Abstract
BACKGROUND:
Our learning about human reproductive development is greatly hampered due to the absence of an adequate model. Animal studies cannot truthfully recapitulate human developmental processes, and studies of human fetal tissues are limited by their availability and ethical restrictions. Innovative three-dimensional (3D) organoid technology utilizing human pluripotent stem cells (hPSCs) offered a new approach to study tissue and organ development in vitro. However, a system for modeling human gonad development has not been established, thus, limiting our ability to study causes of infertility.
METHODS:
In our study we utilized the 3D hPSC organoid culture in mini-spin bioreactors. Relying on intrinsic self-organizing and differentiation capabilities of stem cells, we explored whether organoids could mimic the development of human embryonic and fetal gonad.
RESULTS:
We have developed a simple, bioreactor-based organoid system for modeling early human gonad development. Male hPSC-derived organoids follow the embryonic gonad developmental trajectory and differentiate into multipotent progenitors, which further specialize into testicular supporting and interstitial cells. We demonstrated functional activity of the generated cell types by analyzing the expression of cell type-specific markers. Furthermore, the specification of gonadal progenitors in organoid culture was accompanied by the characteristic architectural tissue organization.
CONCLUSION:
This organoid system opens the opportunity for detailed studies of human gonad and germ cell development that can advance our understanding of sex development disorders. Implementation of human gonad organoid technology could be extended to modeling causes of infertility and regenerative medicine applications.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13770-022-00492-y.
Keywords: Human pluripotent stem cells, Gonad development, Mesonephros, Mini-spin bioreactor, Testis organoid model
Introduction
Our current understanding of human gonad development is largely based on traditional histological analyses of embryonic sections performed over 30 years ago [1]. Despite advances in techniques currently used to study developmental processes, performing research with human embryonic gonads is limited by tissue availability and the related legal and ethical restrictions [2, 3]. Thus, the knowledge of processes driving gonad formation, appearance of specialized cell types and tissue morphogenesis is primarily based on rodent models. Although signaling pathways in mammalian development are conserved, studies in rodents cannot be directly extrapolated to humans due to species-specific differences [4–6]. Recent transcriptomic profiling of human fetal gonads provided important insight into germ cell development and somatic lineage specification [4]. However, the gain- and loss-of-function studies and lineage tracing experiments, which would require genetic modification, cannot be performed with human embryos. Additionally, genetic diversity of the acquired material and accuracy of staging embryonic development can affect reproducibility of results [4]. These limitations underscore the need to create in vitro models.
Human pluripotent stem cells (hPSCs) can self-organize in three-dimensional (3D) culture and follow intrinsic developmental pathways to form fetal-like tissues under favorable culture conditions [5]. To date, hPSC-derived organoids are widely used for developmental studies, disease modeling, and toxicity screening. 3D models have been developed for numerous tissues and organs including brain, liver, and kidney [5]. Engineering gonad organoids will be highly beneficial for studying gonad development and modeling disorders of sex development (DSD) [7]. Congenital reproductive defects and negative impacts on fertility have been linked to parental exposure to environmental toxicants, such as air pollutants, contaminated water, pesticides, steroid hormones and medications [8–10]. In vitro gonadal models can be utilized for developmental and reproductive toxicology [11, 12]. Two publications have described protocols for directed hPSC differentiation into bipotential gonadal progenitors [13, 14]. These progenitors can be further specified into male gonadal cells as in a monolayer culture, as well as after aggregation into 3D organoids, thus, demonstrating the feasibility of creating a gonad organoid model [14].
Establishment of an in vitro system is also crucial for studying human gametogenesis. Research using mice has shown that the capability of primitive germ cells to become meiosis-competent cells (“licensed”) depends on their exposure to a developmentally matched gonadal microenvironment [15]. Transcriptome studies of human primordial germ cells also showed that “licensed” germ cell marker expression is detected only after gonad colonization [15, 16]. Progress in rodent pluripotent stem cell research has led to engineering gonadal microenvironments that can support the development of functional female and male gametes [17–19]. Experiments involving hPSCs succeeded in the production of primordial germ cell-like cells (PGCLCs) [20]. However, more research is needed to engineer an artificial gonad, which will be able to support germ cell development beyond the PGCLC state into functional chromosomally normal gametes [21, 22].
In this study we report a simple, scalable and reproducible organoid system allowing for the investigation of early human gonad development in a 3D environment. We show that hPSC-derived organoids mimic developmental processes from the formation of genital ridge precursors in mesonephros-like organoids to testis-specific tissue organization, accompanied by cell type-specific marker expression.
Materials and methods
Cell lines, animals and human tissues
The use of the human embryonic stem cell (hESC) line H1 (WiCell) was approved by Johns Hopkins University (JHU, Baltimore, MD, USA) institutional stem cell research oversight (ISCRO) committee.
Mouse housing at JHU was performed in accordance with the National Institutes of Health and U.S. Department of Agriculture criteria. Protocols for their care and use were approved by the JHU Institutional Animal Care and Use Committee (IACUC).
Deidentified decedent adult human donor testes (HT10, age 24) were obtained through the Washington Regional Transplant Consortium (WRTC, Annandale, VA, USA). Deidentified decedent prepubertal human donor testes were obtained through the JHU Legacy Gift Rapid Autopsy program (HT13, age 11) and WRTC (HT15, age 5). Deidentified decedent donor testes were designated as ‘‘not human subjects research’’ by the JHU Bloomberg School of Public Health Institutional Review Board.
hESC maintenance and differentiation
hESC (H1, WiCell) maintenance and differentiation were conducted as previously described [23].
Organoid treatment with hormones and growth factors
BMP4 (50 ng/ml) (Peprotech, Cranbury, NJ, USA) and SHH (50 ng/ml) (Peprotech) were added on Day 10–16 (D10-16). For SHH + BMP4 condition SHH was added on D10 and BMP4 on D12. hCG (10 IU/ml) (Sigma-Aldrich, St. Louis, MO, USA), PMSG (25 mIU/ml) (Lee BioSolutions, Maryland Heights, MO, USA) or both were added on D20-24 and D30-38.
ELISA
Conditioned medium was collected every 48 h after hormone addition, centrifuged to remove cell debris, aliquoted and stored at -20ºC until analysis. Testosterone ELISA (Cayman, Ann Arbor, MI, USA) was done according to manufacturer instructions. Absorbance reading was performed using DTX 800 (Beckman Coulter, Indianapolis, IN, USA). Data were calculated using Cayman Excel Workbooks and plotted in GraphPad Prism.
PGCLC generation
PGCLC generation was performed following a published protocol [20].
Semi-quantitative (sq) and quantitative (q) RT-PCR
Total RNA was isolated using PureLink RNA kit (Ambion, Austin, TX, USA) followed by DNase I (RNase-free, Ambion) treatment. First strand cDNA synthesis was carried out using M-MuLV reverse transcriptase and random primer mix (NEB) under the following conditions: 25ºC for 5 min, 42ºC for 1 h, 65ºC for 20 min. sqPCR was set up using AccuStart II PCR SuperMix (Quanta BioSciences, Gaithersburg, MD, USA) [23]. qPCR was performed using Luna Universal qPCR Master Mix (NEB) on the Applied Biosystems 7500 Fast Real-Time PCR system: 95ºC for 1 min, (95ºC for 15 s, 57ºC for 30 s, 60ºC for 30 s) × 40 cycles, melting curve. Data were normalized to ACTB expression. Gene expression was calculated using the 2−ΔCt method [24]. Minus RT control was included for each sample. qPCR fragment sizes were verified in agarose gel, and primers are listed in Table S1.
Western blot analysis
Western blot analysis was performed as previously described [25].
Immunocytochemistry
PGCLC spheroids and single hESCs were fixed in suspension in 10% formalin (Sigma) for 15 min at room temperature, washed in PBS (Life Technologies, Carlsbad, CA, USA) and spun onto glass slides using Shandon Cytospin 4 centrifuge (Thermo Fisher Scientific, Waltham, MA, USA). Immunocytochemistry was performed as previously descried [26]. Cells were mounted with Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Antibodies are listed in Table S2.
Immunohistochemistry
Mouse and human testes were fresh-frozen in O.C.T. compound (ScieGen, Hauppauge, NY, USA). Organoids were fixed in 2.5% formalin in PBS for 20 min at room temperature, washed in PBS and incubated in 20% sucrose in PBS at 4 °C until saturation (~ 2 h). Embryonic day 10.5 (E10.5) mouse embryos were fixed in 2.5% formalin in PBS for 1 h at 4 °C and incubated in 20% sucrose in PBS at 4 °C overnight. After removal of sucrose solution organoids and mouse embryos were frozen in O.C.T. compound. O.C.T. embedded samples were sectioned at 16 µm thickness using the Cryo3 (Sakura Tissue-Tek, Torrance, CA, USA). Sections were mounted on Selectfrost Adhesion (Fisher) microscope slides and stored at − 80 °C until further processing. Mouse and human testis sections were fixed with 2.5% formalin in PBS for 10 min at room temperature and washed in PBS before immunostaining. Immunohistochemistry was carried out as previously described [27]. A minimum of 2 sections per organoid and 8–12 organoids were analyzed for each marker and condition. Antibodies are listed in Table S2.
Microscopy
Live cell and immunofluorescence (IF) image capture and analysis were performed using Keyence BZ-800 and BZ-X800 Viewer and Analyzer software. Adobe Photoshop was used to prepare figure images.
Results
Organoid culture outline
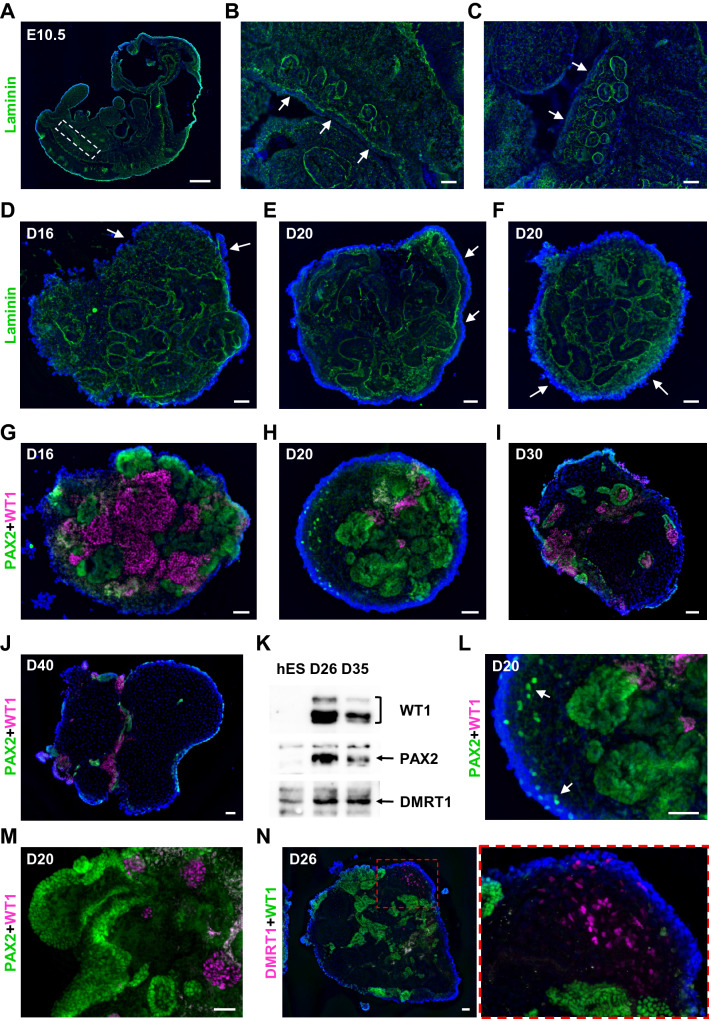
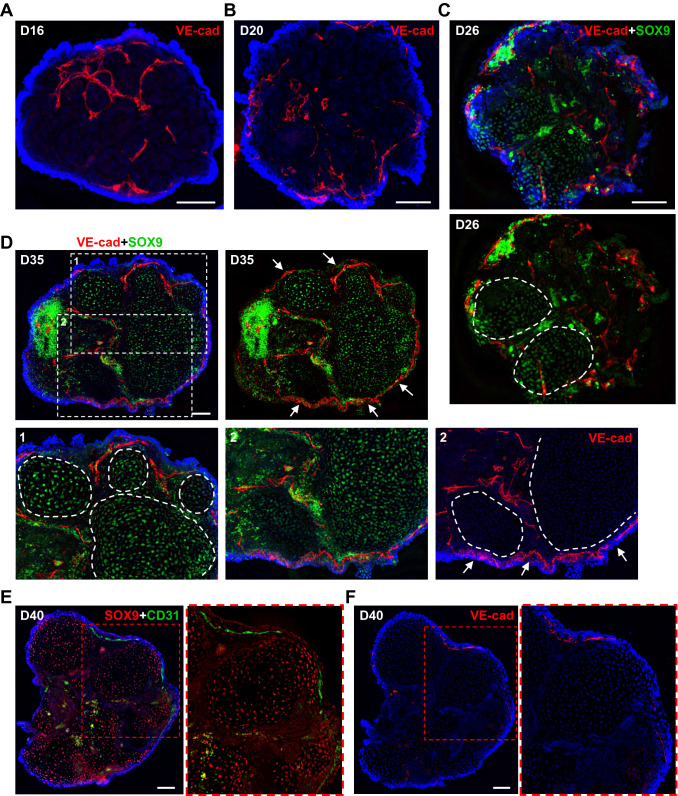
In previous work, we tested a platform for modeling human male gonad development using mini-spin bioreactors and chemically defined 3D organoid culture conditions [23]. Because the study was devoted to designing favorable co-culture conditions for human adult testicular cells and hPSC derivatives in organoids, gonadal hPSC differentiation was not addressed in detail. In the current study, we investigated intrinsic developmental morphogenic and differentiation processes in hPSC-derived organoids during prolonged culture (up to 40 days; Fig. 1A) and provided extensive evaluation of gonadal marker expression (Fig. 1B, Video S1, Video S2). Under these conditions, hPSCs differentiate into mesonephros-like organoids between days 10–16 (D10-16) (Fig. 1B, C). Mesonephros-like organoids then develop a non-tubular progenitor population on their surface within D16-22, followed by dramatic regression of mesonephric tubules and expansion and ingression of progenitor cells (D22-30). Organoids further self-organize into cord-like structures and distinct cell clusters that surround them (D30-40) (Figs. 1B, C and S1A).
Fig. 1.
Outline of bioreactor-based gonad organoid system. A The scheme of hPSC differentiation and organoid culture (detailed in Materials and methods). B Illustration of developmental processes in organoids during long-term culture (refer to Sect. 3.1 for further details). C Representative images of live organoid cultures. Scale bars 200 µm
Where appropriate, we performed comparisons to mouse embryonic gonad development, as well as utilized mouse and human testicular tissues as controls. Whenever possible, we applied complementing assays and used additional antibodies to verify marker specificity and data validity.
Mesonephros contribution to gonad development
Adreno-gonadal primordium is a common source of progenitors for the adrenal gland and bipotential gonad developing on the ventromedial surface of mesonephros [28–30]. It is accepted that intermediate mesoderm (IM) is a source of progenitors developing into all components of the kidney (pronephros, mesonephros and metanephros) [31]. The coelomic epithelium (CE), which lines the surface of all internal organs, originates from the lateral plate mesoderm [32]. Decades of histological observations in different species have concluded that the gonad forms as a result of CE expansion, ingression into mesonephros and differentiation into supporting and steroidogenic lineages, while the mesonephros contributes to the formation of interstitial compartment, testicular morphogenesis and vascularization. However, insufficient research has been performed to determine the origin of CE population [28, 30, 33–35]. Novel methods of analysis such as single-cell transcriptomics and lineage tracing have demonstrated that the mesonephros is the primary source of cells populating genital ridges in chicken and mice [36, 37]. For instance, in chicken embryos, PAX2+ progenitors (PAX2+ /OSR1+ /WNT4+ /DMRT1+) migrate into the developing gonad from the mesonephros and give rise to both, supporting and steroidogenic cell lineages, while CE gives rise to a non-steroidogenic interstitial population [36]. Lineage tracing experiments of WT1+ and IM progenitors in mice confirmed the mesonephric origin of adreno-gonadal precursors [37, 38].
Knowledge about human gonad development is very limited. A study describing detailed histological examination of human embryonic gonads reported: “The primordial sex cords are formed by budding of cells of mesonephric origin from sites where the basal lamina of the mesonephros is eliminated” [1]. In agreement with these findings, a recent study on cynomolgus monkey showed widespread WT1 expression in mesonephric tubules, stroma and CE. Via transcriptome analysis, this study also confirmed that WT1+ gonadal progenitors originate from the IM [37]. PAX2 is a crucial regulator of IM differentiation into all components of the embryonic kidney, as well as the Wolffian (pronephric) and Mullerian (Fallopian tube) ducts [31, 39–41]. WT1 is highly expressed in mesonephros and genital ridges and is essential for gonad and adrenal gland specification [28, 37, 42]. Furthermore, WT1 has been shown to be a negative regulator of PAX2 expression and involved in mesonephros and metanephros development [43].
To investigate if our organoid system mimics early processes of human gonad development, we analyzed morphological changes in the coelomic cell population and basement membrane, as well as assessed the localization of PAX2+ and WT1+ progenitors. In mice, mesonephric tubules are separated from the CE by basement membrane marked by laminin (Fig. 2A, B). In areas with extensive proliferation of CE the continuity of basement membrane is disrupted (Fig. 2C) [30, 44]. Similarly, D16-20 gonad organoids demonstrated the separation of superficial cell layer from mesonephric tubules by laminin basement membrane (Fig. 2D–F). The basement membrane continuity was disrupted in regions with expanding coelomic population, where punctate laminin staining was observed (Fig. 2D–F).
Fig. 2.
Mesonephros contribution to gonad development. A-C Sagittal sections of E10.5 mouse embryo. Forming genital ridge is separated from mesonephros by the basement membrane marked by laminin (green). Gonadal-mesonephros region in E10.5 embryo is boxed in (A). B Continuous basement membrane. C The continuity of basement membrane is disrupted in areas with extensive proliferation of CE. The CE layer is marked by arrows. D-F The separation of coelomic layer from mesonephros by the basement membrane marked by laminin (green) in D16-20 organoids. Arrows mark expanding coelomic layer in the areas with punctate laminin staining (D, F). E CE layer over continuous basement membrane (arrows). G-J The distribution of PAX2+ (green) and WT1+ (magenta, sc-7385) progenitors in D16-40 organoids. K Western blot analysis of WT1 (~ 50-60 kDa, #83535), PAX2 (~ 45 kDa) and DMRT1 (~ 39 kDa) protein expression in hESCs (hES) and D26, D35 organoids (see Fig. S1B for full size). L Single PAX2+ (green) cells migrating into coelomic layer in D20 organoid (arrows) (H). M D20 organoid showing the formation of PAX2+ (green) coelomic population. WT1 is shown in magenta (sc-7385) N DMRT1 (magenta) and WT1 (green, #83535) expression in D26 organoid. Nuclei are shown in blue. Scale bars 500 µm (A), 50 µm (B-N)
PAX2 and WT1 were expressed in a mutually exclusive manner (Fig. 2G–J), which agrees with previously published studies in mice and monkey [37, 43]. PAX2 expression was predominantly allocated to cells in tubular structures. WT1 expression was mostly defined to clustered cells (Fig. 2G). In human embryos, most mesonephric tubules degenerate between gestation weeks 5–12 [45]. Accordingly, we observed significant reduction in mesonephric tubules with organoid age (D16-40), accompanied by a substantial decrease in PAX2 and WT1 marker expression (Fig. 2G–K, Fig. S1B). Similar to studies in chicken, PAX2+ cells were observed migrating into the coelomic area as single cells and forming surface populations in D20 organoids (Fig. 2L, M). Testis-specific marker DMRT1 was readily detected in surface populations of D26 organoids (Figs. 2N and S1C-E) [4, 46].
Our observations suggest that hPSC-derived organoids mimic early stages of gonad development, such as the formation of mesonephros and coelomic cell population that are separated by the basement membrane. Despite the accumulating knowledge based on models in other species, there is no definitive evidence of the adreno-gonadal precursor in humans [36–38, 42]. Lineage tracing experiments using hPSC-based organoids could determine whether PAX2+ cells of mesonephric origin are the precursors of WT1+ cells that contribute to gonad development.
Expression of gonadal ridge markers
Factors necessary for the development of mammalian adreno-gonadal primordium include GATA4, LHX9, NR5A1 (SF1), EMX2 and WT1 [28–30, 42, 44]. Homeobox proteins SIX1 and SIX4 are essential for early proliferation of mouse gonadal precursors [28, 30]. Mutations in GATA4, NR5A1, WT1, as well as SOX9 and SRY result in human gonadal dysgenesis [7, 47].
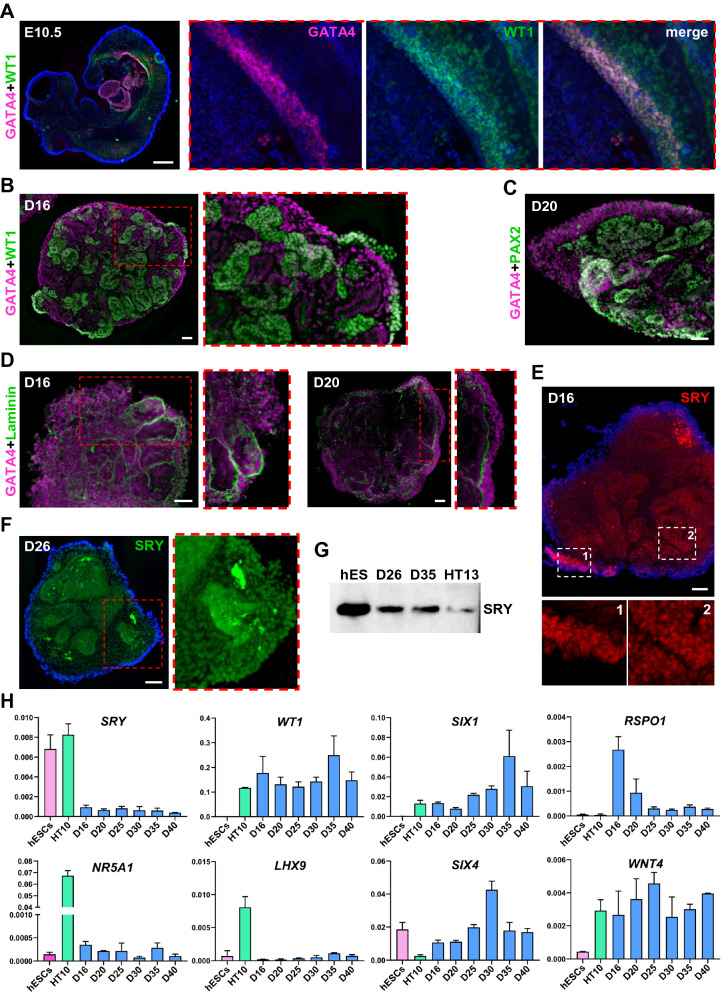
In E10.5 mouse embryos, GATA4+ /WT1+ progenitors define the gonadal ridge located above the mesonephric tubules (Fig. 3A). Expression of GATA4 in sex cords and interstitium of human gonads is evident at 6 weeks post-conception (wpc) [4]. GATA4 and WT1 expression was observed on the surface, as well as inside D16-20 organoids (Figs. 3B, C, S2A-C). Consistent with embryonic gonad development in vivo, GATA4+ population was increased in areas with fragmented laminin staining (Fig. 3D).
Fig. 3.
Expression of gonadal ridge markers. A Sagittal section of E10.5 mouse embryo showing the expression of gonadal ridge markers GATA4 (magenta, #36966) and WT1 (green, sc-7385). B GATA4 (magenta, sc-25310) and WT1 (green, #83535) expression in D16 organoids. C GATA4 (magenta, sc-25310) and PAX2 (green) expression in D20 organoids. D GATA4 (magenta, sc-25310) expression in D16 and D20 organoids coimmunostained with laminin (green). E, F SRY expression in D16 (red) and D26 (green) organoids, respectively. G Western blot analysis of SRY protein (~ 24 kDa) expression in hESCs (hES), D26, D35 organoids and HT13 (age 11) prepubertal human testis (see Fig. S2D for full size). H qRT-PCR analysis of gonadal ridge marker expression in hESCs, HT10 (age 24) adult testis and D16, 20, 25, 30, 35 and 40 organoids. Data are presented as mean with standard deviation from technical triplicates. Nuclei are shown in blue. Scale bars 500 µm (A), 50 µm (B-F)
In human gonads, SRY expression is upregulated at around 6 wpc, followed by the decrease in expression to a base level around 8 wpc and persists into adulthood [4, 28, 29, 48, 49]. In organoid cultures SRY+ cells were present on the periphery and inside D16 organoids, as well as at later stages of differentiation (Fig. 3E, F). We also verified SRY protein size and gene expression (Figs. 3G, H, S2D).
In mice and human, NR5A1 is needed for the development of adrenal and gonadal steroidogenic cells [29, 42]. In contrast, NR5A1 is redundant for human gonadal ridge formation [4]. NR5A1 expression was reported to be low in hPSC-derived gonadal progenitors [14]. In agreement with previous findings, NR5A1 transcript amount in D16-40 organoids was not increased (Figs. 3H, S2E).
Studies in mice have shown that WT1 directly represses NR5A1 expression by binding its promoter region [50]. In the absence of WT1, the expansion of Sertoli cells is abolished and progenitor cells differentiate towards steroidogenic lineage [42, 50]. In D16-40 organoids, WT1 transcript levels were comparable to levels detected in human adult testis (Fig. 3H). Therefore, the high level of WT1 expression might be a reason for NR5A1 downregulation in organoid cultures.
Additional gene expression analysis of D16-40 organoids showed very low level of LHX9 expression, increase in SIX1 expression with organoid age, and levels of SIX4 comparable to hESCs (Fig. 3H). Female gonad marker RSPO1 was downregulated in D25-40 organoids compared to early D16-20 organoids (Fig. 3H). Relative expression of WNT4, a factor responsible for ovarian differentiation, was similar to levels in the human adult testis (Fig. 3H) [28].
In summary, we show that proliferating coelomic cells express common genital ridge markers and migrate inward through disrupted basement membrane, resembling processes described for gonad development in other species. This study can be further detailed to cell population-specific marker analysis using reporter cell lines.
The development of testicular cords and specification of interstitial compartment
In E11.5 mouse genital ridges, upregulation of SRY together with NR5A1 induces the expression of SOX9, which directs gonadal progenitor specification into pre-Sertoli cells, a key cell type supporting germ cells in the developing testis [28, 33, 51, 52]. Initially, proliferating Sertoli cell progenitors form random groups of different sizes and shapes, which further organize into more defined clusters and later into testicular cords. This process is accompanied by the deposition of extracellular matrix (ECM) proteins [33, 53–55]. In human embryonic gonads, SOX9 expression is detectable around 7 wpc followed by upregulation of Sertoli cell markers anti-Mullerian hormone (AMH) and inhibin B (activin B), as well as steroidogenic genes around 8 wpc [4, 28, 49, 56, 57]. Organization of Sertoli cell progenitors into testicular cord-like structures and specification of interstitial cell population occurs at ~ 7–8 wpc [4, 28, 58, 59].
In our culture system, SOX9 expression was not detectable in D16 organoids. However, SOX9+ progenitors were observed in D20 organoids in the superficial layer and, to a less extent, inside organoids (Fig. 4A, B). SOX9+ cell population expanded substantially from D20 to D40 of differentiation, occupying on average ~ 22% of the cross sectioned area in D20-26 organoids compared to ~ 64% in D40 organoids (Fig. 4A–F). Growth of the SOX9+ cell population correlated with qRT-PCR data showing increased SOX9 expression levels with organoid age (Fig. 4G). SOX9 expression increased 5.73-fold in D40 organoids versus (vs) D16 organoids (42.03-fold increase in D40 vs hESCs) (Fig. 4G). We also observed 1.67-fold increase in the expression of interstitial cell marker PDGFRα in D40 organoids compared to D16 (103-fold increase in D40 vs hESCs) (Fig. 4H).
Fig. 4.
Specification of testicular cords and interstitial compartment. A The expansion of SOX9+ (green, #82630) progenitors in D20 organoid surface layer. B The expansion of SOX9+ (green, #82630) progenitors in D20 organoid surface layer and ingression. The section was coimmunostained with GATA4 (red, sc-25310). C The expansion of SOX9+ (red, #82630) cells inside D26 organoids. Note the random distribution of SOX9+ progenitors, characteristic of migratory behavior. D Localization of SOX9+ (green, #82,630) cells in D30 organoid. E, F The expansion and clustering of SOX9+ (red, #14–9765-80) progenitors in D35, D40 organoids, and the deposition of laminin (green) around forming SOX9+ cord-like structures. G, H qRT-PCR analysis of SOX9 and PDGFRα expression in hESCs, HT10 (age 24) adult testis, and D16, 20, 25, 30, 35 and 40 organoids. Data are presented as mean with standard deviation from technical triplicates. I-K The formation of a single or multiple tubules or cord-like structures in D35 and D40 organoids immunostained with SOX9 (green, #82630). Cell debris are marked by asterisk. L Brightfield image of D40 organoids in culture. M, N WT1+ (green, sc-7385) clusters are positioned adjacent to SOX9+ (red, #82630) tubule or cord-like structures in D35 organoids. O, P WT1+ (green, #83535) clusters are positioned adjacent to SOX9+ (red, #14–9765-80) tubule or cord-like structures in D35 organoids. Q, R D40 organoid sections showing WT1+ (red, sc-7385) and PAX2+ (green) cell clusters positioned adjacent to tubule or cord-like structures. Furthermore, WT1+ and PAX2+ progenitors persist on organoid surface (R). Nuclei are shown in blue. Scale bars 50 µm (A-D), 100 µm (I-K, M-R), 200 µm (E, F, L)
Cell proliferation and migration was accompanied by organoid architectural remodeling. Initially disorganized and randomly distributed in D20-30 organoids (Fig. 4A–D), SOX9+ cells acquire characteristic flattened morphology and begin clustering separately from interstitial cells at later stages of differentiation (Fig. 4E, F). The compartmentalization of SOX9+ populations is accompanied by the deposition of laminin, similar to developmental processes described for the mouse gonad (Fig. 4E, F). In D35-40 organoids we observed the formation of large irregularly shaped cord-like structures and tubules (Fig. 4E, F, I–K). Large cell clusters of SOX9+ cells undergo further partitioning defined by circular/concentric cell orientation (Fig. S3A). A single organoid can form one to multiple cord-like structures or tubules (Fig. 4I–L, Fig. S3B). These cell populations are also positive for gonadal marker SRY (Fig. S3C).
Interstitial clusters of either WT1+ or PAX2+ cells were always located at the base of or in between the SOX9+ cord-like structures (Fig. 4M–R). In addition, small populations of WT1+ and PAX2+ progenitors were observed in the superficial layer of D40 organoids, which may be attributed to the persistence of adreno-gonadal progenitors in the coelomic layer (Figs. 4R, 2J) [42].
Our observations demonstrate that this hPSC-based organoid model offers a unique opportunity to explore early events of SOX9+ progenitor specification, formation of cord-like structures and testicular morphogenesis, which we find closely resemble developmental events described for mouse gonads. Understanding embryonic processes leading to gonadal tissue partitioning into testicular cords and the interstitial compartment is crucial for future gonadal tissue engineering and potential applications in regenerative medicine.
The maintenance of spermatogenesis in organoids from adult testicular cells have been unsuccessful, likely because the characteristic tubular architecture was missing and the germ cell niche was not reconstructed [60, 61]. However, studies in mice in vitro and rhesus monkey in vivo have shown that immature prepubertal testicular cells are still capable of self-organization and de novo formation of testicular cords [62, 63]. Little is known which cell types and signaling factors are needed to reverse the capability for gonadal morphogenesis in mature adult testes. hPSC-derived organoid models offer the opportunity to investigate this problem.
Evaluation of AMH and inhibin B expression
Between 5 and 8 wpc human embryonic gonads have both Wolffian and Mullerian ducts [28, 64]. Timely initiation of AMH expression (~ 7–8 wpc) in male gonads is required for regression of the female-specific Mullerian duct [4, 28, 29, 49, 56]. AMH dysregulation during embryonic sexual specification and fetal growth leads to abnormal development of reproductive organs, often requiring surgical intervention after birth [28, 41, 65, 66]. Furthermore, defects such as persistent Müllerian duct syndrome can lead to infertility and cancer [28].
AMH and inhibin B (activin B) are essential for germ cell proliferation and testicular growth, and their levels are important markers of reproductive development [28, 57, 66–68]. Studies in mice have shown that AMH and inhibin B direct endothelial cell migration from the mesonephros to the gonad and establishment of the testis-specific coelomic vessel [28, 69, 70]. Initially gonadotropin-independent, AMH and inhibin B production is regulated by follicle stimulating hormone (FSH) later in development [28, 66, 67].
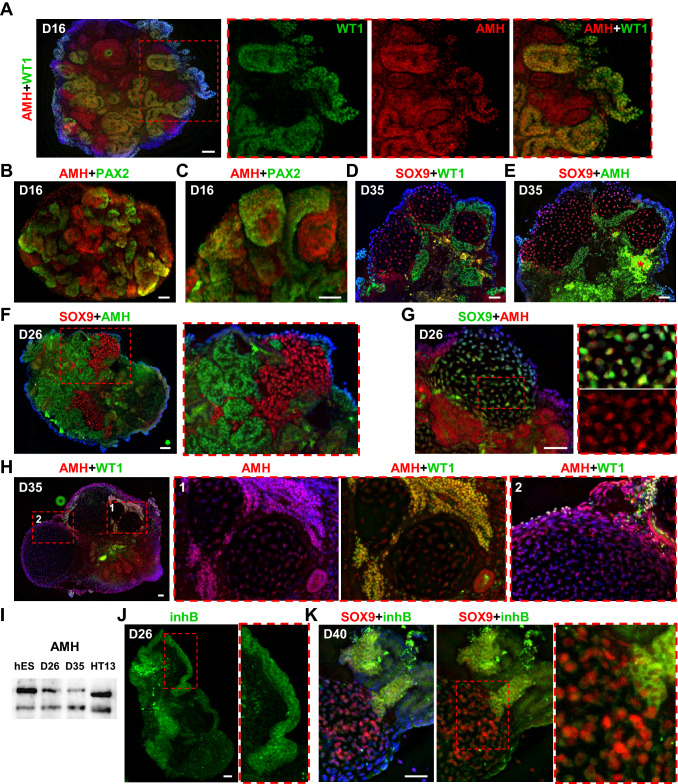
AMH is produced in high amount by immature Sertoli cells and decreases after puberty (Fig. S3D,E) [28]. A single study reported AMH expression in human male fetal testis and mesonephros at 8 wpc [71]. Interestingly, we detected nuclear AMH expression in WT1+ (PAX2-) cells in D16 organoids, long before the appearance of the first SOX9+ cells (Fig. 5A). As with WT1, AMH expression was mutually exclusive with PAX2 expression (Fig. 5B, C, Fig. S3F). We have verified identical localization of WT1+ and AMH+ interstitial cell clusters in sections from the same D35 organoid, where SOX9+ cord-like structures had already formed (Fig. 5D, E). Additionally, we confirmed the results of immunohistochemical (IHC) analysis with different antibodies in D26 and D35 organoids, as well as by western blot (Fig. 5F–I, Fig. S3G, H). IHC analysis of AMH expression in D26 and D35 organoids revealed nuclear localization in WT1+ cell clusters, superficial cells, and nuclear and cytoplasmic expression in SOX9+ cells (Figs. 5F–H and S3G). IF analysis of cell cultures established from a prepubertal human testis also showed nuclear and cytoplasmic AMH staining similar to our results in organoids (Fig. S3I-K).
Fig. 5.
AMH and inhibin B expression in organoids. A Nuclear AMH (red, ab103233) expression in D16 organoids correlates with WT1 (green, sc-7385) expression. B, C Nuclear AMH (red, sc-166752) expression in D16 organoids is mutually exclusive with PAX2 (green) expression. D, E Two sections from the same D35 organoid were immunostained for WT1 (green, #83535) + SOX9 (red, #14–9765-80) (D) and for AMH (green, sc-166752) + SOX9 (red, #82630). The immunostaining shows identical localization of WT1+ and AMH+ cell clusters near SOX9+ tubules. Cell debris are marked by asterisk (E). F D26 organoid section showing nuclear AMH (green, sc-166752) staining in cell clusters surrounding SOX9+ (red, #82630) cord-like structures. G D26 organoid section showing nuclear AMH (red, ab103233) staining in cell clusters surrounding SOX9+ (green, #14–9765-80) tubule and nuclear and cytoplasmic staining in SOX9+ tubular cells. H D35 organoid section showing nuclear AMH (red, ab103233) and WT1 (green, sc-7385) staining in cell clusters surrounding tubules and nuclear and cytoplasmic staining inside tubules. I Western blot analysis of AMH protein (~ 60 kDa) expression in hESCs (hES), D26, D35 organoids and HT13 (age 11) prepubertal testis (see Fig. S3H for full size). J Cytoplasmic inhibin B (inhB, green) expression in the superficial layer of D26 organoid. K Cytoplasmic inhibin B (inhB, green) expression in the interstitial cell clusters and SOX9+ (red, #82630) cells in D40 organoid. Nuclei are shown in blue. Scale bars 50 µm
Inhibin subunits are present in human embryonic gonads and adrenal glands at ~ 7 wpc [57]. Inhibin B localizes to Sertoli and interstitial cells in fetal and prepubertal testes (Supplementary Fig. S3LF) [57]. In D26-40 organoids, cytoplasmic inhibin B expression was detected in the superficial layer and clusters of interstitial cells, as well as weak cytoplasmic staining in SOX9+ cells (Fig. 5J, K).
Parental exposure to man-made chemicals acting as endocrine disruptors can lead to hormonal dysregulation in a fetus resulting in DSD and gonadal tumors [8, 9, 67]. In this study we demonstrate that hPSC-derived organoids produce AMH and inhibin B, which are crucial for male reproductive development and fertility. Therefore, gonad organoids can be utilized further to study hormonal regulation during early human reproductive organ formation and to assess the toxicity of environmental pollutants.
The development of steroidogenic cells
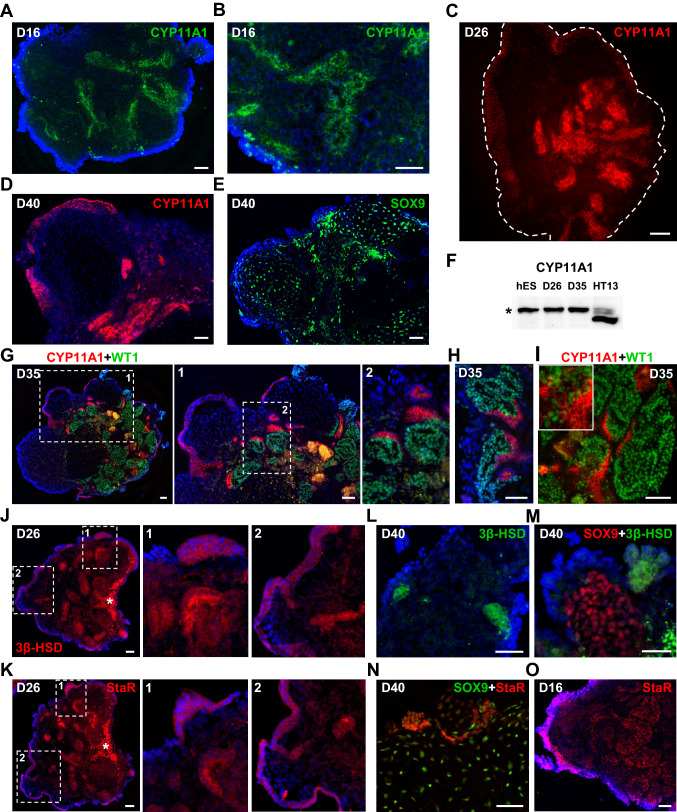
Next, we investigated early markers of steroidogenesis CYP11A1 and StaR, and a later marker, 3β-HSD [28, 72, 73]. The expression of these markers overlaps in the developing adrenal gland and testis [29]. All three markers can be detected in organoid samples by IHC (Fig. 6). Notably, CYP11A1 can be detected in D16 organoids before the appearance of SOX9+ cells (Fig. 6A, B). Similar to localization of WT1+ cells, CYP11A1 expression was present in interstitial cell clusters and cells on the organoid surface, surrounding the expanding SOX9+ population in D26-40 organoids (Fig. 6C–E, S4A-D). Surface CYP11A1+ cells also demonstrated nuclear staining for SRY (Fig. S4E). CYP11A1 protein expression in organoids was confirmed by western blot analysis. The CYP11A1 band detected in organoids is likely the immature form (runs ~ 50 kDa) that includes the N-terminal domain (~ 4 kDa), which is cleaved upon mitochondrial entry (Figs. 6F, S4F) [74]. In contrast, analysis of protein extracted from a prepubertal testis shows bands that correspond to the immature and cleaved forms of CYP11A1 (Fig. 6F, Fig. S4F). WT1 and CYP11A1 expression was mutually exclusive, which agrees with studies in mice outlining the inhibitory role of WT1 on steroidogenic cell differentiation (Fig. 6G, H) [42, 50]. Although, very rare double positive transitioning cells can still be found (Fig. 6I). Analogous to CYP11A1, cytoplasmic StaR and 3β-HSD staining was observed on the surface and in interstitial clusters of D26-40 organoids (Figs. 6J–N, S4G, H). In contrast to CYP11A1 and 3β-HSD localization, we also observed nuclear StaR expression, which correlated with staining pattern observed in human adult testis (Fig. 6N, O, Supplementary Fig. S4I, J).
Fig. 6.
Steroidogenic marker expression in organoid cultures. A, B Cytoplasmic CYP11A1 (green) staining in D16 organoids. C Cytoplasmic CYP11A1 (red) expression in the interstitial clusters and superficial layer of D26 organoids. White dashed line marks organoid borders. D Cytoplasmic CYP11A1 (red) expression in the interstitial clusters and superficial layer surrounding SOX9+ tubular structure in D40 organoid (also see Fig. S4A). E The section from the same D40 organoid in D showing SOX9+ (green, #14–9765-80) staining in tubular structure (also see Fig. S4B). F Western blot analysis of CYP11A1 protein (~ 50 kDa band marked by asterisk) expression in hESCs (hES), D26, D35 organoids and HT13 (age 11) prepubertal testis (see Fig. S4F for full size). G, H Representative images of D35 organoid sections showing mutually exclusive WT1 (green, sc-7385) and CYP11A1 (red) staining. I D35 organoid section demonstrating the presence of transitioning double-positive for CYP11A1 (red) and WT1 (green, sc-7385) cells. J, K Two sections from the same D26 organoid showing positive cytoplasmic staining of the surface layer and interstitial cell clusters for 3β-HSD (red) (J) and StaR (red, sc-166821) (K). Cell debris are marked by asterisk. L, M D40 organoid sections showing positive 3β-HSD (green) cytoplasmic staining in interstitial cell clusters (L) and surface cells (M). SOX9+ (#82630) cells are shown in red (M). N Cytoplasmic and nuclear StaR (red, sc-166821) staining in D40 organoid. SOX9+ (#82630) cells are shown in green. O D16 organoid section showing nuclear StaR (red, sc-25806) expression in superficial layer and interstitial cell clusters. Nuclei are shown in blue. Scale bars 50 µm
At approximately the same time as Sertoli cells begin AMH and inhibin B secretion, fetal Leydig cells initiate testosterone production, which is crucial for development of the male reproductive system [28, 72, 73]. Testosterone secretion has been reported to start as early as ~ 7 wpc with the peak between ~ 14—18 wpc (at ~ 3.5 ng/ml), and then decreasing to basal level (~ 0.5–1 ng/ml) at ~ 22 wpc [28, 75]. The initiation of testosterone biosynthesis is gonadotropin-independent, but around 10 wpc falls under receptor-mediated control by human chorionic gonadotropin (hCG) followed by luteinizing hormone (LH) [28, 73, 76]. To evaluate testosterone biosynthesis in organoid cultures, we performed enzyme immunoassay at D20-40 of differentiation. However, testosterone levels were very low (~ 20–40 pg/ml), and no response to hormone stimulation was observed (Fig. S5) [28, 76].
Comparative transcriptomic analysis of existing hPSC-derived organoids that model organs such as heart, kidney and brain show close matches to the human fetal tissues [5, 77–79]. For example, D26-54 cortical organoids resemble the 8–9 wpc fetal brain, while long-term organoid culture up to D100 shifts transcriptome profile closer to the 17–24 wpc fetal brain [79]. Based on marker expression in our organoids and existing transcriptome profiling of human embryonic and fetal gonads, D20-25 organoids match 7–8 wpc embryonic gonads [4]. We suggest that extended culture up to D40 makes organoid cultures closer to 10 wpc fetal gonads, although detailed gene expression profiling is needed to provide the definitive answer. An increase in testosterone production is expected at the “masculinization window” (~ 10–20 wpc), but we observed testosterone levels lower than the basal level reported from fetal analyses, which correlates with presence of the immature form of CYP11A1 (Figs. 6F, S4F) [28, 73, 75]. This outcome prompts the need for further studies to evaluate whether longer in vitro culture to “age” organoids or exogenous stimuli are required to initiate the process of steroidogenesis.
Organoid vascularization and coelomic vessel formation
Elegant mouse organ culture assays have shown that endothelial cells migrating from adjacent mesonephros are crucial for SOX9+ cell population partitioning and testicular cord formation [28, 53, 54]. A hallmark of testis development is the formation of the coelomic vessel, the main testicular artery [28, 52]. Interstitial gonadal cells produce VEGF, which promotes endothelial cell migration. In turn, endothelial cells secrete PDGF-BB, which is necessary for expansion of the interstitial cell population and testicular morphogenesis [28, 80]. In addition, clustering pre-Sertoli cells secrete ECM proteins that can also restrict and guide endothelial cell migration within the gonad [53].
To date, no research evaluating the role of vascularization in human testis development and morphogenesis has been reported. Histological studies published over a century ago describe the established fetal testis vasculature at ~ 28 wpc, while testicular cords have begun to form ~ 7–8 wpc [4, 28, 58, 59, 81]. These studies also state that there is a remarkable difference in vasculature development between human and other species [81, 82].
In our study, we utilized endothelial cell markers, PECAM1 and VE-cadherin, to evaluate endothelial cell contribution to organoid architecture. Endothelial cells were abundant and distributed around mesonephric tubules and clustered cells in D16-20 organoids (Fig. 7A, B, S6A-C). During expansion of SOX9+ progenitors, endothelial cells began to distribute in between SOX9+ cords and form vasculature under the coelomic layer lining (Figs. 7C–F, S6D-F) [53, 54]. Some organoids also developed a vascular cyst-like outgrowth (up to ~ 7–9%) (Fig. S6G, H). Overall, the process of organoid vascularization resembled the pattern described for the developing mouse testis [53, 54]. However, our previous observations revealed that SOX9+ cells autonomously partition into smaller tubules from large SOX9+ cord-like structures without any intervention from endothelial cells (Fig. 4E, F, Fig. S3A). Therefore, we suggest that endothelial cells likely migrated following paths defined by ECM deposition surrounding SOX9+ cords (Figs. 4E, F, 7C–F, S6D-F).
Fig. 7.
Organoid vascularization. A, B The distribution of VE-cadherin+ (VE-cad, red) endothelial cells in between mesonephric tubules in D16 (A) and D20 (B) organoids. C, D The distribution of VE-cadherin+ (VE-cad, red) endothelial cells in between SOX9+ (green, #14–9765-80) tubules and cord-like structures in D26 (C) and D35 (D) organoids. The formation of surface (coelomic) VE-cadherin+ (VE-cad, red) vasculature in D35 organoids is marked by arrows (D). SOX9+ tubules and cord-like structures are marked by a dashed line. Cell debris are marked by asterisk. E The vasculature formation in D40 organoid detected using anti-CD31 antibody (green). Tubules and cord-like structures demonstrate positive staining for SOX9 (red, #82630). F The section of the same organoid shown in E immunostained with anti-VE-cadherin antibodies (VE-cad, red), confirming the localization of vascular vessel detected using anti-CD31 antibody. Nuclei are shown in blue. Scale bars 100 µm
Recent experiments with reaggregation of dissociated mouse prepubertal testicular cells (5 dpp) showed de novo testicular cord formation [62]. In this case, compartmentalization of supporting and interstitial cells by directional endothelial cell migration from the mesonephros is not possible, although the endothelial cell contribution was not evaluated. Thus, organoid models provide the opportunity to investigate the role of endothelial cells in human gonad morphogenesis.
The addition of exogenous SHH and BMP4 does not influence overall organoid development
Hedgehog-BMP signaling plays an important role in regulation of epithelial-mesenchymal cell interactions during sex-specific formation of the urethra and external genitalia [12, 83]. It is also linked to reproductive system oncogenesis, and there is evidence that suggests Sonic Hedgehog (SHH) is involved in androgen synthesis [83]. Hedgehog and BMP signaling are essential for mouse gonad development and PGC localization [12, 84, 85]. Experiments with chicken embryos revealed that differential expression of BMP4, acting downstream of Hedgehog signaling, is responsible for CE patterning and initiation of gonadogenesis [34]. Moreover, the artificial induction of SHH-BMP4 signaling can trigger ectopic gonad formation on mesonephros [34, 86].
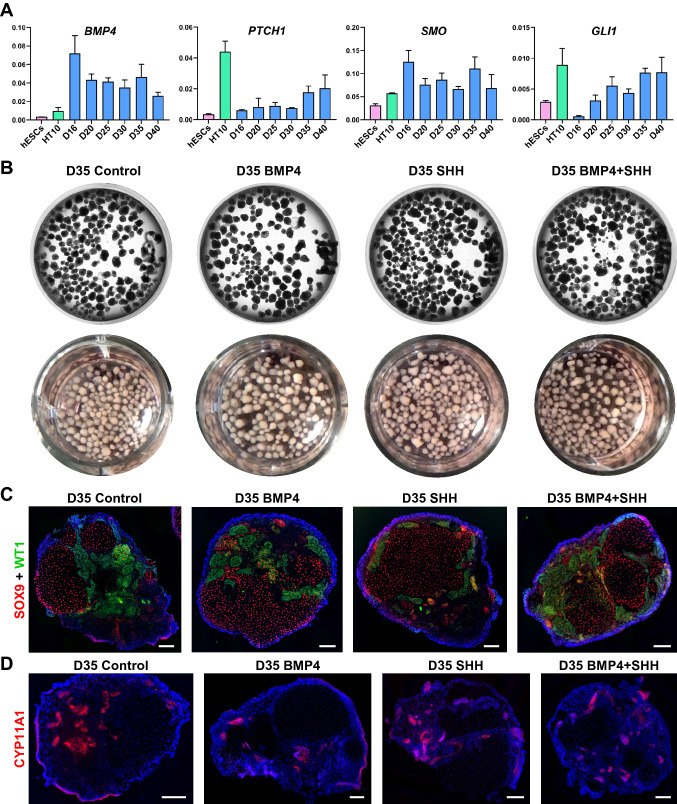
Little is known about the role of Hedgehog-BMP signaling in human gonad development. qRT-PCR analysis showed upregulation of BMP4 and Hedgehog signaling molecules PTCH1, SMO, and GLI1 in organoids cultured from Day 16 to D40 (Fig. 8A). We hypothesized, that similar to studies on chicken embryos, supplementing culture medium with BMP4 and/or SHH would allow for controlled specification of a gonadal ridge progenitor population on the surface of mesonephros-like organoid. To test this hypothesis, we supplemented organoid cultures with 50 ng/ml BMP4, 50 ng/ml SHH, or both during growth of mesonephric tubules and early stages of gonadal progenitor specification (D10 through D18). Because we did not observe the major morphological differences between groups before D20, we continued organoid culture up to D35. After extended culture we compared the expression of gonad-specific cell markers to control organoids (Fig. 8B–D). Similar organoid development was observed in all experimental groups with the formation of SOX9+ cord-like structures, as well as WT1+ and CYP11A1+ interstitial clusters (Fig. 8B–D).
Fig. 8.
Evaluation of the Hedgehog-BMP signaling in organoids. A qRT-PCR analysis of expression of BMP4 and Hedgehog signaling molecules PTCH1, SMO, and GLI1 in hESCs, HT10 (age 24) adult testis, D16, 20, 25, 30, 35 and 40 organoids. Data are presented as mean with standard deviation from technical triplicates. B Phase contrast and digital photography images of D35 live organoid cultures of control, BMP4, SHH and BMP4 + SHH groups in 12 well plate. C, D All groups of organoids (control, BMP4, SHH and BMP4 + SHH) develop WT1+ (green, sc-7385) interstitial cell clusters and SOX9+ (red, #82630) tubules and cord-like structures (C), as well as CYP11A1+ (red) interstitial and surface cell populations (D). Nuclei are shown in blue. Scale bars 100 µm
Despite not seeing an additional stimulatory effect by exogenous factors, we detected persistent expression of key signaling molecules of the Hedgehog-BMP pathway (Fig. 8A). Taking into account the importance of this signaling during sexual morphogenesis, more detailed experiments addressing specific questions are worth pursuing using our organoid culture system [12].
Discussion
Considering the proven success of hPSC-based organoid technology for modeling development and disease of other organs and tissues, hPSCs are best suited to fill the gap in our understanding human reproductive development [5]. A pioneering study by Sepponen et al. established that progenitors expressing bipotential gonad markers can be obtained by spatio-temporal control of BMP and WNT signaling pathways during directed hPSC differentiation in a monolayer culture within 8 days [13]. More recently, Knarston et al. also included FGF9 to direct the differentiation of bipotential gonadal progenitors in a monolayer culture (7 days). They then extended their study for an additional 2 weeks of monolayer or 3D culture on transwell inserts in the presence of PGD2 with the purpose of inducing testicular marker expression [14]. Indeed, under both culture conditions bipotential gonadal progenitors produced derivatives expressing Sertoli cell markers and a subset of cells with steroidogenic identity. Furthermore, SOX9+ cells exhibited clustering and formation of cord-like structures [14].
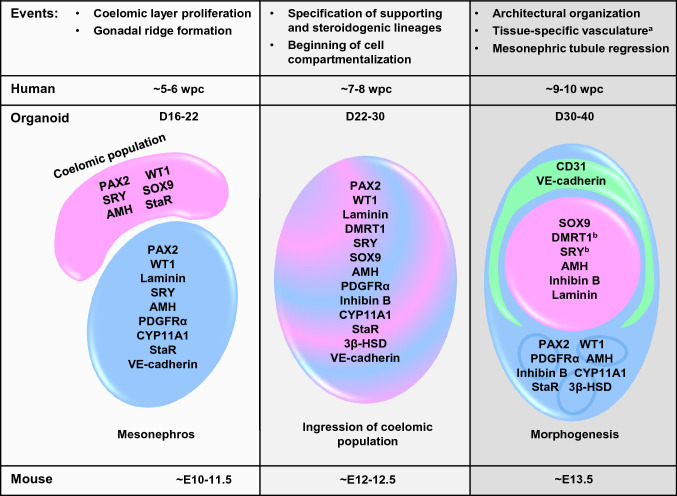
In our study we relied solely on 3D culture and the self-organization and differentiation capabilities of hPSCs, where mesonephros-like organoids (D10-16) were cultured under maintenance conditions for additional 3–4 weeks (up to D40, Fig. 1A). We demonstrate that our hPSC-derived organoid culture system can mimic early processes of human reproductive development, as we were able to observe and analyze the appearance of cells expressing characteristic gonadal markers and tissue-specific morphogenesis in an orderly manner (Fig. 9). We also explored the contribution of vascular cells into testis-specific tissue organization, which has not been investigated before. We detected the autonomous activation of genes involved in Hedgehog-BMP regulatory signaling in organoid cultures. Employment of our scalable mini-spin bioreactor platform allows for multiscale proteomics and transcriptomics studies, which can be used for further characterization and improvement of the gonad organoid model.
Fig. 9.
The timeline and developmental stages in hPSC-derived gonad organoid model. Organoid analysis during extended culture (D16-40) reveals similarity to early gonad development described in human (~ 5–10 wpc) and mice (~ E10-13.5). Formation of coelomic population on the organoid surface (D16-22), progenitor differentiation into supporting and steroidogenic cells, as well as their ingression into mesonephros-like organoids (D22-D30), followed by cell type-specific clustering and architectural reorganization of organoids (D30-40). Each event was accompanied by specific somatic gonadal cell marker expression and compartmentalization as shown. aNot studied in human gonads before ~ 28 wpc, in mouse it is established by E12.5. bDetected by western blot analysis
Our observations line up well with previously published studies of gonad development in mice, monkey and human, suggesting a mesonephric origin (PAX2+ cells) of multipotent WT1+ gonadal progenitors, which are then capable to differentiate into supportive or steroidogenic adreno-gonadal cells [1, 37]. This organoid system will be used for lineage tracing experiments to explore origins of specialized cell types in the developing human gonad and to study processes that drive gonadal morphogenesis. Additionally, the generation of gonad organoids from hPSCs with an integrated system for rapid, reversible and titratable protein degradation will enable the exploration of key protein functions at specific stages of gonad development, such as gonadal progenitor formation, cell type-specific differentiation and hormone production [87]. Utilization of these tools in gonad organoids enables researchers to model diverse DSD phenotypes and study the etiology of DSD [5, 7, 12].
Accumulating knowledge about microenvironment requirements for germ cell development and advances in in vitro culture strategies supporting gametogenesis from mouse PSCs provide strong evidence of feasibility of these techniques for human germ cell studies [4, 15–19]. Previous efforts have shown that co-culture of mouse PSC-derived PGCLCs with immature gonadal somatic cells is crucial for the generation of functional male and female gametes [17, 18]. Moreover, mouse PSC-derived female gonadal somatic cells can successfully replace embryonic gonadal tissue and support gamete development and offspring production [19].
To date, human PGCLCs can be successfully generated from hPSCs in in vitro culture [20]. These cells are characterized by the expression of primitive migratory germ cell markers, such as OCT4, CD38, SOX17, NANOS3, UTF1 and CXCR4 (Fig. S7A-D). However, the production of meiosis-competent cells and fertilization-competent gametes remains a challenge [21, 22]. hPSC-derived gonad organoids will likely serve as an optimal germ cell niche for studying human germ cell development and gametogenesis. The expression of primitive early-stage (OCT4) and post-migratory germ cell (DAZL) markers in gonad organoids was not observed (Fig. S7E-I) [15, 16, 20]. Therefore, in vitro generated PGCLCs could be microinjected into gonad organoids and their developmental potential can be further analyzed [88].
We hope that the results of our pioneering studies, as well as those from other groups, will pave the way for future in depth investigations and help to advance the knowledge of human reproductive development. We believe that hPSC-based gonad organoid systems can become a pivotal tool not only for reproductive development studies, but also for diagnostic assays, toxicity and drug screening, contraception testing, disease modeling and regenerative medicine.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Dr. A. Leung and Dr. V. Busa, as well as Dr. J. Wang and Dr. Honghe Liu for help with qPCR equipment setup and data collection. We would like to thank Dr. B. Zirkin and Dr. J.-Y. Chung for help with ELISA equipment setup and data collection. We thank Dr. I. Rasool from WRTC and Dr. Hooper from JHU Legacy Gift Rapid Autopsy program for coordinating the acquisition of deidentified human testis samples used for this study. Additionally, we would like to acknowledge Dr. M. Matunis for critical discussion of project design. This work was funded by the American Society for Reproductive Medicine to MVP (KY Cha Award in Stem Cell Technology) and National Institute of General Medical Sciences grant to PWJ (R01GM11755).
Declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical statement
The mouse studies were approved by JHU IACUC (MO21H13). The use of human ESC line was approved by JHU ISCRO committee (protocol ISCRO00000643). Deidentified decedent donor testes tissues were designated as “not human subjects research” by JHU (IRB No: 00006700).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marina V. Pryzhkova, Email: marina.pryzhkova.ctr@usuhs.edu
Philip W. Jordan, Email: philip.jordan@usuhs.edu
References
- 1.Satoh M. Histogenesis and organogenesis of the gonad in human embryos. J Anat. 1991;177:85–107. [PMC free article] [PubMed] [Google Scholar]
- 2.Santoro N, Caplan A, Strauss J, Winn VD. A letter to president biden and secretary designate of HHS xavier becerra: remove barriers to federal funding of human embryo and fetal tissue research. Reprod Sci. 2021;28:933–935. doi: 10.1007/s43032-021-00491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDuffie KE, Hyun I, Krogen MM, Dempsey JC, Murry CE, Copp AJ, et al. Rescuing human fetal tissue research in the United States: a call for additional regulatory reform. Stem Cell Reports. 2021;16:2839–2843. doi: 10.1016/j.stemcr.2021.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo J, Sosa E, Chitiashvili T, Nie X, Rojas EJ, Oliver E, et al. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem Cell. 2021 doi: 10.1016/j.stem.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corsini NS, Knoblich JA. Human organoids: New strategies and methods for analyzing human development and disease. Cell. 2022;185:2756–2769. doi: 10.1016/j.cell.2022.06.051. [DOI] [PubMed] [Google Scholar]
- 6.Boitani C, Di Persio S, Esposito V, Vicini E. Spermatogonial cells: mouse, monkey and man comparison. Semin Cell Dev Biol. 2016;59:79–88. doi: 10.1016/j.semcdb.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Zhang D, Su M, Tang R, Luo M, Jiang T, Chen R. DSDatlas: disorders of sex development atlas for reproductive endocrinology-related gene discovery in integrative omics platforms. F S Science. 2022;3:108–117. doi: 10.1016/j.xfss.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson A-M, Eisenberg ML, et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev. 2016;96:55–97. doi: 10.1152/physrev.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho S-M, Cheong A, Adgent MA, Veevers J, Suen AA, Tam NNC, et al. Environmental factors, epigenetics, and developmental origin of reproductive disorders. Reprod Toxicol. 2017;68:85–104. doi: 10.1016/j.reprotox.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messerlian C, Williams PL, Ford JB, Chavarro JE, Mínguez-Alarcón L, Dadd R, et al. The environment and reproductive health (EARTH) study: a prospective preconception cohort. Hum Reprod Open. 2018 doi: 10.1093/hropen/hoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scialli AR, Daston G, Chen C, Coder PS, Euling SY, Foreman J, et al. Rethinking developmental toxicity testing: evolution or revolution? Birth Defects Res. 2018;110:840–850. doi: 10.1002/bdr2.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson HKL, Svingen T. Hedgehog signal disruption, gonadal dysgenesis and reproductive disorders: Is there a link to endocrine disrupting chemicals? Curr Res Toxicol. 2020;1:116–123. doi: 10.1016/j.crtox.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sepponen K, Lundin K, Knuus K, Väyrynen P, Raivio T, Tapanainen JS, et al. The role of sequential BMP signaling in directing human embryonic stem cells to bipotential gonadal cells. J Clin Endocrinol Metab. 2017;102:4303–4314. doi: 10.1210/jc.2017-01469. [DOI] [PubMed] [Google Scholar]
- 14.Knarston IM, Pachernegg S, Robevska G, Ghobrial I, Er PX, Georges E, et al. An in vitro differentiation protocol for human embryonic bipotential gonad and testis cell development. Stem Cell Reports. 2020;15:1377–91. doi: 10.1016/j.stemcr.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y-C, Nicholls PK, Soh YQS, Daniele JR, Junker JP, van Oudenaarden A, et al. Licensing of primordial germ cells for gametogenesis depends on genital ridge signaling. PLoS Genet. 2015;11:e1005019. doi: 10.1371/journal.pgen.1005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo F, Yan L, Guo H, Li L, Hu B, Zhao Y, et al. The transcriptome and DNA methylome landscapes of human primordial germ cells. Cell. 2015;161:1437–1452. doi: 10.1016/j.cell.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q, Wang M, Yuan Y, Wang X, Fu R, Wan H, et al. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell. 2016;18:330–340. doi: 10.1016/j.stem.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539:299–303. doi: 10.1038/nature20104. [DOI] [PubMed] [Google Scholar]
- 19.Yoshino T, Suzuki T, Nagamatsu G, Yabukami H, Ikegaya M, Kishima M, et al. Generation of ovarian follicles from mouse pluripotent stem cells. Science. 2021;373:eabe0237. doi: 10.1126/science.abe0237. [DOI] [PubMed] [Google Scholar]
- 20.Mitsunaga S, Odajima J, Yawata S, Shioda K, Owa C, Isselbacher KJ, et al. Relevance of iPSC-derived human PGC-like cells at the surface of embryoid bodies to prechemotaxis migrating PGCs. Proc Natl Acad Sci U S A. 2017;114:E9913–E9922. doi: 10.1073/pnas.1707779114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handel MA, Eppig JJ, Schimenti JC. Applying “gold standards” to in-vitro-derived germ cells. Cell. 2014;157:1257–1261. doi: 10.1016/j.cell.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saitou M, Hayashi K. Mammalian in vitro gametogenesis. Science. 2021;374:eaaz6830. [DOI] [PubMed]
- 23.Pryzhkova MV, Jordan PW. Adaptation of human testicular niche cells for pluripotent stem cell and testis development research. Tissue Eng Regen Med. 2020;17:223–235. doi: 10.1007/s13770-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 25.Pryzhkova MV, Xu MJ, Jordan PW. Adaptation of the AID system for stem cell andtransgenic mouse research. Stem Cell Res. 2020;49:102078. doi: 10.1016/j.scr.2020.102078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pryzhkova MV, Aria I, Cheng Q, Harris GM, Zan X, Gharib M, et al. Carbon nanotube-based substrates for modulation of human pluripotent stem cell fate. Biomaterials. 2014;35:5098–5109. doi: 10.1016/j.biomaterials.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkins A, Xu MJ, Li M, Rogers NP, Pryzhkova MV, Jordan PW. SMC5/6 is required for replication fork stability and faithful chromosome segregation during neurogenesis. Elife. 2020;9:e61171. doi: 10.7554/eLife.61171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rey R, Josso N, Racine C, et al. Sexual differentiation. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. [Google Scholar]
- 29.Del Valle I, Buonocore F, Duncan AJ, Lin L, Barenco M, Parnaik R, et al. A genomic atlas of human adrenal and gonad development. Wellcome Open Res. 2017;2:25. doi: 10.12688/wellcomeopenres.11253.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piprek RP, Kloc M, Kubiak JZ. Early development of the gonads: origin and differentiation of the somatic cells of the genital ridges. Results Probl Cell Differ. 2016;58:1–22. doi: 10.1007/978-3-319-31973-5_1. [DOI] [PubMed] [Google Scholar]
- 31.Davidson AJ. Mouse kidney development. StemBook. Cambridge (MA): Harvard Stem Cell Institute; 2008. [PubMed]
- 32.Ariza L, Carmona R, Cañete A, Cano E, Muñoz-Chápuli R. Coelomic epithelium-derived cells in visceral morphogenesis. Dev Dyn. 2016;245:307–322. doi: 10.1002/dvdy.24373. [DOI] [PubMed] [Google Scholar]
- 33.Svingen T, Koopman P. Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev. 2013;27:2409–2426. doi: 10.1101/gad.228080.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshino T, Murai H, Saito D. Hedgehog-BMP signalling establishes dorsoventral patterning in lateral plate mesoderm to trigger gonadogenesis in chicken embryos. Nat Commun. 2016;7:12561. doi: 10.1038/ncomms12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romereim SM, Cupp AS. Mesonephric cell migration into the gonads and vascularization are processes crucial for testis development. Results Probl Cell Differ. 2016;58:67–100. doi: 10.1007/978-3-319-31973-5_4. [DOI] [PubMed] [Google Scholar]
- 36.Estermann MA, Williams S, Hirst CE, Roly ZY, Serralbo O, Adhikari D, et al. Insights into gonadal sex differentiation provided by single-cell transcriptomics in the chicken embryo. Cell Rep. 2020;31:107491. doi: 10.1016/j.celrep.2020.03.055. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki K, Oguchi A, Cheng K, Murakawa Y, Okamoto I, Ohta H, et al. The embryonic ontogeny of the gonadal somatic cells in mice and monkeys. Cell Rep. 2021;35:109075. doi: 10.1016/j.celrep.2021.109075. [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Rodriguez K, Yao HH-C. Mapping lineage progression of somatic progenitor cells in the mouse fetal testis. Development. 2016;143:3700–3710. doi: 10.1242/dev.135756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres M, Gómez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- 40.Santana Gonzalez L, Rota IA, Artibani M, Morotti M, Hu Z, Wietek N, et al. Mechanistic drivers of müllerian duct development and differentiation into the oviduct. Front Cell Dev Biol. 2021;9:605301. doi: 10.3389/fcell.2021.605301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunha GR, Robboy SJ, Kurita T, Isaacson D, Shen J, Cao M, et al. Development of the human female reproductive tract. Differentiation. 2018;103:46–65. doi: 10.1016/j.diff.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bandiera R, Vidal VPI, Motamedi FJ, Clarkson M, Sahut-Barnola I, von Gise A, et al. WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland. Dev Cell. 2013;27:5–18. doi: 10.1016/j.devcel.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryan G, Steele-Perkins V, Morris JF, Rauscher FJ, Dressler GR. Repression of Pax-2 by WT1 during normal kidney development. Development. 1995;121:867–875. doi: 10.1242/dev.121.3.867. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y-C, Okumura LM, Page DC. Gata4 is required for formation of the genital ridge in mice. PLoS Genet. 2013;9:e1003629. doi: 10.1371/journal.pgen.1003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smyth IM, Cullen-McEwen LA, Caruana G, Black MJ, Bertram JF. Development of the kidney. In: Fetal and neonatal physiology. Elsevier; 2017. pp. 953–964.e4.
- 46.Zarkower D, Murphy MW. DMRT1: an ancient sexual regulator required for human gonadogenesis. Sex Dev. 2021.10.1159/000518272 [DOI] [PMC free article] [PubMed]
- 47.Domenice S, Arnhold IJP, Costa EMF, Mendonca BB, et al. 46, XY disorders of sexual development. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. [Google Scholar]
- 48.Modi D, Shah C, Sachdeva G, Gadkar S, Bhartiya D, Puri C. Ontogeny and cellular localization of SRY transcripts in the human testes and its detection in spermatozoa. Reproduction. 2005;130:603–613. doi: 10.1530/rep.1.00413. [DOI] [PubMed] [Google Scholar]
- 49.Mamsen LS, Ernst EH, Borup R, Larsen A, Olesen RH, Ernst E, et al. Temporal expression pattern of genes during the period of sex differentiation in human embryonic gonads. Sci Rep. 2017;7:15961. doi: 10.1038/s41598-017-15931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen M, Zhang L, Cui X, Lin X, Li Y, Wang Y, et al. Wt1 directs the lineage specification of sertoli and granulosa cells by repressing Sf1 expression. Development. 2017;144:44–53. doi: 10.1242/dev.144105. [DOI] [PubMed] [Google Scholar]
- 51.Croft B, Ohnesorg T, Hewitt J, Bowles J, Quinn A, Tan J, et al. Human sex reversal is caused by duplication or deletion of core enhancers upstream of SOX9. Nat Commun. 2018;9:5319. doi: 10.1038/s41467-018-07784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- 53.Coveney D, Cool J, Oliver T, Capel B. Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci U S A. 2008;105:7212–7217. doi: 10.1073/pnas.0707674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Combes AN, Wilhelm D, Davidson T, Dejana E, Harley V, Sinclair A, et al. Endothelial cell migration directs testis cord formation. Dev Biol. 2009;326:112–120. doi: 10.1016/j.ydbio.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 55.Combes AN, Lesieur E, Harley VR, Sinclair AH, Little MH, Wilhelm D, et al. Three-dimensional visualization of testis cord morphogenesis, a novel tubulogenic mechanism in development. Dev Dyn. 2009;238:1033–1041. doi: 10.1002/dvdy.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Santa BP, Moniot B, Poulat F, Berta P. Expression and subcellular localization of SF-1, SOX9, WT1, and AMH proteins during early human testicular development. Dev Dyn. 2000;217:293–298. doi: 10.1002/(SICI)1097-0177(200003)217:3<293::AID-DVDY7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 57.Makanji Y, Zhu J, Mishra R, Holmquist C, Wong WPS, Schwartz NB, et al. Inhibin at 90: from discovery to clinical application, a historical review. Endocr Rev. 2014;35:747–794. doi: 10.1210/er.2014-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bendsen E, Byskov AG, Laursen SB, Larsen H-PE, Andersen CY, Westergaard LG. Number of germ cells and somatic cells in human fetal testes during the first weeks after sex differentiation. Hum Reprod. 2003;18:13–18. doi: 10.1093/humrep/deg057. [DOI] [PubMed] [Google Scholar]
- 59.Ostrer H, Huang HY, Masch RJ, Shapiro E. A cellular study of human testis development. Sex Dev. 2007;1:286–292. doi: 10.1159/000108930. [DOI] [PubMed] [Google Scholar]
- 60.Pendergraft SS, Sadri-Ardekani H, Atala A, Bishop CE. Three-dimensional testicular organoid: a novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biol Reprod. 2017;96:720–732. doi: 10.1095/biolreprod.116.143446. [DOI] [PubMed] [Google Scholar]
- 61.Baert Y, De Kock J, Alves-Lopes JP, Söder O, Stukenborg J-B, Goossens E. Primary human testicular cells self-organize into organoids with testicular properties. Stem Cell Rep. 2017;8:30–38. doi: 10.1016/j.stemcr.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edmonds ME, Woodruff TK. Testicular organoid formation is a property of immature somatic cells, which self-assemble and exhibit long-term hormone-responsive endocrine function. Biofabrication. 2020;12:045002. doi: 10.1088/1758-5090/ab9907. [DOI] [PubMed] [Google Scholar]
- 63.Shetty G, Mitchell JM, Lam TNA, Wu Z, Zhang J, Hill L, et al. Donor spermatogenesis in de novo formed seminiferous tubules from transplanted testicular cells in rhesus monkey testis. Hum Reprod. 2018;33:2249–2255. doi: 10.1093/humrep/dey316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Rahilly R. The timing and sequence of events in the development of the human reproductive system during the embryonic period proper. Anat Embryol. 1983;166:247–261. doi: 10.1007/BF00305086. [DOI] [PubMed] [Google Scholar]
- 65.Makiyan Z. Studies of gonadal sex differentiation. Organogenesis. 2016;12:42–51. doi: 10.1080/15476278.2016.1145318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu H-Y, Zhang H-X, Xiao Z, Qiao J, Li R. Regulation of anti-Müllerian hormone (AMH) in males and the associations of serum AMH with the disorders of male fertility. Asian J Androl. 2019;21:109–114. doi: 10.4103/aja.aja_83_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petersen C, Soder O. The sertoli cell–a hormonal target and “super” nurse for germ cells that determines testicular size. Horm Res. 2006;66:153–161. doi: 10.1159/000094142. [DOI] [PubMed] [Google Scholar]
- 68.Demyashkin GA. Inhibin B in seminiferous tubules of human testes in normal spermatogenesis and in idiopathic infertility. Syst Biol Reprod Med. 2019;65:20–28. doi: 10.1080/19396368.2018.1478470. [DOI] [PubMed] [Google Scholar]
- 69.Ross AJ, Tilman C, Yao H, MacLaughlin D, Capel B. AMH induces mesonephric cell migration in XX gonads. Mol Cell Endocrinol. 2003;211:1–7. doi: 10.1016/j.mce.2003.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao HH-C, Aardema J, Holthusen K. Sexually dimorphic regulation of inhibin beta B in establishing gonadal vasculature in mice. Biol Reprod. 2006;74:978–983. doi: 10.1095/biolreprod.105.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mamsen LS, Petersen TS, Jeppesen JV, Møllgård K, Grøndahl ML, Larsen A, et al. Proteolytic processing of anti-Müllerian hormone differs between human fetal testes and adult ovaries. Mol Hum Reprod. 2015;21:571–582. doi: 10.1093/molehr/gav024. [DOI] [PubMed] [Google Scholar]
- 72.Miller WL, Auchus RJ. The “backdoor pathway” of androgen synthesis in human male sexual development. PLoS Biol. 2019;17:e3000198. doi: 10.1371/journal.pbio.3000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scott HM, Mason JI, Sharpe RM. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- 74.Lin YC, Papadopoulos V. Neurosteroidogenic enzymes: CYP11A1 in the central nervous system. Front Neuroendocrinol. 2021;62:100925. doi: 10.1016/j.yfrne.2021.100925. [DOI] [PubMed] [Google Scholar]
- 75.Connan-Perrot S, Léger T, Lelandais P, Desdoits-Lethimonier C, David A, Fowler PA, et al. Six decades of research on human fetal gonadal steroids. Int J Mol Sci. 2021;22:6681. doi: 10.3390/ijms22136681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lambrot R, Coffigny H, Pairault C, Donnadieu AC, Frydman R, Habert R, et al. Use of organ culture to study the human fetal testis development: effect of retinoic acid. J Clin Endocrinol Metab. 2006;91:2696–2703. doi: 10.1210/jc.2005-2113. [DOI] [PubMed] [Google Scholar]
- 77.Lewis-Israeli YR, Wasserman AH, Gabalski MA, Volmert BD, Ming Y, Ball KA, et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat Commun. 2021;12:5142. doi: 10.1038/s41467-021-25329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 79.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. Brain-Region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cool J, DeFalco TJ, Capel B. Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci U S A. 2011;108:167–172. doi: 10.1073/pnas.1010299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hill EC. On the gross development and vascularization of the testis. Am J Anat. 1906;6:439–459. [Google Scholar]
- 82.Hill EC. The vascularization of the human testis. Am J Anat. 1909;9:463–474. [Google Scholar]
- 83.Hyuga T, Alcantara M, Kajioka D, Haraguchi R, Suzuki K, Miyagawa S, et al. Hedgehog signaling for urogenital organogenesis and prostate cancer: an implication for the epithelial-mesenchyme interaction (EMI) Int J Mol Sci. 2019;21:58. doi: 10.3390/ijms21010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wainwright EN, Svingen T, Ng ET, Wicking C, Koopman P. Primary cilia function regulates the length of the embryonic trunk axis and urogenital field in mice. Dev Biol. 2014;395:342–354. doi: 10.1016/j.ydbio.2014.08.037. [DOI] [PubMed] [Google Scholar]
- 85.Dudley B, Palumbo C, Nalepka J, Molyneaux K. BMP signaling controls formation of a primordial germ cell niche within the early genital ridges. Dev Biol. 2010;343:84–93. doi: 10.1016/j.ydbio.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshino T. The role of hedgehog-BMP4 signaling in the patterning of coelomic mesoderm and the onset of gonadogenesis. In: Katabuchi H, Ohba T, Motohara T, editors. Cell biology of the ovary. Singapore: Springer Singapore; 2018. pp. 21–33. [Google Scholar]
- 87.Pryzhkova MV, Xu MJ, Jordan PW. Adaptation of the AID system for stem cell and transgenic mouse research. Stem Cell Res. 2020;49:102078. doi: 10.1016/j.scr.2020.102078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kobayashi M, Kobayashi M, Odajima J, Shioda K, Hwang YS, Sasaki K, et al. Expanding homogeneous culture of human primordial germ cell-like cells maintaining germline features without serum or feeder layers. Stem Cell Rep. 2022;17:507–521. doi: 10.1016/j.stemcr.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.