Abstract
The aim of this study was to evaluate 140 Salmonella Derby isolates collected over a 10-year period from porcine origins (environment, pig carcass, lymph nodes, intestinal content, and pork) for their phenotypic and genotypic antimicrobial resistance, their ability to produce biofilm, and their genetic relatedness. The minimum inhibitory concentration (MIC) was determined using microdilution broth method and antimicrobial resistance genes were investigated by PCR. The quantification of biofilm formation was performed in sterile polystyrene microtiter plates. Genetic relatedness was determined by Xba-I macrorestriction analysis. The highest frequencies of non-wildtype (nWT) populations were observed against tetracycline (75.7%), streptomycin (70%), and colistin (11.4%), whereas wildtype populations were observed against ciprofloxacin, ceftazidime, and gentamicin. The resistance genes found were blaTEM (ampicillin), aadA variant (streptomycin/spectinomycin), tetA (tetracycline), and floR (florfenicol). On 96-well polystyrene microtiter plate, 68.6% of the isolates proved to be biofilm producers. Among 36 S. Derby isolates selected to PFGE analysis, 22 were clustered with 83.6% of similarity. Additionally, 27 isolates were clustered in 11 pulsotypes, which presented more than one strain with 100% of similarity. Most of S. Derby isolates were able to form biofilm and were classified as nWT or resistant to tetracycline, streptomycin, and colistin. PFGE allowed the identification of closely related S. Derby isolates that circulated in pig slaughterhouses and pork derived products along a decade.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-022-00846-7.
Keywords: Adherence, Antimicrobial susceptibility, MIC, Clonal groups, Swine
Introduction
Salmonella enterica is one of the most common foodborne pathogens known, and it is considered one of the leading causes of gastroenteritis in humans in several countries, including Brazil. According to the Brazilian Ministry of Health, Salmonella was responsible for 14.6% of the foodborne outbreaks in 2017 [1]. Salmonellosis is caused by the ingestion of contaminated food, and it has been established that consumption of pork may be responsible for up to 31.1% of all Salmonella infections in humans [2]. In Southern Europe, 43.6% of the confirmed cases of human salmonellosis were related to the ingestion of contaminated pork products [3]. Pigs can harbor Salmonella with no apparent symptoms of any disease. Such clinically unapparent carriers can excrete Salmonella over a long period of time, thereby contaminating their environment and acting as sources of infection for other animals and humans through contamination of pig carcasses on the slaughter line [4].
Salmonella enterica subsp. enterica serovar Derby (S. Derby) is the most frequently serovar reported from pigs and pig carcasses and the sixth most detected in humans in the European Union (EU) [5]. In China, S. Derby isolates represent the third most common serovar isolated from patients with diarrhea after S. Enteritidis and S. Typhimurium. Also, those are typically one of the most commonly isolated from pork [6, 7]. Salmonella Derby has also been found in samples from swine finishing herds, slaughterhouses, and pork in Southern Brazil [8–11].
Antimicrobial resistance has been a worldwide problem since the discovery of antimicrobial agents. The interpretative criterion for antimicrobial resistance generally is based on clinical breakpoint values, which divide bacteria into three categories: susceptible (associated with therapeutic success), intermediate (associated with an indeterminate or uncertain therapeutic effect), and resistant (associated with a high risk of therapeutic failure). However, this categorization is traditionally a clinical one and it is made irrespective of whether or not the organism harbor resistance mechanisms. In order to monitor the resistance development, the concept of “epidemiological cut-off value” (ECOFF) was raised. The purpose of ECOFF values is to separate the wildtype (WT) population (microorganisms without acquired resistance mechanisms) and nWT population (microorganisms with acquired resistance mechanisms) [12, 13].
The ability of Salmonella to form biofilms is seen as an important factor that contributes to its resistance and persistence in both host and non-host environments and is especially important when related to food processing environments [14]. Biofilms are defined as structured communities of bacterial cells embedded in a self-produced polymeric matrix attached to living or non-living surfaces [15, 16]. Bacteria in biofilms are generally well protected against environmental stresses, antimicrobials, disinfectants, and the host immune system, and as a consequence are extremely difficult to eradicate [17, 18]. Several reports have demonstrated the ability of Salmonella from porcine origins to form biofilms under unfavorable conditions, such as pig farms or slaughterhouses and food-processing plants [19–21].
Thus, the aim of this study was to evaluate Salmonella Derby isolates collected over a 10-year period from various porcine origins (environment, pig carcass, lymph nodes, intestinal content, and pork) for (i) their phenotypic and genotypic antimicrobial resistance profile, (ii) their ability to produce biofilm, and (iii) their molecular relationships by macrorestriction analysis.
Material and methods
Origin of the isolates
A total of 140 isolates of Salmonella enterica subsp. enterica serovar Derby were selected from the culture collection of the Laboratory of Preventive Veterinary Medicine (FAVET-UFRGS), Porto Alegre, Southern Brazil. These isolates were found in samples collected from pig slaughterhouses located in Santa Catarina (SC) and Rio Grande do Sul (RS) states, and from pork derived products obtained from supermarkets and butcher shops in Porto Alegre, Brazil, during the period of 1999 to 2010 (Table 1).
Table 1.
Salmonella Derby isolates included in this study
| Sampling | Origin | Number of isolates | Sampling period | Reference |
|---|---|---|---|---|
| I | Mesenteric lymph node | 12 | 1999–2000 | Bessa et al. [48] |
| Intestine fragment | 12 | |||
| II | Submandibular lymph nodes and tonsils | 13 | 2001–2002 |
Ferraz et al. [49] Ferraz et al. [50] |
| Intestinal content | 4 | |||
| Ground meat | 7 | |||
| III | Mesenteric lymph node | 28 | 2005 | Schwarz et al. [51] |
| IV | Fresh pork sausage | 9 | 2005 | Mürmann et al. [10] |
| V | Pig carcass | 21 | 2007–2009 | Silva et al. [52] |
| Intestinal content | 10 | |||
| Lairage environment | 2 | |||
| Floor of slaughterhouse | 1 | |||
| VI | Pig carcass | 16 | 2010 | Pissetti et al. [53] |
| Working table for offal | 2 | |||
| Lairage environment | 3 | |||
| Total | 140 |
Minimum inhibitory concentration (MIC)
The MIC was determined using microdilution broth method according to Clinical and Laboratory Standard Institute [22, 23]. Thus, the following antimicrobials were used: nalidixic acid (Sigma-Aldrich, USA), ampicillin (Sigma-Aldrich, USA), cefotaxime (Sigma-Aldrich, USA), ceftazidime (Sigma-Aldrich, USA), ciprofloxacin (Sigma-Aldrich, USA), colistin (European Pharmacopeia, UK), streptomycin (Carl Roch, Germany), florfenicol (Sigma-Aldrich, USA), gentamicin (Sigma-Aldrich, USA), and tetracycline (Sigma-Aldrich, USA). The established set of tested antimicrobials, concentration range, and ECOFF and breakpoint values were carried out in accordance with EUCAST recommendations [24–26]. In the absence of EUCAST interpretative criteria, CLSI breakpoint values were adopted [23] (Table S1). Escherichia coli ATCC 25,922 was used as a reference strain for quality purposes.
Antimicrobial resistance genes profiling
Salmonella Derby isolates that displayed phenotypic resistance profile against ampicillin (n = 8), florfenicol (n = 1), streptomycin (n = 98), and tetracycline (n = 106) were investigated for the presence of resistance genes previously reported in this genus. Genomic DNA was prepared using the NucleoSpin Tissue Kits (Macherey–Nagel; Düren, Germany). Genes encoding resistance to β-lactams (blaTEM and blaPSE-1), phenicols (floR and catA1), aminoglycosides (strA, strB, aadA, aadB), and tetracyclines (tetA and tetB) were investigated by PCR assays, using the primers described by Lopes et al. [27].
Evaluation of biofilm-forming ability on polystyrene microtiter plates
The quantification of biofilm formation was performed in 230 µL of tryptic soya broth (TSB) with no glucose (Becton Dickinson & Company, USA) in sterile 96-well flat-bottomed polystyrene microtiter plates (Techno Plastic Products, Germany). A quantity of 20 µL of overnight bacterial culture, adjusted at 0.5 on the MacFarland scale, was added into each well. The plates were incubated aerobically at 37 °C for 24 h and at 28 °C for 96 h [28]. After incubation, the content of the plate was drained and the wells washed three times with sterile distilled water. During the washing process, the plates were vigorously shaken for removal of all non-adherent cells. The remaining attached bacteria were fixed with 250 µL of methanol per well. After 15 min, each plate was emptied and its air dried. The plates were stained with 250 µL per well of 2% Crystal Violet for 5 min. Any stain excess was rinsed off using distilled water. Subsequently, the dye bound to adherent cells was resolubilized with 250 µL of 33% (v/v) glacial acetic acid per well. The optical density (O.D.) of each well was measured at 570 nm using a spectrophotometer Strip Reader (EL301, BioTek, USA). Each isolate was tested in triplicate. Staphylococcus epidermidis ATCC 35,984 and Salmonella Typhimurium ATCC 14,028 were used as positive control for biofilm formation, while Salmonella Enteritidis ATCC 13,076 was used as a negative control for biofilm formation. The wells with no inoculum were used as quality control for the medium.
Determination of genetic relatedness using pulsed-field gel electrophoresis (PFGE)
The PFGE was carried out according to PulseNet standardized laboratory protocol for molecular subtyping of Salmonella (https://www.cdc.gov/pulsenet/pathogens/pfge.html). The genomic DNA was digested with 50 units of XbaI (Thermo Scientifics, USA) at 37 °C for 18 h. The respective fragments were separated by pulsed-field gel electrophoresis in certified agarose 1% (BioRad, CA, USA) in a CHEF DR II system (BioRad, CA, USA) at 6 V cm−1 with 0.5 × Tris–borate-EDTA as the running buffer. The pulse times were increased from 2.2 to 63.8 s during 19–20 h period. The XbaI fragments of Salmonella Braenderup H9812 served as size standards. The gel was stained with ethidium bromide (1 μg mL−1, Sigma, St. Louis, USA) and photographed under UV light. The images were recorded for analysis.
Data analysis
For each biofilm microtiter plate, the cut-off O.D. (O.D.c) was defined as three standard deviations above the mean O.D. of the negative control. The isolates were classified into four categories: O.D. ≤ O.D.c = no biofilm producer; O.D.c < O.D. ≤ (2 × O.D.c) = weak biofilm producer; (2 × O.D.c) < O.D. ≤ (4 × O.D.c) = moderate biofilm producer; and (4 × O.D.c) < O.D. = strong biofilm producer. The macrorestriction patterns were analyzed via GelCompar II software (Applied Maths, Belgium), and the similarities between patterns were determined in accordance with the Dice correlation coefficient, with a maximal position tolerance of 1.0% and optimization of 1.0%. The patterns were clustered using the unweighted pair group method with arithmetic averages (UPGMA). Any isolates with one band of difference were considered as different pulsotypes.
Results
Antimicrobial resistance profiling
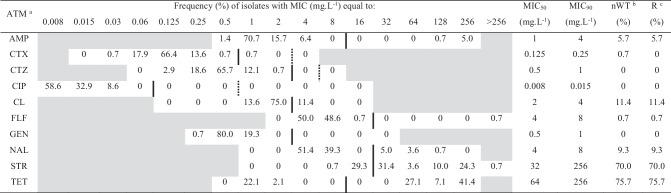
Out of the 140 Salmonella Derby isolates included in this study, 123 (87.8%) were classified as nWT population and only 17 (12.1%) isolates were classified as WT population, according to epidemiological cut-off values. The highest frequencies of nWT populations were observed against tetracycline (75.7%), streptomycin (70%), and colistin (11.4%). There were no detectable nWT population to ciprofloxacin, ceftazidime, and gentamicin (Table 2). The highest values of MIC50 and MIC90 were observed for streptomycin and tetracycline, which exceeded ECOFF values. The antimicrobials cefotaxime and ciprofloxacin exhibited the lowest values of MIC50 and MIC90.
Table 2.
MIC distributions of 10 antimicrobial agents against 140 Salmonella Derby isolates, non-wildtype population, and resistance
aAntimicrobial agents tested: ampicillin (AsMP), cefotaxime (CTX), ceftazidime (CTZ), ciprofloxacin (CIP), colistin (CL), florfenicol (FLF), gentamicin (GEN), nalidixic acid (NAL), streptomycin (STR), and tetracycline (TET)
bNon-wildtype (nWT) isolates, according to ECOFF values
cResistant isolates (R), according to clinical breakpoints
Black vertical lines indicate the ECOFF values and dotted vertical lines indicate the clinical breakpoints, when they are different
Concentrations not included in the test panel are shaded grey
Clinical breakpoints were used to establish the phenotypic profile of the S. Derby isolates. In this study, 123 (87.8%) isolates proved to be resistant to at least one antimicrobial tested, while only 17 (12.1%) isolates were classified as susceptible to all antimicrobials tested. There was no resistance detected for cefotaxime, ceftazidime, ciprofloxacin, and gentamicin. Resistance occurred most frequently to tetracycline (75.7%), streptomycin (70.0%), colistin (11.4%), nalidixic acid (9.3%), ampicillin (5.7%), and florfenicol (0.7%). The resistance profile STR-TET was the most frequent, which was found in 50.0% of the isolates (Table 3). Also, multidrug resistance (resistance to three or more classes of antimicrobial agents) was detected in 12.9% of the isolates. Resistance genes were detected among the resistant S. Derby isolates submitted to PCR analysis. The resistance genes found were blaTEM for ampicillin resistance, aadA variant for streptomycin/spectinomycin resistance, tetA for tetracycline resistance, and floR for florfenicol resistance.
Table 3.
Antimicrobial resistance profiles of Salmonella Derby from pig production chain
| Resistance profilea | No. of isolates | % |
|---|---|---|
| Susceptible | 17 | 12.1 |
| NAL | 2 | 1.5 |
| STR | 10 | 7.1 |
| TET | 14 | 10.0 |
| CL-NAL | 2 | 1.5 |
| CL-TET | 2 | 1.5 |
| NAL-STR | 2 | 1.5 |
| NAL-TET | 3 | 2.1 |
| STR-TET | 70 | 50.0 |
| AMP-CL-NAL | 1 | 0.7 |
| AMP-NAL-TET | 1 | 0.7 |
| AMP-STR-TET | 2 | 1.5 |
| CL-STR-TET | 9 | 6.4 |
| NAL-STR-TET | 1 | 0.7 |
| AMP-CL-STR-TET | 2 | 1.5 |
| AMP-FLF-STR-TET | 1 | 0.7 |
| AMP-NAL-STR-TET | 1 | 0.7 |
| Total | 140 | 100 |
aAMP, ampicillin; CL, colistin; FLF, florfenicol; NAL, nalidixic acid; STR, streptomycin; TET, tetracycline
Biofilm-forming ability of Salmonella Derby
Biofilm formation on polystyrene microtiter plate was observed in 68.6% (96/140) of the S. Derby isolates, where the plates were incubated at 28 °C (Fig. 1). Among biofilm producer isolates, 37.9% (53/140) were classified as weakly adherent, while 30.7% (43/140) were classified as moderately adherent. No strongly adherent isolates were observed at 28 °C. The values of optic density (O.D.) ranged from 0.1203 to 0.8110, with O.D. means of 0.2156 for cut-off (D.O.c), 0.1902 for non-adherent isolates (D.O. < D.O.c), 0.3538 for weakly adherent isolates (D.O.c > D.O. < 2 × D.O.c), and 0.4841 for moderately adherent isolates (2 × D.O.c > D.O. < 4 × D.O.c). It was proven that the temperature set at 37 °C did affect the biofilm formation. Only 5.7% (8/140) of S. Derby isolates were weakly adherent on polystyrene plate when incubated under this condition. The values of O.D. ranged from 0.1233 to 0.3647; the O.D. means of 0.1870 for cut-off (D.O.c), 0.1614 for non-adherent isolates (D.O. < D.O.c), and 0.2082 for weakly adherent isolates (D.O.c > D.O. < 2 × D.O.c). No moderately or strongly adherent isolates were observed at 37 °C.
Fig. 1.
Biofilm-forming ability of Salmonella Derby isolates on polystyrene microtiter plate
Genetic relatedness of Salmonella Derby
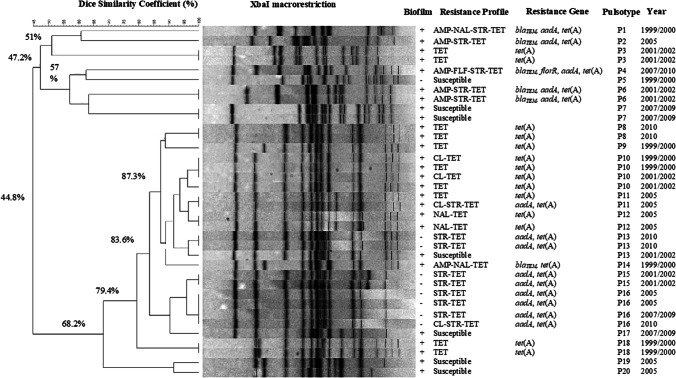
Through the biofilm and antimicrobial resistance profiles observed, 36 isolates were selected for macrorestriction analysis followed by PFGE, and at least one isolate from each phenotypic profile was included. XbaI-macrorestriction generated 20 pulsotypes with a similarity index ranging from 44.8 to 100%, nine of them were unique (Fig. 2). Among the 36 S. Derby isolates, 22 were clustered reporting 83.6% of similarity. Additionally, 27 isolates were clustered in 11 pulsotypes, which presented more than one strain with 100% of similarity (Table 4). The pulsotypes P10, P13, and P16 were found circulating in different sampling periods and from different origins (Table 4).
Fig. 2.
Dendrogram of Salmonella Derby isolates based on XbaI macrorestriction (PFGE) patterns. The similarity analysis was performed using the Dice coefficient and UPGMA method (tolerance 1%)
Table 4.
Characteristics of Salmonella Derby isolates belonged to the same PFGE pulsotype
| PFGE pulsotype | Biofilm | Resistance profilea | No. of isolates | Sampling | Origin |
|---|---|---|---|---|---|
| P3 | + | TET | 2 | II | Submandibular lymph node |
| Ground meat | |||||
| P6 | + | AMP-STR-TET | 2 | II | Submandibular lymph node |
| Ground meat | |||||
| P7 | + | Susceptible | 2 | V | Intestinal content |
| P8 | + | TET | 2 | VI | Pig carcass |
| P10 | + | CL-TET | 2 | I | Mesenteric lymph node |
| II | Tonsil | ||||
| TET | 2 | I | Intestine fragment | ||
| II | Submandibular lymph node | ||||
| P11 | + | TET | 2 | IV | Fresh pork sausage |
| CL-STR-TET | |||||
| P12 | + | NAL-TET | 2 | III | Mesenteric lymph node |
| P13 | - | STR-TET | 2 | VI | Working table for offal |
| + | Susceptible | 1 | II | Ground meat | |
| P15 | - | STR-TET | 2 | II | Intestine fragment |
| P16 | - | STR-TET | 2 | IV | Fresh pork sausage |
| 1 | V | Pig carcass | |||
| CL-STR-TET | 1 | VI | Pig carcass | ||
| P18 | + | TET | 2 | I | Mesenteric lymph node |
aAMP, ampicillin; CL, colistin; NAL, nalidixic acid; STR, streptomycin; TET, tetracycline
Discussion
In this study, the concept of ECOFF value was used for determining nWT populations among 140 S. Derby isolates from pig production chain in a 10-year period. Using the MIC distribution, it was possible to identify 12.1% of the isolates as WT for all antimicrobial agents tested. Among the 140 S. Derby isolates, the highest frequencies of nWT were observed against tetracycline (75.7%), followed by streptomycin (70%), colistin (11.4%), nalidixic acid (9.3%), and ampicillin (5.7%). For ciprofloxacin, ceftazidime, and gentamicin, all of the isolates were classified as WT.
Due to international regulations on the use of antimicrobials in animal production by countries that import meat from Brazil, the use of tetracycline in Brazilian pig farms has decreased considerably lately. Even so, there are still occurrences of high rates of resistance to tetracycline in Salmonella remains [8, 29]. The maintenance of resistance to tetracycline is associated to the presence of tet genes in mobile genetic elements. When other resistance genes are collocated with a tet gene on the same plasmid, such a plasmid can be acquired under the selective pressure imposed by the use of antimicrobial agents other than tetracycline [30]. In the present study, the tet(A) gene was found in all tetracycline resistant isolates (n = 106), whereas the gene tet(B) was not detected. The gene tet(A) is part of a small non-conjugative transposon Tn1721, which are often integrated into conjugative and non-conjugative resistance plasmids in Enterobacteriaceae, and this may contribute to their spread among bacteria populations and environment [31].
A high frequency of nWT was observed against streptomycin (70%). Streptomycin is an aminoglycoside antimicrobial commonly used for treating enteric diseases in cattle, pigs, sheep, and poultry. The most common mechanisms associated with streptomycin resistance are the phosphotransferases APH(6)-Ia and APH(6)-Id, encoded by the strA and strB genes, respectively [31]. The maintenance of resistance to streptomycin is also associated to the presence of aadA genes coding for aminoglycoside-3″-O-adenyltransferases that confer resistance to streptomycin and spectinomycin [32]. In the present study, the aadA variant was found in all streptomycin resistant isolates (n = 98). Gene cassettes carrying variants of the aadA have been constantly found inserted into class 1 or class 2 integrons, or being part of the SGI1- or SGI2-associated multiresistance gene clusters, widely disseminated among Enterobacteriaceae [27, 33]. The other resistance genes such as blaTEM for ampicillin resistance and floR for florfenicol resistance were also detected. The blaTEM gene is usually part of transposon Tn3 in Salmonella isolates [31; 32]. The floR gene can be found in the chromosome or in plasmids of multiresistant Salmonella enterica serovars, including S. Typhimurium DT104 and S. Newport [31]. It is clear that these resistance genes, specially tet(A), are spread between environment, pig carcass, lymph nodes, intestinal content, and pork.
Our study also included colistin in the panel, which is widely used in veterinary medicine for controlling Enterobacteriaceae infections, especially post-weaning diarrhea in pigs due to Escherichia coli, and for prophylaxis purposes [24, 34]. Although the frequency of nWT isolates against colistin was 11.4%, most of isolates (75%) had MIC of 2 mg L−1, very close to the clinical breakpoint and ECOFF value (> 2 mg L−1), classified as borderline susceptible. It has been previously shown that colistin resistance in Enterobacteriaceae is resulting of chromosomal mutations, including mutations in the pmrA/pmrB two-component system that regulate the synthesis and structure of the lipopolysaccharide [35]. Additionally, the plasmid-mediated colistin resistance gene mcr-1 was identified in E. coli strain from a pig in China [36]. The detection of mcr-1 gene in one E. coli isolate classified as wildtype (susceptible) with MIC of 2 mg L−1 was recently reported [37]. These findings indicate that there is a need to review the established epidemiological cut-off value for colistin.
Overall, a low frequency (0.7%) of nWT isolates to third-generation cephalosporins (cefotaxime and ceftazidime) was observed among S. Derby, corroborating previous studies [38, 39]. Also, resistance to fluoroquinolones such as ciprofloxacin was not found. These results represent a favorable situation for the public health panorama, since fluoroquinolones and extended-spectrum cephalosporins are the agents of choice for treating complicated Salmonella infection in humans. The emergence of extended-spectrum β-lactamases (ESBLs) in Enterobacteriaceae is an increasing problem throughout the world, compromising the use of these drugs for treating invasive gastroenteritis in humans. The increasing percentage of Salmonella resistant to fluoroquinolones and cephalosporins is a matter of concern and must be monitored carefully to assess potential risks to human patients, as well as any direct risk to veterinary husbandry [24].
Biofilm formation is one of the processes that may contribute to Salmonella persistence in different environments. The ability to adhere to surfaces and equipment, especially those commonly encountered in the food industry, may offer considerable risks of recurrent contamination affecting the quality and safety of food [40, 41]. The persistence of pathogenic microorganisms in these environments is a major concern due to the increased resistance to sanitizers and antimicrobials, plus conventional cleaning and sanitation carelessness to eradicate them from such surfaces. Studies have highlighted the presence of Salmonella biofilms and its resistance to the most commonly used sanitizers in the food industry [17, 42, 43].
It is interesting to observe that temperature has influenced the biofilm formation on polystyrene microtiter plates. When incubated at 28 °C, 68.6% (96/140) of the S. Derby isolates were positive for biofilm formation on polystyrene. Out of these, 37.9% were classified as weakly biofilm producer and 30.7% as moderately biofilm producer. Our results agree with the literature, which reported Salmonella isolates with biofilm-forming ability classified as weakly adherent on polystyrene microtiter plates [20, 44, 45]. When the test was performed at 37 °C, only 5.7% (8/140) of the isolates were positive for biofilm formation on polystyrene microtiter plate, being categorized as weakly biofilm producers. The biofilm formation behavior of Salmonella serovars from beef processing plants was investigated under a range of temperatures (4 °C, 10 °C, 25 °C, 37 °C, and 42 °C), and the strongest biofilm formation occurring at 25 °C [46]. Our data are also consistent with the research results of Yang et al. [43] who found Salmonella Enteritidis strains forming denser biofilms at 25 °C. In our study, the optimal temperature for the Salmonella isolates to produce biofilm was 28 °C, which is close to ambient temperature on farms, as well as during transportation from the farm to the slaughterhouse and in lairage areas.
PFGE, multiple locus variable number tandem repeat analysis (MLVA), and whole genome sequencing (WGS) are PulseNet’s main subtyping tolls. Based on biofilm and antimicrobial resistance profiles, 36 S. Derby isolates were selected for PFGE analysis. XbaI-macrorestriction of the 36 isolates generated 20 pulsotypes, nine of them being unique (P1, P2, P4, P5, P9, P14, P17, P19, and P20) (Fig. 2). A great cluster of 22 isolates showing 83.6% of similarity was identified. Three pulsotypes (P10, P13, and P16) were found circulating in different sampling periods and from different origins. Four isolates from P10 group showed biofilm-forming ability, tet(A) gene, and were originated from pigs at slaughter, been isolated from mesenteric lymph node, tonsil, intestine fragment, and submandibular lymph node, during the first and second sampling periods (I and II). In contrast to the P10 group, the isolates from P13 were more diverse. One isolate within the P13 group was biofilm producer, susceptible to antimicrobials and originated from ground meat in 2001/2002, while two isolates were non-biofilm producers, harbored aadA and tet(A) resistance genes, and were collected from working table for offal in 2010. A similar degree of homogeneity in the strain characteristics was seen among four isolates assigned to P16. The P16 isolates were from fresh pork sausage and pig carcasses of three different sampling periods (IV, V, and VI), non-biofilm producers, harboring aadA and tet(A) resistance genes. Therefore, closely related of S. Derby isolates were widely distributed and persistent among slaughter-age pigs, environment, and meat products along a decade in Southern Brazil. An integrated production system is characterized by closed herds in multi-site production units, which can provide several opportunities for Salmonella transmission. In this study, animals are originated from integrated production systems and the contamination with the same S. Derby clonal group might have occurred at the farm level, and spread during transport to slaughterhouse, during slaughter process, or in the handling of pork products. Identical Salmonella Derby isolates were also obtained from samples recovered from finishing farms and slaughterhouses in a study performed in Paraná state, Brazil, indicating that genetically close S. Derby isolates are spread in pig production chain in this region [8]. Salmonella isolates with the same PFGE pattern are more likely to have similar antimicrobial resistance profile; however, a correlation among PFGE, biofilm, and antimicrobial resistance profiles was not always observed. In this study, the P13 isolates were biofilm producer and non-biofilm producers with different resistance profiles. Han et al. [47] also reported that a correlation between PFGE clusters with resistant profiles and virulotypes was not observed among Salmonella isolates from a duck slaughterhouse.
Conclusion
Most of S. Derby isolates were able to form biofilm and the highest frequencies of nWT and resistant isolates were observed against tetracycline, streptomycin, and colistin. Through the macrorestriction by PFGE, it was possible to identify closely related S. Derby isolates between different collection periods and distributed among the different origins. In fact, considering the resistance towards antimicrobial agents of various classes and with different mechanisms of action, along with biofilm-forming ability and persistence in the pig production chain, the S. Derby isolates may pose a potential risk to human health when such isolates enter in the food chain.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Karen Apellanis Borges and Thales Quedi Furian for the excellent technical assistance.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by CS, VL, TA, and GVL. The first draft of the manuscript was written by CS and GVL, and all authors commented on previous versions of the manuscript. The manuscript was reviewed by GVL and MC. All authors read and approved the final manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior — Brasil (CAPES) — Finance Code 001.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brazil (Ministério da Saúde) (2019) Surtos de Doenças Transmitidas por Alimentos no Brasil. https://www.saude.gov.br/images/pdf/2019/maio/17/Apresentacao-Surtos-DTA-Maio-2019.pdf/ Accessed 11 May 2022.
- 2.De Knegt LV, Pires SM, Hald T. Attributing foodborne salmonellosis in humans to animal reservoirs in the European Union using a multi-country stochastic model. Epidemiol Infect. 2015;143(6):1175–1186. doi: 10.1017/S0950268814001903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pires SM, De Knegt L, Hald T (2011) Estimation of the relative contribution of different food and animal sources to human Salmonella infections in the European Union. Søborg, Denmark: National Food Institute, Technical University of Denmark EFSA Supporting Publications 2011. 10.2903/sp.efsa.2011.EN-184
- 4.Bonardi S. Salmonella in the pork production chain and its impact on human health in the European Union. Epidemiol Infect. 2017;145(8):1513–1526. doi: 10.1017/S095026881700036X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control) The European Union One Health 2018 Zoonoses Report. EFSA J. 2019;17:e5926. doi: 10.2903/j.efsa.2019.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai YQ, Tao J, Jiao Y, Fei X, Zhou L, Wang Y, Zheng H, Pan Z, Jiao X. Phenotypic characteristics and genotypic correlation between Salmonella isolates from a slaughterhouse and retail markets in Yangzhou, China. Int J Food Microbiol. 2016;222:56–64. doi: 10.1016/j.ijfoodmicro.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Ran L, Wu S, Gao Y, Zhang X, Feng Z, Wang Z, Kan B, Klena JD, Wong D, Angulo FJ, Varma JK. Laboratory based surveillance of nontyphoidal Salmonella infections in China. Foodborne Pathog Dis. 2011;8(8):921–927. doi: 10.1089/fpd.2010.0827. [DOI] [PubMed] [Google Scholar]
- 8.Bersot LS, Cavicchioli VQ, Viana C, Burin RCK, Camargo AC, Pinto JPAN, Nero LA, Destro MT. Prevalence, antimicrobial resistance, and diversity of Salmonella along the pig production chain in Southern Brazil. Pathogens. 2019;8(4):204. doi: 10.3390/pathogens8040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kich JD, Coldebella A, Mores N, Nogueira MG, Cardoso M, Fratamico PM, Call JE, Fedorka-Cray P, Luchansky JB. Prevalence, distribution, and molecular characterization of Salmonella recovered from swine finishing herds and a slaughter facility in Santa Catarina. Brazil Int J Food Microbiol. 2011;151(3):307–313. doi: 10.1016/j.ijfoodmicro.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Mürmann L, Santos MCM, Cardoso MRI. Prevalence, genetic characterization and antimicrobial resistance of Salmonella isolated from fresh pork sausages in Porto Alegre. Brazil Food Control. 2009;20(3):191–195. doi: 10.1016/j.foodcont.2008.04.007. [DOI] [Google Scholar]
- 11.Paim DS, Pissetti C, Vieira TR, Werlang GO, Costa EF, Kich JD, Cardoso M (2019) Enumeration, antimicrobial resistance and typing of Salmonella enterica: profile of strains carried in the intestinal contents of pigs at slaughter in Southern Brazil. Acta Sci Vet 47:1636. 10.22456/1679-9216.89668
- 12.EFSA (European Food Safety Authority) Technical specifications on the harmonized monitoring and reporting of antimicrobial resistance in Salmonella, Campylobacter and indicator Escherichia coli and Enterococcus spp. bacteria transmitted through food. EFSA J. 2012;10:1–64. doi: 10.2903/j.efsa.2012.2742. [DOI] [Google Scholar]
- 13.Kahlmeter G, Brown DFJ, Goldstein FW, Macgowan AP, Muton JW, Österlund A, Rodloff A, Steinbakk M, Urbaskova P, Vatopoulos A. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J Antimicrob Chemother. 2003;52(2):145–148. doi: 10.1093/jac/dkg312. [DOI] [PubMed] [Google Scholar]
- 14.Steenackers H, Hermans K, Vanderleyden J, De Keersmaecker SCJ. Salmonella biofilms: an overview on occurrence, structure, regulation and eradication. Food Res Int. 2012;45(2):502–531. doi: 10.1016/j.foodres.2011.01.038. [DOI] [Google Scholar]
- 15.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15(2):167–219. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackenzie KD, Palmer MB, Köster WL, White AP. Examining the link between biofilm formation and the ability of pathogenic Salmonella strains to colonize multiple host species. Front Vet Sci. 2017;4:138. doi: 10.3389/fvets.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corcoran M, Morris D, De Lappe N, O’Connor J, Lalor P, Dockery P, Cormican M. Commonly used disinfectants fail to eradicate Salmonella enterica biofilms from food contact surface materials. Appl Environ Microbiol. 2014;80(4):1507–1514. doi: 10.1128/AEM.03109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merino L, Procura F, Trejo FM, Bueno DJ, Golowczyc MA. Biofilm formation by Salmonella sp. In the poultry industry: detection, control and eradication strategies. Food Res Int. 2019;119:530–540. doi: 10.1016/j.foodres.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Keelara S, Thakur S, Patel J. Biofilm formation by environmental isolates of Salmonella and their sensitivity to natural antimicrobials. Foodborne Pathog Dis. 2016;13(9):509–516. doi: 10.1089/fpd.2016.2145. [DOI] [PubMed] [Google Scholar]
- 20.Piras F, Fois F, Consolati SG, Mazza R, Mazzette R. Influence of temperature, source, and serotypes on biofilm formation of Salmonella enterica isolates from pig slaughterhouses. J Food Prot. 2015;78(10):1875–1878. doi: 10.4315/0362-028X.JFP-15-085. [DOI] [PubMed] [Google Scholar]
- 21.Tassinari E, Duffy G, Bawn M, Burgess CM, McCabe EM, Lawlor PG, Gardiner G, Kingsley RA. Microevolution of antimicrobial resistance and biofilm formation of Salmonella Typhimurium during persistence on pig farms. Sci Rep. 2019;9(1):8832. doi: 10.1038/s41598-019-45216-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CLSI (Clinical and Laboratory Standards Institute) (2018a) Performance standards for antimicrobial disk and dilution susceptibility test for bacteria isolated from animals; approved standard. CLSI supplement VET08. Wayne, PA, USA: Clinical and Laboratory Standards Institute, 99p.
- 23.CLSI (Clinical and Laboratory Standards Institute) (2018b) Performance standards for antimicrobial susceptibility testing. CLSI document M100-S28. Wayne, PA, USA: Clinical and Laboratory Standards Institute, 282p.
- 24.EFSA (European Food Safety Authority) Technical specifications on harmonized monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food-producing animals and food. EFSA J. 2019;17:e5709. doi: 10.2903/j.efsa.2019.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EUCAST (European Committee on Antimicrobial Susceptibility Testing) (2020a) Antimicrobial wild type distributions of microorganisms. https://mic.eucast.org/Eucast2/ Accessed 15 May 2022.
- 26.EUCAST (European Committee on Antimicrobial Susceptibility Testing) (2020b) Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0, 2020. https://www.eucast.org/clinical_breakpoints/ Accessed 15 May 2022.
- 27.Lopes GV, Michael GB, Cardoso M, Schwarz S. Identification and characterization of Salmonella enterica subsp enterica serovar Derby isolates carrying a new aadA26 gene cassette in a class 1 integron obtained at pig slaughterhouses. FEMS Microbiol Lett. 2014;356(1):71–78. doi: 10.1111/1574-6968.12473. [DOI] [PubMed] [Google Scholar]
- 28.Stepanovic S, Cirkovic I, Ranin L, Svabic-Vlahovic M. Biofilm formation by Salmonella spp and Listeria monocytogenes on plastic surface. Lett Appl Microbiol. 2004;38(5):428–432. doi: 10.1111/j.1472-765X.2004.01513.x. [DOI] [PubMed] [Google Scholar]
- 29.Viana C, Sereno MJ, Pegoraro K, Yamatogi RS, Call DR, Bersot LS, Nero LA. Distribution, diversity, virulence genotypes and antibiotic resistance for Salmonella isolated from a Brazilian pork production chain. Int J Food Microbiol. 2019;310:108310. doi: 10.1016/j.ijfoodmicro.2019.108310. [DOI] [PubMed] [Google Scholar]
- 30.Roberts MC, Schwarz S. Tetracycline and phenicol resistance genes and mechanisms: importance for agriculture, the environment, and humans. J Environ Qual. 2016;45(2):576–592. doi: 10.2134/jeq2015.04.0207. [DOI] [PubMed] [Google Scholar]
- 31.Poirel L, Madec JY, Lupo A, Schink AK, Kieffer N, Nordmann P, Schwarz S. Antimicrobial resistance in Escherichia coli. Microbiol Spectr. 2018;6:4. doi: 10.1128/microbiolspec.ARBA-0026-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michael GB, Schwarz S. Antimicrobial resistance in zoonotic nontyphoidal Salmonella: an alarming trend? Clin Microbiol Infect. 2016;22(12):968–974. doi: 10.1016/j.cmi.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Lopes GV, Michael GB, Cardoso M, Schwarz S. Antimicrobial resistance and class1 integron-associated gene cassettes in Salmonella enterica serovar Typhimurium isolated from pigs at slaughter and abattoir environment. Vet Microbiol. 2016;194:84–92. doi: 10.1016/j.vetmic.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Callens B, Persoons D, Maes D, Laanen M, Postma M, Boyen F, Haesebrouck F, Butaye P, Catry B, Dewulf J. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Prev Vet Med. 2012;106(1):53–62. doi: 10.1016/j.prevetmed.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 35.McPhee JB, Lewenza S, Hancock RE. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol Microbiol. 2003;50(1):205–217. doi: 10.1046/j.1365-2958.2003.03673.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doy Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 37.Rebelo AR, Bortolaia V, Kjeldgaard JS, Pedersen SK, Leekitcharoenphon P, Hansen IM, Guerra B, Malorny B, Borowiak M, Hammerl JA, Battisti A, Franco A, Alba P, Perrin-Guyomard A, Granier SA, Escobar CF, Malhotra-Kumar S, Villa L, Caratolli A, Hendriksen RS. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 2018;23(6):17–00672. doi: 10.2807/1560-7917.ES.2018.23.6.17-00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kijima M, Shirakawa T, Uchiyama M, Kawanishi M, Ozawa M, Koike R. Trends in the serovar and antimicrobial resistance in clinical isolates of Salmonella enterica from cattle and pigs between 2002 and 2016 in Japan. J Appl Microbiol. 2019;127(6):1869–1875. doi: 10.1111/jam.14431. [DOI] [PubMed] [Google Scholar]
- 39.Trongjit S, Angkititrakul S, Tuttle RE, Poungseree J, Padungtod P, Chuanchuen R. Prevalence and antimicrobial resistance in Salmonella enterica isolated from broiler chickens, pigs and meat products in Thailand-Cambodia border provinces. Microbiol Immunol. 2017;61(1):23–33. doi: 10.1111/1348-0421.12462. [DOI] [PubMed] [Google Scholar]
- 40.Kumar CG, Anand SK. Significance of microbial biofilms in food industry: a review. Int J Food Microbiol. 1998;42(1–2):9–27. doi: 10.1016/s0168-1605(98)00060-9. [DOI] [PubMed] [Google Scholar]
- 41.Simões M, Simões L, Vieira MJ. A review of current and emergent biofilm control strategies. LWT-Food Sci Technol. 2010;43(4):573–583. doi: 10.1016/j.lwt.2009.12.008. [DOI] [Google Scholar]
- 42.Joseph B, Otta SK, Karunasagar I, Karunasagar I. Biofilm formation by Salmonella spp on food contact surfaces and their sensitivity to sanitizers. Int J Food Microbiol. 2001;64(3):367–372. doi: 10.1016/s0168-1605(00)00466-9. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Miks-Krajnik M, Zheng Q, Lee SB, Lee SC, Yuk H. Biofilm formation of Salmonella Enteritidis under food-related environmental stress conditions and its subsequent resistance to chlorine treatment. Food Microbiol. 2016;54:98–105. doi: 10.1016/j.fm.2015.10.010. [DOI] [Google Scholar]
- 44.Ghasemmahdi H, Tajik H, Moradi M, Mardani K, Modaresi R, Badali A, Dilmaghani M. Antibiotic resistance pattern and biofilm formation ability of clinically isolates of Salmonella enterica serotype typhimurium. Int J Enteric Pathog. 2015;3(2):e27372. doi: 10.17795/ijep27372. [DOI] [Google Scholar]
- 45.Nair A, Rawool DB, Doijadb S, Poharkar K, Mohana V, Barbuddhe SB, Kolhe R, Kurkure NV, Kumar A, Malik SVS, Balasaravanan T. Biofilm formation and genetic diversity of Salmonella isolates recovered from clinical, food, poultry and environmental sources. Infect Genet Evol. 2015;36:424–433. doi: 10.1016/j.meegid.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Yin B, Zhu L, Zhang Y, Dong P, Mao Y, Liang R, Niu L, Luo X. The characterization of biofilm formation and detection of biofilm-related genes in Salmonella isolated from beef processing plants. Foodborne Pathog Dis. 2018;15(10):660–667. doi: 10.1089/jpd.2018.2466. [DOI] [PubMed] [Google Scholar]
- 47.Han X, Peng J, Guan X, Li J, Huang X, Liu S, Liu S, Wen Y, Zhao Q, Huang X, Yan Q, Huang Y, Cao S, Wu R, Ma X, Zou L. Genetic and antimicrobial resistance of Salmonella spp isolated from ducks along the slaughter line in southwestern China. Food Control. 2020;107:106805. doi: 10.1016/j.foodcont.2019.106805. [DOI] [Google Scholar]
- 48.Bessa MC, Costa M, Cardoso M. Prevalência de Salmonella sp em suínos abatidos em frigoríficos sob inspeção federal no Rio Grande do Sul. Pesq Vet Bras. 2004;24(2):80–84. doi: 10.1590/S0103-84782008005000035. [DOI] [Google Scholar]
- 49.Ferraz SM, Schwarz P, Canal CW, Cardoso MRI. Prevalência de suínos portadores de Salmonella sp ao abate e contaminação de embutidos tipo frescal. Acta Sci Vet. 2004;32(2):141–147. doi: 10.22456/1679-9216.16836. [DOI] [Google Scholar]
- 50.Ferraz SM, Schwarz P, Canal CW, Cardoso MRI. Presença de Salmonella sp no trato intestinal/linfonodos submandibulares de suínos ao abate. Arq Bras Med Vet Zootec. 2004;56(3):300–306. doi: 10.1590/S0102-09352004000300003. [DOI] [Google Scholar]
- 51.Schwarz P, Kich JD, Kolb J, Cardoso M. Use of an avirulent live Salmonella Choleraesuis vaccine to reduce the prevalence of Salmonella carrier pigs at slaughter. Vet Rec. 2011;169(21):553. doi: 10.1136/vr.d5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva LE, Dias V, Ferronatto A, Guerra P, Berno L, Triches N, Kich JD, Corbellini LG, Cardoso M. Longitudinal dissemination of Salmonella enterica clonal groups through the slaughter process of Salmonella-positive pig batches. J Food Protection. 2012;75(9):1580–1588. doi: 10.4315/0362-028X.JFP-11-515. [DOI] [PubMed] [Google Scholar]
- 53.Pissetti C, Werlang GO, Biesus LL, Kich JD, Cardoso M. Detecção de Salmonella enterica e Listeria monocytogenes em carcaças suínas na etapa de pré-resfriamento. Acta Sci Vet. 2012;40:1071. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.