Abstract
The antibody response to the L1 stage of Trichinella spiralis has been described as biphasic. Worms resident in the intestine during the first week of infection stimulate an antibody response against a subset of larval proteins. L1 larvae in the muscle at the end stage of infection stimulate a second antibody response against tyvelose-bearing glycoproteins. Antityvelose antibodies protect rats against challenge infection with larvae. The aim of this study was to characterize the rat B-cell response against larval antigens during the intestinal phase of T. spiralis infection and to test the antiparasitic effects of such antibodies. Strain PVG rats were infected orally with 500 larvae. Antibodies specific for phosphorylcholine-bearing proteins of L1 larvae first appeared in serum 9 days postinfection. Absorption experiments showed that the majority of antilarval antibodies produced in rats 16 days after infection with T. spiralis were specific for phosphorylcholine-bearing proteins. A fraction of these antibodies bound to free phosphorylcholine. Immunoglobulin G2c (IgG2c) producing cells in the mesenteric lymph node dominated this early antibody response. IgG2c is associated with T-independent immune responses in the rat; however, a comparison of athymic rats with euthymic controls suggested that only a small fraction of the phosphorylcholine-related antibody response against T. spiralis was T independent. Phosphorylcholine is a common epitope in antigens of bacteria and nematode parasites and has been shown to be a target of protective immunity in certain bacteria. A monoclonal IgG2c antibody was prepared from infected rats and shown to be specific for phosphorylcholine. Monoclonal phosphorylcholine-specific IgG2c failed to protect rats against intestinal infection with T. spiralis. Therefore, our findings do not support a role for phosphorylcholine-bearing antigens in immune defense against T. spiralis; however, the potency of the immune response induced suggests an immunomodulatory role for the lymphocytes involved.
Trichinella spiralis parasitizes many mammalian species and has served as a model for the study of parasitic nematodes and mucosal immunity. The rat is a natural host for T. spiralis. Infective larvae (L1 stage) are freed in the stomach when a rat consumes an infected carcass. Larvae enter the small intestine, where they undergo a series of four molts in a period of 30 h. Worms reside in an epithelial habitat (41). Adult worms reproduce, and newborn larvae migrate from the gut to striated muscle, where they invade cells and mature to the infectious L1 stage (reviewed by Despommier [10]).
Different antigens are expressed during each life stage of T. spiralis. Philipp et al. (28) showed that the proteins displayed on the epicuticle change with each molt. Jungery and Ogilvie (16) reported that the host antibody response mirrors the changes in antigen expression. In addition to the antigenic variation observed in different life stages, Denkers et al. reported that the antibody response directed against L1 larvae is biphasic (8). Of the two responses, the one that has been studied most intensively is induced late in infection by muscle-stage L1 larvae. Antibodies are induced by glycans attached to a number of larval proteins found in the secretory cells, or stichocytes, as well as on the body surface (4, 12). These glycans are tri- and tetra-antennary structures that are capped with 3,6-dideoxy-d-arabino-hexose (tyvelose) (11, 31, 40). Tyvelose forms the immunodominant epitope. This response is important to the survival of host and parasite, as shown by passive immunization of rats with antityvelose MAbs. Antibodies eliminate up to 99% of an oral challenge dose of infective T. spiralis larvae within a few hours. IgG1 and IgG2c appear to be the most protective antibody subclasses (1, 3, 5).
The other phase of the antilarval response is less well characterized, but indirect evidence suggests that it is specific for phosphorylcholine-bearing glycoproteins (35). Phosphorylcholine is widely distributed in tissues, including the cuticle, epidermis, hypodermis, hemolymph, and intestinal gland (15). Ubeira et al. (36) investigated the splenic B-cell response induced by phosphorylcholine antigens in mice infected with Trichinella; however, the more relevant, intestinal B-cell response has not been studied. Phosphorylcholine immunogens have been found in bacteria and nematodes and serve as targets of protective immunity against certain bacteria (19). In this report, we describe the rat B-cell response against phosphorylcholine-bearing glycoproteins during the intestinal phase of T. spiralis infection as well as results of experiments to test the antiparasitic effects of such antibodies.
(Some of these data were presented at the 75th Annual Meeting of the Conference of Research Workers in Animal Diseases, Chicago, Ill. [abstract no. 142], and at the 9th International Congress on Immunology, San Francisco, Calif. [program no. 931].)
MATERIALS AND METHODS
Abbreviations.
ASC, antibody-secreting cells; BSA, bovine serum albumin; CLN, cervical lymph node; DPBS, Dulbecco’s phosphate-buffered saline; ECL, enhanced chemiluminescence; ELISA, enzyme-linked immunosorbent assay; ELISPOT, enzyme-linked immunospot; HRP, horseradish peroxidase; IFN-γ, gamma interferon; Ig, immunoglobulin; IL, interleukin; MAb, monoclonal antibody; MLN, mesenteric lymph node; TI-2, thymus-independent type 2.
Rats.
Strain AO and strain PVG rats were produced and maintained under specific-pathogen-free conditions. Outbred, male NIH-RNU (athymic) and NIH-RNU/+ (euthymic) rats were obtained from the Frederick Cancer Research and Development Center and were barrier maintained. All rats were housed in the James A. Baker Institute vivarium according to American Association for Accreditation of Laboratory Animal Care guidelines.
Parasite.
T. spiralis (pig strain) was maintained in irradiated adult rats. Infectious larvae were harvested from muscle by digestion in a solution of 1% pepsin–1% HCl at 37°C. Experimental rats were infected orally with larvae suspended in a volume of 0.2 to 0.5 ml of 0.6% nutrient broth–2% gelatin (2).
Antigen.
Crude larval antigen and excretory/secretory antigens of muscle larvae were prepared as previously described (4). Phosphorylcholine-bearing antigens were isolated from crude antigen by affinity chromatography with MAb 6G3 (see below).
Antibodies.
Normal rat serum was collected by cardiac puncture from adult rats anesthetized with ether. Sera were collected at the times specified (see Results) from rats infected with 500 or 1,000 T. spiralis larvae. Monoclonal rat antibody 9E6 (IgG2c) is specific for tyvelose-capped glycans of T. spiralis (3, 11).
Mouse MAbs specific for rat IgA, IgM, and HRP-conjugated polyclonal anti-rat IgG2c or IgE were obtained from Serotec (Harlan, Indianapolis, Ind.). Mouse MAbs specific for rat IgG2a, IgG2b, and IgG1 were a gift from T. Springer (Harvard University, Boston, Mass.) (34).
Monoclonal rat antibody 6G3 was produced by somatic cell hybridization (17). MLN cells were collected from a PVG rat which had been infected orally with 2,500 larvae 10 days previously. The MLN cells were processed in the manner described below (“Preparation of Cells”). Rat lymphocytes and mouse myeloma cells (SP2/0) were fused at a ratio of 2:1. Fused cells were plated in 96-well plates on rat peritoneal exudate cells. Hybridomas were screened by ELISA in order to detect IgG2c specific for crude T. spiralis larval antigen. Positive hybridomas were cloned by limiting dilution on rat peritoneal exudate cells. Ascites fluid was produced in nude mice by Harlan Sprague-Dawley (Indianapolis, Ind.).
ELISA.
Crude larval antigen (5 μg/ml, 50 μl/well) was incubated in polyvinyl microtiter plates overnight at 4°C. Wells were blocked with DPBS containing 0.2% gelatin for 15 min. The remainder of the procedure was performed as described previously (3), except that for hybridoma screening, antibody binding was detected with peroxidase-conjugated rabbit antibody specific for rat IgG2c (Serotec).
In one experiment, pooled serum (collected from rats 16 days postinfection) or MAb 6G3 was absorbed with MAb 6G3 affinity-purified antigens or with BSA by incubating antibody solutions on antigen-coated (5 μg of protein/ml) petri dishes for 2 h at 4°C. Absorbed antibody preparations were tested by ELISA for binding to crude larval antigen. Antibody binding was detected by using peroxidase-conjugated goat antibody specific for rat IgG (heavy- and light-chain reactive) (3).
Preparation of cells.
MLNs, CLNs, spleens, and blood were collected from infected rats at intervals following infection. Lymph nodes and spleens were dissected to remove the fat, cut into several pieces, and pressed through a sterile sieve. Cell suspensions were filtered through a cell strainer (Becton Dickinson, Lincoln Park, N.J.), diluted to 25 ml in DPBS–0.05% BSA (Sigma, St. Louis, Mo.), and centrifuged at 153 × g for 10 min at 4°C. This step was repeated twice. The cells were resuspended, counted (Coulter Counter ZM; Coulter Electronics, Hialeah, Fla.), centrifuged, and resuspended at 107 cells/ml in RPMI medium containing 5% fetal calf serum, 50 U of penicillin per ml, 50 μg of streptomycin per ml, and 1 mM sodium pyruvate (all from Gibco, Grand Island, N.Y.), as well as 2 mM l-glutamine and 50 μM 2-mercaptoethanol (Sigma).
ELISPOT.
Tissue culture plates (96 well) with nitrocellulose filters (Millipore, Bedford, Mass.) were incubated for 24 h at 4°C with 50 μl of crude antigen (100 μg/ml) in DPBS. The filters were washed with DPBS and then blocked for 15 min in DPBS–1% dry milk. The plates were washed twice with DPBS, and 100 μl of complete RPMI medium was added to each well, together with 100 μl of the appropriate cell suspension. Cells were tested at three dilutions. Each dilution was tested in duplicate. The plates were left at room temperature for 5 min and then incubated for 3 h at 37°C in 5% CO2. The filters were washed three times with DPBS and incubated with unconjugated mouse anti-rat isotype antibodies or with HRP-conjugated anti-IgG2c or anti-IgE for 1 h at 4°C with agitation. Filters were washed three times in DPBS–0.05% BSA. For detection of IgG1, IgG2a, IgG2b, IgM, and IgA, filters were next incubated with agitation for 1 h at 4°C with goat anti-mouse IgG conjugated to HRP (Southern Biotech, Birmingham, Al.) diluted in DPBS–0.05% BSA plus 10% normal rat serum. For detection of IgE, the signal was amplified further by an additional incubation with an HRP-conjugated antiperoxidase antibody. The filters were washed three times in DPBS–0.05% Tween 20 (Sigma) and incubated with 4-chloro-naphthol (Sigma) for 30 min at room temperature with agitation.
Dark purple spots, representing ASC, were counted with a Nikon dissecting microscope (6×) fitted with a Sony DXC-107 video camera attached to a video display. Wells were counted if they contained between 1 and 200 spots per well. Data were expressed as the mean numbers of ASC per tissue ± 1 standard deviation. Hybridoma cells that produce anti-T. spiralis MAbs of known isotypes (3) were used as positive controls and to confirm the specificity of the assay.
Polyacrylamide gel electrophoresis and Western blotting.
Ten percent polyacrylamide gels with 3% stacking gels in standard format or miniformat (as noted) were used. Samples were boiled for 3 to 5 min prior to loading in sample preparation buffer (0.0625 M Tris–2% sodium dodecyl sulfate–10% glycerol [pH 6.8]) containing 5% 2-mercaptoethanol. Prestained molecular weight standards (Diversified Biotech, Newton Center, Mass.) were used. Gel electrophoresis and Western transfer to nitrocellulose were performed according to the methods described by Burnette (7). Nitrocellulose was blocked with DPBS–0.2% Tween–7.5% dry milk–2% BSA (Sigma). Antibodies were diluted in DPBS–0.2% Tween–2% dry milk–0.25% BSA. The nitrocellulose was washed in DPBS–0.2% Tween. ECL reagent (Amersham Life Science, Little Chalfont, Buckinghamshire, England) was prepared according to the manufacturer’s instructions. In one experiment, primary antibodies were prepared in the presence of phosphorylcholine chloride (Sigma).
Passive immunization of rats.
Fourteen-day-old AO rats were injected intraperitoneally with antibody (2.5 mg/20 g of body weight) 90 min prior to oral infection with 185 larvae. Twelve rats each were treated with serum immunoglobulin from uninfected rats, antityvelose MAb 9E6 (IgG2c), or MAb 6G3 (IgG2c). Worms were harvested from intestines on days 1, 8, and 10 after infection according to methods that have been described (2).
Statistical analyses.
Student’s t test was used to compare means for treatment groups. Analysis of variance and Tukey’s least significant difference test were used to detect differences among three or more means. Differences were considered significant when P was <0.05.
RESULTS
IgG2c ASC dominated the early immune response.
We used crude larval antigen in the ELISPOT. Repeated attempts to detect ASC specific for larval excretory/secretory antigens (which are largely tyvelose-bearing glycoproteins) during the intestinal stage of infection were unsuccessful. Thus, antigens recognized in crude larval homogenates by ASC were distinct from those found in larval excretory/secretory antigens and were not tyvelose-bearing glycoproteins. The presence of tyvelose-bearing proteins in crude antigen was confirmed by ELISPOT with specific hybridomas (not shown).
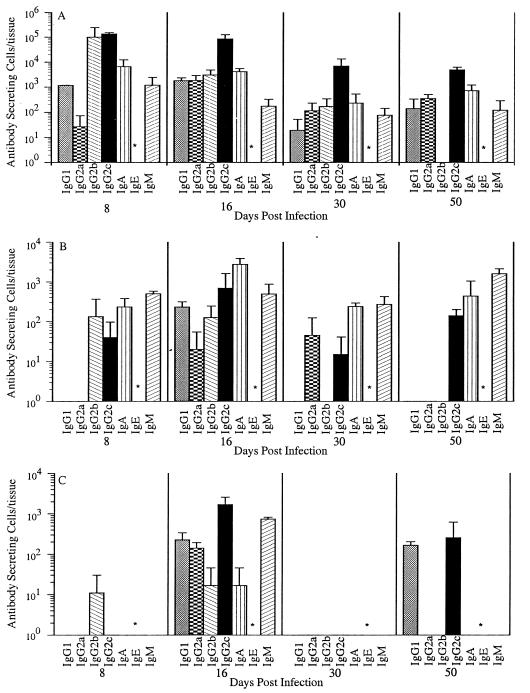
We assayed parasite-specific ASC in three lymphoid tissues on days 8, 16, 30, and 50 postinfection (Fig. 1). The MLN dominated the antibody response, generating 50 times the ASC found in spleen or CLN. Over 105 T. spiralis-specific ASC were detected in MLN 8 and 16 days after infection. The peak activity in the spleen and CLN did not occur until day 16. ASC numbers in all tissues decreased more than 10-fold between days 16 and 30.
FIG. 1.
Kinetics of the T. spiralis-specific ASC response in three lymphoid tissues: MLN (A), CLN (B), and spleen (C). PVG rats were infected with an oral dose of 867 T. spiralis muscle larvae. The numbers of specific ASC were determined by ELISPOT on days 8, 16, 30, and 50 postinfection. Values expressed are the means + standard deviations (for three rats or, on day 50, two rats). ∗, IgE was not assayed in this experiment.
IgG2c was the dominant isotype in the MLN, comprising 55% of the total ASC on day 8 and 89% of the total ASC on day 16 (Fig. 1A). IgG2b made a large contribution on day 8 (41% of the total ASC) but was more variable and declined quickly. IgA ASC contributed 4.3% of the total ASC on day 16. IgM, IgG1, and IgG2a ASC together comprised less than 4% of the total ASC at any given time. Parasite-specific IgE ASC were not assayed in this experiment.
The ASC response in the CLN was similar in isotype distribution but muted in comparison to that of the MLN (Fig. 1C). IgG2c accounted for 60% of the total ASC on day 16. No ASC were detected on day 30. On day 50, specific ASC again were detected, coincident with the onset of the antityvelose response described previously (1).
The isotype profile of ASC in the spleen was distinct from those of the MLN and CLN (Fig. 1B). IgA ASC accounted for 64% of the total on day 16. IgA, IgM, and IgG2c ASC declined from day 16 to day 30 and then rebounded on day 50.
The kinetics of the early immune response were confirmed in three additional experiments. In one experiment, parasite-specific IgE ASC were rare but detectable in the MLN (less than 0.1% of the total ASC) on day 16. In a third experiment, we detected low numbers of IgE ASC in the CLN 47 days postinfection (data not shown).
The ASC response was largely T dependent.
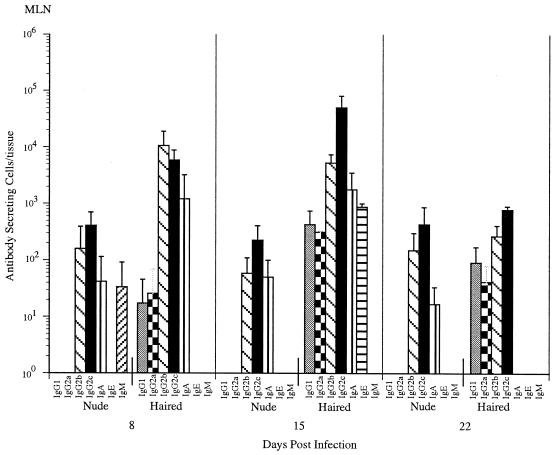
Euthymic NIH rats produced an antibody response with an isotype distribution similar to that of the PVG rats, although it was not of equal magnitude (Fig. 2). Nude rats mounted a weak (less than 10% of the haired-rat response) but constant ASC response in the MLN, with relatively constant numbers of ASC at 8, 15, and 22 days postinfection. The isotypes produced by nude rats were the same as those produced by euthymic rats: IgG2b, IgG2c, and IgA. The overall level of activity in the spleens of rats in this experiment was too small to allow any conclusions to be drawn (data not shown). When euthymic rats were infected orally with larvae, they eliminated nearly all of the worms by 15 days postinfection. As expected based on previously reported studies (27, 37), athymic rats failed to eliminate significant numbers of adult worms by 22 days postinfection (data not shown), confirming the T-cell dependence of adult worm rejection and showing that the weak B-cell response did not promote worm rejection.
FIG. 2.
Comparison of the kinetics of the T. spiralis-specific ASC responses in the MLNs of euthymic (haired) and athymic (nude) rats. Rats were infected with an oral dose of 500 T. spiralis muscle larvae. The numbers of specific ASC were determined by ELISPOT on days 8 and 16 postinfection. Values expressed are the means + standard deviations (n = three rats).
Isotype profiles of specific antibodies in sera from T. spiralis-infected PVG rats correlated with ASC numbers.
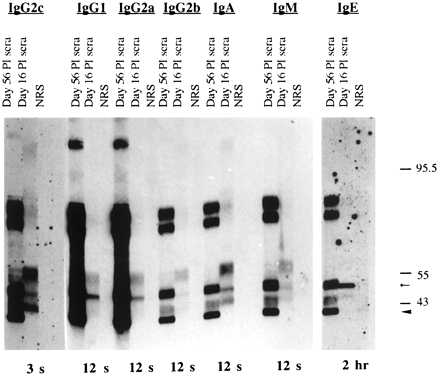
Western blots of crude larval antigens were probed with pooled serum antibodies collected from uninfected rats, rats infected with 1,000 larvae (56 days postinfection), or rats infected with 500 larvae (16 days postinfection) (Fig. 3). Antibodies in the sera collected 16 days postinfection bound to at least three proteins (reduced) of 40 to 60 kDa. The difference in binding specificities between antibodies collected on days 16 and 56 of infection illustrates the switch from phosphorylcholine antigens to tyvelose antigens that takes place in the biphasic antibody response (8). The predominance of IgG1, IgG2a, and IgG2c among antityvelose antibodies detected on day 56 postinfection corresponds with ELISA data reported previously (3).
FIG. 3.
Isotype profile of specific antibodies in sera of PVG rats on days 16 and 56 postinfection (PI). Western-blotted T. spiralis crude antigen was probed with pooled sera from normal rats (NRS), rats infected with 1,000 larvae 56 days prior to bleeding, and rats infected with 500 larvae 16 days prior to bleeding. Antibody binding was detected with HRP-conjugated antibodies developed in an ECL system, as described in Materials and Methods. Molecular mass standards are indicated in kilodaltons. Autoradiograph exposure time for each isotype is shown at the bottom of the figure. The small arrow marks migration of the 49-kDa phosphorylcholine-bearing protein. The large arrowhead marks migration of the smallest tyvelose-bearing glycoprotein.
The isotype profile of pooled antibodies in sera collected 16 days postinfection correlated with ELISPOT data obtained from the same rats. IgG2c was the predominant isotype. IgG2b had a weak signal, correlating with a variable and volatile IgG2b ASC response. The IgE signal was extremely weak, requiring a 2-h exposure to develop, compared to the 3- to 12-s exposures for the other isotypes. The specificities of the isotypes were similar, but the intensity of binding to different proteins varied. IgE was distinctive in that it bound to a single protein species.
Serum antibodies from infected rats and MAb 6G3 were specific for phosphorylcholine.
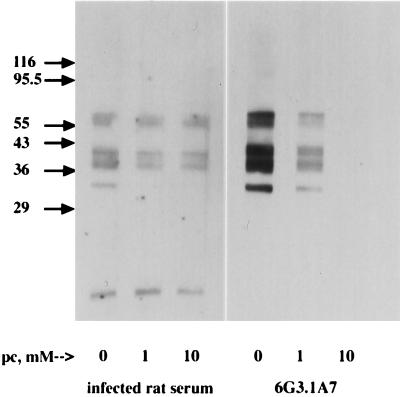
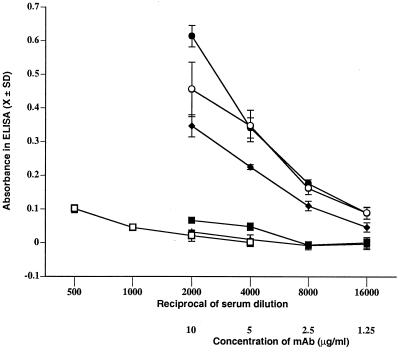
Based on indirect evidence, Takahashi has concluded that the antibody response induced in the first 2 weeks of infection with T. spiralis is specific for phosphorylcholine-bearing proteins (35). In order to confirm the specificity of the antibodies produced in infected rats, we performed blocking experiments. Incubation of MAb 6G3 with 10 mM phosphorylcholine blocked binding of the antibody to Western blots of crude larval antigen (Fig. 4), proving the specificity of the antibody. In contrast, phosphorylcholine completely blocked serum antibody binding to only one of several proteins, suggesting that other epitopes on these proteins were being recognized (Fig. 4). We confirmed the specificity of serum antibodies by absorbing rat sera with phosphorylcholine-bearing proteins that were affinity purified from crude antigen by using MAb 6G3. Absorption removed all serum antibodies capable of binding crude antigens in ELISA (Fig. 5), confirming the phosphorylcholine-bearing antigens to be dominant in this phase of the antilarval response.
FIG. 4.
Soluble phosphorylcholine (pc) blocked binding of MAbs and polyclonal antibodies to crude larval antigens. Crude antigens were resolved on 10% acrylamide minigels, blotted, immunostained, and developed with ECL. Phosphorylcholine was titrated in the presence of the primary antibodies: 6G3 hybridoma culture supernatant (diluted 1:40) (20-s exposure) or pooled serum from rats infected 16 days prior with 500 larvae (diluted 1:5,000) (1-min exposure). Similarly diluted serum from uninfected rats showed no reaction. Molecular mass standards are indicated in kilodaltons.
FIG. 5.
Serum antibodies in rats infected 16 days with T. spiralis were specific for phosphorylcholine-bearing antigens. Pooled serum collected from rats 16 days postinfection or antibody 6G3 was absorbed with phosphorylcholine-bearing antigens or BSA as described elsewhere and then titrated in an ELISA on crude larval antigens. Absorbances are graphed for pooled serum collected from rats 16 days postinfection with 500 T. spiralis absorbed with phosphorylcholine antigens (■), absorbed with BSA (●), or unabsorbed (○); for pooled sera collected from uninfected rats (□); and for MAb 6G3 absorbed with phosphorylcholine antigens (▴) or BSA (⧫).
Antiphosphorylcholine MAb 6G3 was not protective.
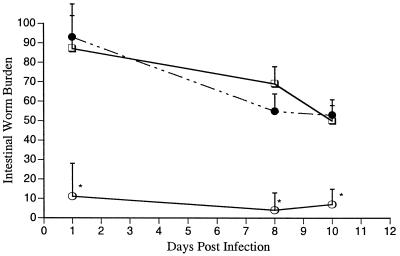
In order to test the effects of antiphosphorylcholine antibody on intestinal T. spiralis, we used a passive immunization and challenge protocol in suckling rats. We have shown this system to be highly sensitive in revealing protective activity of rat antibodies against intestinal infection with the parasite (3). Passive immunization with MAb 6G3 failed to accelerate expulsion of intestinal larvae or adult worms from rat pups challenged with T. spiralis (Fig. 6). We used antityvelose antibody 9E6 (IgG2c) as an isotype-matched, positive control in this experiment. As described previously, passive immunization with 9E6 caused rats to eliminate more than 90% of the challenge larvae within 24 h (3). Antibody 9E6 had no effect on the few adult worms that developed in passively immunized rats.
FIG. 6.
Comparison of intestinal worm burdens in rat pups that were passively immunized with 6G3 MAb (●), 9E6, tyvelose-specific MAb (○), or serum antibodies from an uninfected rat (□). PVG rats were infected with an oral dose of 185 T. spiralis muscle larvae. The numbers of worms per intestine were counted on 1, 8, and 10 days postinfection. Values are the means + standard deviations (four or five rats). ∗, 9E6-treated rats bore significantly fewer worms than the two other treatment groups (Tukey’s least significant difference test, P < 0.05).
DISCUSSION
MLNs are sites of intense B- and T-lymphocyte activity between 8 and 16 days after infection with T. spiralis in rats and mice (13, 22). The increased cellular activity in the MLN is correlated with the expulsion of the adult worms. T lymphocytes from this tissue (13) and from lymph vessels draining the intestine (18) are protective in adoptive transfer experiments. We found that the isotype distribution among ASC in lymph nodes draining the intestine correlated well with specific antibody isotypes in serum, suggesting that in addition to the T-cell response, the MLN is playing a major role in the serum antibody response to infection.
Intestinal nematodes have long been known to induce strong IgE responses in rodents. Although we expected to measure large numbers of IgE ASC in our experiments, such cells were rare in the tissues examined from infected PVG rats. We obtained similar results from other rat strains, including Brown Norway (data not shown), a strain that is reported to produce strong allergic responses (42). It is possible that IgE ASC may reside in tissues that we did not evaluate, such as intestinal lamina propria. Circulating IgE levels similarly were barely detectable. Interpretation of our results is aided by the findings of Ramaswamy and coworkers, which showed that IgE is transported from serum to the gut lumen in T. spiralis-infected rats (29). This transport depletes the serum of circulating IgE (but not IgG) between 4 and 14 days postinfection, times that correlate with our assessments. Thus, we cannot rule out the possibility that IgE was produced by B cells in tissues we did not assay or that it was cleared from the circulation so that our sampling methods failed to detect it.
The dominant isotype in the antiphosphorylcholine antigen response against T. spiralis was IgG2c. In the rat, polysaccharide and hapten-polysaccharide antigens (TI-2 antigens) stimulate IgG2c predominantly (9). Our studies of athymic rats showed that less than 10% of the early antibody response against T. spiralis was T-lymphocyte independent. This result is compatible with findings for nude mice, where IgG3 (the mouse homologue of rat IgG2c [6, 9]) responses to TI-2 antigens are reduced by more than 90% compared to those in euthymic controls (32). Biological activities unique to rat IgG2c are not known.
Other investigations have shown that anti-Ig antibodies conjugated to dextran mimic the B-cell-stimulatory activity of TI-2 antigens in mice (reviewed by Mond et al. [23]). The influences of cytokines on this stimulation have been investigated. Anti-Ig–dextran conjugates trigger B-cell proliferation in vitro, and addition of IL-5 induces these cells to produce IgG3. Furthermore, IgG3 production is enhanced 10-fold by the addition of IFN-γ together with IL-5 (33). These findings can be applied to the analysis of the immune response against T. spiralis in rats. Ramaswamy et al. (30) showed that IFN-γ was released into the intestinal lymph in a cyclical manner, increasing at 35, 75, and 180 h following infection. IL-5 was detected as early as 20 h following infection and subsequently was present together with IFN-γ. Thus, IL-5 and IFN-γ are present in intestinal lymphoid tissue at the time that worms are releasing their phosphorylcholine-bearing antigens in the intestine. These cytokines may drive the production of IgG2c against phosphorylcholine-bearing antigens of T. spiralis.
Phosphorylcholine epitopes in other nematodes have been described to have immunomodulatory effects. A phosphorylcholine-bearing glycoprotein of the filarial nematode Acanthocheilonema viteae has been shown to inhibit B-cell activation (14). In addition, phosphorylcholine has been implicated in the induction of IL-10 secretion by B-1 cells from mice immunized with microfilarial lysates of Brugia malayi (26). Although we have not investigated B-1-cell proliferation or cytokine production in T. spiralis-infected rats, the magnitude of the response to phosphorylcholine-bearing antigens suggests that it is likely to influence the other immune responses induced by the parasite.
Bacterial organisms incorporate phosphorylcholine into teichoic acids (24), lipopolysaccharide (39), protein, and pili (38). Using mice transgenic for an antiphosphorylcholine antibody, Lim and Choy demonstrated that phosphorylcholine is a target of protective immunity against Streptococcus pneumoniae (20). In these studies, transgenic adult mice were not resistant to T. spiralis infection. Because it was found that suckling rats are more sensitive to the effects of protective antilarval antibodies than adult rats (3, 5), we tested phosphorylcholine-specific antibody for protection in suckling rats. Antiphosphorylcholine IgG2c did not alter the establishment or development of T. spiralis in the intestine. Previously, it was shown that protective, antityvelose IgG2c antibodies bind to tyvelose-bearing surface and secreted products of L1 larvae (4, 11, 12) and that secreted products are the primary targets in protection (21). In contrast, phosphorylcholine antigens are sequestered in the body of the larva and are not secreted by this stage (reference 25 and unpublished observations). Sequestration would explain both the failure of these antigens to induce an immune response during the muscle stage of infection and also their failure to serve as targets in protective intestinal immunity. It seems plausible, although not proven, that phosphorylcholine antigens would be released during molting. Although phosphorylcholine antigens have been detected in adult worms (25), we found that antiphosphorylcholine IgG2c antibody did not accelerate expulsion of adult worms from the gut. Thus, phosphorylcholine antigens are highly immunogenic but do not appear to serve as targets of antibody-mediated, protective immunity against intestinal T. spiralis.
In summary, we have described an IgG2c-dominated antibody response in the intestines of rats infected with the parasitic nematode, T. spiralis. This response is directed against previously described phosphorylcholine-bearing glycoproteins produced by the first larval stage and is distinct from the well-characterized antityvelose response which is induced by muscle-stage larvae. The antiphosphorylcholine response is largely T dependent and, although it is induced in the intestine, does not appear to directly influence the outcome of intestinal infection. Unique biological activities of rat IgG2c are not known. The strong representation of IgG2c in two distinct antibody responses directed against T. spiralis larvae supports a conclusion that this isotype, as well as the cells that produce it, has a significant and unique role in host responses to infection with this nematode.
ACKNOWLEDGMENTS
This work was supported by USPHS grant AI 14490, a grant from the Cooperative State Research, Education and Extension Service, U.S. Department of Agriculture, under project no. 473401, and a Fogarty Senior International Fellowship (J.A.A.). P.J.P. and A.B.B. were supported by a grant from the Howard Hughes Medical Institute (Cornell Hughes Scholars Program), and E.A.S. was supported by a fellowship from the College of Veterinary Medicine, Cornell University.
We thank F. Ubeira for helpful discussion and A. Hesser for assistance in preparing the manuscript.
REFERENCES
- 1.Appleton J A, McGregor D D. Characterization of the immune mediator of rapid expulsion of Trichinella spiralis in suckling rats. Immunology. 1987;62:477–484. [PMC free article] [PubMed] [Google Scholar]
- 2.Appleton J A, McGregor D D. Life-phase specific induction and expression of rapid expulsion in rats suckling Trichinella spiralis-infected dams. Immunology. 1985;55:225–232. [PMC free article] [PubMed] [Google Scholar]
- 3.Appleton J A, Schain L R, McGregor D D. Rapid expulsion of Trichinella spiralis in suckling rats: mediation by monoclonal antibodies. Immunology. 1988;65:487–492. [PMC free article] [PubMed] [Google Scholar]
- 4.Appleton J A, Usack L. Identification of potential antigenic targets for rapid expulsion of Trichinella spiralis. Mol Biochem Parasitol. 1993;58:53–62. doi: 10.1016/0166-6851(93)90090-k. [DOI] [PubMed] [Google Scholar]
- 5.Bell R G, Appleton J A, Negrao-Correa D A, Adams L S. Rapid expulsion of Trichinella spiralis in adult rats mediated by monoclonal antibodies of distinct IgG isotypes. Immunology. 1992;75:520–527. [PMC free article] [PubMed] [Google Scholar]
- 6.Brüggemann M, Delmastro-Galfrè P, Waldmann H, Calabi F. Sequence of a rat immunoglobulin γ2c heavy chain constant region cDNA: extensive homology to mouse γ3. Eur J Immunol. 1988;18:317–319. doi: 10.1002/eji.1830180222. [DOI] [PubMed] [Google Scholar]
- 7.Burnette W N. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 8.Denkers E Y, Wassom D L, Krco C J, Hayes C E. The mouse antibody response to Trichinella spiralis defines a single, immunodominant epitope shared by multiple antigens. J Immunol. 1990;144:3152–3159. [PubMed] [Google Scholar]
- 9.der Balian G, Slack J, Clevinger B L, Bazin H, Davie J M. Subclass restriction of murine antibodies. III. Antigens that stimulate IgG3 in mice stimulate IgG2c in rats. J Exp Med. 1980;152:209–218. doi: 10.1084/jem.152.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Despommier D D. Biology. In: Campbell W C, editor. Trichinella and trichinosis. New York, N.Y: Plenum Press; 1983. pp. 75–80. [Google Scholar]
- 11.Ellis L A, McVay C S, Probert M A, Zhang J, Bundle D R, Appleton J A. Terminal β-linked tyvelose creates unique epitopes in Trichinella spiralis glycan antigens. Glycobiology. 1997;7:383–390. doi: 10.1093/glycob/7.3.383. [DOI] [PubMed] [Google Scholar]
- 12.Ellis L A, Reason A J, Morris H R, Dell A, Iglesias R, Ubeira F M, Appleton J A. Glycans as targets for monoclonal antibodies that protect rats against Trichinella spiralis. Glycobiology. 1994;4:585–592. doi: 10.1093/glycob/4.5.585. [DOI] [PubMed] [Google Scholar]
- 13.Grencis R K, Wakelin D. Short lived, dividing cells mediate adoptive transfer of immunity to Trichinella spiralis in mice. I. Availability of cells in primary and secondary infections in relation to cellular changes in the mesenteric lymph node. Immunology. 1982;46:443–450. [PMC free article] [PubMed] [Google Scholar]
- 14.Harnett W, Harnett M M. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory-secretory product. J Immunol. 1993;151:4829–4837. [PubMed] [Google Scholar]
- 15.Hernández S, Romarís F, Acosta I, Gutiérrez P N, Ubeira F M. Ultrastructural colocalization of phosphorylcholine and a phosphorylcholine-associated epitope in first-stage larvae of Trichinella spiralis. Parasitol Res. 1995;81:643–650. doi: 10.1007/BF00931840. [DOI] [PubMed] [Google Scholar]
- 16.Jungery M, Ogilvie B M. Antibody response to stage-specific Trichinella spiralis surface antigens in strong and weak responder mouse strains. J Immunol. 1982;129:839–843. [PubMed] [Google Scholar]
- 17.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 18.Korenaga M, Wang C H, Bell R G, Zhu D, Ahmad A. Intestinal immunity to Trichinella spiralis is a property of OX8−OX22− T-helper cells that are generated in the intestine. Immunology. 1989;66:588–594. [PMC free article] [PubMed] [Google Scholar]
- 19.Lim P L, Choy W F. A thymus-independent (type 1) phosphorylcholine antigen isolated from Trichinella spiralis protects mice against pneumococcal infection. Immunology. 1990;69:443–448. [PMC free article] [PubMed] [Google Scholar]
- 20.Lim P L, Choy W F, Chan S T H, Leung D T, Ng S S. Transgene-encoded antiphosphorylcholine (T15+) antibodies protect CBA/N (xid) mice against infection with Streptococcus pneumoniae but not Trichinella spiralis. Infect Immun. 1994;62:1658–1661. doi: 10.1128/iai.62.5.1658-1661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McVay C S, Tsung A, Appleton J. Participation of parasite surface glycoproteins in antibody-mediated protection of epithelial cells against Trichinella spiralis. Infect Immun. 1998;66:1941–1945. doi: 10.1128/iai.66.5.1941-1945.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molinari J A, Hess J A, Wesley R K. Sequential mesenteric lymph node histological response following Trichinella spiralis infection. Int Arch Allergy Appl Immunol. 1982;69:81–85. doi: 10.1159/000233150. [DOI] [PubMed] [Google Scholar]
- 23.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 24.Mosser J L, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an enzyme. J Biol Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 25.Ortega-Pierres M G, Yepez-Mulia L, Homan W, Gamble H R, Lim P L, Takahashi Y, Wassom D L, Appleton J A. Workshop on a detailed characterization of Trichinella spiralis antigens: a platform for future studies on antigens and antibodies to this parasite. Parasite Immunol. 1996;18:273–284. doi: 10.1046/j.1365-3024.1996.d01-103.x. [DOI] [PubMed] [Google Scholar]
- 26.Palanivel V, Posey C, Horauf A M, Solbach W, Piessens W F, Harn D A. B-cell outgrowth and ligand-specific production of IL-10 correlate with Th2 dominance in certain parasitic diseases. Exp Parasitol. 1996;84:168–177. doi: 10.1006/expr.1996.0102. [DOI] [PubMed] [Google Scholar]
- 27.Perrudet B A, Boussac A Y, Ruitenberg E J, Kruizinga W, Elgersma A. Protection to challenge with Trichinella spiralis after primary oral infection in congenitally athymic (nude) mice. J Parasitol. 1983;69:253–255. [PubMed] [Google Scholar]
- 28.Philipp M, Parkhouse R M, Ogilvie B M. Changing proteins on the surface of a parasitic nematode. Nature. 1980;287:538–540. doi: 10.1038/287538a0. [DOI] [PubMed] [Google Scholar]
- 29.Ramaswamy K, Hakimi J, Bell R G. Evidence for an interleukin 4-inducible immunoglobulin E uptake and transport mechanism in the intestine. J Exp Med. 1994;180:1793–1803. doi: 10.1084/jem.180.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramaswamy K, Negrao-Correa D, Bell R. Local intestinal immune responses to infections with Trichinella spiralis. J Immunol. 1996;156:4238–4337. [PubMed] [Google Scholar]
- 31.Reason A J, Ellis L A, Appleton J A, Wisnewski N, Grieve R B, McNeil M, Wassom D L, Morris H R, Dell A. Novel tyvelose-containing tri- and tetra-antennary N-glycans in the immunodominant antigens of the intracellular parasite Trichinella spiralis. Glycobiology. 1994;4:593–603. doi: 10.1093/glycob/4.5.593. [DOI] [PubMed] [Google Scholar]
- 32.Slack J H, Davie J M. Subclass restriction of murine antibodies. V. The IgG plaque-forming cell response to thymus-independent and thymus-dependent antigens in athymic and euthymic mice. Cell Immunol. 1982;68:139–145. doi: 10.1016/0008-8749(82)90096-x. [DOI] [PubMed] [Google Scholar]
- 33.Snapper C M, McIntyre T M, Mandler R, Pecanha L M, Finkelman F D, Lees A, Mond J J. Induction of IgG3 secretion by interferon-gamma: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J Exp Med. 1992;175:1367–1371. doi: 10.1084/jem.175.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Springer T A, Bhattacharya A, Cardoza J T, Sanchez-Madrid F. Monoclonal antibodies specific for rat IgG1, IgG2a, and IgG2b subclasses, and kappa chain monotypic and allotypic determinants: reagents for use with rat monoclonal antibodies. Hybridoma. 1982;1:257–273. doi: 10.1089/hyb.1.1982.1.257. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi Y. Antigens of Trichinella spiralis. Parasitol Today. 1997;13:104–106. doi: 10.1016/s0169-4758(97)01008-9. [DOI] [PubMed] [Google Scholar]
- 36.Ubeira F M, Leiro J, Santamarina M T, Villa T G, Sanmartín-Durán M L. Immune response to Trichinella epitopes: the antiphosphorylcholine plaque-forming cell response during the biological cycle. Parasitology. 1987;94:543–553. doi: 10.1017/s0031182000055888. [DOI] [PubMed] [Google Scholar]
- 37.Vos J G, Ruitenberg E J, Van Basten N, Buys J, Elgersma A, Kruizinga W. The athymic nude rat. IV. Immunocytochemical study to detect T-cells, and immunological and histopathological reactions against Trichinella spiralis. Parasite Immunol. 1983;5:195–215. doi: 10.1111/j.1365-3024.1983.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 38.Weiser J N, Goldberg J B, Pan N, Wilson L, Virji M. The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae. Infect Immun. 1998;66:4263–4267. doi: 10.1128/iai.66.9.4263-4267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiser J N, Pan N, McGowan K L, Musher D, Martin A, Richards J. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wisnewski N, McNeil M, Grieve R B, Wassom D L. Characterization of novel fucosyl- and tyvelosyl-containing glycoconjugates from Trichinella spiralis muscle stage larvae. Mol Biochem Parasitol. 1993;61:25–35. doi: 10.1016/0166-6851(93)90155-q. [DOI] [PubMed] [Google Scholar]
- 41.Wright K D. Trichinella spiralis: an intracellular parasite in the intestinal phase. J Parasitol. 1979;65:441–445. [PubMed] [Google Scholar]
- 42.Yoo T J, Kuo C Y. Cellular and reaginic immune responses to ragweed antigen E in inbred rats. Int Arch Allergy Appl Immunol. 1980;61:259–270. doi: 10.1159/000232444. [DOI] [PubMed] [Google Scholar]