Abstract
This research aimed to identify the diversity of bacterial species of the genus Staphylococcus spp. in subclinical mastitis in dairy herds in the state of Piauí, Northeastern Brazil, and to evaluate the phenotypic and genotypic resistance profile. Samples were obtained from a total of 17 dairy farms, amounting to 321 positive samples in the California Mastitis Test. Staphylococcus spp. were identified by matrix-assisted laser desorption ionization time-of-flight mass spectroscopy. Subsequently, an antibiogram was performed, and a polymerase chain reaction was carried out to screen for resistance genes in the isolates. Among all the isolates, 59.45% (110/185) belonged to the Staphylococcus genus. Moreover, the following Staphylococcus spp. were identified Staphylococcus aureus, 68.1% (75/110); Staphylococcus chromogenes, 12.7% (14/110); Staphylococcus epidermidis, 5.4% (6/110); Staphylococcus sciuri, 4.5% (5/110); Staphylococcus warneri, 2.7% (3/110); Staphylococcus haemolyticus, 1.8% (2/110); Staphylococcus hominis, 1.8% (2/110); Staphylococcus arlettae, 0.9% (1/110); Staphylococcus capitis, 0.9% (1/110); and Staphylococcus gallinarum, 0.9% (1/110). The antibiogram showed a high frequency of resistance to penicillin and ampicillin, 70.0% (77/110) and 61.8% (68/110), respectively, and a low frequency of resistance to gentamicin and vancomycin, 10.9% (12/110) and 11.8% (13/110), respectively. In the genotypic tests for the different species of Staphylococcus spp., the occurrence of the blaZ gene was observed in 60.9% (67/110) of the isolates, followed by tetL and tetM, both with 20.0% (22/110) each, and the mecA and vanB genes were detected in 0.9% (1/110) of the samples. The identification of all Staphylococcus species isolated from subclinical mastitis cases and the phenotypic and genotypic resistance characterization in these isolates is of great importance for dairy farming in the state of Piauí, as well as for public health.
Keywords: Antimicrobial resistance, Dairy cattle, Infection, MALDI-TOF MS
Introduction
Bovine mastitis, characterized by an inflammatory reaction in the mammary glands, is considered one of the major diseases that affect dairy cows. It can cause several productive and economic losses, such as reduced production of milk, increased expenses for the treatment, and in some cases, early disposal of animals [1–4].
Regarding the causative agents of bovine mastitis, the genus Staphylococcus is one of the most researched pathogens regarding the etiology of bovine mastitis, especially to subclinical mastitis. The importance of this genus is associated with its high resistance to antimicrobials, enterotoxaemia cases in humans, and disease development in animals [5–7]. Initially, studies on bovine mastitis had focused on S. aureus. However, recently, there has been an increase in research related to resistant coagulase-negative staphylococci (CoNS), with emphasis on Staphylococcus epidermidis and Staphylococcus chromogenes [8–12].
In recent years, antimicrobial resistance has become a major problem for the treatment of both animal and human diseases, as it is already possible to identify several species of bacteria carrying genes that confer resistance to some antimicrobials, such as the blaZ, mecA, and mecC (resistance to β-lactams) [13–15], tetL and tetM (tetracycline resistance) [13, 16], and vanA and vanB (vancomycin resistance) [17]. For example, gram-positive bacteria, such as Staphylococcus spp., can transmit these genes horizontally to other Staphylococcus spp. and/or other gram-positive bacteria, whether of human or animal origin [16].
Several studies have investigated the etiology of bovine mastitis and the resistance profile of the causative agents in various Brazilian states, such as Rio de Janeiro [18], Pará [19], São Paulo [20], Minas Gerais [13], Pernambuco [21, 22], Mato Grosso [23], and Paraná [10]. However, there are still various lacunes in other states, such as Piauí state, where there are no recordings of the said illness in the dairy region, nor records of the resistance profile of bacteria causing bovine mastitis. Likewise, this research aimed to identify the diversity of bacterial species of the genus Staphylococcus spp. in subclinical mastitis in dairy herds in the state of Piauí, Northeastern Brazil, and to evaluate the phenotypic and genotypic resistance profile.
Material and methods
Sampling and sample collection
Farms were chosen non-probabilistically for convenience. Milk samples were collected from 17 farms, being 3 in the municipality of Luiz Correia, 3 in Buriti dos Lopes and 11 in the municipality of Parnaíba, both municipalities located in the dairy basin of Piauí, Northeast Brazil. Initially, the California Mastitis Test (CMT) was performed on 680 breast quarters (170 Girolando cows) as per the protocol provided by Schalm and Noorlander [24]. The teats had been previously sanitized for subsequent testing. Milk samples were collected from the glands that displayed two (+ +) or three (+ + +) crosses in the CMT result, totaling 321 positive samples. Subsequently, these samples were stored in sterile bottles, placed in isothermal boxes, refrigerated, and sent for microbiological and molecular analyses in the Laboratory of Animals Infectious Diseases of the Federal Rural University of Pernambuco.
Microbiological isolation and identification of Staphylococcus spp.
Milk samples were cultivated in Base Agar supplemented with 7% sheep blood. The plates were incubated in a bacteriological incubator at 37 °C for 72 h and evaluated every 24 h. In post bacterial growth, the colonies were characterized by their morphology and morphotintorial characteristics via Gram staining, followed by biochemical tests of catalases in Staphylococcus spp. Subsequently, species identification was performed by the matrix-assisted laser desorption ionization time-of-flight mass spectroscopy (MALDI-TOF MS) (Bruker Daltonics) of the Institute of Pharmacology and Molecular Biology of the Federal University of São Paulo, as described by Wolters et al. [25].
Evaluation of antimicrobial resistance in Staphylococcus spp.
The phenotypic profile of antimicrobial resistance in Staphylococcus spp. was determined using the agar disk diffusion method [26]. The antimicrobials used were ampicillin (10 µg), penicillin G (10 UI), cefoxitin (30 µg), gentamicin (10 µg), oxacillin (1 µg), tetracycline (30 µg), erythromycin (15 µg), and vancomycin (30 µg), as per the recommendations in the Clinical and Laboratory Standards Institute guidelines [27].
Evaluation of resistance genes in Staphylococcus spp.
To obtain bacterial DNA, Staphylococcus spp. colonies were subjected to the DNA extraction method described by Fan et al. [28]. The genotypical resistance profile was evaluated for the genes tetM and tetL for tetracycline, blaZ for penicillin, vanA and vanB for vancomycin, and mecC and mecA for methicillin (Table 1).
Table 1.
Genes, oligonucleotide sequences, size of amplified fragments, and reference
| Gene | Sequence (5′ – 3′) | Fragment size (pb) | References | |
|---|---|---|---|---|
| blaZ |
F: AAGAGATTTGCCTATGCTTC R: GGCAATATGATCAAGATAC |
517 | [29] | |
| tetL |
F: TCGTTAGCGTGCTGTCATTC R: GTATCCCACCAATGTAGCCG |
267 | [30] | |
| tetM |
F: GTGGACAAAGGTACAACGAG R: CGGTAAAGTTCGTCACACAC |
406 | [30] | |
| mecA |
F: TGGTATGTGGAAGTTAGATTGGGAT R:CTAATCTCATATGTGTTCCTGTATTGGC |
155 | [31] | |
| mecC |
F: CATTAAAATCAGAGCGAGGC R: TGGCTGAACCCATTTTTGAT |
188 | [32] | |
| vanA |
F: GGGAAAACGACAATTGC R: GTACAATGCGGCCGTTA |
732 | [33] | |
| vanB |
F: GTGACAAACCGGAGGCGAGGA R: CCGCCATCCTCCTGCAAAAAA |
430 | [34] | |
Conventional polymerase chain reaction (PCR) was employed to amplify specific regions of these genes according to their thermal profiles with some modifications in the reagent concentrations. To this end, the final volume of each reaction was 12.5 µl, containing 100 ng of template DNA, 10 pmol of forward and reverse primers, and 6.25 µl of Go-TaqGreen Master Mix (Promega). Bacterial strains harboring these genes were used as a positive control, and ultrapure Milli-Q water was used as a negative control. The PCR products were stained with Blue Green (LGC Biotechnology) and subjected to electrophoresis in 1.5% agarose gel for 50 min at 100 V. The separated DNA bands were visualized and photographed by a photo documenter under ultraviolet light.
Statistical analysis
The results of microbiological analysis, polymerase chain reaction, and disk diffusion technique were expressed in relative and absolute frequencies [35].
Results
In this study, 57.63% of the CMT-positive samples (185/321) were also positive in the microbiological examination, and 59.45% (110/185) of the positive isolates obtained belonged to Staphylococcus spp. The MALDI-TOF MS analysis detected the following Staphylococcus spp. among the positive isolates: S. aureus, 68.1% (75/110); S. chromogenes, 12.7% (14/110); S. epidermidis, 5.4% (6/110); Staphylococcus sciuri, 4.5% (5/110); Staphylococcus warneri, 2.7% (3/110); Staphylococcus haemolyticus, 1.8% (2/110); Staphylococcus hominis, 1.8% (2/110); Staphylococcus arlettae, 0.9% (1/110); Staphylococcus capitis, 0.9% (1/110); and Staphylococcus gallinarum, 0.9% (1/110).
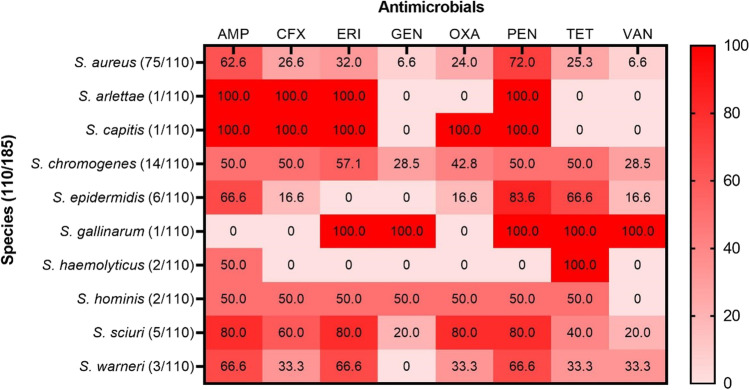
The results of the phenotypic resistance test, evaluating the resistance of Staphylococcus spp. to antimicrobials, demonstrated that 70.0% (77/110) and 61.8% (68/110) of Staphylococcus spp. isolates were resistant to penicillin and ampicillin, respectively. On the other hand, only 10.9% (12/110) and 11.8% (13/110) of the isolates were resistant to gentamicin and vancomycin, respectively. The distribution of phenotypic resistance of Staphylococcus species against each antimicrobial tested is described in Fig. 1.
Fig. 1.
Relative frequencies of phenotypic antimicrobial resistance for different species of Staphylococcus. Color variations show different percentages. 0 = no resistant samples, (AMP) ampicillin, (CFX) cefoxitin, (ERI) erythromycin, (GEN) gentamicin, (OXA) oxacillin, (PEN) penicillin, (TET) tetracycline, (VAN) vancomycin
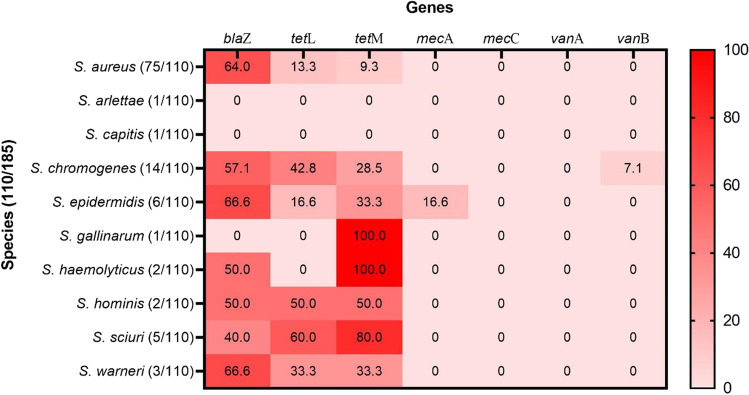
The genotypic resistance test in Staphylococcus spp. revealed the presence of the blaZ gene in 60.9% (67/110) of the isolates and the tetL and tetM in 20% (22/110) of the isolates, each. Additionally, 0.9% (1/110) of the isolates presented the mecA and vanB genes, while none of them had the mecC and vanA genes (Fig. 2).
Fig. 2.
Relative frequency of resistance genes for different species of Staphylococcus. Color variations show different percentages. 0 = absence of resistance gene
Of the samples carrying the blaZ gene, 83.5% (56/67) showed phenotypic resistance to penicillin; 63.6% (14/22) of the tetL positive samples were resistant to tetracycline in the disc-diffusion test, and 59.0% (13/22) of the positive tetL samples were also phenotypically resistant to tetracycline. One positive mecA sample (0.9%; 1/110) also presented phenotypical resistance to oxacillin and to cefoxitin, and one vanB positive sample (0.9% 1/110) was resistant to vancomycin in the phenotypical test.
Among the Staphylococcus spp. isolates, 10.9% (12/110) had both tetracycline resistance genes (tetL and tetM) and 7.2% (8/110) presented multiple genotypical resistance, presenting three resistance genes (blaZ, tetL, and tetM).
Discussion
This is the first study evaluating the occurrence of bovine subclinical mastitis caused by Staphylococcus spp. and investigating the phenotypic and genotypic resistance profile of these bacteria in Piauí, Brazil. Our findings concurred with that of most etiological studies (those conducted in Brazil and globally) on bovine subclinical mastitis that Staphylococcus spp. were predominant (59.45%; 110/185) in the milk samples extracted from cows affected with subclinical mastitis [7, 8, 12, 19, 21, 36–39]. This study can be considered the most widespread study in identifying all Staphylococcus spp. (S. arlettae, S. capitis, S. chromogenes, S. gallinarum, S. haemolyticus, S. hominis, S. sciuri, and S. warneri) responsible for causing bovine subclinical mastitis in the Northeast region of Brazil; most studies have only identified S. aureus and coagulase-negative staphylococci (CoNS) as the causative agents in this region [21, 22, 36, 39]. These species, while never identified in Northeast Brazil, have been identified in the South and Southeast regions of Brazil [38]. Additionally, our findings emphasize the importance of the MALDI-TOF MS technique in investigating the etiology of bovine subclinical mastitis, as it is quick, cost-efficient, and easily operatable [40].
Post identifying the bacterial species, we generated the phenotypic resistance profile of the Staphylococcus spp. isolates using the disk diffusion test. We observed that resistance to penicillin and ampicillin was above 60.0%. This observation can be attributed to the wide-scale use of these drugs in the treatment of this disease [21, 41]. On the other hand, less than 40.0% of the isolates displayed resistance against other antimicrobials.
Several factors are pointed out as possible causes of the emergence of bacterial isolates resistant to antimicrobials in the agricultural production environment, among them the high use of antimicrobials or their indiscriminate use stand out [42, 43]. Both characterize the acquired form of resistance, in which a bacterial population that was naturally susceptible to the antimicrobial becomes resistant due to mutations in chromosomal genes or due to the acquisition of external genetic determinants of resistance [44]. Particularly for beta-lactams (such as penicillin and ampicillin), antimicrobials with the highest percentage of resistant isolates detected in this study, two mechanisms responsible for resistance are frequently reported. The first is the production of enzymes that inactivate antimicrobials, resulting in the destruction of the beta-lactam ring; and the second is the modification of the antimicrobial target, causing a decrease, or total loss, of the affinity between the drug and its binding site [15, 45, 46]. These mechanisms were studied and detected in the present study, demonstrating their presence in Staphylococcus spp. that causes of subclinical mastitis in the state of Piaui.
Regarding gentamicin, 10.9% of the isolates were resistant, this low resistance of the Staphylococcus species isolated in this study may be related to its little use in dairy cattle, due to its toxic potential for animals and the prolonged residual power in milk [47, 48] and according to Awandkar et al. [49], the low preference to gentamicin in veterinary therapy may be the reason behind this high sensitivity.
Regarding phenotypic resistance and the search for penicillin resistance genes, 70% (77/110) of Staphylococcus spp. were resistant in the disk-diffusion test, and of these, 87.0% (67/77) carried the blaZ gene, being 64.0% (48/75) of the S. aureus species and 45.7% (16/35) of the CoNS group. This high number of blaZ-carrying Staphylococcus spp. have already been observed in studies conducted in Brazil, such as Krewer et al. [50], with 93.1% (203/218), Martini et al. [13] with 97.7% (88/90), Santos et al. [21] with 68.9% (111/161), and Silva et al. [22] with 74.07% (20/27), and in other regions of the world such as the USA, with 53.48% (46/86) by Ruegg et al. [51] and in China, with 94.6% (35/37) by Yang et al. [52]. Only S. arlettae, S. capitis, and S. gallinarum did not presented the blaZ gene. The gene blaZ increases the production of β-lactamases in a cell; thus, inactivating β-lactams and conferring resistance against these compounds in bacteria [53]. Resistance to β-lactams can be mainly attributed to the indiscriminate use of these antimicrobials in mastitis treatment [21] and the increased occurrence of the blaZ gene in several Staphylococcus spp. as was observed in this study.
Concerning the genes that confer resistance against tetracycline, 20.0% (22/110) of the Staphylococcus spp. isolates were positive for the tetL gene and 20.0% (22/110) for the tetM gene. In S. aureus, the frequency was 13.3% (10/75) for tetL and 9.3% (7/75) for tetM. A higher frequency was observed in the CoNS group, with 35.2% (12/35) for tetL and 42.8% (15/35) for tetM, with emphasis on S. chromogenes and S. sciuri. This study is the first in reporting the occurrence of tetracycline resistance genes tetL and tetM in CoNS in bovine subclinical mastitis isolates in the Northeast region of Brazil. Presently, there are only a few studies reporting these in S. aureus; however, they are restricted to the Southeast region of Brazil [13, 16], where frequencies of occurrence of these genes were observed to be 8.8% for tetL and 2.2% for tetM [13] and 1.61% for tetL and 3.22% for tetM [16]. It is important to highlight that the tetL and tetM have distinct mechanisms, one, caused by the tetL gene, is an antimicrobial efflux system, and the other, caused, by the tetM gene, caused ribosome protection, this portrays the bacterial versatility regarding resistance acquisition [30].
We found that only one (1/110) isolate of S. epidermidis harbored the mecA gene. To date, only one related study has demonstrated the presence of this gene in S. aureus and CoNS isolates from milk, environmental, and human samples in mastitis cases in the Northeast region of Brazil (Pernambuco) [14]. Moreover, studies have highlighted that methicillin-resistant CoNS are globally recognized as a major cause of persistent infections in humans and animals, particularly S. epidermidis, S. haemolyticus, and Staphylococcus lugdunensis [54–56].
In the present study, no Staphylococcus spp. presented the mecC gene, although this gene has been the target of several studies in Brazil, there are only two studies with positive samples [15, 57]. In the Americas, one of the first reports of finding this gene in a case of bovine mastitis was in an isolate of Staphylococcus saprophyticus in Argentina [58]. However, in Brazil, there are only two reports on the occurrence of this gene in a Staphylococcus spp. isolate from a case of bovine mastitis; the first in the state of Pernambuco in the northeast of the country [15] and the second in the Southeast region of Brazil [57], both reports identified the mecC gene in S. aureus isolates.
Regarding the presence of vancomycin resistance genes (vanA and vanB), the presence of the vanB gene was observed in one isolate (1/110), a Staphylococcus chromogenes, and the absence of the vanA gene in all isolates. There are reports of the occurrence of the vanA (15/178) and vanB (1/178) genes in Brazil in Staphylococcus species isolated from the milk of goats with mastitis [59], but until the completion of this research, there are no reports of the occurrence of these genes in Staphylococcus species or in other species of bacteria isolated from bovine mastitis in Brazil. This finding is unprecedented in Brazil and is alarming for public health issues, configuring as the first record of this gene (vanB) in a bacteria isolated from bovine mastitis.
The findings of mecA and vanB positive bacteria in bovine mastitis samples if of major impact, especially for public health, since, consuming milk that has not been correctly processed may cause infection in humans. The presence of mecA and vanB carrying bacteria is indicative of possible horizontal transmission of resistant bacteria from humans to animals, since, methicillin and vancomycin are not used in the treatment of animal infection. Another worrying fact is the occurrence of both mecA and vanB genes in the same study since vancomycin is considered the first choice of antibiotics used in treatment against methicillin-resistant Staphylococcus (MRS) [16, 60–66].
In late years, strains have been identified carrying two or more resistance genes, showcasing that bacterium may present different resistance mechanisms [13, 52]. It was noted that some bacteria in our study also harbored more than one resistance gene (blaZ, tetL, and tetM). The mecA and vanB genes were not associated with other genes; however, it was observed that both were expressed in the phenotypical resistance test.
Some strains carrying these genes did not display similar behavior in the disk-diffusion test, since bacterial strains may carry a resistance gene but may not express this gene; the phenotypic expression of the gene depends on several factors such as environmental conditions and the genetic context [67].
Conclusion
The identification of all Staphylococcus species in the present study related to mastitis cases, as well as its characterization of the phenotypic and genotypic resistance profile of these isolates for some classes of antimicrobials, has a high impact on the dairy region, since it will allow for the elaboration of control measures against this disease. Also, it is worrying that the circulation of antimicrobial-resistant samples in dairy farming, considering that antimicrobials, such as vancomycin and methicillin, are not used in the treatment of animal infections in Brazil.
Author contribution
RPO: study design, collections, processing, identification of isolates, PCR analysis, and writing. JGS: idealization of the study, processing, and identification of isolates. BBA: sample processing and gene identification by PCR. RGC and MAJ: identification of isolates by MALDI-TOF MS. JF: identification of tet and van genes, yielding controls and sending them for analysis. MPOF: idealization of the study and collection of samples. RAM: idealization of the study, processing, identification of grants, and writing. All authors were essential for the study, thank you all.
Data availability
Data sharing not applicable, all data generated are described in this study.
Declarations
Ethics approval
This study was approved by the Animal Use Ethics Committee of the Federal Rural University of Pernambuco under license number 79/2018.
Consent to participate
No humans participated in the study.
Consent for publication
The authors are giving their consent to the publisher to publish their manuscript upon acceptance.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mehmeti I, Behluli B, Mestani M, Ademi A, Nes IF, Diep DB. Antimicrobial resistance levels amongst staphylococci isolated from clinical cases of bovine mastitis in Kosovo. J Infect Dev Ctries. 2016;10:1081–1087. doi: 10.3855/jidc.7912. [DOI] [PubMed] [Google Scholar]
- 2.Liang D, Arnold LM, Stowe CJ, Harmon RJ, Bewley JM. Estimating US dairy clinical disease costs with a stochastic simulation model. J Dairy Sci. 2017;100:1472–1486. doi: 10.3168/jds.2016-11565. [DOI] [PubMed] [Google Scholar]
- 3.Keane OM. Symposium review: intramammary infections-Major pathogens and strain-associated complexity. J Dairy Sci. 2019;102:4713–4726. doi: 10.3168/jds.2018-15326. [DOI] [PubMed] [Google Scholar]
- 4.El Garch F, Youala M, Simjee S, Moyaert H, Klee R, Truszkowska B, Rose M, Hocquet D, Valot B, Morrissey I, de Jong A; VetPath Study Group (2020) Antimicrobial susceptibility of nine udder pathogens recovered from bovine clinical mastitis milk in Europe 2015-2016: VetPath results. Vet Microbiol 245:108644. 10.1016/j.vetmic.2020.108644 [DOI] [PubMed]
- 5.Gomes F, Henriques M. Control of bovine mastitis: old and recent therapeutic approaches. Curr Microbiol. 2016;72:377–382. doi: 10.1007/s00284-015-0958-8. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Lin X, Jiang T, Peng Z, Xu J, Yi L, Li F, Fanning S, Baloch Z. Prevalence and characterization of Staphylococcus aureus cultured from raw milk taken from dairy cows with mastitis in Beijing. China Front Microbiol. 2018;9:1123. doi: 10.3389/fmicb.2018.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren Q, Liao G, Wu Z, Lv J, Chen W. Prevalence and characterization of Staphylococcus aureus isolates from subclinical bovine mastitis in southern Xinjiang, China. J Dairy Sci. 2020;103:3368–3380. doi: 10.3168/jds.2019-17420. [DOI] [PubMed] [Google Scholar]
- 8.Gooraninejad S, Ghorbanpoor M, Salati AP. Antibiotic susceptibility of staphylococci isolated from bovine subclinical mastitis. Pak J Biol Sci. 2007;10:2781–2783. doi: 10.3923/pjbs.2007.2781.2783. [DOI] [PubMed] [Google Scholar]
- 9.Fessler A, Scott C, Kadlec K, Ehricht R, Monecke S, Schwarz S. Characterization of methicillin-resistant Staphylococcus aureus ST398 from cases of bovine mastitis. J Antimicrob Chemother. 2010;65:619–625. doi: 10.1093/jac/dkq021. [DOI] [PubMed] [Google Scholar]
- 10.Saab AB, Zamprogna TO, Lucas TM, Martini KC, Mello PL, Silva AV, Martins LA. Prevalence and etiology of bovine mastitis in the Nova Tebas, Paraná. Semina Ciênc Agrár. 2014;35:835–843. doi: 10.5433/1679-0359.2014v35n2p835. [DOI] [Google Scholar]
- 11.Klaas IC, Zadoks RN. An update on environmental mastitis: challenging perceptions. Transbound Emerg Dis. 2018;1:166–185. doi: 10.1111/tbed.12704. [DOI] [PubMed] [Google Scholar]
- 12.Antók FI, Mayrhofer R, Marbach H, Masengesho JC, Keinprecht H, Nyirimbuga V, Fischer O, Lepuschitz S, Ruppitsch W, Ehling-Schulz M, Feßler AT, Schwarz S, Monecke S, Ehricht R, Grunert T, Spergser J, Loncaric I. Characterization of antibiotic and biocide resistance genes and virulence factors of Staphylococcus species associated with bovine mastitis in Rwanda. Antibiotics (Basel) 2019;9:1. doi: 10.3390/antibiotics9010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martini CL, Lange CC, Brito MA, Ribeiro JB, Mendonça LC, Vaz EK. Characterisation of penicillin and tetracycline resistance in Staphylococcus aureus isolated from bovine milk samples in Minas Gerais. Brazil J Dairy Res. 2017;84:202–205. doi: 10.1017/S0022029917000061. [DOI] [PubMed] [Google Scholar]
- 14.Silva GJ, Camargo AC, Melo RPB, Aragão BB, Oliveira JMB, Sena MJ, Nero LA, Mota RA (2022) mecA positive Staphylococcus spp. in bovine mastitis, milkers, milking environment, and the circulation of different MRSA clones at dairy cows farms in the Northeast region of Brazil. Ciênc Rural 52(3):1–9. 10.1590/0103-8478cr20210008
- 15.Silva JG, Araujo WJ, Leite EL, Dias LM, Vasconcelos PC, Silva NMV, Oliveira RP, Sena MJ, Oliveira CJB, Mota RA. First report of a livestock-associated methicillin-resistant Staphylococcus aureus ST126 harbouring the mecC variant in Brazil. Transbound Emerg Dis. 2020;68:1019–1025. doi: 10.1111/tbed.13771. [DOI] [PubMed] [Google Scholar]
- 16.Pérez VKC, Custódio DAC, Silva EMM, de Oliveira J, Guimarães AS, Brito MAVP, Souza-Filho AF, Heinemann MB, Lage AP, Dorneles EMS. Virulence factors and antimicrobial resistance in Staphylococcus aureus isolated from bovine mastitis in Brazil. Braz J Microbiol. 2020;51:2111–2122. doi: 10.1007/s42770-020-00363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abd El-Aziz NK, Abd El-Hamid MI, Bendary MM, El-Azazy AA, Ammar AM. Existence of vancomycin resistance among methicillin resistant S. aureus recovered from animal and human sources in Egypt. Slov Vet Res. 2018;55:221–230. doi: 10.26873/SVR-649-2018. [DOI] [Google Scholar]
- 18.Coelho SM, Pereira IA, Soares LC, Pribul BR, Souza MM. Short communication: profile of virulence factors of Staphylococcus aureus isolated from subclinical bovine mastitis in the state of Rio de Janeiro, Brazil. J Dairy Sci. 2011;94:3305–3310. doi: 10.3168/jds.2010-3229. [DOI] [PubMed] [Google Scholar]
- 19.Oliveira CMC, Sousa MGS, Silva NDS, Mendonça CL, Silveira JAS, Oaigen RP, Barbosa JD. Prevalência e etiologia da mastite bovina na bacia leiteira de Rondon do Pará, estado do Pará. Pesq Veta Bras. 2011;31:104–110. doi: 10.1590/S0100-736X2011000200002. [DOI] [Google Scholar]
- 20.Melo P, Ferreira LM, Filho AN, Zafalon LF, Vicente HI, de Souza V. Comparison of methods for the detection of biofilm formation by Staphylococcus aureus isolated from bovine subclinical mastitis. Braz J Microbiol. 2013;44:119–124. doi: 10.1590/S1517-83822013005000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos AS, Lima DCV, Abad ACA, Silva JG, Oliveira JMB, Oliveira PRF, Amorim VS, Costa MM, Mota RA. High frequency of beta-lactam resistance among Staphylococcus aureus isolated from bovine mastitis in Northeast of Brazil. J\ Bacteriol Parasitol. 2018;18:01–06. [Google Scholar]
- 22.Silva ATF, da Silva JG, Aragão BB, Peixoto RM, Mota RA. Occurrence of β-lactam-resistant Staphylococcus aureus in milk from primiparous dairy cows in the northeastern region of Brazil. Trop Anim Health Prod. 2020;52:2303–2307. doi: 10.1007/s11250-020-02259-w. [DOI] [PubMed] [Google Scholar]
- 23.Martins RP, da Silva JAG, Nakazato L, Dutra V, de Almeida Filho ES. Prevalence and infectious etiology of bovine mastitis in the microregion of Cuiabá, MT, Brazil. Ciênc Anim Bras. 2010;11:181–187. doi: 10.5216/cab.v11i1.5085. [DOI] [Google Scholar]
- 24.Schalm OW, Noorlander DO. Experiments and observations leading to development of the California mastitis test. J Am Vet Med Assoc. 1957;130(5):199–204. [PubMed] [Google Scholar]
- 25.Wolters M, Rohde H, Maier T, Belmar-Campos C, Franke G, Scherpe S, Aepfelbacher M, Christner M. MALDI-TOF MS fingerprinting allows for discrimination of major methicillin-resistant Staphylococcus aureus lineages. Int J Med Microbiol. 2011;301:64–68. doi: 10.1016/j.ijmm.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496. doi: 10.1093/ajcp/45.4_ts.493. [DOI] [PubMed] [Google Scholar]
- 27.CLSI, PerforCLSI (2018) Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute. Retrieved from http://www.clsi.orgmance Standards for Antimicrobial Susceptibility Testing, Performance Standards for Antimicrobial Susceptibility Testing. Accessed July 2021
- 28.Fan HH, Kleven SH, Jackwood MW. Application of polymerase chain reaction with arbitrary primers to strain identification of Mycoplasma gallisepticum. Avian Dis. 1995;39(4):729–735. doi: 10.2307/1592409. [DOI] [PubMed] [Google Scholar]
- 29.Sawant AA, Gillespie BE, Oliver SP. Antimicrobial susceptibility of coagulase-negative Staphylococcus species isolated from bovine milk. Vet Microbiol. 2009;134:73–81. doi: 10.1016/j.vetmic.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Ng LK, Martin I, Alfa M, Mulvey M. Multiplex PCR for the detection of tetracycline resistant genes. Mol Cell Probes. 2001;15:209–215. doi: 10.1006/mcpr.2001.0363. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa S, Taneike I, Mimura D, Iwakura N, Nakayama T, Emura T, Kitatsuji M, Fujimoto A, Yamamoto T. Gene sequences and specific detection for Panton-Valentine leukocidin. Biochem Biophys Res Commun. 2005;328:995–1002. doi: 10.1016/j.bbrc.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 32.Paterson GK, Larsen AR, Robb A, Edwards GE, Pennycott TW, Foster G, Mot D, Hermans K, Baert K, Peacock SJ, Parkhill J, Zadoks RN, Holmes MA. The newly described mecA homologue, mecALGA251, is present in methicillin-resistant Staphylococcus aureus isolates from a diverse range of host species. J Antimicrob Chemother. 2012;67:2809–2813. doi: 10.1093/jac/dks329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutka-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995;33:24–27. doi: 10.1128/jcm.33.1.24-27.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark NC, Cooksey RC, Hill BC, Swenson JM, Tenover F. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob Agents Chemother. 1993;37:2311–2317. doi: 10.1128/AAC.37.11.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Field A. Discovering statistics using IBM SPSS statistics. Sage; 2013. [Google Scholar]
- 36.Mota RA, Medeiros ES, Santos MV, Pinheiro Júnior JW, Moura APB, Coutinho LCA. Participação de Staphylococcus spp na etiologia das mastites em bovinos leiteiros no estado de Pernambuco (Brasil) Ciênc Anim Bras. 2012;13:124–130. doi: 10.5216/cab.v13i1.3790. [DOI] [Google Scholar]
- 37.Aslantaş Ö, Demir C. Investigation of the antibiotic resistance and biofilm-forming ability of Staphylococcus aureus from subclinical bovine mastitis cases. J Dairy Sci. 2016;99:8607–8613. doi: 10.3168/jds.2016-11310. [DOI] [PubMed] [Google Scholar]
- 38.Mello PL, Riboli DFM, Martins LA, Brito MAVP, Victória C, Calixto Romero L, de Souza Ribeiro, da Cunha ML. Staphylococcus spp. isolated from bovine subclinical mastitis in different regions of Brazil: molecular typing and biofilm gene expression analysis by RT-qPCR. Antibiotics (Basel) 2020;9:888. doi: 10.3390/antibiotics9120888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva JGD, Barros M, Santos NDL, Paiva PMG, Napoleão TH, Sena MJ, Costa MMD, Oliveira HP, Moreira MAS, Mota RA. Antimicrobial activity of polypyrrole nanoparticles and aqueous extract of Moringa oleifera against Staphylococcus spp. carriers of multi-drug efflux system genes isolated from dairy farms. J Dairy Res. 2020;87:309–314. doi: 10.1017/S0022029920000874. [DOI] [PubMed] [Google Scholar]
- 40.Pasternak J. Novas metodologias de identificação de micro-organismos: MALDI-TOF. Einstein (São Paulo) 2012;10:118–119. doi: 10.1590/S1679-45082012000100026. [DOI] [PubMed] [Google Scholar]
- 41.Klimiene I, Virgailis M, Pavilonis A, Siugzdiniene R, Mockeliunas R, Ruzauskas M. Phenotypical and genotypical antimicrobial resistance of coagulase-negative staphylococci isolated from cow mastitis. Pol J Vet Sci. 2016;19:639–646. doi: 10.1515/pjvs-2016-0080. [DOI] [PubMed] [Google Scholar]
- 42.Raia Junior RB (2001) Influência da mastite na ocorrência de resíduos de antimicrobianos no leite. Dissertação (Mestrado) Universidade de São Paulo, São Paulo. 87 f
- 43.Guimarães FF (2011) Perfil de sensibilidade microbiana, pesquisa de gene mecA de resistência à meticilina e detecção molecular de genes codificadores de enterotoxinas, em espécies de estafilococos coagulase positiva e negativa isolados de mastites bovinas. Dissertação (Mestrado em Medicina Veterinária). Universidade Estadual Paulista Julio de Mesquita Filho
- 44.Munita JM, Arias CA (2016) Mechanisms of antibiotic resistance. Microbiol Spectr 4(2):1–2. 10.1128/microbiolspec.VMBF-0016-2015 [DOI] [PMC free article] [PubMed]
- 45.Costa SS, Viveiros M, Amaral L, Couto I. Multidrug Efflux Pumps em Staphylococcus aureus: uma Atualização. Open Microbiol J. 2013;7:59–71. doi: 10.2174/1874285801307010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar S, Mukherjee MM, Varela MF. Modulation of bacterial multidrug resistance efflux pumps of the major facilitator superfamily. Int J Bacteriol. 2013;2013:204141. doi: 10.1155/2013/204141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan X, Jiang YW, Huang YJ, Hu SH. Persistence of gentamicin residues in milk after the intramammary treatment of lactating cows for mastitis. J Zhejiang Univ Sci B. 2009;10(4):280–284. doi: 10.1631/jzus.B0820198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma D, Manimaran A, Kumaresan A, Sivaram M, Rajendran D. Antimicrobials use and their indications in dairy farm and individual farmer production conditions in southern India. Trop Anim Health Prod. 2021;54(1):29. doi: 10.1007/s11250-021-03025-2. [DOI] [PubMed] [Google Scholar]
- 49.Awandkar SP, Kulkarni MB, Khode NV. Bacteria from bovine clinical mastitis showed multiple drug resistance. Vet Res Commun. 2022;46(1):147–158. doi: 10.1007/s11259-021-09838-8. [DOI] [PubMed] [Google Scholar]
- 50.Krewer C, Santos Amanso E, Veneroni Gouveia G, de Lima SR, da Costa MM, Aparecido Mota R. Resistance to antimicrobials and biofilm formation in Staphylococcus spp. isolated from bovine mastitis in the Northeast of Brazil. Trop Anim Health Prod. 2015;47:511–518. doi: 10.1007/s11250-014-0752-9. [DOI] [PubMed] [Google Scholar]
- 51.Ruegg PL, Oliveira L, Jin W, Okwumabua O. Phenotypic antimicrobial susceptibility and occurrence of selected resistance genes in gram-positive mastitis pathogens isolated from Wisconsin dairy cows. J Dairy Sci. 2015;98:4521–4534. doi: 10.3168/jds.2014-9137. [DOI] [PubMed] [Google Scholar]
- 52.Yang F, Wang Q, Wang X, Wang L, Xiao M, Li X, Li H. Prevalence of blaZ gene and other virulence genes in penicillin-resistant Staphylococcus aureus isolated from bovine mastitis cases in Gansu, China. Turk J Vet Anim Sci. 2015;39:634–636. doi: 10.3906/vet-1504-81. [DOI] [Google Scholar]
- 53.Li S, Rong H, Zhang X, Zhang Z, Wang C, Tan R, Wang Y, Zheng T, Zhu T. Meta-analysis of topical vancomycin powder for microbial profile in spinal surgical site infections. Eur Spine J. 2019;28:2972–2980. doi: 10.1007/s00586-019-06143-6. [DOI] [PubMed] [Google Scholar]
- 54.Arnold AR, Burnham CA, Ford BA, Lawhon SD, McAllister SK, Lonsway D, Albrecht V, Jerris RC, Rasheed JK, Limbago B, Burd EM, Westblade LF. Evaluation of an immunochromatographic assay for rapid detection of penicillin-binding protein 2a in human and animal Staphylococcus intermedius group, Staphylococcus lugdunensis, and Staphylococcus schleiferi clinical isolates. J Clin Microbiol. 2016;54:745–748. doi: 10.1128/JCM.02869-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdel-Moein KA, Zaher HM. Occurrence of multidrug-resistant methicillin-resistant Staphylococcus aureus among healthy farm animals: a public health concern. Int J Vet Sci Med. 2019;7:55–60. doi: 10.1080/23144599.2019.1689630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chon JW, Lee UJ, Bensen R, West S, Paredes A, Lim J, Khan S, Hart ME, Phillips KS, Sung K. Virulence characteristics of mecA-positive multidrug-resistant clinical coagulase-negative staphylococci. Microorganisms. 2020;8:659. doi: 10.3390/microorganisms8050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alves MFNF, Penna B, Pereira RF, Geraldo RB, Folly E, Castro HC, Aguiar-Alves F. First report of meticillin-resistant Staphylococcus aureus harboring mecC gene in milk samples from cows with mastitis in southeastern Brazil. Braz J Microbiol. 2020;51:2175–2179. doi: 10.1007/s42770-020-00385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srednik ME, Archambault M, Jacques M, Gentilini ER. Detection of a mecC-positive Staphylococcus saprophyticus from bovine mastitis in Argentina. J Glob Antimicrob Resist. 2017;10:261–263. doi: 10.1016/j.jgar.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 59.Aragão BB, Trajano SC, de Oliveira RP, Sobral da Silva DM, de Carvalho RG, Juliano MA, Pinheiro Junior JW, Mota RA. Multiresistant zoonotic pathogens isolated from goat milk in Northeastern Brazil. Comp Immunol Microbio lInfect Dis. 2021;79:101701. doi: 10.1016/j.cimid.2021.101701. [DOI] [PubMed] [Google Scholar]
- 60.Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, Kolonay JF, Shetty J, Killgore GE, Tenover FC. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science. 2003;302:1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 61.Luna CM, Rodríguez-Noriega E, Bavestrello L, Gotuzzo E. Treatment of methicillin-resistant Staphylococcus aureus in Latin America. Braz J Infect Dis. 2010;2:S119–S127. doi: 10.1590/s1413-86702010000800007. [DOI] [PubMed] [Google Scholar]
- 62.Smith JR, Barber KE, Hallesy J, Raut A, Rybak MJ. Telavancin demonstrates activity against methicillin-resistant Staphylococcus aureus isolates with reduced susceptibility to vancomycin, daptomycin, and linezolid in broth microdilution MIC and one-compartment pharmacokinetic/pharmacodynamic models. Antimicrob Agents Chemother. 2015;59:5529–5534. doi: 10.1128/AAC.00773-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruniera FR, Ferreira FM, Saviolli LR, Bacci MR, Feder D, da Luz Gonçalves Pedreira M, SorginiPeterlini MA, Azzalis LA, Campos Junqueira VB, Fonseca FL. The use of vancomycin with its therapeutic and adverse effects: a review. Eur Rev Med Pharmacol Sci. 2015;19:694–700. [PubMed] [Google Scholar]
- 64.Geriak M, Haddad F, Rizvi K, Rose W, Kullar R, LaPlante K, Yu M, Vasina L, Ouellette K, Zervos M, Nizet V, Sakoulas G. Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2019;635:e02483–e2518. doi: 10.1128/AAC.02483-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li XZ, Mehrotra M, Ghimire S, Adewoye L. Beta-lactam resistance and beta-lactamases in bacteria of animal origin. Vet Microbiol. 2007;121:197–214. doi: 10.1016/j.vetmic.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 66.Ohata K, Kitagawa J, Niwa T, Takahashi-Yamauchi T, Harada S, Matsumoto T, Nakamura N, Nakamura H, Kanemura N, Shimizu M, Suzuki A. Comparison of breakthrough Gram-positive cocci infection during vancomycin vs teicoplanin therapy in patients receiving haematopoietic stem cell transplantation. J Clin Pharm Ther. 2020;45:1342–1348. doi: 10.1111/jcpt.13215. [DOI] [PubMed] [Google Scholar]
- 67.Hughes D, Andersson DI. Evolutionary trajectories to antibiotic resistance. Annu Rev Microbiol. 2017;71:579–596. doi: 10.1146/annurev-micro-090816-093813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable, all data generated are described in this study.