Abstract
In recent years, ample research has focused on applying wild (especially non-Saccharomyces) yeasts in producing alcoholic beverages. Common characteristics of wild yeast strains include simultaneous high production of fruity and floral aroma compounds and low ethanol production. In this study, mead starter cultures were selected based on preliminary screening of wild yeast strains from a Brazilian culture collection (n = 63) for their ability to produce aroma-active compounds. The selected strains included one strain of Saccharomyces cerevisiae and three non-Saccharomyces strains (Pichia jadinii, Torulaspora delbrueckii, and Kluyveromyces lactis). These strains were used to ferment honey must prepared with Aroeira honey, adjusted to 24°Brix, which took 36 days to complete. Single culture fermentations and co-fermentations with S. cerevisiae and non-Saccharomyces strains were carried out. The quality of the produced beverages was evaluated by sugar consumption and production of alcohols and organic acids, analyzed with high-performance liquid chromatography. The volatile organic compound composition was analyzed with gas chromatography-mass spectrometry. Meads with various ethanol amounts (4.7–11.0% v/v) and residual sugar contents (70.81–160.25 g l−1) were produced. In addition, in both single-strain fermentation and co-fermentation with S. cerevisiae, meads produced with either Torulaspora delbrueckii or Kluyveromyces lactis had a roughly three-fold higher content of honey-aroma compound phenethyl acetate and a higher hedonic impression score than meads produced with only S. cerevisiae. These results demonstrated non-Saccharomyces yeasts’ ability to increase aroma complexity and improve the sensory quality of low-alcoholic meads.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-022-00840-z.
Keywords: Culture collection, Alcoholic fermentation, Mead, Wild yeasts, Torulaspora delbrueckii, Kluyveromyces lactis
Introduction
Mead is a traditional, honey-based beverage and was among the most commonly consumed alcoholic drinks of the Egyptian, Ancient Greek, Roman civilizations, and medieval Europe[1]. Today, the popularity of mead has increased in the wake of the craft beer movement.
The beverage is produced by fermentation of a diluted honey solution, with the optional addition of salts supporting yeasts’ growth. The ratio of honey to water in the initial must vary from 2:1 to 1:3 or less, each producing a distinct mead type[2]. The final beverage can have an alcohol content between 4 and 18% v/v depending on the initial concentration of honey and the extent of fermentation. Meads can be dry or sweet, with residual sugar concentrations ranging from 2.5 to 28% w/w[3].
Yeasts’ primary role during mead production is metabolizing honey’s fermentable sugars into ethanol. In the process, various compounds are produced, including higher alcohols and esters. In an earlier study, 16 yeast-derived metabolites were identified that contribute to the sensory quality of mead[4]. Other studies established that aroma production by Saccharomyces cerevisiae starter cultures in mead was influenced by nitrogen supplementation[5] and inoculum size[6]. More recent studies also established a role for wild yeasts (including yeasts other than S. cerevisiae), such as Torulaspora delbrueckii[7], ethanol tolerant wild yeasts[8], and lactic acid-producing yeasts[9] of several different genera.
A growing interest in wild yeasts has spurred research on their application in alcoholic beverages. Non-Saccharomyces starter cultures are known for producing high amounts of certain aroma-active compounds, including esters associated with floral and fruity aromas[10, 11], and produce low-alcoholic beverages with an attractive aroma profile[12, 13]. A recent review highlighted interactions between wild strains of Saccharomyces and non-Saccharomyces yeasts that commonly occur in spontaneously fermented beverages and, in some cases, lead to an improved flavor profile[14].
Wild yeast starter cultures may be suitable for mead production for several reasons. First, commercial mead is habitually sold with a relatively high residual sugar concentration. This means that incomplete fermentation is not necessarily a problem for this beverage. Second, the floral and fruity aromas produced by wild yeasts may complement meads’ aroma profile since those aromas are associated with superior honey quality[15]. In other beverages, the same aroma compounds can be perceived as overwhelming when present in high amounts[16], but this may not be the case for mead.
This study aimed to select a wild yeast starter culture for mead production. Starter culture strains were selected in a two-step procedure, in which the first step screened strains for their ability to produce relevant aroma-active compounds. The second step applied the selected yeast cultures to mead production as single culture fermentations and co-fermentations of Saccharomyces and non-Saccharomyces strains. A similar multi-step approach for selecting non-Saccharomyces yeast starter cultures to be applied in alcoholic beverages was previously followed by Yin et al.[17]. This procedure ensures the selection of yeasts with distinct aroma profiles and other properties required for successful alcoholic fermentation (such as ethanol- and osmotolerance)[18, 19].
The quality of the beverage was assessed by analyzing its chemical composition and its sensory quality. Based on these results, it is discussed how wild yeast starter cultures modulate the aroma and sensory profile of low-alcoholic mead.
Materials and methods
Strains
All yeast isolates characterized in this study (n = 63) come from the Culture Collection of Agricultural Microbiology (CCMA), World Federation of Culture Collections accession number 1083, belonging to the Laboratory of Microbial Physiology and Genetics of the Universidade Federal de Lavras (UFLA), MG, Brazil. The yeasts have been isolated from various agricultural substrates, including indigenous beverages, coffee, cocoa, sugar cane, silage, and soil. These strains are identified based on phenotypic analyses, MALDI-TOF MS, and/or sequencing of rDNA regions. A complete list can be found in Table 1 of the Supplementary Materials, including their origin and identification information.
Storage and reactivation
Yeast stocks were stored at − 80 °C in 20% (w/v) glycerol. For reactivation, a loop of the stock material was streaked on a YEPG agar plate (1% (w/v) yeast extract, 2% soy peptone, 2% glucose, 2% agar), and incubated at 28 °C for at least 16 h, depending on the growth of the yeast.
Culture medium fermentations
To make an initial assessment of the aroma-producing ability of the yeast strains, they were inoculated in a culture medium. Analysis of their volatile compounds facilitated the subsequent selection of strains for the mead fermentations.
Pre-cultures were prepared by inoculating a 1.5 ml microtube containing 1 ml of YEPG broth (2% (w/v) yeast extract, 1% soy peptone, 2% glucose) with a loop of freshly reactivated yeast cell material from a single colony and incubated at 28 °C for 24 h without agitation.
To perform the culture medium fermentations, 50 ml tubes were filled with 45 ml of modified YEPG (0.5% (w/v) yeast extract, 0.5% soy peptone, 4% glucose)[10]. Each tube was inoculated with 450 μl of the pre-culture and incubated at 25 °C for 72 h in well-closed tubes without agitation. After 72 h, the cultures were centrifuged at 9000 rpm, 4 °C for 10 min, and supernatants were frozen at − 18 °C before analyzing their volatile composition.
Honey must fermentations
Honey produced by Apis mellifera from Myracrodruon urundeuva, locally known as aroeira honey[20], was obtained from a beekeeper in Taiobeiras, Minas Gerais, Brazil. With modifications, meads were prepared similarly as described by Sroka and Tuszyński[21]. The honey was diluted with mineral water (Ingai, Brazil) until the solution reached a Brix reading of 24 degrees. The must was supplemented with 0.45 g l−1 of diammonium phosphate (DAP) to prevent nitrogen limitation. No salts or vitamins were added, as benefits ascribed to these supplements lack empirical support[22]. The must was pasteurized in a water bath at 60 °C for 25 min. One-and-a-half-liter bottles were equipped with an airlock and filled with 1 l of the honey must.
Starter cultures were selected based on the results of the culture medium fermentations (see Results and discussion). The selected strains included one Saccharomyces cerevisiae and three non-Saccharomyces strains. Mead beverages were prepared with these individual strains and combinations of the Saccharomyces strain and individual non-Saccharomyces strains.
Pre-cultures were made by inoculating reactivated yeasts of selected strains from a single colony in the honey must and incubating for 24 h at 28 °C. These were enumerated in a Neubauer chamber under a light microscope with a 400 × magnification. Non-viable cells were stained with methylene blue, and daughter cells with less than half the parent cell size were not counted[23]. An appropriate amount of pre-culture was added to the honey must achieve an initial cell count of 105 cfu ml−1. Half this amount (5 × 104 cfu ml−1) was added for each strain in the mixed inoculations.
The bottles were incubated at 25 °C. Fermentations were monitored by CO2 release (weight loss of the bottles) and were completed after 36 days until the weight of all bottles differed by less than 2 g after measuring at two-day intervals. Samples (15 ml) were taken after 24-h, 48-h, then at 48-h intervals (first 10 days), then at 96-h intervals until the end of fermentation for analysis of organic acids, sugars, and ethanol content (all time points) and volatile composition (final time point). Samples were frozen at − 18 °C until further analysis. Fermentations were carried out in duplicate, but samples were taken from only one bottle (alternating) to limit the impact of sampling on the fermentation process at each intermediate time point.
Solid-phase micro-extraction
Volatile compounds were extracted as previously described[24] with minor modifications, using a manual headspace-solid phase micro-extraction procedure (HS–SPME) with a divinylbenzene/carboxen/polydimethylsiloxane 50/30 μm SPME fiber (Supelco Co., Bellefonte, PA, USA). Two ml of liquid sample was mixed with 0.5 g of sodium chloride to improve extraction efficiency[25] and placed in a 15 ml hermetically sealed vial. After equilibration at 60 °C for 15 min, the volatile compounds were extracted at 60 °C for 30 min. Desorption time on the column was 3 min.
Gas chromatography-mass spectrometry
Samples from culture medium fermentations and honey must fermentations were analyzed by gas chromatography-mass spectrometry (GC–MS) to determine their volatile compound composition. Operating conditions were as previously described[24]. A GCMS-QP2010 (Shimadzu), equipped with an OV Carbowax column (30 m × 0.25 mm × 0.25 μm) was used. The oven temperature was maintained at 50 °C for 5 min, then raised to 190 °C at 3 °C/min and maintained at 190 °C for 10 min. The injector and detector were maintained at 230 and 240 °C, respectively. The He carrier gas was maintained at a flow rate of 1.2 ml/min. Compound identification compared mass spectra to the NIST 11 library and the retention index based on an alkane series to data reported in the literature, as described elsewhere[26].
High-performance liquid chromatography
High-performance liquid chromatography samples were analyzed to determine the concentration of residual sugars, ethanol, and organic acids. They were prepared by centrifuging twice at 9000 rpm, 4 °C for 10 min, and filtering the second supernatant with a 0.22 μm nitrocellulose membrane. HPLC operating conditions, compound identification, and quantification were as previously described[27] with minor modifications. A Shimadzu liquid chromatography system (Shimadzu Corp., Japan) equipped with a dual detection system consisting of a UV–Vis detector (SPD 10Ai) and a refractive index detector (RID-10Ai) was used. A Shimadzu ion exclusion column, Shim-pack SCR-101 H (7.9 mm × 30 cm), was operated at 30 °C. A perchloric acid solution with pH 2.11 was used as the eluent at a 0.6 ml/min flow rate. Compounds were detected via RID (sugars, ethanol) or UV–Vis (organic acids).
Data analysis
GC–MS data were analyzed using Shimadzu’s proprietary software. Identifications were based on mass spectra from NIST 11 and Kovat’s retention index. Statistical analyses (ANOVA and Tukey’s HSD post hoc test) were performed using Python with Statsmodels. Heatmaps were produced using the web-based tool Morpheus (https://software.broadinstitute.org/morpheus) using z-values as input and clustering based on Pearson correlation. A principal component analysis (PCA) was carried out using R library ggfortify after log-normalization, centering, and scaling of the data[28].
Sensory analysis
A panel was composed of twenty-one untrained panelists aged 21–58, both male and female, who indicated that they enjoy drinking alcoholic beverages. Meads were served at room temperature using 15 ml servings in plastic cups. Samples were assigned random numeric codes, served in random order, and evaluated in two rounds, as Dashko et al. described[29]. Panelists were asked to taste each sample first and rank their overall impression on a structured 9-point hedonic scale[30]. Afterward, each sample was tested again to score taste attributes (sweet, sour) and flavor attributes (alcoholic, fruity, honey, floral, herbal, woody and intensity) on an intensity scale from 1 to 9. Attributes were based on those proposed by Castro-Vázquez et al.[31] to describe the sensory characteristics of honey but were adjusted to mead samples. Finally, panelists rinsed their mouths with water in between samples.
Results and discussion
Screening of volatile organic compound production in culture medium
This study selected wild yeast starter cultures for mead with a two-step selection procedure. As a first step, the metabolic fingerprint of 63 wild yeast isolates from the CCMA collection has been determined in lab-scale fermentations on a culture medium. The objective was to select yeasts with various flavor profiles characterized by the production of metabolites that may contribute to a pleasant aroma for application in producing alcoholic beverages such as mead.
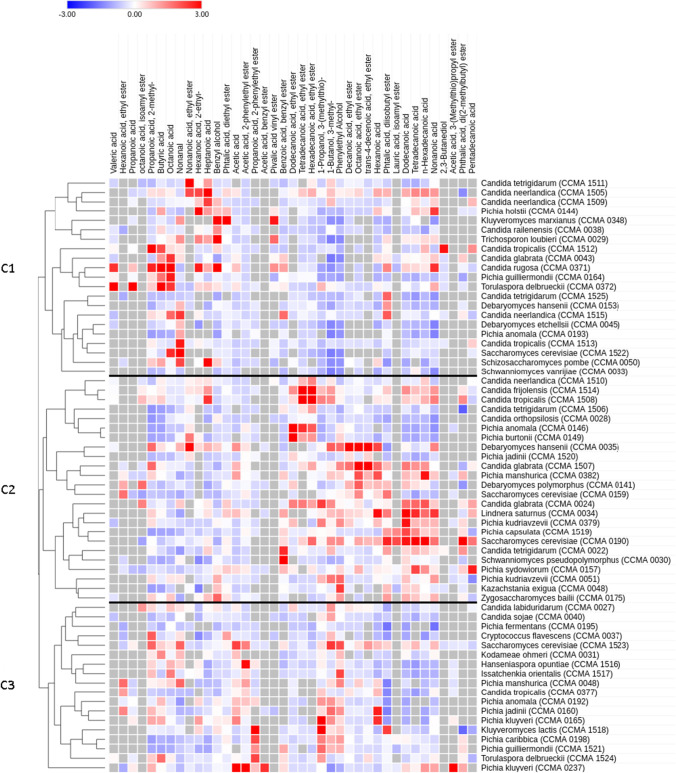
Across samples, 39 volatile organic compounds (VOC’s) were identified: 18 esters, 9 fatty acids, 6 acids, 5 alcohols, and 1 aldehyde. Results of the screening are presented in a heatmap (Fig. 1). In this figure, red squares indicate a production above the average among the yeasts in this screening for a particular aroma compound, whereas blue squares indicate below-average production. Compounds not detected for a given yeast strain were marked with a grey square.
Fig. 1.
Heatmap of volatile organic compounds produced by yeasts from the CCMA during culture medium fermentation. Numbers are z-values of chromatographic peak areas, and yeasts are clustered by Pearson correlation; main clusters are labeled C1-C3. Red squares indicate above-average production, blue squares below average, grey squares indicate undetected compounds
Several compounds produced during the culture medium fermentations are known to be aroma-active. Ethyl esters (such as ethyl hexanoate) are associated with various types of fruity odors. In contrast, aromatic alcohols and derived esters produced (phenethyl alcohol, phenethyl acetate, phenethyl propanoate, benzyl alcohol, and benzyl benzoate) represent floral, rose, honey, and or balsamic odors. Also, organic acids with cheesy and sweaty odors (propanoic acid, 2-methylpropanoic acid, valeric acid, butanoic, hexanoic, heptanoic, and nonanoic acid) were found, alongside the higher alcohol methionol, which is associated with a sulfurous odor[32].
The strains were clustered based on Pearson correlation to analyze the distribution of metabolite profiles among genera and species, which resulted in three main clusters. First, based on the similarity of their aroma profiles, the clustering method did not present a clear-cut separation according to genera or species. For example, strains of Pichia spp., Candida spp., and S. cerevisiae can be found among all three clusters. This finding might result from habitat adaptations as individual strains were isolated from substrates as diverse as coffee, cocoa, Amerindian beverages, silage, and soil. Previous studies on aroma production by yeasts also observed significant intraspecies variation[10].
Evaluating the VOC screening results, strains with an above-average production of the desirable aromas are primarily present in C3 of the heatmap in Fig. 1. This section contains several yeast species previously described as high producers of desirable aroma compounds during wine fermentation, including Torulaspora delbrueckii[33, 34] and Hanseniaspora opuntiae[35]. According to their position in this cluster, strains with closely related aroma production may produce aroma compounds in quantities relevant to alcoholic fermentations.
Selection of strains for mead fermentations
Increasing fruity and floral aroma has repeatedly been stated to be an objective of applying non-conventional yeasts in alcoholic fermentation[36, 37]. In this study, floral and fruity notes are also considered desirable since they are associated with superior honey quality[15] and may enhance the honey aroma of the mead beverage. In addition, cheese and lactic odors, which may be impacted by some of the organic and fatty acids encountered in this study, were deemed unpleasant during a sensory evaluation of mead[31].
Three non-Saccharomyces yeasts were selected for their ability to grow on honey must and complementary aroma profile to Saccharomyces cerevisiae. This way, it could be evaluated if co-fermentation with Saccharomyces and non-Saccharomyces wild yeasts positively influences the final beverage. The selected yeasts were Pichia jadinii CCMA 0160 (hereafter PJ) for its high production of ethyl esters, Torulaspora delbrueckii CCMA 1524 (TD), and Kluyveromyces lactis CCMA 1518 (KL) for their high production of phenethyl esters. S. cerevisiae CCMA 1523 (SC) was initially isolated from low-alcoholic cassava fermentation; the aroma screening stood out for its high phenyl ethanol production.
The selected yeast strains (n = 4) were used as single-strain starter cultures in the mead production. Each of the non-Saccharomyces yeast strains was also applied in co-culture with SC (SC + PJ, SC + TD, SC + KL).
A small-scale intermediate test confirmed that all selected strains could grow on honey must and that the main flavor characteristics for which they were selected were reproduced in mead (data not shown).
Consumption of sugars and production of ethanol, glycerol, and organic acids
The fermentations were monitored via the release of CO2 (Fig. 1 of Supplementary Materials), which revealed that the fermentations had lag phases between 24–96 h and took 36 days to complete.
Upon completion of fermentation, consumption of sugars and production of glycerol and ethanol were determined (Table 1). They must contain the fermentable sugars glucose, fructose, and maltose in a total concentration of 243.41 g l−1. Honey of Myracrodruon urundeuva flowers contained negligible amounts of sucrose due to invertase in the honey[20], and in the honey-must prepared for this study, it was not detected. All yeasts showed a slight preference for glucose consumption over fructose, as is commonly observed for Saccharomyces, Pichia, and Torulaspora wine yeasts[38, 39]. Maltose remained mostly unfermented.
Table 1.
Concentrations (g l−1, mean ± sd) of sugars and alcohols in honey must and in mead after 36 days of fermentation. SC Saccharomyces cerevisiae, PJ Pichia jadinii, TD Torulaspora delbrueckii, KL Kluyveromyces lactis
| Must | SC | PJ | TD | KL | SC + PJ | SC + TD | SC + KL | |
|---|---|---|---|---|---|---|---|---|
| Glucose | 92.33 ± 2.56 | 15.04 ± 4.70b | 5.22 ± 0.39 b | 48.93 ± 8.66a | 38.89 ± 8.00a | 6.72 ± 1.21 b | 15.68 ± 4.04b | 15.71 ± 4.28b |
| Fructose | 115.16 ± 3.24 | 42.66 ± 8.42bcd | 33.79 ± 3.92 cd | 81.02 ± 6.93ab | 70.33 ± 9.84abc | 33.99 ± 0.23 cd | 42.28 ± 8.74bcd | 42.99 ± 8.05bcd |
| Maltose | 35.92 ± 0.88 | 30.37 ± 4.10 | 32.17 ± 5.56 | 30.30 ± 1.89 | 29.48 ± 2.23 | 30.10 ± 1.56 | 30.52 ± 3.37 | 29.68 ± 1.11 |
| Ethanol | nd | 71.11 ± 7.16ab | 87.15 ± 17.03a | 37.48 ± 8.21b | 46.83 ± 1.38b | 68.68 ± 13.54ab | 73.01 ± 6.43ab | 67.63 ± 2.72ab |
| Glycerol | nd | 5.49 ± 0.97 a | 4.98 ± 0.57 ab | 3.65 ± 0.41 ab | 2.75 ± 0.78 b | 4.66 ± 0.42 ab | 5.72 ± 0.60 a | 5.05 ± 0.48 ab |
nd not detected; different letters in the same row mean samples are significantly different according to One-way ANOVA with Tukey’s HSD post hoc test (P < 0.05)
The mead beverages had 70.81 g l−1 and 160.25 g l−1 of residual sugars and between 37.48 g l−1and 87.15 g l−1 of ethanol (corresponding to 4.7–11.0% v/v). The highest ethanol concentration was found in meads produced with PJ, although it was not significantly different from meads obtained with SC and mixed starter cultures. Meads obtained with single-strain starter cultures of TD and KL contained the lowest ethanol and significantly higher residual sugars than all other samples.
PJ’s high ethanol production (in a strain initially isolated from coffee) was remarkable. While strains of S. cerevisiae usually produce higher amounts of ethanol than non-conventional yeasts, previous studies identified strains of non-conventional yeast species, including Pichia kudriavzevii and Pichia anomala, as high ethanol producers, some with potential for bioethanol production[40, 41]. In mead, a wild strain of Lachancea cidri has been reported to achieve an ethanol content of 9.7% v/v[8].
The primary organic acids that were produced during the fermentation were citric acid (6.00–12.33 g l−1) and acetic acid (0.24–0.38 g l−1) (Fig. 2 of Supplementary Materials). Succinic acid and malic acid were present in the honey-must and persisted during the fermentation, although the latter’s concentration decreased over time.
Mead is a beverage that can be produced in a variety of styles. Previous studies on commercial meads’ composition found that most meads contain approximately 13% v/v of ethanol (in a range from 4.0 to 20.8% v/v) and between 25 and 278 g l−1 of residual sugar. [3, 42]. Therefore, the mead beverages produced for this study can be characterized as low-alcoholic, while the amounts of residual sugar are within the typical range for commercial meads.
Production of aroma compounds
Volatile organic compounds present in the meads obtained in this study were analyzed using gas chromatography-mass spectrometry (Table 2). A total of 40 compounds were detected, of which 19 also occurred in the must.
Table 2.
Volatile organic compounds detected in mead samples by HS-SPME/GC–MS (logarithmic peak areas, mean ± sd). SC Saccharomyces cerevisiae, PJ Pichia jadinii, TD Torulaspora delbrueckii, KL Kluyveromyces lactis
| Compound | Must | SC | PJ | TD | KL | SC + PJ | SC + TD | SC + KL | ANOVA |
|---|---|---|---|---|---|---|---|---|---|
| Alcohols | |||||||||
| 1-Butanol, 3-methyl | 6.14 ± 0.25ab | 5.96 ± 0.15ab | 5.59 ± 0.07b | 5.82 ± 0.05ab | 6.09 ± 0.18ab | 5.81 ± 0.18ab | 6.21 ± 0.00a | * | |
| 2,3-Butanediol | 4.75 ± 0.31a | 4.38 ± 0.00a | nd | 4.22 ± 0.18a | 5.05 ± 0.20a | 4.89 ± 0.10a | 4.80 ± 0.06a | *** | |
| Benzyl alcohol | X | 6.13 ± 0.20 | 4.97 ± 0.10 | 5.49 ± 0.05 | 5.80 ± 0.05 | 5.87 ± 0.59 | 5.41 ± 0.17 | 5.71 ± 0.59 | NS |
| Phenethyl alcohol | X | 7.29 ± 0.03 | 6.99 ± 0.09 | 7.08 ± 0.22 | 7.08 ± 0.16 | 7.48 ± 0.11 | 7.45 ± 0.14 | 7.46 ± 0.08 | * |
| p-anisylalcohol | X | 5.17 ± 0.04 | 4.60 ± 0.26 | 5.27 ± 0.37 | 5.38 ± 0.56 | 5.61 ± 0.21 | 5.47 ± 0.39 | 5.24 ± 0.03 | NS |
| 2-(4-methoxyphenyl)-ethanol | 5.09 ± 0.04 | 4.58 ± 0.00 | 4.84 ± 0.45 | 4.68 ± 0.51 | 5.48 ± 0.12 | 5.35 ± 0.39 | 5.15 ± 0.25 | NS | |
| Acids | |||||||||
| Butanoic acid, 3-methyl | X | 5.22 ± 0.00 | 4.81 ± 0.10 | 4.83 ± 0.07 | 4.92 ± 0.03 | 5.18 ± 0.05 | 4.92 ± 0.16 | 5.35 ± 0.46 | NS |
| Benzeneacetic acid, 4-methoxy | 5.78 ± 0.13a | 5.36 ± 0.00ab | 4.88 ± 0.34b | 5.59 ± 2.30ab | 5.94 ± 0.05a | 5.45 ± 0.26ab | 5.90 ± 0.15a | ** | |
| Octanoic acid | X | 6.27 ± 0.09a | 6.04 ± 0.13a | 5.97 ± 0.17ab | 5.34 ± 0.34b | 6.57 ± 0.11a | 6.20 ± 0.06a | 6.36 ± 0.14a | ** |
| Nonanoic acid | X | 5.22 ± 0.19 | 4.76 ± 0.27 | 4.88 ± 0.23 | 4.82 ± 0.37 | 5.29 ± 0.16 | 4.95 ± 0.18 | 5.27 ± 0.05 | NS |
| N-decanoic acid | X | 6.26 ± 0.15ab | 6.10 ± 0.17ab | 5.74 ± 0.30bc | 5.10 ± 0.19c | 6.67 ± 0.28a | 6.20 ± 0.01ab | 6.31 ± 0.06ab | ** |
| Benzoic acid | X | 5.43 ± 0.00 | 4.45 ± 0.03 | 5.25 ± 0.97 | 4.63 ± 0.13 | 5.38 ± 0.04 | 5.12 ± 0.22 | 4.84 ± 0.25 | NS |
| Dodecanoic acid | X | 5.48 ± 0.07ab | 5.13 ± 0.16ab | 5.10 ± 0.31ab | 4.92 ± 0.20b | 5.77 ± 0.25a | 5.37 ± 0.03ab | 5.61 ± 0.08ab | * |
| Tetradecanoic acid | X | 4.70 ± 0.26 | 4.33 ± 0.09 | 4.18 ± 0.44 | 4.23 ± 0.32 | 4.79 ± 0.37 | 4.63 ± 0.08 | 4.72 ± 0.07 | NS |
| Esters | |||||||||
| Octanoic acid, ethyl ester | 5.61 ± 0.08ab | 5.70 ± 0.08ab | 5.27 ± 0.33ab | 5.19 ± 0.20b | 5.95 ± 0.03a | 5.68 ± 0.00ab | 5.81 ± 0.25ab | * | |
| Decanoic acid, ethyl ester | 6.12 ± 0.00a | 6.15 ± 0.19a | 5.65 ± 0.21ab | 4.79 ± 0.15b | 6.46 ± 0.28a | 5.66 ± 0.65ab | 6.26 ± 0.10a | * | |
| Ethyl 9-decenoate | 5.41 ± 0.33 | 5.01 ± 0.52 | 4.89 ± 0.19 | 4.58 ± 0.29 | 5.32 ± 0.08 | 5.18 ± 0.14 | 5.14 ± 0.07 | NS | |
| Benzeneacetic acid, ethyl ester | 5.28 ± 0.09ab | 4.90 ± 0.13c | 4.91 ± 0.00c | 4.95 ± 0.00bc | 5.21 ± 0.05abc | 5.03 ± 0.09abc | 5.39 ± 0.10ab | ** | |
| Acetic acid, 2-phenylethyl ester | 6.09 ± 0.01bc | 5.74 ± 0.00c | 6.66 ± 0.07a | 6.61 ± 0.13a | 6.16 ± 0.14bc | 6.37 ± 0.19abc | 6.59 ± 0.14a | ** | |
| Dodecanoic acid, ethyl ester | 5.92 ± 0.09ab | 5.72 ± 0.18ab | 5.49 ± 0.02ab | 5.15 ± 0.01b | 6.09 ± 0.39a | 5.67 ± 0.37ab | 6.04 ± 0.04a | * | |
| Tetradecanoic acid, ethyl ester | 5.21 ± 0.19ab | 4.72 ± 0.17b | 5.01 ± 0.06ab | 5.28 ± 0.05ab | 5.40 ± 0.10ab | 5.39 ± 0.30ab | 5.50 ± 0.15a | * | |
| Pentadecanoic acid, ethyl ester | 4.93 ± 0.35 | 4.32 ± 0.33 | 4.25 ± 0.36 | 4.56 ± 0.36 | 4.99 ± 0.24 | 4.95 ± 0.03 | 4.92 ± 0.26 | NS | |
| Ethyl anisate | 5.29 ± 0.11a | 4.74 ± 0.06b | 4.84 ± 0.08b | 5.14 ± 0.13ab | 5.44 ± 0.02a | 5.23 ± 0.15a | 5.48 ± 0.12a | ** | |
| Ethyl 4-methoxyphenylacetate | X | 5.88 ± 0.01ab | 5.43 ± 0.13b | 5.45 ± 0.18b | 5.70 ± 0.22ab | 6.21 ± 0.08a | 5.92 ± 0.12ab | 6.02 ± 0.19ab | ** |
| Hexadecanoic acid, ethyl ester | X | 5.27 ± 0.23abc | 4.82 ± 0.07c | 4.77 ± 0.13c | 5.03 ± 0.12bc | 5.61 ± 0.10ab | 5.43 ± 0.15ab | 5.63 ± 0.09ab | ** |
| Ethyl 9-hexadecenoate | 5.08 ± 0.19a | nd | 4.58 ± 0.19a | 4.74 ± 0.09a | nd | 5.07 ± 0.37a | 5.25 ± 0.18a | *** | |
| Decanedioic acid, diethyl ester | 4.89 ± 0.01abc | 4.39 ± 0.13 cd | 4.25 ± 0.22d | 4.47 ± 0.14 cd | 5.21 ± 0.17ab | 4.98 ± 0.15ab | 5.17 ± 0.01ab | ** | |
| 2-decenedioic acid, diethyl ester | 4.75 ± 0.03a | nd | nd | 4.39 ± 0.41a | 5.02 ± 0.11a | 4.78 ± 0.32a | 4.90 ± 0.09a | *** | |
| Lactones | |||||||||
| γ-Nonalactone | 4.92 ± 0.09ab | 4.43 ± 0.14c | 4.60 ± 0.17abc | 4.72 ± 0.11abc | 5.08 ± 0.11ab | 4.81 ± 0.03abc | 4.99 ± 0.08ab | ** | |
| γ-Decalactone | 4.76 ± 0.09ab | 4.31 ± 0.10c | 4.44 ± 0.14bc | 4.60 ± 0.05abc | 4.96 ± 0.11ab | 4.61 ± 0.05abc | 4.88 ± 0.17ab | ** | |
| Terpenoids | |||||||||
| Trans-linalool oxide | X | 5.32 ± 0.07a | 5.09 ± 0.03ab | 4.91 ± 0.01b | 5.09 ± 0.10ab | 5.36 ± 0.05a | 5.14 ± 0.16ab | 5.40 ± 0.00a | ** |
| Cis-linalool oxide | X | 4.66 ± 0.18a | 4.35 ± 0.06a | nd | 4.48 ± 0.04a | 4.68 ± 0.10a | nd | 4.72 ± 0.07a | *** |
| ß-linalool | X | 5.01 ± 0.01 | 4.90 ± 0.10 | 4.95 ± 0.12 | 4.99 ± 0.09 | 5.00 ± 0.10 | 4.98 ± 0.13 | 5.11 ± 0.01 | NS |
| Hotrienol | X | 5.25 ± 0.10 | 5.20 ± 0.05 | 5.04 ± 0.08 | 5.11 ± 0.16 | 5.20 ± 0.52 | 5.30 ± 0.19 | 5.43 ± 0.09 | NS |
| Epoxylinalol | X | 4.88 ± 0.04ab | 4.45 ± 0.06b | 4.67 ± 0.20ab | 4.69 ± 0.17ab | 5.00 ± 0.07a | 4.78 ± 0.04ab | 4.94 ± 0.10a | * |
| Others | |||||||||
| ß-Damascenone | 4.81 ± 0.11a | 4.61 ± 0.04a | 4.67 ± 0.07a | 4.61 ± 0.26a | nd | nd | 5.00 ± 0.13a | *** | |
| Acetoin | 6.03 ± 0.00a | nd | nd | nd | 5.33 ± 0.28a | 5.36 ± 0.38a | 5.87 ± 0.42a | *** | |
| 1-Propanol, 3-ethoxy | nd | nd | 4.40 ± 0.39a | 4.56 ± 0.09a | nd | 4.67 ± 0.08a | 4.70 ± 0.04a | *** | |
| Benzaldehyde | X | 5.66 ± 0.06 | 5.49 ± 0.44 | 5.44 ± 0.15 | 5.28 ± 0.67 | 5.29 ± 0.25 | 5.98 ± 0.12 | 5.63 ± 0.20 | NS |
| p-Anisaldehyde | X | 5.76 ± 0.09 | 5.04 ± 0.19 | 5.20 ± 0.28 | 5.51 ± 0.20 | 5.77 ± 0.02 | 5.72 ± 0.23 | 5.80 ± 0.21 | * |
X = compound is present in must; nd not detected; ANOVA analysis of variance: NS not significant. * = P < 0.05. ** = P < 0.01. *** = P < 0.001; different letters in same row mean samples are significantly different according to Tukey’s HSD post hoc test (P < 0.05)
A principal component analysis (PCA) was performed on the volatile composition of meads (Fig. 2). Meads produced with SC and all mixed starter cultures occur in a single cluster, separated from single strain starter cultures of PJ, TD, and KL along PC1 (explaining 60.85% of variance), whereas KL and TD are separated from PJ and SC along PC2 (explaining 15.85% of variance).
Fig. 2.
Principal component analysis biplot of mead samples based on logarithmic peak areas of volatile organic compounds detected by GC–MS. SC = Saccharomyces cerevisiae, PJ = Pichia jadinii, TD = Torulaspora delbrueckii, KL = Kluyveromyces lactis
Based on the chromatographic data, the majority of compounds was produced in larger amounts by SC and its co-cultures than by the non-Saccharomyces yeasts. This is confirmed by the PCA biplot, where most compounds are associated with the SC cluster. However, a number of compounds are associated with the non-Saccharomyces yeasts, in particular TD and KL.
Looking at specific aroma compounds produced by individual yeasts, TD and KL produced 372 and 331% more phenethyl acetate than SC but lower fatty acid ethyl esters. Such differences can influence meads’ aroma profile as phenethyl acetate contributes to floral aromas (tobacco, rose) and may therefore amplify the honey and floral aroma of the mead.
The meads produced by TD were also characterized by the absence of 2,3-butanediol (buttery notes) and low production of 3-methyl-1-butanol, which can cause off-flavors at sufficiently high concentrations[43].
P. jadinii produced relatively high amounts of fatty acid ethyl and low acetate esters. Compared with S. cerevisiae, it produced lower amounts of several essential aroma compounds, including phenethyl alcohol (rose aroma), and γ-decalactone (peach aroma). Although it has a strong fermentation capacity, it is not matched by a high production of aroma compounds, which may result in beverages with low complexity.
In mixed fermentations, SC had a more significant influence on the aroma profile of mixed starter cultures than the non-conventional yeasts. This finding could mean that SC negatively influenced the growth and performance of non-Saccharomyces strains. Although it was not within the scope of this study to investigate such interactions, others reported that wild strains of Saccharomyces cerevisiae could inhibit the growth of non-Saccharomyces yeasts by various mechanisms, ranging from ethanol production and competition for nutrients to the production of killer toxins[14].
In some cases, aroma profiles of individual yeast strains did enrich each other in the mixed starter culture. For example, the meads produced with SC and TD contained high ethyl octanoate (characteristic of SC) and phenethyl acetate (characteristic of TD). Similar results were observed for mixed cultures of SC and KL; ethyl octanoate production in the mixed culture of SC and KL was higher than that of KL (by 417%) and that of SC (increasing by 58%). Ethyl octanoate is known to have a fruity, flowery aroma[44]. It has a low odor threshold, and its occurrence has a meaningful impact on the characteristics of alcoholic beverages[45].
Adding complexity to the aroma profile is one of the main reasons to use mixed starter cultures, which could occur if both strains persist during the fermentation and carry out their specific metabolic activities [46].
In some cases, mixed cultures produce higher or lower amounts of specific metabolites than expected based on single strain fermentations, for example, due to synergistic effects caused by extracellular enzymes[47] or shifts in the extracellular environment caused by the co-inoculated strain[48].
Sensory analysis
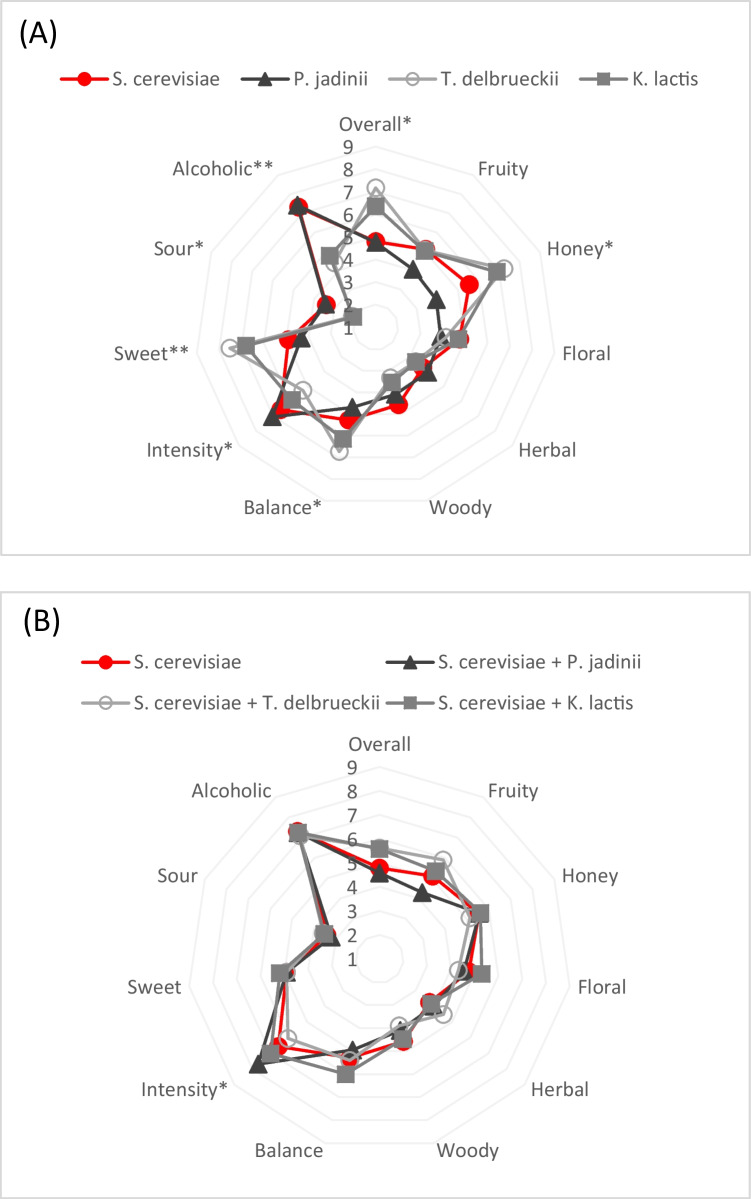
A sensory analysis was performed to evaluate the intensity of several taste and flavor attributes in the meads and the panelists’ overall hedonic impression. The results enable comparisons between single strain starter cultures (Fig. 3a) and mixed starter cultures (Fig. 3b). Differences were more pronounced and statistically significant between single-strain starter cultures than mixed starter cultures.
Fig. 3.
Average scores (scale 1–9) as judged by a panel (n = 21), including overall impression and intensity of taste and flavor attributes, comparing meads produced with single strain S. cerevisiae with other single culture inoculations (A) and mixed culture inoculations (B). Stars next to attribute labels indicate significance level according to ANOVA (*: P < 0.01, **: P < 0.001)
Mead produced by a single strain starter culture of TD obtained the most robust overall impression and balance score, followed by mead produced with KL. Panelists identified these meads as sweeter, less sour, and less alcoholic than other mead samples, agreeing with the actual composition of sugars, ethanol, and acids reported here. The meads fermented with SC obtained a lower overall score than those fermented with SC + TD and SC + KL. Because each of these meads had similar ethanol and residual sugar content, this indicates that the aroma compounds produced by the non-Saccharomyces yeasts contributed to their greater acceptability.
The high ANOVA significance level of sweetness and alcohol (P < 0.001) might indicate that panelists were more familiar with these descriptors. However, untrained panels often find it difficult to distinguish specific odor attributes in complex beverages[49], perhaps explaining why perceived differences in odor attributes such as fruity, floral, and herbal were not statistically significant.
Although it is possible that fermentation-derived aroma compounds such as ethyl octanoate and phenethyl acetate caused the higher acceptability of meads fermented by non-Saccharomyces yeasts, its high residual sweetness may also have contributed to this result. Previous studies on the influence of sweetness and ethanol content on mead acceptability found that the sweetest meads included had the highest acceptability[50, 51]. Sweetness is an essential characteristic of mead and depends on the initial ratio of honey and water used and the extent of fermentation. Preferences may differ by region; in one study, commercial meads from South Africa contained an average of 67–77 g l−1 of residual sugar, whereas commercial Slovak meads contained 137–200 g l−1 [42]. The Brazilian panelists who evaluated this study’s meads may share the preference for sweeter meads, or dryer mead may be an acquired taste to which they had no previous exposure.
Conclusions
The two-step screening method used in this study proved to be a suitable selection mechanism for wild yeast starter cultures in mead. Clustering yeasts based on the correlation between aroma profiles facilitated the selection of yeasts with interesting aroma profiles for alcoholic fermentation.
The yeasts T. delbrueckii and K. lactis are promising candidates for inclusion in starter cultures. Meads produced by these yeasts, alone or in co-fermentation with S. cerevisiae, had higher contents of the honey aroma-compound phenethyl acetate and higher overall sensory impression scores than meads produced by S. cerevisiae on their own.
Overall, the results demonstrate that fermenting mead with wild yeast strains can produce a beverage with a low to moderate ethanol content, high residual sugar content, and a high content of floral and fruity aroma compounds. Using mixed starter cultures of wild Saccharomyces and non-Saccharomyces yeasts increased the complexity of the aroma profile.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Rosane Freitas Schwan and Joshua Johannes van Mullem contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Joshua Johannes van Mullem and Jing Zhang. Joshua Johannes van Mullem wrote the first draft of the manuscript, and all authors commented on previous versions. Rosane Freitas Schwan acquired funding. The research was supervised by Rosane Freitas Schwan and Disney Ribeiro Dias. All authors read and approved the final manuscript.
Funding
This work was supported by the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joshua Johannes Van Mullem, Email: josvanmullem@gmail.com.
Jing Zhang, Email: jinghuanzhang0712@outlook.com.
Disney Ribeiro Dias, Email: diasdr@ufla.br.
Rosane Freitas Schwan, Email: rschwan@ufla.br.
References
- 1.Vidrih R, Hribar J (2016) Mead: The Oldest Alcoholic Beverage. In: Traditional Foods. Boston, MA: Springer US 325–338. 10.1007/978-1-4899-7648-2_26
- 2.Ramalhosa E, Gomes T, Pereira AP, Dias T, Estevinho LM. Mead production: tradition versus modernity. Adv Food Nutr Res. 2011;63:101–118. doi: 10.1016/B978-0-12-384927-4.00004-X. [DOI] [PubMed] [Google Scholar]
- 3.Steinkraus KH, Morse RA. Chemical Analysis of Honey Wines. J Apic Res. 1973;12(3):191–195. doi: 10.1080/00218839.1973.11099749. [DOI] [Google Scholar]
- 4.Mendes-Ferreira A, Cosme F, Barbosa C, Falco V, Inês A, Mendes-Faia A. Optimization of honey-must preparation and alcoholic fermentation by Saccharomyces cerevisiae for mead production. Int J Food Microbiol. 2010;144(1):193–198. doi: 10.1016/J.IJFOODMICRO.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Pereira AP, Mendes-Ferreira A, Oliveira JM, Estevinho LM, Mendes-Faia A. Mead production: effect of nitrogen supplementation on growth, fermentation profile and aroma formation by yeasts in mead fermentation. J Inst Brew. 2015;121(1):122–128. doi: 10.1002/jib.184. [DOI] [Google Scholar]
- 6.Pereira AP, Mendes-Ferreira A, Oliveira JM, Estevinho LM, Mendes-Faia A. High-cell-density fermentation of Saccharomyces cerevisiae for the optimisation of mead production. Food Microbiol. 2013;33(1):114–123. doi: 10.1016/J.FM.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Barry JP, Metz MS, Hughey J, Quirk A, Bochman ML (2018) Two novel strains of Torulaspora delbrueckii isolated from the honey bee microbiome and their use in honey fermentation. Fermentation 4(2). 10.3390/fermentation4020022
- 8.Villarreal P, Quintrel PA, Olivares‐Muñoz S, Ruiz JI, Nespolo RF, Cubillos FA (2021) Identification of new ethanol‐tolerant yeast strains with fermentation potential from central Patagonia. Yeast yea.3662. 10.1002/yea.3662 [DOI] [PubMed]
- 9.Peepall C, Nickens DG, Vinciguerra J, Bochman ML. An organoleptic survey of meads made with lactic acid-producing yeasts. Food Microbiol. 2019;82:398–408. doi: 10.1016/j.fm.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Gamero A, Quintilla R, Groenewald M, Alkema W, Boekhout T, Hazelwood L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016;60:147–159. doi: 10.1016/J.FM.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Holt S, Mukherjee V, Lievens B, Verstrepen KJ, Thevelein JM. Bioflavoring by non-conventional yeasts in sequential beer fermentations. Food Microbiol. 2018;72:55–66. doi: 10.1016/J.FM.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Gamero A, Ren X, Lamboni Y, de Jong C, Smid EJ, Linnemann AR. Development of a low-alcoholic fermented beverage employing cashew apple juice and non-conventional yeasts. Fermentation. 2019;5(3):71. doi: 10.3390/fermentation5030071. [DOI] [Google Scholar]
- 13.Contreras A, Hidalgo C, Henschke PA, Chambers PJ, Curtin C, Varela C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl Environ Microbiol. 2014;80(5):1670–1678. doi: 10.1128/AEM.03780-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres-Guardado R, Esteve-Zarzoso B, Reguant C, Bordons A. Microbial interactions in alcoholic beverages. Int Microbiol. 2021 doi: 10.1007/s10123-021-00200-1. [DOI] [PubMed] [Google Scholar]
- 15.Anupama D, Bhat K, Sapna V. Sensory and physico-chemical properties of commercial samples of honey. Food Res Int. 2003;36(2):183–191. doi: 10.1016/S0963-9969(02)00135-7. [DOI] [Google Scholar]
- 16.Lilly M, Bauer FF, Lambrechts MG, Swiegers JH, Cozzolino D, Pretorius IS. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast. 2006;23(9):641–659. doi: 10.1002/yea.1382. [DOI] [PubMed] [Google Scholar]
- 17.Yin L, Wang C, Zhu X, et al. A multi-step screening approach of suitable non-Saccharomyces yeast for the fermentation of hawthorn wine. LWT. 2020;127:109432. doi: 10.1016/j.lwt.2020.109432. [DOI] [Google Scholar]
- 18.Sottil C, Salor-Torregrosa JM, Moreno-Garcia J, et al. Using Torulaspora delbrueckii, Saccharomyces cerevisiae and Saccharomyces bayanus wine yeasts as starter cultures for fermentation and quality improvement of mead. Eur Food Res Technol. 2019;245(12):2705–2714. doi: 10.1007/s00217-019-03384-z. [DOI] [Google Scholar]
- 19.Fernandes T, Silva-Sousa F, Pereira F, et al. Biotechnological Importance of Torulaspora delbrueckii: from the obscurity to the spotlight. J Fungi. 2021;7(9):712. doi: 10.3390/jof7090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastos EMAF, Calaça PSST, Simeão CMG, Cunha MRR. Characterization of the honey from Myracrodruon urundeuva (Anacardiceae-Aroeira) in the Dry Forest of northern of Minas Gerais/Brazil. Adv Agric Sci. 2016;4(4):64–71. [Google Scholar]
- 21.Sroka P, Tuszyński T. Changes in organic acid contents during mead wort fermentation. Food Chem. 2007;104(3):1250–1257. doi: 10.1016/j.foodchem.2007.01.046. [DOI] [Google Scholar]
- 22.Pereira AP, Mendes-Ferreira A, Estevinho LM, Mendes-Faia A. Improvement of mead fermentation by honey-must supplementation. J Inst Brew. 2015;121(3):405–410. doi: 10.1002/jib.239. [DOI] [Google Scholar]
- 23.Simões LA, de Souza AC, Schwan RF, Dias DR (2021) Enumerating Distinct Yeast in the Same Food Sample. In; 111–123. 10.1007/978-1-0716-1932-2_11
- 24.Ribeiro LS, da Cruz Pedrozo Miguel MG, Evangelista SR, et al. Behavior of yeast inoculated during semi-dry coffee fermentation and the effect on chemical and sensorial properties of the final beverage. Food Res Int. 2017;92:26–32. doi: 10.1016/J.FOODRES.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Ducki S, Miralles-Garcia J, Zumbé A, Tornero A, Storey DM. Evaluation of solid-phase micro-extraction coupled to gas chromatography–mass spectrometry for the headspace analysis of volatile compounds in cocoa products. Talanta. 2008;74(5):1166–1174. doi: 10.1016/J.TALANTA.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Bressani APP, Martinez SJ, Evangelista SR, Dias DR, Schwan RF. Characteristics of fermented coffee inoculated with yeast starter cultures using different inoculation methods. LWT. 2018;92:212–219. doi: 10.1016/J.LWT.2018.02.029. [DOI] [Google Scholar]
- 27.Evangelista SR, da Cruz Pedrozo Miguel MG, de Souza Cordeiro C, Silva CF, Marques Pinheiro AC, Schwan RF. Inoculation of starter cultures in a semi-dry coffee (Coffea arabica) fermentation process. Food Microbiol. 2014;44:87–95. doi: 10.1016/j.fm.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Tang Y, Horikoshi M, Li W. Ggfortify: unified interface to visualize statistical results of popular r packages. R J. 2016;8(2):478–489. doi: 10.32614/rj-2016-060. [DOI] [Google Scholar]
- 29.Dashko S, Zhou N, Tinta T, et al. Use of non-conventional yeast improves the wine aroma profile of Ribolla Gialla. J Ind Microbiol Biotechnol. 2015;42(7):997–1010. doi: 10.1007/s10295-015-1620-y. [DOI] [PubMed] [Google Scholar]
- 30.Peryam D, Pilgrim F. Hedonic scale method of measuring food preferences. Food Technol. 1957;11:9–14. [Google Scholar]
- 31.Castro-Vázquez L, Díaz-Maroto MC, González-Viñas MA, Pérez-Coello MS. Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem. 2009;112(4):1022–1030. doi: 10.1016/J.FOODCHEM.2008.06.036. [DOI] [Google Scholar]
- 32.Garg N, Sethupathy A, Tuwani R, et al. FlavorDB: a database of flavor molecules. Nucleic Acids Res. 2018;46(D1):D1210–D1216. doi: 10.1093/nar/gkx957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Breda V, Jolly N, van Wyk J. Characterisation of commercial and natural Torulaspora delbrueckii wine yeast strains. Int J Food Microbiol. 2013;163(2–3):80–88. doi: 10.1016/J.IJFOODMICRO.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Azzolini M, Tosi E, Lorenzini M, Finato F, Zapparoli G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J Microbiol Biotechnol. 2015;31(2):277–293. doi: 10.1007/s11274-014-1774-1. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira Assis M, Pereira A, Santos C, Rosa CA, de Oliveira Mamede ME. Impact of a non-saccharomyces yeast isolated in the equatorial region in the acceptance of Wine Aroma. Food Nutr Sci. 2014;5:759–769. doi: 10.4236/fns.2014.59086. [DOI] [Google Scholar]
- 36.Basso RF, Alcarde AR, Portugal CB. Could non-Saccharomyces yeasts contribute on innovative brewing fermentations? Food Res Int. 2016;86:112–120. doi: 10.1016/J.FOODRES.2016.06.002. [DOI] [Google Scholar]
- 37.Gutiérrez A, Boekhout T, Gojkovic Z, Katz M. Evaluation of non- Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J Inst Brew. 2018;124(4):389–402. doi: 10.1002/jib.512. [DOI] [Google Scholar]
- 38.Tronchoni J, Gamero A, Arroyo-López FN, Barrio E, Querol A. Differences in the glucose and fructose consumption profiles in diverse Saccharomyces wine species and their hybrids during grape juice fermentation. Int J Food Microbiol. 2009;134(3):237–243. doi: 10.1016/J.IJFOODMICRO.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Mestre Furlani MV, Maturano YP, Combina M, Mercado LA, Toro ME, Vazquez F (2017) Selection of non-Saccharomyces yeasts to be used in grape musts with high alcoholic potential: a strategy to obtain wines with reduced ethanol content. FEMS Yeast Res 17(2). 10.1093/femsyr/fox010 [DOI] [PubMed]
- 40.Ruyters S, Mukherjee V, Verstrepen KJ, Thevelein JM, Willems KA, Lievens B. Assessing the potential of wild yeasts for bioethanol production. J Ind Microbiol Biotechnol. 2015;42(1):39–48. doi: 10.1007/s10295-014-1544-y. [DOI] [PubMed] [Google Scholar]
- 41.Zha Y, Hossain AH, Tobola F, Sedee N, Havekes M, Punt PJ. Pichia anomala 29X: a resistant strain for lignocellulosic biomass hydrolysate fermentation. FEMS Yeast Res. 2013;13(7):609–617. doi: 10.1111/1567-1364.12062. [DOI] [PubMed] [Google Scholar]
- 42.Šmogrovičová D, Nádaský P, Lich RT, Wilhelmi BS, Cambray G. Analytical and aroma profiles of Slovak and South African meads. Czech J Food Sci. 2012;30(3):241–246. doi: 10.1080/13669870903136175. [DOI] [Google Scholar]
- 43.Bartowsky EJ, Pretorius IS (2009) Microbial formation and modification of flavor and off-flavor compounds in wine. In: Biology of Microorganisms on Grapes, in Must and in Wine. Berlin, Heidelberg: Springer Berlin Heidelberg 209–231. 10.1007/978-3-540-85463-0_11
- 44.Fahlbusch K-G, Hammerschmidt F-J, Panten J et al (2003) Flavors and Fragrances. In: ‘Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA 10.1002/14356007.a11_141
- 45.Xu Y, Wang D, Li G, et al. Flavor contribution of esters in lager beers and an analysis of their flavor thresholds. J Am Soc Brew Chem. 2017;75(3):201–206. doi: 10.1094/ASBCJ-2017-3007-01. [DOI] [Google Scholar]
- 46.Ciani M, Comitini F. Yeast interactions in multi-starter wine fermentation. Curr Opin Food Sci. 2015;1:1–6. doi: 10.1016/J.COFS.2014.07.001. [DOI] [Google Scholar]
- 47.Maturano YP, Rodríguez Assaf LA, Toro ME, et al. Multi-enzyme production by pure and mixed cultures of Saccharomyces and non-Saccharomyces yeasts during wine fermentation. Int J Food Microbiol. 2012;155(1–2):43–50. doi: 10.1016/J.IJFOODMICRO.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Zara G, Mannazzu I, Del Caro A, et al. Wine quality improvement through the combined utilisation of yeast hulls and C andida zemplinina / S accharomyces cerevisiae mixed starter cultures. Aust J Grape Wine Res. 2014;20(2):199–207. doi: 10.1111/ajgw.12078. [DOI] [Google Scholar]
- 49.Hopfer H, Heymann H. Judging wine quality: do we need experts, consumers or trained panelists? Food Qual Prefer. 2014;32:221–233. doi: 10.1016/J.FOODQUAL.2013.10.004. [DOI] [Google Scholar]
- 50.Gomes T, Dias T, Cadavez V, et al. Influence of sweetness and ethanol content on mead acceptability. Polish J Food Nutr Sci. 2015;65(2):137–142. doi: 10.1515/pjfns-2015-0006. [DOI] [Google Scholar]
- 51.Vidrih R, Hribar J. Studies on the sensory properties of mead and the formation of aroma compounds related to the type of honey. Acta Aliment. 2007;36(2):151–162. doi: 10.1556/AAlim.36.2007.2.2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Not applicable.