each nanoparticle (n = 4, *p < 0.05 versus all other groups)
Cytotoxic effects of AuNP, GS, and SGS on hMSCs
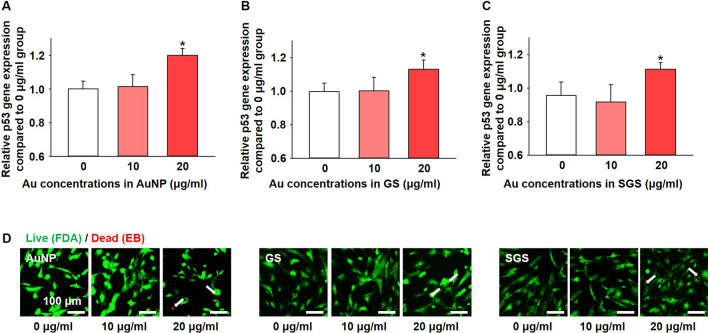
The concentration of each particle was estimated in terms of cytotoxicity. After culturing hMSCs with each nanoparticle for 24 h, cytotoxicity was confirmed. All nanoparticles applied in this experiment did not show any cytotoxic effects until the concentration of each particle reached 10 μg/mL as confirmed by the expression of pro-apoptotic gene, p53. However, all particles significantly increased p53 expression when their concentration reached 20 μg/mL (Fig. 3A–C). Live and dead assay (FDA/EB staining, Fig. 3D) showed similar results with p53 expression according to the concentration change of each particle.
Fig. 3.
Cytotoxic effect of AuNPs, GSs, and SGSs at different concentrations for 24 h culturing with hMSCs. A–C. Expression levels of pro-apoptotic factor, p53, according to different concentrations of each nanoparticle (n = 4, * p < 0.05 versus all other groups). D Cell viability of hMSCs evaluated by live and dead (fluorescein diacetate (FDA)/ethidium bromide (EB)) assay according to the different concentrations of each nanoparticle. Green (FDA-positive signal) indicates live cells and red (EB-positive signal) indicates dead cells (white arrows). Scale bars indicate 100 μm
Temperature increment and following in vivo therapeutic effect using SGSs-laden hMSCs
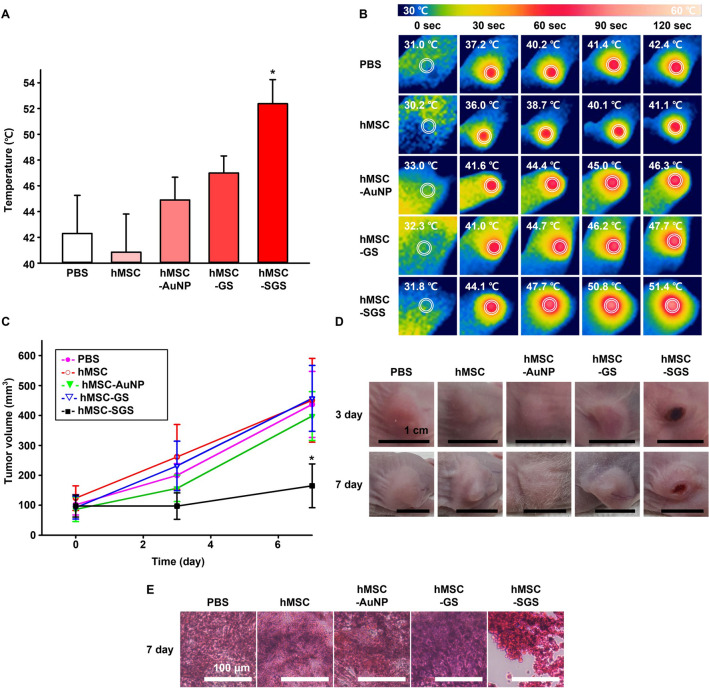
PBS, hMSC, AuNPs-laden hMSCs (hMSC-AuNP), GSs-laden hMSCs (hMSC-GS), and SGSs-laden hMSCs (hMSC-SGS) were intravenously injected to tumor bearing mice. Tumor sites in mice were irradiated with laser at 3 days after intravenous injections. The laser irradiation resulted in temperature change to 42.3 °C in PBS, 40.9 °C in hMSC, 44.9 °C in hMSC-AuNP, 47.0 °C in hMSC-GS, and 52.4 °C in hMSC-SGS groups (Fig. 4A, B). Compared to all other groups, the hMSC-SGS group showed the highest temperature increment. Volume of tumor in the hMSC-SGS group showed significantly more decrement at 7 days after laser irradiation compared to those of all other groups (Fig. 4C, D). Histological analysis based on H&E staining showed that most tumor tissues were destroyed after injecting SGSs-laden hMSCs, more than in all other groups (Fig. 4E).
Fig. 4.
In vivo therapeutic effect of SGSs-laden hMSCs on tumor bearing mouse model after intravenous injection. A,B Temperature increment and representative real-time infrared thermal images of tumor sites at three days after hMSCs or various nanoparticles-laden hMSCs injections with laser irradiation up to 2 min. C Changes in tumor volume for 7 days after various treatments (n = 4, * p < 0.05 versus all other groups). D Representative images of tumors at days 3 and 7 after various treatments. E Representative hematoxylin and eosin staining images of tumor site at 7 days after various treatments
Discussion
Previously, many studies have applied hMSCs and nanomaterials to tumor therapy to enhance tumor targeting efficiency and therapeutic effect of PT [23, 24]. Despite enhanced tumor targeting efficiency based on hMSCs and nanomaterials, low number of nanomaterials that can generate heat to induce PT has remained as a problem to overcome [6]. To this end, we utilized Si capping strategy to elevate the amount of AuNPs in combination with hMSCs. It is known that hMSCs can intracellularly deliver materials to tumor through blood circulation efficiently. Therefore, we hypothesized that increasing the amount of AuNP in hMSCs could enhance the photothermal therapeutic effect. Thus, we synthesized SGS to prevent AuNP loss known to decrease the PT effect.
Three nanoparticles (AuNP, GS, and SGS) were synthesized and confirmed to show photothermal effects (Figs. 1, 2). However, due to the loss of AuNP from the Si surface of GS, total amounts of AuNP were different. Even with a gentle shake, the number of AuNPs decreased sharply in GS than in SGS. The loss of nanoparticles occurs due to mild physical stress such as gentle shaking, indicating that the loss might occur in the medium during the uptake process and also in the blood vessels after in vivo injection. Physical stress can be applied to the nanoparticles while the medium is shaken or circulated in the blood vessels. Although both GS and SGS showed similar photothermal effects, such effects were only possible when the concentration of each particle was prepared with an identical amount of gold. Calculating the concentration of each particle based on the Au amount in each particle led to different Si concentrations in hMSCs. However, the cytotoxicity was not very different from each group. Due to easy detachment of AuNPs from the Si surface by Si degradation, amounts of AuNPs in GS and SGS groups were significantly different. Since the Si part in each particle can undergo degradation by cells and amount of AuNPs varies with exogenous physical stress, optimizing the concentration of each particle should have been based on the amount of Au instead of Si. Regardless of the amount of SiNPs, p53, an apoptosis factor, was significantly elevated in 20 μg/mL. All nanoparticles were non-toxic at 10 μg/mL (Fig. 3). Live and dead assay results also showed similar results. EB-positive cells were found only when concentrations of GS and SGS particles reached 20 μg/mL (Fig. 3). Based on the concentration of AuNPs, 10 μg/mL was selected as an optimal particle concentration for treating hMSCs for in vivo experiment.
As shown in the in vivo experimental results, only the hMSC-SGS group showed significant therapeutic effect. When laser irradiation was performed at three days after intravenous injection by group, the temperature was overwhelmingly higher in the hMSC-SGS group than in all other groups. Two minutes after laser irradiation, only the hMSC-SGS group reached a temperature higher than 50 °C (Fig. 4A, B). Previous studies have shown that tissue temperatures above 48 °C can cause irreversible damage towards tumor [25]. As presented in Fig. 4, hMSC-AuNP and hMSC-GS groups showed temperature increment lower than 50 °C. No significant difference in temperature was found when they were compared to PBS or hMSC groups. This result suggested that photothermal effects in hMSC-AuNP and hMSC-GS groups were not sufficient to affect the decrement of tumor volume. Correlated results were observed in volume ratio of tumor in each group. The tumor volume of hMSC-SGS group showed significant difference from other experimental groups, indicating that a structure for preventing AuNP loss would be critical to achieve sufficient photothermal effect (Fig. 4C). Representative images in Fig. 4D further consolidated the photothermal effect in the hMSC-SGS group. Tumor volume in the hMSC-SGS group was the lowest, presenting part of skin color in black associated with photothermal effect (Fig. 4D). Dead cells in tumor tissue were also observed in the hMSC-SGS group by histology (Fig. 4E). Combining results in Fig. 4 demonstrated that Si shell in SGS showed robust photothermal effect in vivo due to its outstanding shielding strategy to prevent AuNPs loss by Si degradation.
In this study, we synthesized SGS for advanced PT to treat tumors efficiently. SGSs were introduced to hMSCs to prevent AuNPs loss and enhance the photothermal effect. Although detailed and advanced shielding mechanisms need to be discovered in the future, Si capping strategy that can improve both intracellular nanoparticle delivery and PT effect may suggest a new strategy in stem cell mediated photothermal therapy.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (NRF-2018M3A9E2023255, NRF-2020M2D9A3094171, NRF-2019R1C1C1007384, NRF-2022R1A2B5B02001237, and NRF-2021M3H4A4079509). This research was also supported by the Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare, 21A0102L1-11).
Declarations
Conflict of interest
The authors declare no conflicts of interest relevant to this article.
Ethical approval
All animal treatments and experimental procedures were approved by the Institutional Animal Care and Use Committee of Sungkyunkwan University (No. SKKUIACUC 2021-05-46-1).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jung Hwan Park and Hyun-Seok Choe have contributed equally to this work.
Contributor Information
Jae-Hyuk Kim, Email: Jaehyuk.kim@pusan.ac.kr.
Suk Ho Bhang, Email: sukhobhang@gmail.com.
References
- 1.Sun R, Chen H, Sutrisno L, Kawazoe N, Chen G. Nanomaterials and their composite scaffolds for photothermal therapy and tissue engineering applications. Sci Technol Adv Mater. 2021;22:404–428. doi: 10.1080/14686996.2021.1924044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han HS, Choi KY. Advances in nanomaterial-mediated photothermal cancer therapies: toward clinical applications. Biomedicines. 2021;9:305. doi: 10.3390/biomedicines9030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poursalehi Z, Salehi R, Samadi N, Rasta SH, Mansoori B, Majdi H. A simple strategy for chemo-photothermal ablation of breast cancer cells by novel smart gold nanoparticles. Photodiagnosis Photodyn Ther. 2019;28:25–37. doi: 10.1016/j.pdpdt.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Roh YH, Eom JY, Choi DG, Moon JY, Shim MS, Bong KW. Gold nanorods-encapsulated thermosensitive drug carriers for NIR light-responsive anticancer therapy. J Ind Eng Chem. 2021;98:211–216. doi: 10.1016/j.jiec.2021.03.052. [DOI] [Google Scholar]
- 5.Park S, Lee WJ, Park S, Choi D, Kim S, Park N. Reversibly pH-responsive gold nanoparticles and their applications for photothermal cancer therapy. Sci Rep. 2019;9:20180. doi: 10.1038/s41598-019-56754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang X, Guo X, Niu X, An W, Li S, Liu Z, et al. Photothermal therapeutic application of gold nanorods-porphyrin-trastuzumab complexes in HER2-positive breast cancer. Sci Rep. 2017;7:42069. doi: 10.1038/srep42069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emami F, Banstola A, Jeong J, Yook S. Cetuximab-anchored gold nanorod mediated photothermal ablation of breast cancer cell in spheroid model embedded with tumor associated macrophage. J Ind Eng Chem. 2022;106:177–188. doi: 10.1016/j.jiec.2021.10.029. [DOI] [Google Scholar]
- 8.Vines JB, Yoon J, Ryu N, Lim D, Park H. Gold nanoparticles for photothermal cancer therapy. Front Chem. 2019;7:167. doi: 10.3389/fchem.2019.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han H, Joe A, Jang E. Reduced cytotoxicity of CTAB-templated silica layer on gold nanorod using fluorescence dyes and its application in cancer theranostics. J Ind Eng Chem. 2021;96:202–212. doi: 10.1016/j.jiec.2021.01.020. [DOI] [Google Scholar]
- 10.Jeong G, Castels H, Kang I, Aliya B, Jang YC. Nanometerial for skeletal muscle regeneration. Tissue Eng Regen Med. 2022;19:253–261. doi: 10.1007/s13770-022-00446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H, Kim SW, Kwon DY, Kang HW, Jung M, et al. Near-infrared transillumination and photodynamic therapy using hypericin in animal laryngeal tumors. Tissue Eng Regen Med. 2021;18:941–951. doi: 10.1007/s13770-021-00377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, et al. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small. 2011;7:169–183. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]
- 13.Kang S, Bhang SH, Hwang S, Yoon J, Song J, Jang H, et al. Mesenchymal stem cells aggregate and deliver gold nanoparticles to tumors for photothermal therapy. ACS Nano. 2015;9:9678–9690. doi: 10.1021/acsnano.5b02207. [DOI] [PubMed] [Google Scholar]
- 14.Torre PDL, Perez-Lorenzo MJ, Alcazar-Garrido-A FAI. Cell-based nanoparticles delivery systems for targeted cancer therapy: Lessons from anti-angiogenesis treatments. Molecules. 2020;25:715. doi: 10.3390/molecules25030715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon D, Kim H, Lee E, Park MH, Chung S, et al. Study on chemotaxis and chemokinesis of bone marrow-derived mesenchymal stem cells in hydrogel-based 3D microfluidic devices. Biomater Res. 2016;20:25. doi: 10.1186/s40824-016-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chulpanova DS, Kitaeva KV, Tazetdinova LG, James V, Rizvanov AA, et al. Application of mesenchymal stem cells for therapeutic agent delivery in anti-tumor treatment. Front Pharmacol. 2018;9:259. doi: 10.3389/fphar.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao S, Yue W, Cai S, Tang Q, Lu W, Huang L, et al. Improvement of gold nanorods in photothermal therapy: Recent progress and perspective. Front Pharmacol. 2021;12:664123. doi: 10.3389/fphar.2021.664123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohammad-Ali S, Barbara H, Hélder AS. Nanostructured porous Si-based nanoparticles for targeted drug delivery. Biomatter. 2012;2:296–312. doi: 10.4161/biom.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallet-Regí M. Nanostructured mesoporous silica matrices in nanomedicine. J Intern Med. 2010;267:22–43. doi: 10.1111/j.1365-2796.2009.02190.x. [DOI] [PubMed] [Google Scholar]
- 20.Santos HA, Bimbo LM, Lehto VP, Airaksinen AJ, Salonen J, Hirvonen J. Multifunctional porous silicon for therapeutic drug delivery and imaging. Curr Drug Discov Technol. 2011;8:228–249. doi: 10.2174/157016311796799053. [DOI] [PubMed] [Google Scholar]
- 21.Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75. doi: 10.1039/df9511100055. [DOI] [Google Scholar]
- 22.Lee HL, Wei H, Kim K, Choe HS, Park H, Yu T, et al. Versatile yolk–shell encapsulation: Catalytic, photothermal, and sensing demonstration. Small. 2020;16:2002311. doi: 10.1002/smll.202002311. [DOI] [PubMed] [Google Scholar]
- 23.Huang RY, Lin YH, Lin SY, Li YN, Chiang CS, Chang CW. Magnetic ternary nanohybrids for nonviral gene delivery of stem cells and applications on cancer therapy. Theranostics. 2019;9:2411–2423. doi: 10.7150/thno.29326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang L, Xu C, Xu P, Qin Y, Chen M, Feng Q, et al. Intelligent photosensitive mesenchymal stem cells and cell-derived microvesicles for photothermal therapy of prostate cancer. Nanotheranostics. 2018;3:41–53. doi: 10.7150/ntno.28450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaque D, Maestro LM, Rosal BD, Haro-Gonzalez P, Benayas A, Plaza JL, et al. Nanoparticles for photothermal therapies. Nanoscale. 2014;6:9494. doi: 10.1039/C4NR00708E. [DOI] [PubMed] [Google Scholar]