Abstract
Background:
Liver inflammation is the main cause of severe liver diseases, including liver fibrosis, steatohepatitis, cirrhosis and hepatocellular carcinoma. Cell therapy topics are receiving increasingly more attention. The therapeutic applications of mesenchymal stem cells (MSC) have become one of the most discussed issues. While other stem cells have therapeutic effects, they have only one or two clinical applications. MSCs are responsible for repairing a variety of tissue injuries. Moreover, MSCs could be derived from several sources, including adipose tissue. MSCs are usually more abundant and easier to obtain compared to other stem cells.
Methods:
To prove the concept that MSCs have homing ability to the injured tissue and assist in tissue repair, we examined the effects of intravenous injected adipose-derived mesenchymal stem cells (ADSCs) in a N-nitrosodiethylamine (DEN)-induced liver injury rat model.
Results:
The significant repairing ability of ADSCs was observed. The levels of fibrosis, apoptosis, and tumorigenesis in the DEN-injured liver tissues all decreased after ADSC treatment. Furthermore, to enhance the therapeutic effects of ADSCs, we pretreated them with L-theanine, which promotes the hepatocyte growth factor secretion of ADSC, and therefore improved the healing effects on injured liver tissue.
Conclusion:
ADSCs, especially L-theanine-pretreated ADSCs, have anti-inflammation, anti-apoptosis, and anti-tumorigenesis effects on the N-nitrosodiethylamine-induced liver injury rat model.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13770-022-00472-2.
Keywords: Mesenchymal stem cell, Adipose tissue-derived stem cell, Cell therapy, Tissue repair, Liver inflammation
Introduction
Liver inflammation that leads to liver fibrosis accounts for the cause of most liver diseases, including cirrhosis and hepatocellular carcinoma (HCC). HCC is the most common primary liver malignant disease. Its incidence and mortality rank in the top ten in both male and female cancers. Cirrhosis, even worse, is a global health concern which causes 2% of annual deaths worldwide [1]. Over the past few decades, the causes of cirrhosis or liver cancer have shifted from viral infections to metabolic problems such as alcoholic hepatitis or non-alcoholic steatohepatitis [2, 3]. Due to the pressured and irregular modern lifestyle, the rising incidences of these liver inflammation diseases have been noticed. Repeated inflammation causes ROS production and cell apoptosis. Therefore, different from viral hepatitis which depends on anti-viral therapies, the treatments for metabolic hepatitis depend on inflammation control.
Previous studies pointed out that mesenchymal stem cells (MSCs) could serve multiple positive functions in tissue regeneration. MSC actively secretes several essential growth factors, including stromal cell-derived factor 1(SDF-1), vascular endothelial growth factor (VEGF)-A, and hepatic growth factor (HGF), etc. [4, 5]. The secretion of these growth factors implies that MSC can aid in the regeneration and repair of injured tissue by promoting angiogenesis, preventing apoptosis, and stimulating native cell proliferation. MSC application to liver damage was previously proposed [6, 7]. MSC could migrate into injured tissue, contribute to the paracrine system and provide immunomodulatory effects. To demonstrate and enhance the therapeutic effects of adipose tissue-derived MSCs (ADSCs) in liver inflammatory injury, we generated a rat hepatitis model induced by N-nitrosodiethylamine (DEN) [8, 9]. The ADSC effects in injured liver were examined. DEN is a potent hepatocarcinogenic nitrosamine which produces the pro-mutagenic products, O6-ethyldeoxyguanosine and O4 and O6-ethyldeoxythymidine, leading to cell apoptosis and tissue inflammation [8, 10, 11]. Here we demonstrate that ADSC reduced DEN-induced liver injury by decreasing fibrosis levels and apoptosis. The liver tumorigenesis marker and alpha-fetoprotein (AFP) levels were also reduced. Moreover, L-theanine-pretreatment promoted HGF secretion by ADSCs to enhance liver repair.
Materials and methods
Animal model
Male Wistar rats (200–250 g) at the age of 7 weeks were provided by BioLASCO Taiwan Co., Ltd. (Ilan, Taiwan). Rats were housed at the Experimental Animal Center, National Taiwan Normal University, with a constant temperature and a consistent light cycle (light from 07:00 to 18:00). Food and water were provided ad libitum. All surgical and experimental procedures were approved by Institutional Animal Care and Use Committee (IACUC) of National Taiwan University (College of Medicine and College of Public Health) (Approval number 105035). Procedures were designed in accordance with the National Science Council of Republic of China guidelines (NSC 1997). Different liver dysfunctions were induced by a toxic reagent, N-nitrosodiethylamine (DEN, Sigma-Aldrich, St. Louis, MO, USA), in 2, 4, and 8 weeks.
Sixty animals were randomly divided into ten groups, group 1: control group (n = 6); group 2: DEN treatment for 2 weeks (n = 6); group 3: DEN treatment for 4 weeks (n = 6); group 4: DEN treatment for 8 weeks (n = 6); group 5: DEN treatment for 2 weeks followed by ADSC treatment for 4 weeks (n = 6); group 6: DEN treatment for 4 weeks followed by ADSC treatment for 4 weeks (n = 6); group 7: DEN treatment for 8 weeks followed by ADSC treatment for 4 weeks (n = 6); group 8: DEN treatment for 2 weeks followed by L-theanine-pretreated ADSC treatment for 4 weeks (n = 6); group 9: DEN treatment was given for 4 weeks followed by L-theanine-pretreated ADSC treatment for 4 weeks (n = 6); (n = 6); group 10: DEN treatment for 8 weeks followed by L-theanine-pretreated ADSC treatment for 4 weeks (n = 6).
Five hundred ppm DEN in water was given as an inducer of liver injury for 2, 4, or 8 weeks. One million first passaged ADSCs (1 × 106 P1) were administered intravenously after 2, 4, or 8 weeks of DEN treatment. The rats were sacrificed 4 weeks after ADSC or L-theanine-pretreated ADSC treatment.
Adipose tissue-derived mesenchymal stem cells (ADSC)
Male Wistar rats (200–250 g) at the age of 7 weeks were purchased from BioLASCO Taiwan Co., Ltd. Rat ADSCs were isolated from adipocytes as described previously [12], and all procedures were approved by the IACUC of National Taiwan University College of Medicine and College of Public Health. Adipocytes obtained from rats were washed with phosphate-buffered saline (PBS) with 1% penicillin/streptomycin and minced into small pieces around 1 × 1 cm2. Adjacent microvascular tissue was carefully removed. The adipose tissue was enzymatically dissociated by adding isometric 0.15% type I collagenase (Gibco, now part of Thermo Fisher Scientific, Waltham, MA, USA) for 15 min in a 37°C incubator supplemented with 5% CO2. The dissolved tissue was then centrifuged at 15,000 rpm for 5 min. The cell pellet was re-suspended and transferred into a T75 culture flask. Cells attached to the flask bottom were observed after 24 h of culturing (P0).
Primary ADSCs were obtained from Wistar rats, seeded into a T75 culture flask and grown in complete culture medium (CCM: α-MEM (α-minimal essential medium; Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS), 100 unit/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine). Media were changed every 2 days. The incubator environment is a gas mixture composed of 94% N2, 5% CO2, and 1% O2, combining with two air sensors, one for CO2 and the other for O2. The O2 concentration is achieved and maintained by nitrogen gas (N2) delivery generated from a liquid nitrogen tank or a tank containing pure N2. If the O2 percentage rises above the desired level, N2 gas is automatically injected into the system to replace the excess O2.
To detect the ADSC surface markers, we performed immunocytochemistry staining followed by flow cytometry analysis. ADSCs (from passage 1 to passage 4) were seeded into a T75 culture flask for 24 h and then were re-suspended to single-cell suspension followed by fixation with 4% paraformaldehyde for 30 min. For flow cytometry analysis, the ADSCs were incubated with 2 μL of PE-conjugated mouse Anti-Rat CD90 (positive marker, BD Biosciences, San Jose, CA, USA) and FITC-conjugated mouse anti-rat CD45 (negative marker, BD Biosciences) antibodies. After incubating for 30 min, ADSCs were washed with PBS three times and analyzed using flow cytometry (BD FACSAria III; BD Biosciences).
L-theanine-pretreated ADSC
L-theanine is a component discovered in the extract of tea leaves. It is an amino acid structurally similar to one of the neurotransmitters, glutamate. Its oxidative ability and preventive effects on cognitive dysfunction are well recognized [13]. To investigate whether L-theanine could enhance the homing effects of ADSCs, we pre-incubated ADSCs with L-theanine (Taiyo Kagaki CO LTD, Yokkaichi, Japan) for 2 h and then replaced the medium to the regular one for the following 24 h.
Cell counting kit-8 (CCK8) proliferation assay, trypan blue staining and bicinchoninic acid (BCA) protein assay
To examine the effects of L-theanine treatment on ADSCs, we investigated the proliferation, percentage of cell death, and protein secretion ability of ADSCs with or without L-theanine treatment by CCK8 proliferation assay (Dojindo, Munich, Germany), trypan blue staining (Sigma-Aldrich), and BCA protein assay (Thermo Fisher Scientific) respectively. All assays were performed according to the manufacturer’s instruction. In brief, 2 × 103 ADSC cells were seeded in 96-wells for all three assays. For proliferation assay, eight microliter of CCK8 reagent were added into 100 μL culture medium on the first (0 h) and the third day (48 h) after seeding, and were incubated for 3.5 h before the OD450 nm measurement. For cell death, 0.08% trypan blue were used to stain the ADSC cells for 15 min on the third day after seeding. To examine the paracrine ability of ADSCs, the total protein concentration of ADSC culture medium with or without L-theanine treatment was measured by BCA protein assay kit. 1:50 dilution of BCA reagent was incubated with culture medium for 15 min before OD562 nm measurement.
Cytokine array
To further analyze the difference between ADSCs and L-theanine-pretreated ADSCs, the ADSC culture media in passage 1 cultured with or without L-theanine for 2 h were collected and analyzed by cytokine array (Glass Slide-based Antibody Arrays L-Series, RayBio, GA, USA). With the standard curve of each cytokine, the concentration (pg/mL) of the cytokines can be determined. The increased folds of cytokines induced by L-theanine treatment were calculated by dividing the cytokine concentration in the L-theanine treated ADSC medium with that in the non-treated ADSC control medium.
Masson’s staining
The histologic features were assessed for fibrosis by Masson’s trichrome staining. The staining procedure was performed according to manufacturer’s instruction (Abcam, Cambridge, UK). In brief, deparaffinized sections were fixed by preheated Bouin’s solution and then stained with Weigert’s iron hematoxylin reagents. Slides were then incubated with Biebrich scarlet-acid fuchsin solution and differentiated in phosphomolybdic/phosphotungstic acid solution. Finally, the slides were stained with aniline blue solution and treated with acetate. The stained slides were then observed and analyzed by light microscopy.
Immunohistochemistry
The immunohistochemistry staining was performed as described previously [14]. In brief, hepatic sections were deparaffinized in xylene for 10 min and drenched in ethanol for rehydration at room temperature, followed by immunohistochemically staining to present in vivo tumorigenesis and apoptosis markers. In brief, primary antibody was applied overnight at 4 °C. The tissue sections were then washed with phosphate buffer saline tween-20 (PBST) three times before incubating with secondary antibodies for 1 h at room temperature. The slides were applied using Dako Liquid diaminobenzene, DAB (ImmPACT DAB Peroxidase Substrate; Vector, Burlingame, CA, USA) for 1–5 min, and washed with double-distilled water (ddH2O), then immersed with hematoxylene for 1–3 min. The slides were dehydrated in ethanol series and mounted in mounting medium (Leica, Wetzlar, Germany).
The primary antibodies used for tumorigenesis were anti-AFP (1:500, Abcam). Antibodies used for apoptotic markers included rabbit polyclonal anti-B-cell lymphoma 2 (Bcl-2, 1:200, Proteintech, Chicago, IL, USA), rabbit polyclonal anti-Bcl-2-associated X protein (Bax, 1:200, Cell Signaling Technology, Denver, MA, USA), and rabbit polyclonal anti-caspase3 (1:200, Chemicon, CA, USA). The anti-HGF antibody was purchased from Invitrogen (part of Thermo Fisher Scientific, 1:100).
The protein expression percentages were calculated as the percentage of the stained area using Image J software (Research Services Branch of the National Institute of Mental Health, Bethesda, MD, USA).
Western blotting
The protein concentrations of liver extracts were determined using a BioRad Protein Assay (BioRad Laboratories, Hercules, CA, USA). Twenty micrograms of protein were electrophoresed and the Bax, caspase 3 and Bcl-2 expressions were evaluated using Western immunoblotting and densitometry as described previously [15]. Briefly, the tissue proteins were homogenized with a pre-chilled mortar and pestle in extraction buffer, which consists of 10 mM Tris-HCl (pH 7.6), 140 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1% Nonidet P-40, 0.5% deoxycholate, 2% β-mercaptoethanol, 10 μg/ml pepstatin A, and 10 μg/ml aprotinin. The mixtures were homogenized completely by vortexing and kept at 4 °C for 30 min. After centrifugation at 12,000 rpm, 4 °C for 12 min, the supernatants were collected and analyzed using western blotting.
The polyclonal rabbit anti-Bax, caspase 3, Bcl-2 (Chemicon), and monoclonal mouse anti-actin (Sigma-Aldrich) were used at 1:400 dilution for incubation. The band density with the appropriate molecular weight were determined semi-quantitatively with densitometry using Image J software.
Statistical analysis
All values were expressed as mean ± standard error mean (SEM). Differences between groups were evaluated using paired t-test. Intergroup comparisons were made by Student’s t-test. Differences were regarded as significant if p < 0.05 is obtained.
Results
P1 ADSC purification and culture
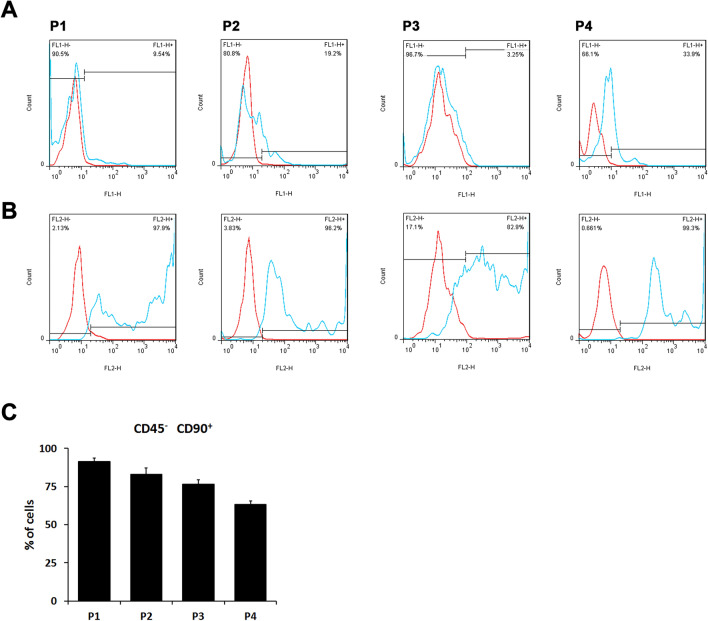
The ADSCs were isolated from adipocytes as described in materials and methods. To confirm and identify the ADSCs, cells isolated from adipocytes were cultured for 48 h and then stained with anti-CD90 and CD45 antibodies. The labeled cells were analyzed by flow cytometry. CD90 and CD45 were served as positive and negative surface markers for ADSC, respectively. The majority of our isolated cells expressed CD90 but not CD45 throughout passages 1 to 4. The CD90 positive cells occupied 95.6 ± 1.0% of the examined cells in passage 1; 96.6 ± 2.0% in passage 2; 93.8 ± 6.7% in passage 3; 89.2 ± 10.9% in passage 4. On the other hand, the CD45 negative cells accounted for 96.4 ± 2.6%, 89.3.6 ± 4.5%, 90.2 ± 10.1% and 90.3 ± 9.4% throughout cell passages 1–4, respectively (Fig. 1A). The cells qualified for both CD90 positive and CD45 negative conditions were 90.8 ± 1.5% in the passage 1 cells, and then dropped sequentially to passage 4 (90.3 ± 9.4% in P2, 84.3 ± 4.1% in P3, and 74.4 ± 11.3% in P4) (Fig. 1B). Therefore, we used the cells from passage 1 for the following experiments.
Fig. 1.
Characterization of adipose tissue-isolated MSCs (ADSCs). Primary rat MSCs isolated from adipose tissue were stained with IgG control (red lines) or A the FITC-conjugated anti-rat CD45 monoclonal antibody (blue lines) and B the PE-conjugated anti-rat CD90 monoclonal antibody (blue lines) throughout ADSC passages 1–4 (P1-P4. The freshly isolated ADSC is passage 0, P0). The black lines indicated the dividing boundary of negative (IgG control) and positive staining signals. C The percentage of CD90 positive and CD45 negative cells in different primary ADSC culture passages. In passage 1 isolated cells, the percentage of ADSCs were the highest among the first four passages
L-theanine treatment increases the ADSC proliferation and protein secretion, and decreases the death rate of ADSC
To enhance the ADSC properties, pretreatments of natural compounds had been investigated [16]. L-theanine is a component discovered in the extract of tea leaves and a structural analog of the neurotransmitter, glutamate. Its oxidative ability and preventive effects on cognitive dysfunction are well recognized [13]. To investigate the effects of L-theanine on ADSCs in vitro, we examined the following three in vitro assays, including the proliferation effects by CCK8 proliferation assays; the cell viability by trypan blue staining assay; and the paracrine secretion ability by BCA protein assay. As shown in Fig. 2A, L-theanine treatment slightly increased the proliferation rates of ADSC compared to no treatment control. Dying cells were detected 48 h post seeding and the decreased dead cell proportion of ADSCs with L-theanine treatment were observed (Fig. 2B). Moreover, to examine the general paracrine ability of stem cells, total protein concentrations of ADSC culture media with or without L-theanine treatment were also measured. The trend of higher total protein amount in the culture medium of L-theanine-treated ADSCs were noticed (Fig. 2C).
Fig. 2.
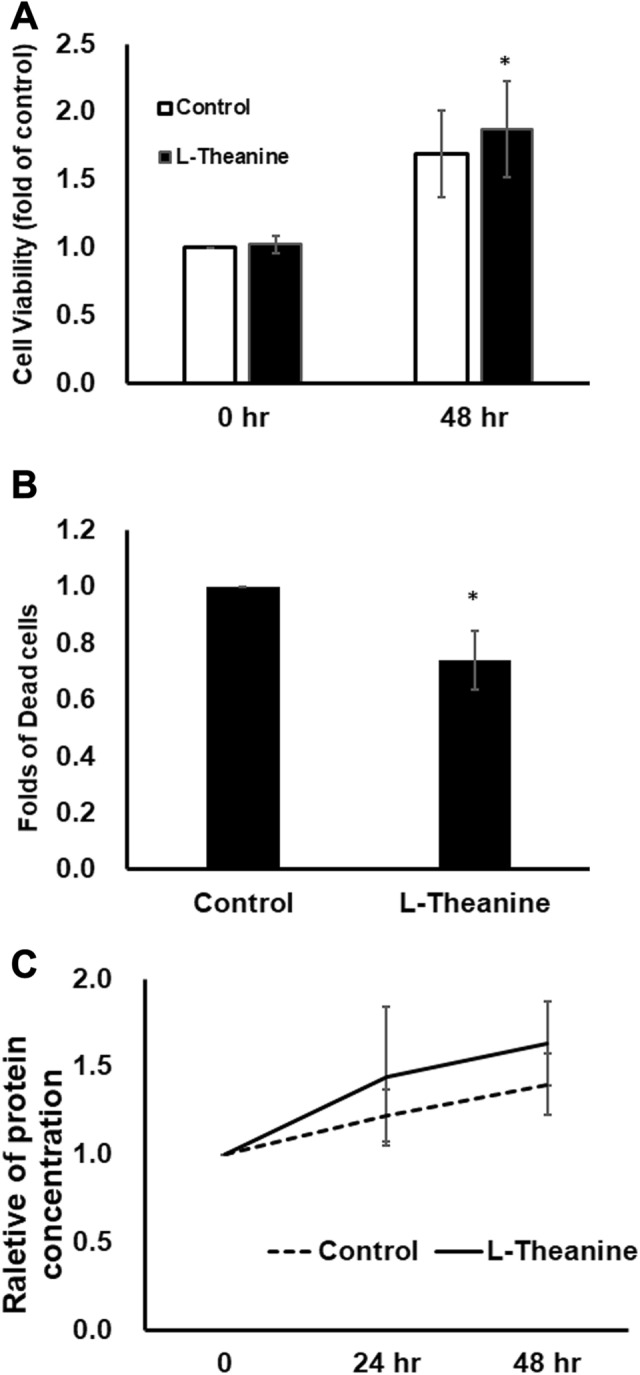
L-theanine treatment increased the ADSC proliferation and protein secretion, and decreased the death rate of ADSCs. A Cell proliferation of ADSC with (black bar) or without (white bar) L-theanine treatment were examined by CCK8 proliferation kit at 0 h and 48 h post cell seeding. B The percentage of dead ADSC cells after 48 h-culture were detected by trypan blue staining. The data showed the dead cell percentage of ADSC with L-theanine treatment compared to ADSC without treatment. C The total protein amounts of cultural media were determined by BCA protein assay
ADSC treatment recovers the appearance of DEN-damaged liver
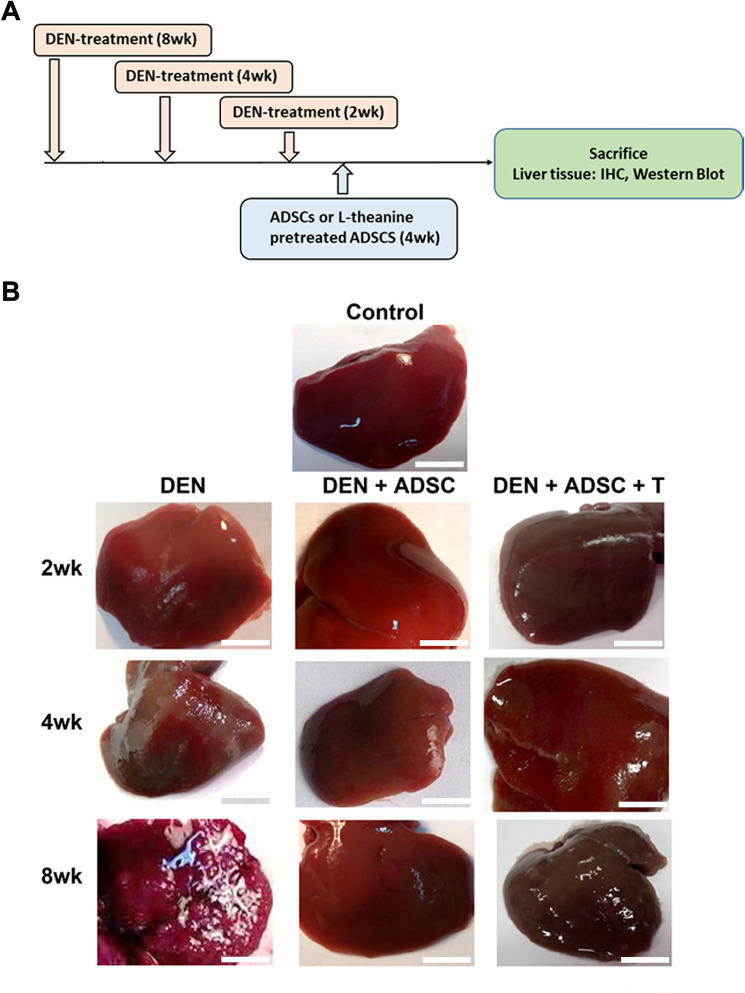
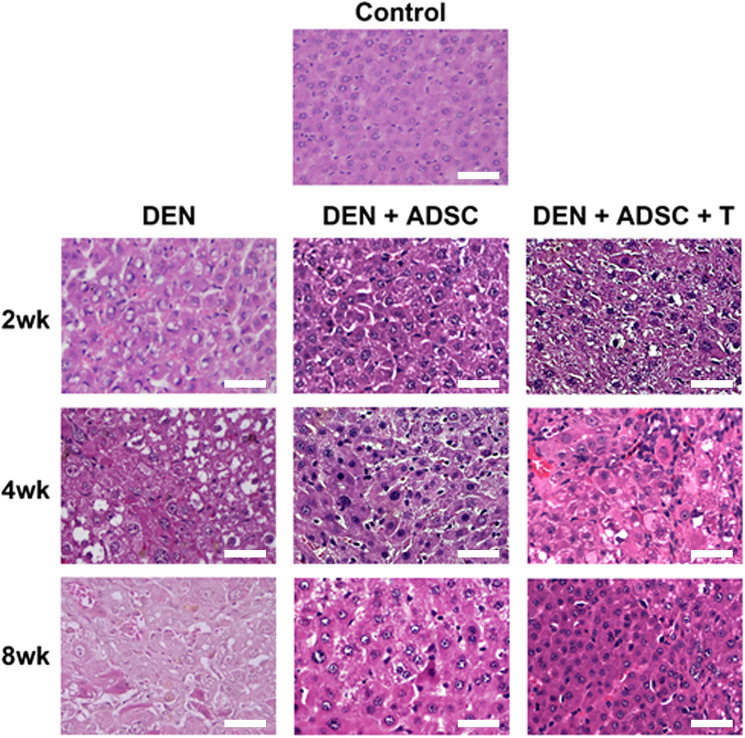
To explore the ADSC effects on liver damage in vivo, DEN-induced liver-injury rat model were subjected for ADSC treatments as demonstrated in Fig. 3A. The liver tissues were collected and the appearances were observed. The appearance of DEN-treated liver had rough and gloomy surfaces. However, after ADSC or L-theanine-pretreated ADSC treatments, the damaged liver recovered its smooth surface (Fig. 3B). To examine the pathological effects of DEN and ADSC treatments on liver tissue, hematoxylin and eosin (HE) staining were performed and results were shown in Fig. 4. The DEN-treated liver tissue showed shattered pattern compared to the control group. After ADSC or L-theanine-pretreated ADSC treatment, the tissue returned to organized patterns (Fig. 4).
Fig. 3.
DEN-induced liver fibrosis rat model. A The time line of establishment of DEN-induced liver injury rat model. Liver injury was induced by DEN in the 7, 11 and 13-week-old rats. 1 × 106 ADSC or L-theanine preconditioned ADSC cells/rat were given to rats by intravenous injection after DEN injection for 2, 4 or 8 weeks. Liver tissue and blood were collected four weeks after ADSC injection for further evaluation. B Observation of experimental animal liver tissues. DEN 2wk, 4wk and 8wk: Liver appearance from DEN-treated rats for 2, 4, or 8 weeks, respectively. DEN+ADSC: Liver appearance from rats treated with ADSC for another 4 weeks. DEN+ADSC+T: Liver appearance from rats with ADSCs pretreated with L-theanine for 2 h one day before applying to animal. The scale bar represents 1 cm
Fig. 4.
Hemotoxylin and eosin liver slice staining in experimental animals. Liver tissue slices from sacrificed rats were treated with hematoxylin and eosin staining. 2wk, 4wk and 8wk: Liver tissue slices with DEN treatment for 2, 4, or 8 weeks, respectively. DEN+ADSC: DEN-injected liver tissue slices treated with ADSC for another 4 weeks. DEN+ADSC+T: DEN-injected liver tissue slices with ADSCs which pretreated with L-theanine for 2 h one day before applying to animal. The scale bar represents 50 μm
ADSC treatment reduces fibrosis, tumorigenesis, apoptosis and tumorigenesis in DEN-injured liver
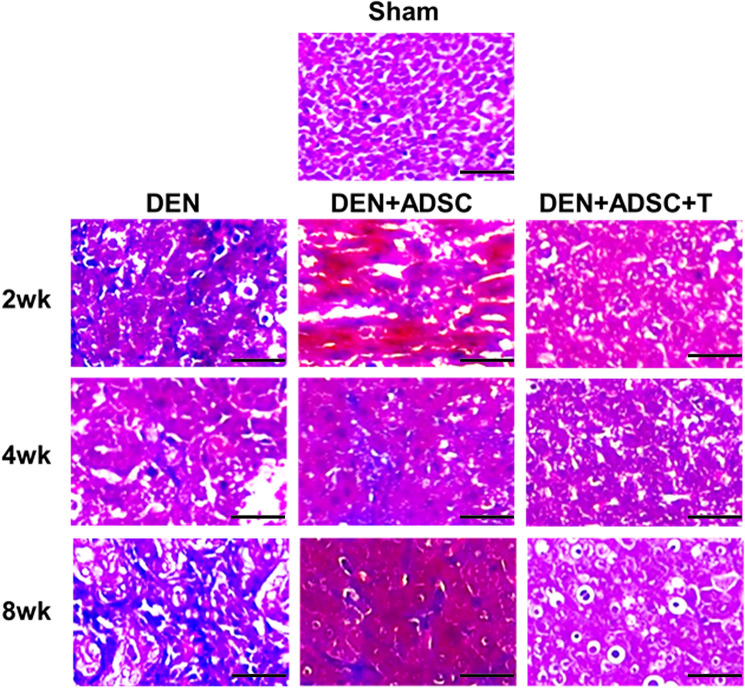
To explore whether DEN-injured liver developed hepatic fibrosis, we investigated the fibrosis status using Masson’s staining which stains connective tissues. The results showed that after DEN treatment, compared to the control group, the positive blue staining for connective tissue were increased. The blue staining levels in the groups treated with ADSCs were reduced compared to the DEN-only groups. The groups treated with L-theanine-pretreated ADSCs showed the best rescue effects in fibrosis (Fig. 5).
Fig. 5.
Masson’s Trichrome liver tissue slice staining in the experimental animals. Liver tissue slices from sacrificed rats were treated with Masson’s Trichrome staining. 2wk, 4wk and 8wk: Liver tissue slides with DEN treatment for 2, 4, or 8 weeks, respectively. DEN+ADSC: DEN-injected liver tissue slides treated with ADSC for another 4 weeks. DEN+ADSC+T: DEN-injected liver tissue slides with ADSCs which pretreated with L-theanine. The scale bar represents 25 μm
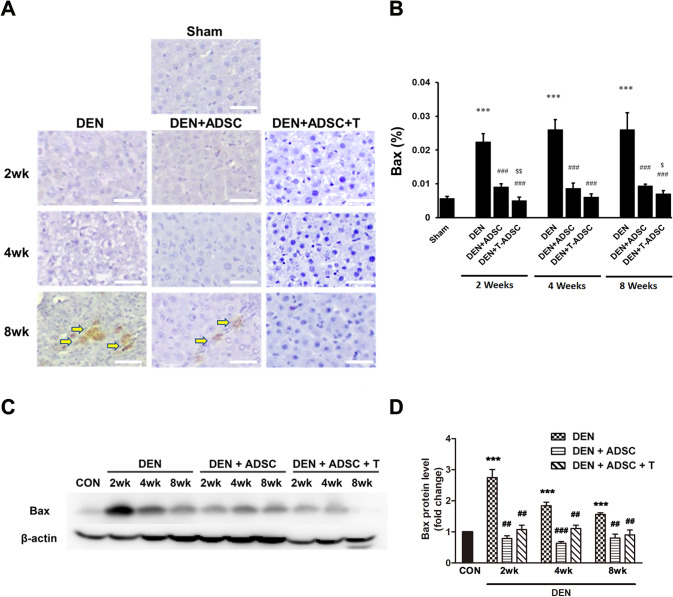
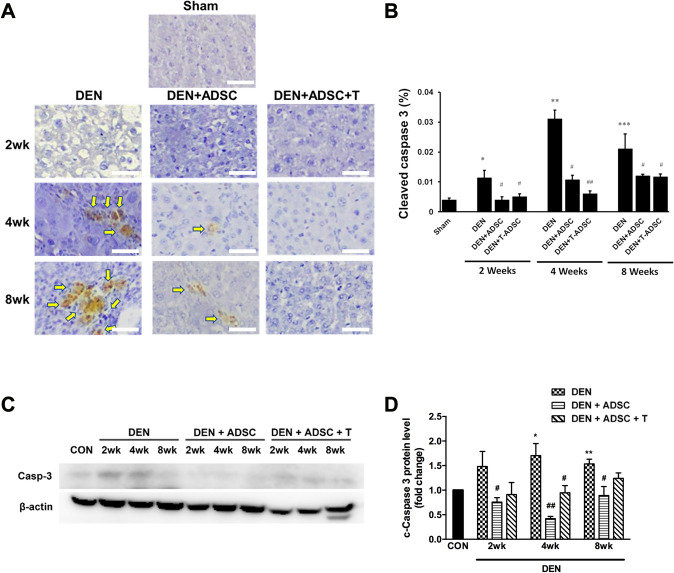
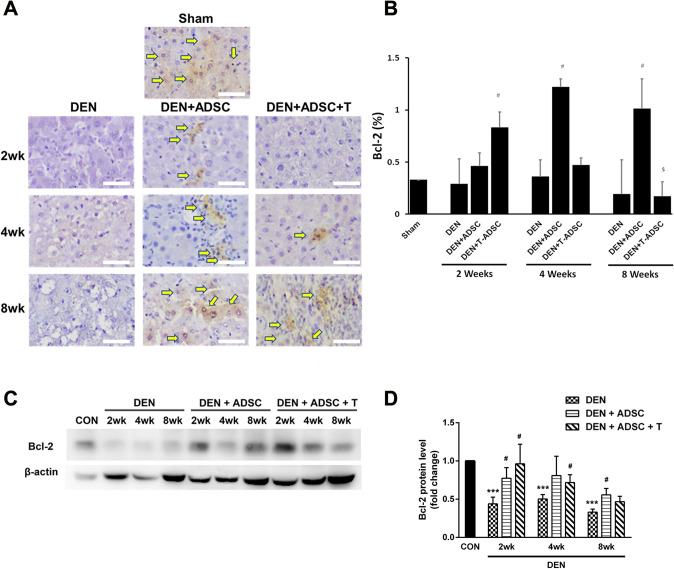
To investigate how DEN influence liver injury, the apoptosis markers, such as Bax, Caspase 3 and Bcl-2, were also examined. Both immunohistochemistry and western blotting results showed a significant increase of Bax and cleaved Caspase 3 levels after DEN treatments (Figs. 6, and 7). On the other hand, DEN-induced apoptosis inhibition was observed in ADSC and L-theanine-pretreated ADSC groups (Figs. 6 and 7). Oppositely, the expression of anti-apoptotic factor, Bcl-2, was decreased after DEN treatment according to both IHC and Western blot analysis. The Bcl-2 level was restored and even increased after ADSC or L-theanine-pretreated ADSC injection (Fig. 8).
Fig. 6.
Bax levels were elevated in DEN-injected liver tissues and ADSCs treatments recovered this effects. A Immunohistochemical Bax staining in experimental animal liver tissues with DEN treatment for 2, 4, or 8 weeks (DEN), respectively (2wk, 4wk, 8wk). DEN+ADSC: DEN-injected liver tissue slices treated with ADSC for another 4 weeks. DEN+ADSC+T: DEN-injected liver tissue slices with ADSCs pretreated with L-theanine. Arrow points to the brown positive Bax signal stain. The scale bar represents 25 μm. B Quantification of immunohistochemical Bax staining in experimental animal liver tissues. C Western blotting analysis and D quantification of Bax expression in experimental animal liver tissues. Compared to Sham, ***: p < 0.001. Compared to DEN, ##: p < 0.01. ###: p < 0.001. $Compared to DEN + ADSC, p < 0.05. Compared to DEN + ADSC, $$: p < 0.01
Fig. 7.
Caspase 3 levels were elevated in DEN-injected liver tissues and ADSCs treatments reduced caspase 3 levels. A Immunohistochemical caspase 3 staining in experimental animal liver tissues with DEN treatment for 2, 4, or 8 weeks (DEN), respectively (2wk, 4wk, 8wk). DEN+ADSC: DEN-injected liver tissue slices treated with ADSC for another 4 weeks. DEN+ADSC+T: DEN-injected liver tissue slices with ADSCs pretreated with L-theanine. Arrow points to the brown positive caspase 3 signal stain. The scale bar represents 25 μm. B Quantification of immunohistochemical caspase 3 staining in experimental animal liver tissues. C Western blotting analysis and D quantification of caspase 3 expression in experimental animal liver tissues. Compared to Sham, *: p < 0.05. **: p < 0.01. ***: p < 0.001. Compared to DEN, #: p < 0.05. ##: p < 0.01
Fig. 8.
Bcl-2 levels were decreased in DEN-injected liver tissues and ADSCs treatments increased Bcl-2 levels. A Immunohistochemical Bcl-2 staining in experimental animal liver tissues with DEN treatment for 2, 4, or 8 weeks (DEN), respectively (2wk, 4wk, 8wk). DEN+ADSC: DEN-injected liver tissue slices treated with ADSC for another 4 weeks. DEN+ADSC+T: DEN-injected liver tissue slices with ADSCs pretreated with L-theanine. Arrow point to the brown positive Bcl-2 signal stain. The scale bar represents 25 μm. B Quantification of immunohistochemical Bcl-2 staining in experimental animal liver tissues. C Western blotting analysis and D quantification of Bcl-2 expression in experimental animal liver tissues. Compared to Sham, ***: p < 0.001. Compared to DEN, #: p < 0.05. Compared to DEN + ADSC, $: p < 0.05
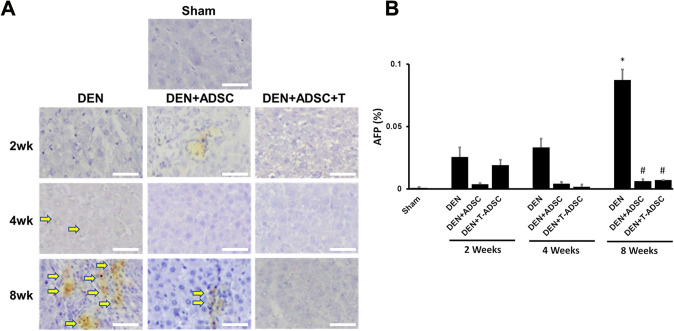
Accompanied with liver injury and fibrosis, repeated inflammation will cause tumorigenesis in the liver, and lead to the development of hepatocellular carcinoma (HCC). The clinical correlation between alpha-fetoprotein (AFP) with HCC were studied thoroughly [17]. It has been widely accepted for clinical application that the AFP serum-level serves as a diagnostic and prognostic marker for HCC. In order to examined the tumorigenesis levels of our DEN-treated animal model, we performed immunohistochemistry staining for AFP. As expected, the DEN-treated group for 8 weeks showed the strongest AFP staining. After ADSC or L-theanine-pretreated ADSC treatment, the AFP expression in the liver tissue was significantly decreased (Fig. 9).
Fig. 9.
AFP levels were elevated in DEN-injected liver tissues and ADSCs treatments reduced this effect. A Immunohistochemical AFP staining in experimental animal liver tissues with DEN treatment for 2, 4, or 8 weeks (DEN), respectively (2wk, 4wk, 8wk). DEN+ADSC: DEN-injected liver tissue slices treated with ADSC for another 4 weeks. DEN+ADSC+T: DEN-injected liver tissue slices with ADSCs pretreated with L-theanine. Arrow points to the brown positive AFP signal stain. The scale bar represents 25 μm. B Quantification of immunohistochemical AFP staining in experimental animal liver tissues. Compared to Sham, *: p < 0.05. Compared to DEN, #: p < 0.05
L-theanine treatment promotes HGF secretion by ADSCs
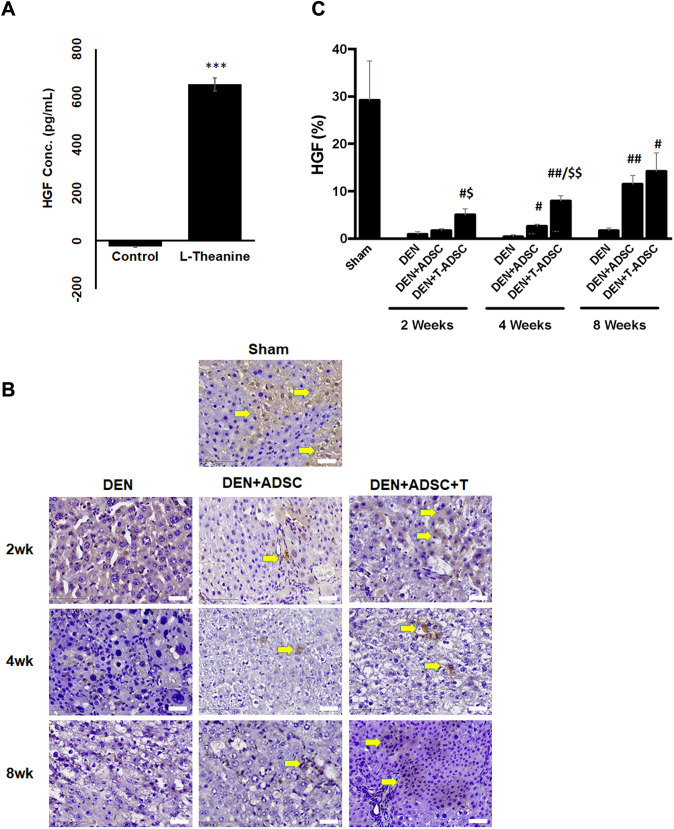
The paracrine effects of MSCs were previously noted, and proposed as contributing to tissue repair [4]. To investigate if the ADSC L-theanine treatment promotes specific ADSC paracrine, we collected the culture media from regular cultured ADSCs and the one with L-theanine-pretreated for 2 h. These media were analyzed using the cytokine array to identify the cytokines with differential expression, and the results were listed in supplementary table 1. We found that the amount of hepatic growth factor (HGF) was increased at least 24 folds in L-theanine-pretreated ADSC culture medium compared to the one without pretreatments (Fig. 10A). Moreover, the higher levels of HGF were also detected in the liver sections of L-theanine-pretreated ADSC groups compared to the one without treatment (Fig. 10B, C). HGF is an important growth factor for the liver and may assist in injured liver repair by promoting cell proliferation, recruiting inflammation modulatory cells and anti-apoptosis [18]. It serves as an autocrine to promote the proliferation of MSC itself [5, 19]. Its function in MSC-mediated tissue repair had also been reported [20, 21]. Overall, our data support that L-theanine-pretreated ADSCs secret higher levels of HGF autocrine and have better therapeutic effects on injured or inflammatory liver tissues (Fig. 11).
Fig. 10.
L-theanine treatment increased HGF secretion by ADSCs. A Quantitative results of cytokine array for secreted HGF from ADSCs with and without L-theanine treatment. ***Compared to control, p < 0.001. B Immunohistochemical staining of HGF in the liver sections of experimental animals with DEN treatment for 2, 4, or 8 weeks (DEN), respectively (2wk, 4wk, 8wk). DEN + ADSC: liver sections of DEN treatment followed with 4 weeks of ADSC treatment. DEN + ADSC + T: DEN-injected liver tissue slices with ADSCs pretreated with L-theanine. Arrow points to the brown positive HGF signal stain. The scale bar represents 25 μm. C Quantification of immunohistochemical HGF staining. Compared to DEN, #: p < 0.05, ##: p < 0.01. Compared to DEN + ADSC, $: p < 0.05. $$: p < 0.01
Fig. 11.
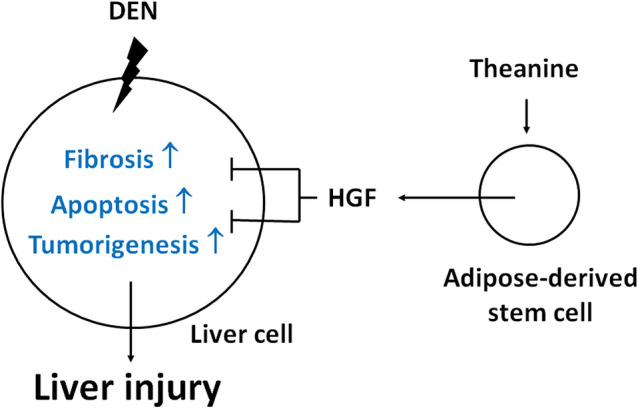
Graphic summary of this study. In DEN-induced liver injury rat model, the fibrosis, apoptosis and tumorigenesis markers were all elevated in the liver tissue. Intravenous ADSC injection can alleviate these phenomena. L-theanine treatment will induce more HGF secretion by ADSC, therefore enhances the ADSC therapeutic effects
Discussion
The liver is one of the important organs in maintaining normal body operations. Liver damage will induce abnormal body operation, leading to reduced quality of life. In this study, DEN treatment is capable of activating certain cellular signaling associated with hepatic dysfunction, including histological alteration (Fig. 4), fibrotic substances deposition (Fig. 5) and apoptotic markers activation (Figs. 6, 7, 8). These findings are consistent with the results from previous studies [22, 23].
According to our results, transplanting both of ADSC (regular adipose-derived stem cells) and L-theanine-pretreated ADSC show protective effect on DEN induced liver injury via suppression of fibrosis and apoptosis pathways. We know that DEN-induced hepatocyte injury may lead to tumorigenesis since mutant hepatocytes are highly proliferative in the DEN stressed microenvironment [23]. Meanwhile, hepatocyte apoptosis is observed due to mutant hepatocytes elimination induced by DEN. The compensatory proliferation of mutant hepatocytes accompanies prolonged hepatocyte apoptosis. On the other hand, hepatocyte apoptosis reduction shows a positive effect on tumorigenesis decrease in liver injury [23, 24]. Interestingly, apoptosis suppression in L-theanine-pretreated ADSC group is more significant than ADSC group (Fig. 6B 2 weeks, DEN + T-ADSC < DEN + ADSC, p < 0.01; 8 weeks DEN + T-ADSC < DEN + ADSC, p < 0.05; and as shown in Fig. 7B, 4 weeks DEN + ADSC < DEN, p < 0.05; DEN + T-ADSC < DEN, p < 0.01). These results imply that the anti-apoptotic effect on ADSC pre-treated with theanine is better than ADSC without theanine pre-incubation in recovering of DEN-induced liver injury.
Several studies had shown that MSC-treated HCC models had significant effects on suppressing AFP expression [25, 26]. Both in vitro and in vivo models had similar observation. HCC cells co-cultured with ADSC or cultured in ADSC-conditioned medium had lower AFP secretion [26]. Administration of MSC could suppress chemically-induced HCC in rats so that the serum AFP levels were significantly decreased in these models [26]. Here we also detected lower immunohistochemical signals of AFP in our injured liver tissue with ADSC treatment (Fig. 9). Immunohistochemical detection of AFP in biopsy tissues had been correlated to higher serum AFP levels [27], therefore our results confirm the effects of ADSC on hepatocyte tumorigenesis.
Several papers stated that paracrine secretion of growth factors from mesenchymal stem cells plays a major role in tissue regeneration [28, 29]. Therefore, this study speculates that theanine pre-incubation may increase paracrine secretion of growth factors for ADSC. After investigation of conditioned medium collecting from ADSC and L-theanine-pretreated ADSC using protein array, we found that a higher HGF level (hepatocyte growth factor) was detected in conditioned medium from L-theanine-pretreated ADSC than ADSC (Fig. 10). Moreover, the higher HGF signals were also detected in tissues with L-theanine-pretreated ADSC compared to no L-theanine treatment (Fig. 10B, C). This result indicated that theanine pre-incubation increases HGF secretion from ADSC. Choi et al. pointed out that high HFG expression can be measured in conditioned medium collected from stem cells cultured in 3-dimentional environment, and HFG secretion showed a protective effect on liver injury through inhibition of fibrosis pathway [30]. In addition, Moon et al. indicated that high HGF expression was found in HGF-transfected mesenchymal stem cells. The HGF-transfected mesenchymal stem cells improved liver damage induced by DEN through apoptosis signaling blockage [31]. Other reports also showed that MSC-secreted HGF improved tissue repair and function [20, 21].
In conclusion, the therapeutic effect of L-theanine pretreated ADSCs on liver injury induced by DEN can be summarized as follows: (1) DEN-induced liver injury is involved in pathological pathway activation including fibrosis and apoptosis; (2) paracrine secretion of HGF is higher in L-theanine-pretreated ADSC than ADSC; (3) the hepatic protective effect of L-theanine-pretreated ADSC is better than ADSC in DEN-induced liver injury due to fibrosis and apoptosis suppression via increased paracrine HGF secretion. A graphic summary of this study is shown in Fig. 11. In the future, a human study may be designed for further investigation of L-theanine-pretreated ADSC clinical use in the treatment of liver injury.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Instrumentation Center (National Taiwan Normal University) for the image acquisition and flow cytometry analysis. This work was partly supported from MOST-106-2320-B-003-002 -MY3 (Dr. Chiang-Ting Chien), MOST-105-2320-B-003-007 (Dr. Yun-Ju Lai) and NTUS-innovation cooperation 10912041004 (Dr. Yun-Ju Lai).
Declarations
Conflict of interest
The authors of this study state that there is no conflict of interests in this study.
Ethical statement
All surgical and experimental procedures were approved by National Taiwan Normal University Institutional Animal Care and Use Committee (Approval number 105035) and were in accordance with the guidelines of the National Science Council of Republic of China (NSC 1997).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tung-Sheng Chen, Email: tschen@ntnu.edu.tw.
Chiang-Ting Chien, Email: ctchien@ntnu.edu.tw.
References
- 1.Wang PL, Flemming JA. Addressing the global cirrhosis epidemic: one size will not fit all. Lancet Gastroenterol Hepatol. 2020;5:230–231. doi: 10.1016/S2468-1253(19)30382-6. [DOI] [PubMed] [Google Scholar]
- 2.Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127:55–64. doi: 10.1172/JCI88881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 4.Liang X, Ding Y, Zhang Y, Tse HF. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 5.Leuning DG, Beijer NRM, du Fossé NA, Vermeulen S, Lievers E, van Kooten C, et al. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci Rep. 2018;8:7716. doi: 10.1038/s41598-018-25700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Meng Y, Han Z, Ye F, Wei L, Zong C. Mesenchymal stem cell therapy for liver disease: full of chances and challenges. Cell Biosci. 2020;10:123. doi: 10.1186/s13578-020-00480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Yang Y, Fan L, Zhang F, Li L. The clinical application of mesenchymal stem cells in liver disease: the current situation and potential future. Ann Transl Med. 2020;8:565. doi: 10.21037/atm.2020.03.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolba R, Kraus T, Liedtke C, Schwarz M, Weiskirchen R. Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab Anim. 2015;49:59–69. doi: 10.1177/0023677215570086. [DOI] [PubMed] [Google Scholar]
- 9.Helms TH, Mullins RD, Thomas-Ahner JM, Kulp SK, Campbell MJ, Lucas F, et al. Inhibition of androgen/AR signaling inhibits diethylnitrosamine (DEN) induced tumour initiation and remodels liver immune cell networks. Sci Rep. 2021;11:3646. doi: 10.1038/s41598-021-82252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn B, Han BS, Kim DJ, Ohshima H. Immunohistochemical localization of inducible nitric oxide synthase and 3-nitrotyrosine in rat liver tumors induced by N-nitrosodiethylamine. Carcinogenesis. 1999;20:1337–1344. doi: 10.1093/carcin/20.7.1337. [DOI] [PubMed] [Google Scholar]
- 11.Boitier E, Merad-Boudia M, Guguen-Guillouzo C, Defer N, Ceballos-Picot I, Leroux J, et al. Impairment of the mitochondrial respiratory chain activity in diethylnitrosamine-induced rat hepatomas: possible involvement of oxygen free radicals. Cancer Res. 1995;55:3028–3035. [PubMed] [Google Scholar]
- 12.Chen TS, Ju DT, Day CH, Yeh TL, Chen RJ, Viswanadha VP, et al. Protective effect of autologous transplantation of resveratrol preconditioned adipose-derived stem cells in the treatment of diabetic liver dysfunction in rat model. J Tissue Eng Regen Med. 2019;13:1629–1640. doi: 10.1002/term.2917. [DOI] [PubMed] [Google Scholar]
- 13.Hidese S, Ogawa S, Ota M, Ishida I, Yasukawa Z, Ozeki M, et al. Effects of L-theanine administration on stress-related symptoms and cognitive functions in healthy adults: a randomized controlled trial. Nutrients. 2019;11:2362. doi: 10.3390/nu11102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu WY, Lee SP, Chiang BJ, Lin WY, Chien CT. Urothelial calcium-sensing receptor modulates micturition function via mediating detrusor activity and ameliorates bladder hyperactivity in rats. Pharmaceuticals (Basel) 2021;14:960. doi: 10.3390/ph14100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung SD, Lai TY, Chien CT, Yu HJ. Activating Nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS One. 2012;7:e47299. doi: 10.1371/journal.pone.0047299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen TS, Liao WY, Huang CW, Chang CH. Adipose-derived stem cells preincubated with green tea EGCG enhance pancreatic tissue regeneration in rats with type 1 diabetes through ROS/Sirt1 signaling regulation. Int J Mol Sci. 2022;23:3165. doi: 10.3390/ijms23063165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai DS, Zhang C, Chen P, Jin SJ, Jiang GQ. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep. 2017;7:12870. doi: 10.1038/s41598-017-12834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forte G, Minieri M, Cossa P, Antenucci D, Sala M, Gnocchi V, et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells. 2006;24:23–33. doi: 10.1634/stemcells.2004-0176. [DOI] [PubMed] [Google Scholar]
- 20.Song P, Han T, Xiang X, Wang Y, Fang H, Niu Y, et al. The role of hepatocyte growth factor in mesenchymal stem cell-induced recovery in spinal cord injured rats. Stem Cell Res Ther. 2020;11:178. doi: 10.1186/s13287-020-01691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Chen QH, Liu AR, Xu XP, Han JB, Qiu HB. Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res Ther. 2015;6:250. doi: 10.1186/s13287-015-0257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latief U, Ahmad R. β-carotene inhibits NF-kappaB and restrains diethylnitrosamine-induced hepatic inflammation in Wistar rats. Int J Vitam Nutr Res. 2020:1–10. [DOI] [PubMed]
- 23.Nozaki Y, Hikita H, Tanaka S, Fukumoto K, Urabe M, Sato K, et al. Persistent hepatocyte apoptosis promotes tumorigenesis from diethylnitrosamine-transformed hepatocytes through increased oxidative stress, independent of compensatory liver regeneration. Sci Rep. 2021;11:3363. doi: 10.1038/s41598-021-83082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wree A, Johnson CD, Font-Burgada J, Eguchi A, Povero D, Karin N, et al. Hepatocyte-specific Bid depletion reduces tumor development by suppressing inflammation-related compensatory proliferation. Cell Death Differ. 2015;22:1985–1994. doi: 10.1038/cdd.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serhal R, Saliba N, Hilal G, Moussa M, Hassan GS, Atat OE, et al. Effect of adipose-derived mesenchymal stem cells on hepatocellular carcinoma: in vitro inhibition of carcinogenesis. World J Gastroenterol. 2019;25:567–583. doi: 10.3748/wjg.v25.i5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel aziz MT, El Asmar MF, Atta HM, Mahfouz S, Fouad HH, Roshdy NK, et al. Efficacy of mesenchymal stem cells in suppression of hepatocarcinorigenesis in rats: possible role of Wnt signaling. J Exp Clin Cancer Res. 2011;30:49. [DOI] [PMC free article] [PubMed]
- 27.Sato K, Tanaka M, Kusaba T, Fukuda H, Tanikawa K. Immunohistochemical demonstration of alpha-fetoprotein in small hepatocellular carcinoma. Oncol Rep. 1998;5:355–358. [PubMed] [Google Scholar]
- 28.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Miguel-Gómez L, Ferrero H, López-Martínez S, Campo H, López- Pérez N, Faus A, et al. Stem cell paracrine actions in tissue regeneration and potential therapeutic effect in human endometrium: a retrospective study. BJOG. 2020;127:551–560. doi: 10.1111/1471-0528.16078. [DOI] [PubMed] [Google Scholar]
- 30.Choi JS, Park YJ, Kim SW. Three-dimensional sifferentiated human mesenchymal stem cells exhibit robust antifibrotic potential and ameliorates mouse liver fibrosis. Cell Transplant. 2021;30:963689720987525. doi: 10.1177/0963689720987525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon SH, Lee CM, Park SH, Nam MJ. Effects of hepatocyte growth factor gene-transfected mesenchymal stem cells on dimethylnitrosamine-induced liver fibrosis in rats. Growth Factors. 2019;37:105–119. doi: 10.1080/08977194.2019.1652399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.