Abstract
This research evaluates the bioactivity of twelve endophytic fungi successfully isolated and characterised from Gynura procumbens. The fungal extracts displayed inhibitory activity against Staphylococcus aureus, Pseudomonas aeruginosa, Methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli and Salmonella typhi with the MIC and MBC of 5000 µg/mL. High antioxidant activity using DPPH free radical scavenging assay with inhibition of 86.6% and IC50 value of 104.25 ± 18.51 µg/mL were exhibited by ethyl acetate extract of Macrophomina phaseolina SN6. In contrast, the highest scavenging activity percentage of methanolic extract was exhibited by Mycoleptodiscus indicus SN4 (50.0%). Besides that, the highest ferric reducing antioxidant power (FRAP) value of ethyl acetate and methanolic extract was recorded from M. phaseolina SN6 (239.9 mg Fe (II)/g) and M. indicus SN4 (44.7 mg Fe (II)/g), respectively. Total phenolic content (TPC) and total flavonoid content (TFC) of ethyl acetate and methanolic fungal extracts were determined using Folin-Ciocalteu and aluminium chloride, respectively. The highest TPC for ethyl acetate and methanolic extracts were exhibited by Colletotrichum gloeosporioides SN11 (87.0 mg GAE/g) and M. indicus SN4 (35.0 mg GAE/g), whereas the highest TFC of ethyl acetate and methanolic extracts were showed by M. phaseolina SN6 (122.8 mg QCE/g) and M. indicus SN4 (60.4 mg QCE/g), respectively. Bioactive metabolites of isoelemicin (50.8%), terpinen-4-ol (21.5%), eucalyptol (24.3%), oleic acid (19.8%) and β-pinene (10.9%) were detected. Owing to the higher content of phytochemicals represented in the ethyl acetate extract of M. phaseolina, SN6 is therefore identified to be a superior candidate in exhibiting strong antioxidant and antimicrobial properties be fit for further pharmaceutical studies.
Keywords: Antioxidant activity, Antibacterial activity, Bioactive compounds, Endophytic fungi, Gynura procumbens, Macrophomina phaseolina
Introduction
The emergence of new diseases, development of different drug resistant pathogenic microorganisms and the resurgence of infections that are more complex and rapidly mutating viral variants have become pressing health problem and concern worldwide [1]. These factors have elevated the need for novel, safe and effective bioactive compounds to provide aid and comfort in all aspects of life. Over the years, medicinal plants have been used and grown rapidly throughout the world in parallel with the increasing demand for natural drugs and bioactive compounds in the effort to curb those maladies [2]. However, the dependence on only one possible source can lead to excessive collection, exploitation, and gradual exhaustion of medicinal plants. According to Chen et al. [3], one third of the estimated medicinal plant species are threaten with extinction from overharvesting and natural anthropogenic habitat destruction. Besides, medicinal plants also pose several limitations as a feasible source for bioactive compounds as they are slow growing and yield small quantity of compounds [4].
The issue regarding the possible extinction of medicinal plants must be taken into attention as it will not only eradicate the plants from earth forever, but also lead to the extinction of yet uncharacterized endophytic fungi associated with the respective plants [5]. Therefore, the discovery of an alternative source that are feasible to produce the bioactive compounds to meet the current demand is in need and this can be achieved by exploiting the ability of microorganisms known as endophytic fungi [6]. Endophytic fungi are symbiotic microorganisms that reside within healthy plant tissues without causing any damage or symptoms of disease towards the host plants [7]. These ubiquitous microorganisms can be found in all plant species and inhabit almost all plant tissues, including the leaves, stems, barks, roots, and rhizomes. They promote plant growth and tolerance towards biotic and abiotic stresses in return of the protection and nutrients contributed by the host plants [4].
Besides that, endophytic fungi are also known to possess the ability to produce diverse natural products, including potent and life-saving drugs [6]. Primarily, fungal endophytes produce a plethora of bioactive compounds that have anticancer, antimicrobial, antioxidant, antidiabetic, anti-inflammatory, and immunosuppressive properties [4]. In addition, several studies have documented those endophytic fungi could produce similar plant-derived bioactive compounds as their host plants. For instance, an anticancer drug called paclitaxel (Taxol) discovered from an endophytic fungus Taxomyces andreanae showed similarities to paclitaxel from the host plant, Taxus brevifolia (yew tree) [2]. These bioactive compounds can be essentially exploited and applied by human as important pharmaceutical and medicinal resources [8]. Currently, there are approximately one million terrestrial endophytes and yet only less than 10% of them have been investigated [9].
On another note, each individual plant of the approximately 300,000 plant species forming the vegetal biodiversity of earth is a host to one or more endophytes and can consequently constitutes to the finding of unknown yet interesting fungal endophytes [9]. In fact, most of the existing plants are relatively not completely studied especially in relation to the endophytic fungi living in association with them [10]. Thus, there is a need to expand the investigation on endophytic fungi to other plant species. In the present study, a medicinal plant known as G. procumbens was chosen for the investigation of their associated fungal endophytes. To the best of our knowledge, there is no specific research study conducted on the endophytic fungi of G. procumbens in respect to their biological activities and bioactive compounds has been reported, even though the phytochemistry, pharmacological and ethnopharmacological usage of G. procumbens have been documented by several authors [11–15].
The medicinal plant, G. procumbens, belongs to Asteraceae family and is commonly found in tropical Asian countries such as Malaysia, Indonesia, Thailand, Vietnam, and China. It is known to possess great therapeutic potential and is widely used in the treatment of various health ailments such as kidney discomfort, rheumatism, diabetes mellitus, hypertension, and rashes [11]. According to Mou and Dash [12], some of these traditional claims have been validated in scientific studies and proven to be positively exhibiting antidiabetic, anticancer, antihypertensive, anti-inflammatory and antihyperlipidemic activities. In previous studies, this plant was found to exhibit high total phenolic and flavonoid content, potent antioxidant activity and wide range of inhibitory activity against pathogenic microorganisms [13, 14]. Furthermore, the leaf of this medicinal plant also yields diversified phytoconstituents such as flavonoids, steroids, alkaloids, terpenoids and phenolics [15].
To date, many studies have shown the potential of endophytic fungi from medicinal plants in producing useful bioactive compounds with diverse biotechnological applications [1–5]. Therefore, the present study was aimed to isolate and identify endophytic fungi associated with the selected medicinal plant, G. procumbens, with the main objective to investigate the possible biological properties and bioactive compounds of the fungal extracts that may contribute to the development of a more suitable approach for further applications in various industries. The current study is expected to provide insights and adequate understanding on the biological properties and bioactive compounds of endophytic fungi isolated from this medicinal plant.
Materials and methods
Collection of plant samples
Healthy and matured Gynura procumbens (Sambung Nyawa) plants were collected from Kampung Tabuan Melayu (1.5398° N, 110.3816° E), Kuching, Sarawak, Malaysia. The taxonomic identity of the plant was authenticated by the plant taxonomy expert, Dr Qammil Muzzamimil Abdullah @ Meekiong Kalu from the Department of Plant Resource Science and Management, Universiti Malaysia Sarawak (UNIMAS). Plant samples were brought to the laboratory in sterile zip bags and used for isolation within 24 h of collection [10].
Isolation and identification of endophytic fungi
The plant leaves were washed thoroughly under running tap water for 10 min. The slightly modified surface sterilization method described by Yadav et al. [16] was performed. Leaf samples were sterilized in series with 70% ethanol for 1 min, 1% sodium hypochlorite for 2 min and 70% ethanol for 2 min. It was further cleansed with three sets of sterile distilled water and dried with sterile filter paper. After that, the leaves were cut into approximately 1 cm in size using sterile blade and forceps. Three explants were inoculated accordingly on Petri plates containing potato dextrose agar (PDA) supplemented with 50 mg/L chloramphenicol. Surface sterilization validation plates were set up by inoculating 100 µL aliquots of water samples from the final rinse of surface sterilization process in Petri plates containing similar medium [17]. The Petri plates were incubated at room temperature for approximately 1 week [18]. Sub-culturing of endophytic fungal isolates was conducted after 1 week of endophytic fungal growth to obtain pure isolates [19].
The morphological characteristics of endophytic fungal isolates were observed by viewing the top and bottom sides of culture plates. Then, microscopic characteristics were observed by preparing slides by tease mount method using lactophenol cotton blue staining and viewed under microscope [20]. Further molecular identification involving the extraction of DNA, polymerase chain reaction (PCR) amplification [21] using universal primer pair of ITS1 and ITS4 under the following conditions: initial denaturation at 95 °C for 5 min, 30 cycles of denaturation at 95 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 7 min. PCR products were then purified and sequenced. The sequence obtained for each sample was used as query sequence to search similar sequences in GenBank. Subsequently, the sequences were aligned and analysed using Basic Local Alignment Search Tool (BLAST) of National Centre for Biotechnology Information (NCBI, USA) (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The search was conducted for the close matched sequence in GenBank database. A phylogenetic tree was constructed using Molecular Evolutionary Genetic Analysis 7.0 (MEGA 7.0) (Pennsylvania State University, USA) software using the neighbour-joining tree with 1000 replicates as bootstrap value according to the slightly modified method of Yenn et al. [22]. The DNA sequences were deposited at GenBank, NCBI and accession numbers obtained.
Fermentation and metabolite extraction of bioactive compounds
The fermentation process was conducted by inoculating plugs of endophytic fungi into 250-mL Erlenmeyer flask containing 150 mL of potato dextrose broth (PDB) and incubated at room temperature under periodical shaking at 150 rpm for 21 days [19]. Then, the culture broth was filtered with sterile filter paper to remove the mycelial mats and the filtrate was extracted with ethyl acetate. Equal volume of ethyl acetate and filtrate was transferred into a separatory funnel and shaked for 10 min. The solution was kept for 5 min until two clear immiscible layers formed and the upper layer was collected. The residual mycelial mats were maintained in methanol for 1 week and filtered using sterile filter paper. Ethyl acetate and methanol were evaporated under reduced pressure at 50 °C and 150 rpm in rotary vacuum evaporator to obtain dried crude extracts [23].
Antimicrobial activity by broth microdilution assay
Five test microorganisms were obtained from the Faculty of Medicine and Health Sciences, Universiti Malaysia Sarawak (UNIMAS). The microorganisms were gram-positive bacteria; Staphylococcus aureus (ATCC 25,923), MRSA and gram-negative bacteria; Escherichia coli (ATCC 25,922), Pseudomonas aeruginosa (ATCC 27,853), Salmonella typhi. The bacterial suspension with turbidity equal to 0.5 McFarland was prepared. Subsequently, 1:150 dilution in Mueller Hinton broth (MHB) was performed to obtain a suspension of 1 × 106 CFU/mL [24]. The fungal extracts were dissolved in 1% dimethyl sulfoxide (DMSO) and standardized to 10 mg/mL. The minimum inhibitory concentration (MIC) of the extracts was determined using a sterile 96-well microtiter plate. The microplate wells were filled with 50 μL of MHB starting from the second to the twelfth well. Then, 100 μL of fungal extract was transferred into the first well and a two-fold serial dilution was conducted until the eleventh well. After that, 50 μL of the test microorganism suspension was inoculated into each well of the microplate. A positive control; 1 mg/mL tetracycline and negative control; 1% (v/v) DMSO were prepared. The microplate was incubated at 37 °C for 24 h. The minimum bactericidal concentration (MBC) of the extract was determined by sub-culturing an aliquot of sample from wells that yielded a negative microbial growth on the surface of Mueller Hinton agar (MHA). The assay was conducted in triplicates [25].
Antioxidant properties
The DPPH radical scavenging activity of the extracts was conducted according to Rahim et al. [26] with slight modifications. In this assay, ascorbic acid was used as a standard. One milligram per millilitre of ascorbic acid and working standards of between 1000 and 3.91 μg/mL were prepared. The calibration curve of ascorbic acid was plotted. After that, 1 mg/mL of fungal extract was prepared and 400 µL of the extract was transferred into a test tube. Then, serial dilution of the extract was conducted until the concentration of 3.91 μg/mL and each test tube was at a final volume of 200 µL. Next, 800 µL of DPPH solution was added into the test tube. The mixture was shaken and left to stand in the dark for 30 min. Subsequently, the absorbance of the solution was measured at 517 nm using UV/Vis spectrophotometer (Metertech SP-803 PLUS). After that, the scavenging activity of the extract on DPPH radical was calculated using the formula (Eq. 1.0) below:
| 1 |
The DPPH radical scavenging value was expressed as IC50 value using GraphPad Prism 8. The fungal extract samples in this assay were prepared and measured in triplicates.
The FRAP assay was conducted based on the procedure of Benzie and Strain [27]. Initially, the FRAP reagent containing 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM hydrochloric acid (HCl) and 20 mM of iron (III) chloride (FeCl3.6H2O) was freshly prepared and prewarmed at 37 ºC in the ratio of 10:1:1 (v/v/v) respectively. Then, 50 µL of endophytic fungal extract (62.5–1000 µg/mL) was mixed with 1500 µL FRAP reagent in proportions of 1:30. The reaction mixture was incubated at 37 ºC for 4 min in the dark and the absorbance was recorded at 593 nm. Iron (II) sulphate (FeSO4) solution (0.02–0.10 mg/mL) and ascorbic acid (62.5–1000 µg/mL) was used a standard and positive control, respectively. The results of the assay were expressed as mg Fe (II)/g. The assay was conducted in triplicates.
Total phenolic and flavonoid contents
Total phenolic content of the extracts was measured using Folin-Ciocalteu method as described by Rahim et al. [26] with slight modifications. In this assay, gallic acid was used as a standard. One milligram per millilitre of gallic acid and working standards of between 0.02 and 0.10 mg/mL were prepared. The standard calibration curve of gallic acid was plotted. After that, 1 mg/mL of fungal extract was prepared and 100 μL of extract was transferred into a test tube. Subsequently, 0.75 mL of 10% Folin-Ciocalteu reagent was added to the test tube. The mixture was left in the dark for 5 min at room temperature. Then, 0.75 mL of 6% (w/v) sodium carbonate was added to the mixture. The mixture was left in the dark for 90 min at room temperature. After the incubation period, the absorbance was read at 725 nm using UV/Vis spectrophotometer (Metertech SP-803 PLUS). This assay was done in triplicates.
Total flavonoid content was determined using aluminium trichloride method according to Rebaya et al. [28] with slight modifications. In this assay, quercetin was used as a standard. One milligram per millilitre of quercetin and working standards of between 0.02 and 0.10 mg/mL were prepared. The standard calibration curve of quercetin was plotted. After that, 1 mg/mL of extract was prepared and 200 μL of extract was added into a test tube. Then, 75 μL of 5% sodium nitrite solution was added into the test tube. The mixture was left to stand for 6 min. Subsequently, 150 μL of 10% aluminium trichoride was added and incubated for 5 min. This was followed by the addition of 750 μL of 1 M NaOH. The final volume of the solution was adjusted to 2500 μL using distilled water and incubated in the dark for 15 min. After the incubation period, the absorbance was measured at 510 nm using UV/Vis spectrophotometer (Metertech SP-803 PLUS). The samples in this experiment were prepared and measured in triplicates.
Characterisation by gas chromatography mass spectrometry
The gas chromatography mass spectrometry (GC–MS) analysis was carried out using Sharma et al. [29] procedure with slight modifications. In this analysis, Shimadzu GC–MS-QP 2010 Plus fitted with BPX-5 (30 m × 0.25 mm × 0.25 μm) capillary column coupled with a series of mass selective detector was used. The instrument was set to an initial temperature of 50 °C which was maintained for 5 min before the oven temperature was increased to 280 °C at the rate of 10 °C/min and maintained for 10 min. Both the injection port and detector temperature were maintained as 300 °C with pressure maintained at 53.5 kPa. Helium was used as a carrier gas at a flow rate of 1 mL/min and the ionization voltage was 70 eV. Fungal crude extract was then injected in splitless mode at a volume of 1 µL. Meanwhile, the mass spectral scan range was set at 45–1000 (m/z) with a scan speed of 3333. The compound eluted were spotted using ion mass spectrophotometer and PDA detector, absorbance at nm ranging from 1 to 25 min. The identification of bioactive compounds present in the extract was conducted by comparing the mass spectra with the data from National Institute of Standards and Technology (NIST14) mass spectral libraries. The name of compound, area (%) retention time, molecular formula and weight including the structure of the endophytic fungal compounds was identified and ascertained.
Results
Identification of endophytic fungi
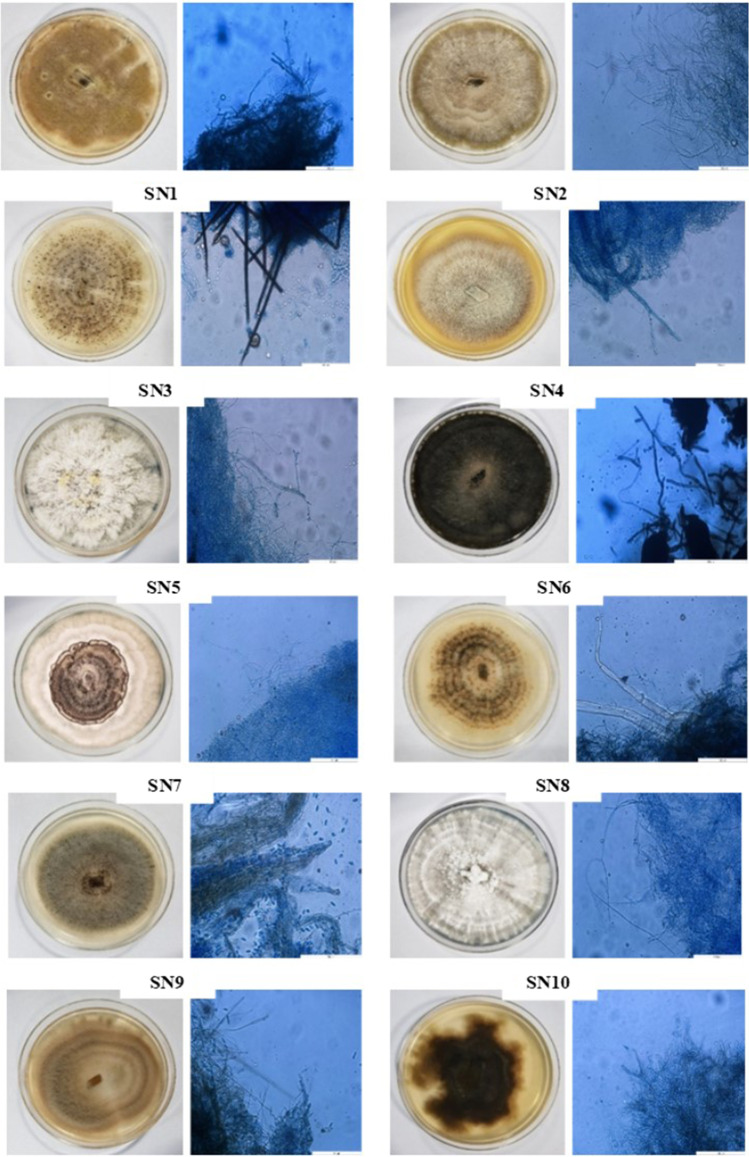
A total of 12 morphologically different endophytic fungi were successfully isolated from the leaves of G. procumbens. Each isolate was given a specific code, SN1 to SN12 for identification purposes. The morphological and microscopic views and characteristics of isolated endophytic fungal isolates were shown and explained in Table 1 and Fig. 1.
Table 1.
The morphological and microscopic characteristics of endophytic fungal isolates
| Code | Morphological characteristics | Microscopic characteristics |
|---|---|---|
| SN1 | Olive green colour colony with brown backside, filamentous form, filiform margin, flat elevation | Septate hyphae, sterile mycelia |
| SN2 | Cream colour colony with brown backside, circular form, undulate margin, flat elevation | Septate hyphae, sterile mycelia |
| SN3 | Grey colour colony with tinges of black, circular form, curled margin, flat elevation | Septate hyphae, falcate conidia |
| SN4 | Cream colour colony with brown backside, circular form, undulate margin, flat elevation | Septate hyphae, sterile mycelia |
| SN5 | White colour colony, filamentous form, filiform margin, raised elevation | Septate hyphae, sterile mycelia |
| SN6 | Black colour colony, circular form, entire margin, flat elevation | Septate hyphae, sterile mycelia |
| SN7 | White colour with black centre colony, circular form, undulate margin, flat elevation | Septate hyphae, sterile mycelia |
| SN8 | Black colour colony with tinges of orange, circular form, curled margin, flat elevation | Septate hyphae, sterile mycelia |
| SN9 | Grey colour and cottony colony, circular form, curled margin, raised elevation | Septate hyphae, cylindrical with rounded end conidia |
| SN10 | White colour colony, circular form, curled margin, flat elevation | Septate hyphae, sterile mycelia |
| SN11 | Brown colour colony, circular form, curled margin, flat elevation | Septate hyphae, cylindrical with pointed end conidia |
| SN12 | Black colour colony, irregular form, undulate margin, flat elevation | Septate hyphae, sterile mycelia |
Fig. 1.
The morphological and microscopic (magnification 400 ×) views of endophytic fungal isolates. Scale bar, 100 μm; 50 μm (e)
Based on the results of BLAST analysis, it was revealed that the endophytic fungal isolates sequences showed 95–99% sequence similarity with the relevant sequences in GenBank. All isolates were identified to belong in phylum Ascomycota, with genus Colletotrichum as the main genus found from the plant. The rDNA-ITS sequences of each isolates obtained were deposited in GenBank.
In addition, a phylogenetic tree comprising of the endophytic fungal isolates was constructed using MEGA 7.0 software. Generally, a phylogenetic tree is commonly used to represent the evolutionary relationships between organisms with common ancestors [30]. In the current study, neighbour-joining method with 1000 replicates of bootstrap values was inferred to study the evolutionary history of the isolated endophytic fungi (Fig. 2). It was noted that the evolutionary distances between fungal isolates were checked using Maximum Composite Likelihood method and the analysis involved 25 nucleotide sequences in total.
Fig. 2.
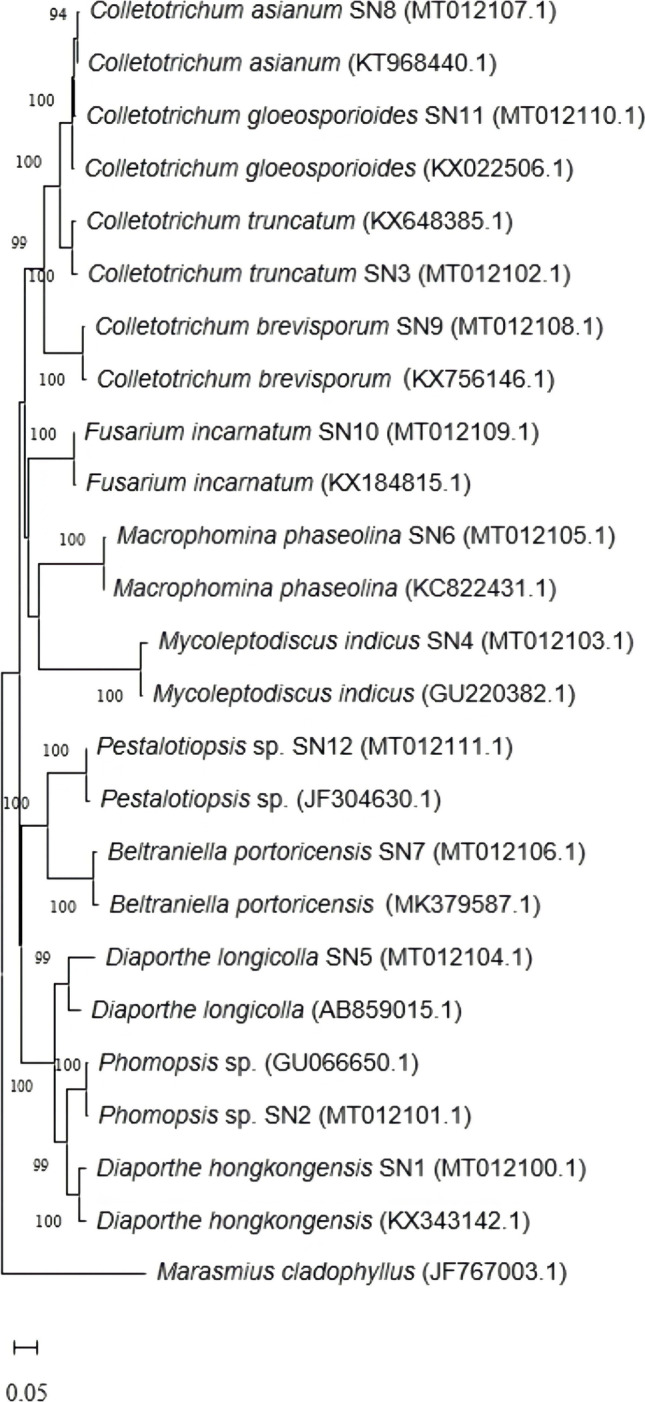
The phylogenetic tree of the endophytic fungi isolated from G. procumbens constructed using MEGA 7.0 using neighbour-joining statistical method with number of bootstrap replications of 1000
As shown in Fig. 2, the phylogenetic clustering of fungal isolates consistently matched their identification at the species level. The phylogenetic tree revealed eight clades representing eight different genera belonging to phylum Ascomycota. Furthermore, distinct clustering of between 90 and 100% similarities was also showed through the results of the phylogenetic analysis and reconstruction. A precise view of the evolutionary relationships among isolates was successfully provided with ITS rRNA genes that are known to have great features of being universally distributed, functionally constant, sufficiently conserved, and of adequate length [31].
Antimicrobial activity by broth microdilution assay
As summarised in Table 2, both ethyl acetate and methanolic extracts of each fungal isolate were exhibiting antibacterial activity against at least on two pathogenic microorganisms. The ethyl acetate extracts indicated a broad-spectrum of inhibitory potential against the microorganisms. For instance, the ethyl acetate extracts of M. phaseolina SN6 and C. gloeosporioides SN11 showed antibacterial activity against all pathogenic bacteria with the MIC and MBC of 5000 µg/mL. Whereas Phomopsis sp. SN2 (MIC, MBC: 5000 µg/mL) showed its antibacterial potential against all bacteria except for S. typhi. In contrary, the methanolic extracts of M. phaseolina SN6 (MIC, MBC: 5000 µg/mL) were found to be only inhibiting the activity of S. aureus, MRSA and P. aeruginosa. While M. indicus SN 4 was also observed to be showing activity against S. aureus (MIC: 5000 µg/mL), MRSA (MIC: 5000 µg/mL) and P. aeruginosa (MIC, MBC: 5000 µg/mL). Lastly, D. hongkongensis SN1 (MIC: 5000 µg/mL) exhibited the least spectrum of antibacterial activity where it only inhibits two types of bacteria which were S. aureus and MRSA.
Table 2.
The MIC and MBC of ethyl acetate and methanolic extracts of endophytic fungal isolates
| Endophytic fungal isolates | Test microorganisms | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | MRSA | P. aeruginosa | E. coli | S. typhi | ||||||
| MIC1 | MBC1 | MIC1 | MBC1 | MIC1 | MBC1 | MIC1 | MBC1 | MIC1 | MBC1 | |
| Tetracycline | 62.5 | 62.5 | 62.5 | 62.5 | 62.5 | 250 | 62.5 | 62.5 | 62.5 | 250 |
| Phomopsis sp.* | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | - | - |
| M. phaseolina* | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 |
| C. gloeosporioides* | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 |
| D. hongkongensis** | 5000 | - | 5000 | - | - | - | - | - | - | - |
| M. indicus** | 5000 | - | 5000 | - | 5000 | 5000 | - | - | - | - |
| M. phaseolina** | 5000 | 5000 | 5000 | 5000 | 5000 | 5000 | - | - | - | - |
*, ethyl acetate extracts; **, methanol extracts; 1 Data are presented in µg/mL (n = 3); -, no activity observed
Antioxidant activities of endophytic fungal extracts
The scavenging activity percentage of ethyl acetate and methanolic endophytic fungal extracts from G. procumbens and a positive control, ascorbic acid against DPPH radical. The highest scavenging activity for ethyl acetate extracts was observed from M. phaseolina SN6 (86.60 ± 3.20%), followed by C. gloeosporioides SN11 (78.71 ± 15.91%) and Phomopsis sp. SN2 (68.68 ± 9.63%). Whereas the scavenging activity of methanolic extracts decreased in the following order: M. indicus SN4 (49.95 ± 11.58%) > M. phaseolina SN6 (21.08 ± 3.08%) > D. hongkongensis SN1 (20.37 ± 11.46%). The positive control, ascorbic acid exhibited strong antioxidant activity with the percentage of radical reduction of 95.63 ± 0.20%.
Table 3 presents the IC50 values observed from the ethyl acetate extracts of all the 12 endophytic fungal isolates from G. procumbens together with the positive control, ascorbic acid. A lower IC50 value corresponds to higher radical scavenging activity and potent antioxidant activity. According to the Table 4, the highest antioxidant activity was shown by M. phaseolina SN6 (104.25 ± 18.51 µg/mL), followed by Phomopsis sp. SN2 (250.43 ± 7.15 µg/mL) and C. gloeosporioides SN11 (309.07 ± 179.08 µg/mL). It was noted that the positive control (16.34 ± 2.06 µg/mL) used, ascorbic acid exhibits powerful antioxidant potential and superior than the extracts studied. The IC50 values for methanolic fungal extracts were not included as the their scavenging activity percentage did not reach 50%.
Table 3.
The IC50 DPPH and IC50 FRAP of ethyl acetate extracts of all the twelve endophytic fungal isolates together with the positive control, ascorbic acid
| Probable fungal species | IC50 DPPH (µg/mL) |
IC50 FRAP FE (µg/mL) |
|---|---|---|
| Diaporthe hongkongensis SN1 | 983.89 ± 542.35ab | 143.11 ± 156.60a |
| Phomopsis sp. SN2 | 250.43 ± 7.15b | 45.23 ± 6.97a |
| Colletotrichum truncatum SN3 | 2437.40 ± 207.44ab | 3208.23 ± 283.00a |
| Mycoleptodiscus indicus SN4 | 895.67 ± 655.74ab | 540.94 ± 386.27a |
| Diaporthe longicolla SN5 | 1759.35 ± 823.92ab | 1031.68 ± 547.70a |
| Macrophomina phaseolina SN6 | 104.25 ± 18.51b | 222.15 ± 49.90a |
| Beltraniella portoricensis SN7 | 2078.39 ± 519.03ab | 1739.09 ± 49.43a |
| Colletotrichum asianum SN8 | 849.45 ± 156.85ac | 493.20 ± 78.89a |
| Colletotrichum brevisporum SN9 | 1214.49 ± 611.96ab | 741.99 ± 306.13a |
| Fusarium incarnatum SN10 | 3400.05 ± 490.06ab | 3871.51 ± 1016.73a |
| Colletotrichum gloeosporioides SN11 | 309.07 ± 179.08ab | 376.51 ± 259.21a |
| Pestalotiopsis sp. SN12 | 2038.28 ± 439.30ab | 1745.27 ± 51.37a |
| Ascorbic acid (control) | 16.34 ± 2.06 | 87.28 ± 25.37 |
Values are mean ± standard deviation (n = 3) followed by superscript letters in each column. Different superscript letters indicate significant difference at p < 0.05
Table 4.
Antimicrobial and antioxidant activities, total phenolic and flavonoid contents of the selected endophytic fungi isolated from G. procumbens
| Extract/ standard compound | Antimicrobial activity | Antioxidant activity | Total phenolic content (mg GAE/g) |
Total flavonoid content (mg QCE/g) |
||
|---|---|---|---|---|---|---|
| Minimum inhibitory concentration (MIC) (μg/mL) | Minimum bactericidal concentration (MBC) (μg/mL) | DPPH (IC50 µg/mL) |
FRAP (IC50 Fe µg/mL) |
|||
| Ascorbic acid | ND | ND | 16.34 ± 2.06 | 87.28 ± 25.37 | ND | ND |
| Diaporthe hongkongensis SN1 | 2500 -5000 | 5000 | 983.89 ± 542.35 | 143.11 ± 156.60 | 15.70 ± 7.10 | 35.50 ± 10.00 |
| Phomopsis sp. SN2 | 5000 | 5000 | 250.43 ± 7.15 | 45.23 ± 6.97 | 78.70 ± 13.60 | 60.10 ± 12.30 |
| Mycoleptodiscus indicus SN4 | 2500 | 2500—5000 | 895.67 ± 655.74 | 540.94 ± 386.27 | 85.30 ± 16.00 | 78.60 ± 11.90 |
| Macrophomina phaseolina SN6 | 2500 – 5000 | 5000 | 104.25 ± 18.51 | 222.15 ± 49.90 | 55.00 ± 5.60 | 122.80 ± 11.00 |
| Colletotrichum gloeosporioides SN11 | 5000 | 5000 | 309.07 ± 179.08 | 376.51 ± 259.21 | 87.00 ± 14.80 | 62.80 ± 10.00 |
The ethyl acetate and methanolic fungal extracts isolated from G. procumbens displayed antioxidant activity through their reducing capacity. Both extracts showed similar increasing trend in activity with increase in extract concentration. In the assay, the highest reduction potential at 239.9 ± 66.2 mg Fe (II)/g was observed from M. phaseolina SN6 for ethyl acetate extracts. It was followed by C. gloeosporioides SN11 (172.7 ± 91.8 mg Fe (II)/g) and Phomopsis sp. SN2 (123.2 ± 21.3 mg Fe (II)/g) as the isolate with the lowest reduction potential. In contrast, for methanolic extracts, the highest antioxidant activity was shown by M. indicus SN4 (44.7 ± 7.7 mg Fe (II)/g), followed by M. phaseolina SN6 (28.5 ± 8.6 mg Fe (II)/g) and the lowest antioxidant activity was exhibited by D. hongkongensis SN1 (23.5 ± 11.6 mg Fe (II)/g).
Total phenolic and flavonoid contents of endophytic fungi extracts
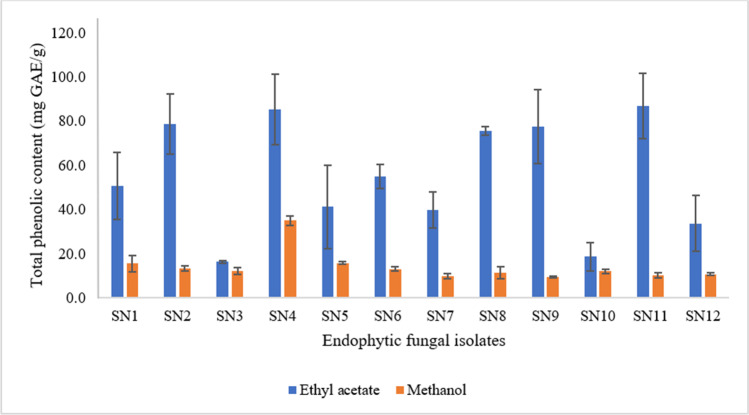
The total phenolic content (TPC) and total flavonoid content (TFC) found in the two different extracts of all 12 isolated fungal endophytes of G. procumbens are shown in Fig. 3 and Fig. 4. Among the 12 fungal isolates, the ethyl acetate extracts of C. gloeosporioides SN11 were found to contain the highest phenolic content with a total of 87.0 ± 14.8 mg GAE/g. Comparatively similar, 85.3 ± 16.0 mg GAE/g of phenolic content was also found in the ethyl acetate extract of M. indicus SN4. The phenolic content in the methanolic extract of M. indicus SN4 was also the highest (35.0 ± 2.2 mg GAE/g) among the methanolic extract of the 12 isolates. The methanolic extract of C. brevisporum SN9 on the other hand contained the lowest amount of phenolic content (9.5 ± 0.4 mg GAE/g).
Fig. 3.
The total phenolic content (TPC) of ethyl acetate and methanolic extracts of the twelve endophytic fungal isolates
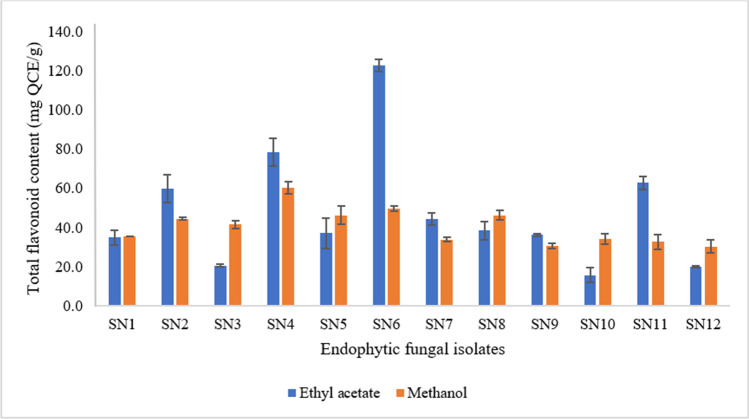
Fig. 4.
The total flavonoid content (TFC) of ethyl acetate and methanolic extracts of the twelve endophytic fungal isolates
The highest flavonoid content (Fig. 4) was found in the ethyl acetate extract of M. phaseolina SN6 (122.8 ± 11.0 mg QCE/g), followed by M. indicus SN4 (78.6 ± 11.9 mg QCE/g) and C. gloeosporioides SN11 (62.8 ± 10.0 mg QCE/g). The lowest TFC was observed in the ethyl acetate extract from F. incarnatum SN10 (15.8 ± 6.4 mg QCE/g).
Table 4 summarised the overall antimicrobial and antioxidant activities, total phenolic and flavonoid contents of the selected endophytic fungi isolated from G. procumbens. It was observed that the endophytic fungi ethyl acetate extracts were able to provide a broad-spectrum inhibitory potential and exhibit antibacterial activity with three fungal extracts were showing the ability to inhibit all pathogenic microorganisms tested. The fungal extracts were Diaporthe hongkongensis SN1, Macrophomina phaseolina SN6, and Colletotrichum gloeosporioides SN11. Macrophomina phaseolina SN6 showed the highest anti-oxidative activity (IC50 of 104.25 ± 18.51 μg/mL) in the DPPH assay and a high FRAP value (239.90 ± 66.20 μg/mg) (Table 5). The ethyl acetate of the fungal extract contained both phenolics and flavonoids in concentrations of 55.00 ± 5.60 mg GAE/g and 122.80 ± 11.00 mg QCE/g, respectively. These phenol and flavonoid chemical contents might play roles in the antimicrobial and anti-oxidative activities of the fungal extract, M. phaseolina SN6.
Table 5.
Tentative identification on the identified bioactive compounds of endophytic fungal extracts from G. procumbens
| Name of compound | Area (%) | Retention time | Molecular formula | Molecular weight | Mass over charge m/z |
Biological properties | References |
|---|---|---|---|---|---|---|---|
| Isoelemicin | 50.84 | 32.535 | C12H16O3 | 208.110 | 208 | Antioxidant, antibacterial | Torbati et al. 2014 |
| Terpinel-4-ol | 21.46 | 13.937 | C10H18O | 154.136 | 154 | Antimicrobial, anticancer, antioxidant | Shapira et al. 2016, Khaleel et al. 2018 |
| Eucalyptol | 24.26 | 11.173 | C10H18O | 154.136 | 154 | Anti-inflammatory, antioxidant, antimicrobial, antitumorigenic | Bhowal & Gopal, 2015, Seol & Kim, 2016 |
| Oleic acid | 19.75 | 24.979 | C18H34O2 | 282.256 | 282 | Antibacterial, antioxidant |
Abubakar & Majinda, 2016 Wei et al. 2016 |
| β-pinene | 10.88 | 9.826 | C10H16 | 136.125 | 136 | Antimicrobial | Ghaffari et al. 2019 |
| γ-terpinene | 10.18 | 11.621 | C10H16 | 136.125 | 136 | Antimicrobial, antioxidant, | Giweli et al., 2012 |
| 4-carene | 6.65 | 10.666 | C10H16 | 136.124 | 136 | Antioxidant, antimicrobial, cytoprotective | Smeriglio et al., 2017 |
| Albuterol | 5.32 | 34.856 | C13H21NO3 | 239.152 | 239 | Anti-inflammatory, antioxidant | Uzkeser et al., 2012 |
| Octadecanoic acid | 3.45 | 23.305 | C18H36O2 | 284.271 | 284 | Antimicrobial | Abubakar & Majinda, 2016 |
| Caryophyllene | 2.52 | 17.266 | C15H24 | 204.187 | 204 | Anticancer, analgesic | Fidyt et al., 2016 |
| Aromadendrene | 1.89 | 17.534 | C15H24 | 204.188 | 204 | Analgesic, antinociceptive, antimicrobial | Sadiq et al., 2018, Boiteux et al., 2018 |
| Globulol | 1.48 | 19.485 | C15H26O | 222.198 | 222 | Antimicrobial | Tan et al., 2008 |
Characterisation of bioactive compounds by gas chromatography mass spectrometry analysis
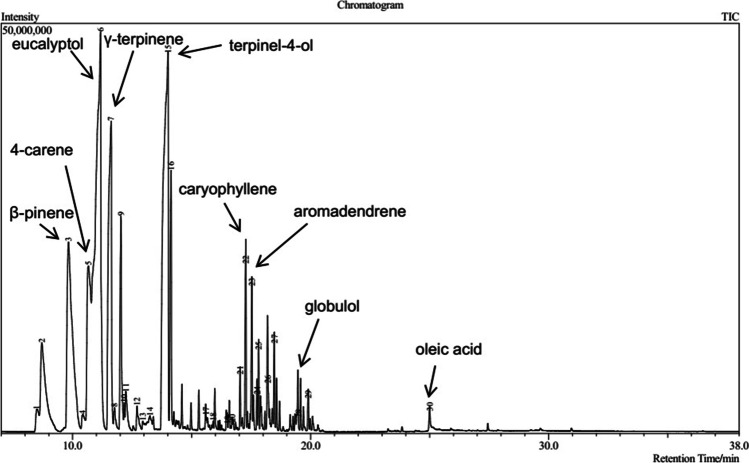
The bioactive compounds of ethyl acetate and methanolic extracts of the endophytic fungal isolates from G. procumbens were identified using GC–MS analysis. The GC–MS chromatogram of one of the methanolic extract which was D. hongkongensis SN1 is presented in Fig. 5.
Fig. 5.
The GC–MS chromatogram from the methanolic extract of D. hongkongensis SN1
Based on the data collected from the chromatograms and spectra of mass spectrometry, 12 bioactive compounds in the ethyl acetate and methanolic extracts of the endophytic fungal isolates were identified. The area (%), retention time, molecular formula and biological properties of the identified bioactive compounds are summarised in Table 5. The extracts consist mainly of isoelemicin (50.84%), terpinel-4-ol (21.46%), eucalyptol (24.26%), oleic acid (19.75%), β-pinene (10.88%), γ-terpinene (10.18%) and few other minor compounds such as 4-carene (6.65%), albuterol (5.32%), octadecanoic acid (3.45%), caryophyllene (2.52%), aromadendrene (1.89%) and globulol (1.48%).
Discussion
Endophytic fungi are a good source for identifying novel bioactive products with potentially beneficial biological properties. The plant tissues of leaves are excellent reservoirs of endophytic fungi [32]. Most of the recovered endophytic fungi commonly belong to phylum Ascomycota and are frequently reported as endophytes of tropical plants [19, 33]. Most of the genera including Diaporthe, Colletotrichum and Macrophomina are common as plant pathogens though non-pathogenic forms of these also exist. Several reports stated that endophytes remain latent and symptomless inside the host plant only until the environmental conditions are favourable for them to turn into aggressive saprophytes or opportunistic pathogens [17, 18, 23, 30–32]. The current endophytic fungi were isolated from a healthy symptomless plant after thorough surface sterilization which confirmed the isolated fungi were in endophytic form during the isolation period [32]. Besides that, many endophytic fungi did not produce spores on PDA media. Thus, molecular identification based on rDNA sequencing was employed to identify species that could not be categorized based on their morphological and microscopic characteristics [34]. The endophytic fungal species obtained herein were consistent with the commonly isolated species from other medicinal plants documented by previous studies [17, 18, 23, 32]. Furthermore, to the best of our knowledge, this is the first report of endophytic fungi isolated from G. procumbens.
The broth microdilution assay is one of the most basic antimicrobial susceptibility testing methods and commonly used in the determination of antibacterial activity. This method is simple, reliable and requires only small number of reagents and space [24]. Furthermore, by using this method, it allows the determination of MIC and MBC of the tested samples. Basically, the MIC is the lowest concentration of an antimicrobial agent that completely inhibits growth of the tested pathogenic microorganism as observed by naked eyes [24].
The MBC is defined as the lowest concentration of an antimicrobial agent at which 99.9% of the final inoculum is completely inhibited [25]. The MBC can be determined by sub-culturing a sample from wells yielding a negative microbial growth after incubation on the surface of non-selective agar [24]. Based on our results, there were varying degrees of antibacterial potency displayed by the fungal extracts which could be in consequence to the number and concentration of the bioactive compounds they contain. Thus, the extracts with high antibacterial potential could be affirmed to probably contain several bioactive compounds or contain high concentration of the available compounds. Other endophytic fungal extracts which showed low or no antibacterial potency may have bioactive compounds that are most probably in smaller amounts [18]. In addition, the reason for the different sensitivity between Gram-positive and Gram-negative bacteria could be ascribed to the morphological differences between these microorganisms. Gram-negative bacteria have an outer polysaccharide membrane which carries the structural lipopolysaccharide components. This makes the cell wall impermeable to lipophilic solutes. In contrary, Gram-positive should be more susceptible having only an outer peptidoglycan layer which is not an effective permeability barrier [35].
In the determination of antioxidant activity, the DPPH assay has been extensively used to evaluate antioxidant activity in vitro. It is a robust, simple, rapid, and inexpensive method that is sufficiently sensitive in detecting activity even at low concentrations [36, 37]. This assay involves the use of the free radical, DPPH that is widely used to test the potential of a sample to act as free radical scavenger or hydrogen donor [37]. Basically, when an antioxidant reacts with DPPH, it becomes paired off in the presence of a hydrogen donor and is reduced to DPPH-H form which results in the decrease of absorbance and decolourization of the solution from yellow to purple colour with respect to the number of electrons captured [37]. The increase in the curves of the graph of DPPH assay illustrates that more concentrated solutions of the compounds have greater radical scavenging activity [36]. The values obtained and calculated into scavenging activity percentage have enabled the curves to be traced into an exponential shape with the presence of a stationary phase which means the almost total reduction of DPPH in its non-radical form [38]. Furthermore, the IC50 value, defined as the concentration of antioxidant required for 50% reduction of DPPH radicals, is a parameter commonly used to measure the antioxidant potency of a particular extract [37].
The FRAP assay is generally recognized as a validated clinical method for calibrating antioxidant potential of plasma. The application of this assay was eventually extended to biological systems such as plant, foods, biological fluids, plant extracts and now fungal extracts [39]. It is a simple, rapid, reliable, and inexpensive direct method of measuring the total antioxidant activity of reductive antioxidants in a sample [40]. In this discolouration assay, ferric ions are reduced to ferrous ions in the presence of an antioxidant which cause a colour change to a blue coloured TPTZ-Fe (II) complex [35]. The DPPH and FRAP assays are both electrons transfer based assays; however, both assays employ different chromogenic redox reagents with different standard potentials. Basically, the DPPH assay acts by radical reduction, uses preformed radicals and determines the decrease in absorbance, while, the FRAP assay measures the formed ferrous ions and determines the increase in absorbance [5]. In addition, FRAP assay primarily measured only the hydrophilic antioxidants present in a sample, while DPPH assay is more sensitive to antioxidants that are soluble in organic solvents especially alcohols [5].
According to our results, there were significant differences in the scavenging activity and IC50 values of the extracts. Rahim et al. [26] explained that the variations might be influenced by the intrinsic characteristics of fungal bioactive compounds and application of different extracting solvents. The extract with higher scavenging activity might be due to the presence of higher amount of hydrogen donating components extracted from the fungal isolate. The various extracts may contain active substances including phenolic compounds that had a high hydrogen-donating capacity to scavenge DPPH radicals as possible mechanism for their antioxidant activities [36]. This also presumably indicates the presence of a higher content of protic flavonoids in the extract, facilitating hydrogen atom transfer to take place [35]. Moreover, the yield and antioxidant activities of extracts generally rely on the nature of the extracting solvent due to the presence of distinct antioxidant compounds with different chemical characteristics and polarities [39]. In the case of FRAP assay, similar results as the DPPH assay were observed and this revealed that the various extracts do possess hydrogen-donating capacity which subsequently facilitate the reducing power of the extracts [40].
The phenolic and flavonoid content of the fungal extracts were determined in our study. In TPC assay, Folin-Ciocalteu reagent, a mixture of phosphotungstic (H3PW12O40) and phosphomolybdic (H3PMo12O40) acids is reduced to blue oxides of tungstene (W8O23) and molybdene (Mo8O23) during phenol oxidation [39]. Under basic reaction, a phenol loses an H+ ion to produce a phenolate ion which reduces Folic-Ciocalteu reagent [39]. The phenolic compounds constitute one of the major groups of compounds acting as primary antioxidants or free radical terminators. This is due to their redox properties which play an important role in adsorbing and neutralising free radicals, quenching singlet or triplet oxygen, decomposing peroxides and acts as reducing agents and hydrogen donors [40, 41]. Flavonoids, on the other hand, are the most common and widely distributed group of phenolic compounds which usually are effective antioxidants [40, 41]. The basis of TFC assay is the fact that aluminium ion (Al3+) forms complexes with C-4 keto and either C-3 or C-5 hydroxyl, or with ortho hydroxyl groups in the A or B ring [39]. Flavonoids are low molecular weight polyphenolic compound having common benzopyrone structure and showing enormous biological and pharmacological activities in biological systems [40].
Furthermore, flavonoid compounds provide fungi with natural security against free radicals, metal chelators and pro-oxidants by acting as reductant, radical scavenger, and metallic ion chelator [41]. Besides that, Gonzalez-Palma et al. [42] stated that the phytochemicals content depends on the type of sample, solvent and temperature used for extraction. The temperature of more than 50 °C may not be suitable for extracting the desired compounds. Moreover, exposing the samples to direct sunlight or relevant high temperature may cause some phenolic compounds to degrade rapidly [37]. According to Rahim et al. [26], different extracting solvents influenced different yields of phytochemicals content. These findings are comparable to previous reports stating higher yields of phenols and flavonoids when using semi-polar to polar solvents [36]. Ethyl acetate extraction is the most efficient method of isolating fungal bioactive compounds as it selectively extracts low molecular weight phenols and high molecular weight polyphenols [19, 39]. It was also reported that ethyl acetate allowed the highest phenolic content and selective removal of non-phenolic compounds [43]. Besides, as phenolics including many flavonoids contain polar phenolic hydroxyl groups, their high extraction into methanol is also reasonable [39].
On top of that, TPC and TFC assays were stated to give a crude estimate of the phenolic and flavonoid present in an extract, whereas the antioxidant assays are not only specific to these two phytochemicals. Besides, various phenolic compounds respond differently in antioxidant assay depending on the number of phenolic groups they have. It was further suggested that negative correlation between the phytochemicals content and antioxidant activity may be due to the phytochemicals content that does not incorporate necessarily all the antioxidants that may be present in an extract. These may explain the negative correlation between the phytochemicals content and antioxidant assay observed in present study [44].
The GC–MS is mainly used for the separation and analysis of multi component mixtures such as essential oils, hydrocarbons and solvents [45]. It is commonly applied for the separation of complex mixtures with low polarity or volatile substances [46]. Besides, it is widely used for quantitative and qualitative analysis of mixtures, purification of compounds and determination of such thermo chemical constants. Intrinsically, with the use of the high sensitivity flame ionization detector and the electron capture detector, gas chromatography can quantitatively determine materials present at very low concentrations [45]. The increased fragmentation afforded by GC–MS allows for the identification of unknowns by searching a mass spectral database [46]. In the current study, various terpenoids, terpenes, monoterpenes, sesquiterpenes, alcohols, fatty acids and derivatives were detected in the endophytic fungal extracts. Based to the GC–MS results, the strong antioxidant activity and the presence of antibacterial inhibition activity against pathogenic microorganisms of the ethyl acetate fungal extracts are most probably due to its richness of active compounds which possess antioxidant and antibacterial effects.
Conclusion
In conclusion, these findings showed that the leaves of G. procumbens harbours various morphologically distinct endophytic fungi that are equipped with potentially beneficial biological properties and bioactive compounds. Based on the results and discussion, the ethyl acetate extracts of endophytic fungal isolates were observed to be exhibiting potent antioxidant activity and able to inhibit several bacterial pathogenic microorganisms such as S. aureus, MRSA, P. aeruginosa, E. coli and S. typhi. Furthermore, the endophytic fungal extracts possessed high content of phenolic and flavonoid and produced various bioactive compounds that were documented to be equipped with beneficial biological properties such as antioxidant, antimicrobial, anticancer, antifungal, anti-inflammatory and so forth. Among all extracts, the ethyl acetate extract of M. phaseolina was found to have high content of phenolic and flavonoid, exhibiting strong antioxidant activity, able to inhibit pathogenic microorganisms and contain beneficial bioactive compounds with varying biological property. This result of ethyl acetate extract of M. phaseolina has been proven and found to be similar with the result documented by Chowdary and Kaushik [47]. However, further studies on the purified endophytic fungal extracts will need to be conducted in order to ascertain fully its bioactivity potentials and toxicity effects. Therefore, these findings are hoped to be of great significance with beneficial insights which would contribute for the betterment and further applications in various industries especially pharmaceutical and medicinal industries.
Acknowledgements
The authors are thankful to Universiti Malaysia Sarawak and Tun Zaidi Research Chair (TZC), Universiti Malaysia Sarawak for providing the laboratory facilities and financial support during this research investigation.
Author contribution
Haifa Arghnia A. Jamal, Ahmad Husaini and Ngieng Ngui Sing brought up the idea, performed the literature search and data analysis and drafted the manuscript. Hairul Azman Roslan, Azham Zulkharnain and Wahab Abideen Akinkunmi proofread and critically revised the manuscript. All authors read and approved the final manuscript.
Funding
The research project was funded under the Tun Zaidi Chair Research Grant Scheme (TZC) (F07(ZRC04)/1237/2015(01)).
Data availability
All data generated or analysed during this study are included in this published article.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Luiz Henrique Rosa
The original online version of this article was revised: Due to wrong affiliation of Ahmad Husaini
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/11/2022
Affiliation of Ahmad Husaini has been corrected.
Change history
11/15/2022
A Correction to this paper has been published: 10.1007/s42770-022-00873-4
Contributor Information
Haifa Arghnia A. Jamal, Email: haifaarghnia@gmail.com
Ahmad Husaini, Email: haahmad@unimas.my.
Ngieng Ngui Sing, Email: snngui@unimas.my.
Hairul Azman Roslan, Email: rhairul@unimas.my.
Azham Zulkharnain, Email: azham@shibaura-it.ac.jp.
Wahab Abideen Akinkunmi, Email: abideen.wahab@uniosun.edu.ng.
References
- 1.Bhardwaj A, Agrawal P. A review of fungal endophytes: as a store house of bioactive compound. World J Pharmacy and Pharm Sci. 2014;3(9):228–237. [Google Scholar]
- 2.Venieraki A, Dimou M, Katinakis P. Endophytic fungi residing in medicinal plants have the ability to produce the same or similar pharmacologically active secondary metabolites as their host. Hellenic Plant Protection J. 2017;10:51–56. doi: 10.1515/hppj-2017-0006. [DOI] [Google Scholar]
- 3.Chen L, Zhang QY, Jia M, Ming QL, Yue W, Rahman K, Qin LP, Han T. Endophytic fungi with antitumor activities: their occurrence and anticancer compounds. Crit Rev Microbiol. 2016;42(3):454–473. doi: 10.3109/1040841X.2014.959892. [DOI] [PubMed] [Google Scholar]
- 4.Yap LS, Lee WL, Ting ASY (2017) Endophytes from Malaysian medicinal plants as sources for discovering anticancer agents. Medicinal Plants and Fungi: Recent Advances in Research and Development, Medicinal and Aromatic Plants of the World Volume 4, Singapore: Springer Nature Singapore Pte Ltd.
- 5.Bhagobaty RK, Joshi SR. Enzymatic activity of fungi endophytic on five medicinal plant species of the pristine sacred forests of Meghalaya, India. Biotech Bioprocess Eng. 2012;17:33–40. doi: 10.1007/s12257-011-0453-4. [DOI] [Google Scholar]
- 6.Kuralarasi R, Lingakumar K. Isolation and antibacterial activity of endophytic fungi from Madhuca longifolia bark. J Medicinal Plants Stud. 2018;6(1):36–39. [Google Scholar]
- 7.Manganyi MC, Regnier T, Tchatchouang CDK, Bezuidenhout CC, Ateba CN. Antibacterial activity of endophytic fungi isolated from Sceletium tortuosum L (Kougoed) Annals of Microbiology. 2019;69(6):659–663. doi: 10.1007/s13213-019-1444-5. [DOI] [Google Scholar]
- 8.Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, Qin LP. A friendly relationship between endophytic fungi and medicinal plants. Front Microbiol. 2016;7:1–14. doi: 10.3389/fmicb.2016.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan X, Zhou Y, Zhou X, Xia X, Wei Y, He L, Tang H, Yu L. Diversity and bioactive potential of culturable fungal endophytes of Dysosma versipellis; a rare medicinal plant endemic to China. Sci Rep. 2018;8(5929):1–9. doi: 10.1038/s41598-018-24313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nongkhlaw FMW, Joshi SR. Investigation on the bioactivity of culturable endophytic and epiphytic bacteria associated with ethnomedicinal plants. The Journal of Infection in Developing Countries. 2015;9(9):954–961. doi: 10.3855/jidc.4967. [DOI] [PubMed] [Google Scholar]
- 11.Tan HL, Chan KG, Pusparajah P, Lee LH, Goh BH. Gynura procumbens: an overview of the biological activities. Front Pharmacol. 2016;7(52):1–14. doi: 10.3389/fphar.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mou KM, Dash PR. A comprehensive review on Gynura procumbens leaves. Int J Pharmacogn. 2016;3(4):167–174. [Google Scholar]
- 13.Rahman AFMM, Asad MSA. Chemical and biological investigations of the leaves of Gynura procumbens. Int J Biosci. 2013;3(4):36–43. [Google Scholar]
- 14.Afandi A, Zulkiffli MH, Sadikun A, Ismail S. Antioxidant properties of Gynura procumbens extracts and their inhibitory effects on two major human recombinant cytochrome P450s using a high throughput luminescence assay. Asian J Pharm Clin Res. 2014;7(5):36–41. [Google Scholar]
- 15.Kaewseejan N, Puangpronpitag D, Nakornriab M. Evaluation of phytochemical composition and antibacterial property of Gynura procumbens extract. Asian J Plant Sci. 2012;11(2):77–82. doi: 10.3923/ajps.2012.77.82. [DOI] [Google Scholar]
- 16.Yadav R, Singh AV, Joshi S, Kumar M. Antifungal and enzyme activity of endophytic fungi isolated from Ocimum sanctum and Aloe Vera. African J Microbiol Res. 2015;9(29):1783–1788. doi: 10.5897/AJMR2015.7451. [DOI] [Google Scholar]
- 17.Bezerra JDP, Nascimento CCF, Barbosa RN, Silva DCV, Svedese VM, Silva-Nogueira EB, Gomes BS, Paiva LM, Souza-Motta CM. Endophytic fungi from medicinal plant Bauhinia forficata: diversity and biotechnological potential. Braz J Microbiol. 2015;46(1):49–57. doi: 10.1590/S1517-838246120130657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolulope RA, Adeyemi AI, Erute MA, Abiodun TS. Isolation and screening of endophytic fungi from three plants used in traditional medicine in Nigeria for antimicrobial activity. International Journal of Green Pharmacy. 2015;9(1):58–62. doi: 10.4103/0973-8258.150929. [DOI] [Google Scholar]
- 19.Bhardwaj A, Sharma D, Jadon N, Agrawal PK. Antimicrobial and phytochemical screening of endophytic fungi isolated from spikes of Pinus roxburghii. Arch Clin Microbiol. 2015;6(3):1–9. [Google Scholar]
- 20.Deepthi VC, Sumathi S, Faisal M, Elyas KK. Isolation and identification of endophytic fungi with antimicrobial activities from the leaves of Elaeocarpus sphaericus (Gaertn) K Schum and Myristica fragrans Houtt. Int J Pharma Sci and Res. 2018;9(7):2783–2791. [Google Scholar]
- 21.Cubero OF, Crespo A, Fatehi J, Bridge PD. DNA extraction and PCR amplification method suitable for fresh, herbarium-stored, lichenized and other fungi. Plant Syst Evol. 1999;216:243–249. doi: 10.1007/BF01084401. [DOI] [Google Scholar]
- 22.Yenn TW, Ngim AS, Ibrahim D, Zakaria L. Antimicrobial activity of Penicillium minioluteum ED24, an endophytic fungus residing in Orthosiphon stamineus Benth. World J Pharm Pharmaceutical Sci. 2014;3(3):121–132. [Google Scholar]
- 23.Selvi K, Balagengatharathilagam P. Isolation and screening of endophytic fungi from medicinal plants of Virudhunagar district for antimicrobial activity. Int J Sci Nature. 2014;5(1):147–155. [Google Scholar]
- 24.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Analysis. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bastos EGP, Aguiar AA, Oliveira AIT, Silva JFM, Pimenta RS. Antimicrobial evaluation of endophytic fungi extracts isolated from Casearia sylvestris. J Med Plants Res. 2017;11(43):683–689. doi: 10.5897/JMPR2017.6510. [DOI] [Google Scholar]
- 26.Rahim KK, Almey AAA, Khan AJ, Zahir SI, Suleiman MK, Aisyah MR. Total phenolic content and antioxidant activity of methanolic and ethanolic extracts of aromatic plants’ leaves. Int Food Res J. 2010;17:1077–1084. [Google Scholar]
- 27.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Chem. 1996;239(292):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 28.Rebaya A, Belghith SI, Baghdikian B, Leddet VM, Mabrouki M, Olivier E, Cherif JK, Ayadi MT. Total phenolic, total flavonoid, tannin content, and antioxidant activity of Halimium halimifolium (Cistaceae) J Appl Pharma Sci. 2014;5(1):52–57. [Google Scholar]
- 29.Sharma D, Pramanik A, Agrawal PK. Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D. Don Biotech. 2016;6(210):1–14. doi: 10.1007/s13205-016-0518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaithra M, Vanitha S, Ramanathan A, Jegadeeshwari V, Rajesh V, Hegde V, Apshara E. Morphological and molecular characterization of endophytic fungi associated with cocoa (Theobroma cacao L) in India. Current J Appl Sci Tech. 2020;38(6):1–8. doi: 10.9734/cjast/2019/v38i630447. [DOI] [Google Scholar]
- 31.Alsohaili SA, Bani-Hasan BM (2018) Morphological and molecular identification of fungi isolated from different environmental sources in the northern eastern desert of Jordan. Jordan Journal of Biological Sciences 11(3).
- 32.Jariwala M, Desai B. Isolation and identification of endophytic fungi from various medicinal plants. BMR Microbiology. 2018;4(1):1–7. [Google Scholar]
- 33.Chagas MBDO, Santos IPD, Silva LCND, Correia MTDS, Araújo JMD, Cavalcanti MDS, Lima VLDM. Antimicrobial activity of cultivable endophytic fungi associated with Hancornia speciosa Gomes Bark. The Open Microbiol J. 2017;11:179–188. doi: 10.2174/1874285801711010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J, Wu Y, He Z, Li M, Zhu K, Gao B. Diversity and antifungal activity of endophytic fungi associated with Camellia oleifera. Microbiol. 2018;46(2):85–91. doi: 10.1080/12298093.2018.1454008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadrati N, Daoud H, Zerroug A, Dahamna S, Bouharati S. Screening of antimicrobial and antioxidant secondary metabolites from endophytic fungi isolated from wheat (Triticum durum) J Plant Protection Res. 2013;53(2):128–136. doi: 10.2478/jppr-2013-0019. [DOI] [Google Scholar]
- 36.Liu X, Jia J, Jing X, Li G. Antioxidant activities of extracts from sarcocarp of Cotoneaster multiflorus. J Chem. 2018;2018:1–7. [Google Scholar]
- 37.Shekhar TC, Anju G. Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn leaves. American J Ethnomedicine. 2014;1(4):244–249. [Google Scholar]
- 38.Sofiane I, Ratiba S, Miguel CMD, Nuria C. Phytochemical composition and evaluation of the antioxidant activity of the ethanolic extract of Calendula suffruticosa subsp suffruticosa Vahl. Pharmacognosy Journal. 2018;10(1):64–70. [Google Scholar]
- 39.Hameed J, Hussain SA, Yang J, Ijaz MU, Liu Q, Suleria HAR, Song Y. Antioxidants potential of the filamentous fungi (Mucor circinelloides) Nutrients. 2017;9(1101):1–20. doi: 10.3390/nu9101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sudha G, Vadivukkarasi S, Shree RBI, Lakshmanan P. Antioxidant activity of various extracts from an edible mushroom Pleurotus eous. Food Sci Biotech. 2012;21(3):661–668. doi: 10.1007/s10068-012-0086-1. [DOI] [Google Scholar]
- 41.Benzie IFF, Devaki M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: concepts, procedures, limitations and applications. Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications. 1. West Sussex, UK: John Wiley & Sons Ltd.; 2017. [Google Scholar]
- 42.González-Palma I, Escalona-Buendía HB, Ponce-Alquicira E, Téllez-Téllez M, Gupta VK, Díaz-Godínez G, Soriano-Santos J. Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Front Microbiol. 2016;7(1099):1–9. doi: 10.3389/fmicb.2016.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed M, Hussain M, Dhar MK, Kaul S. Comparative analysis of phenolics, flavonoids, and antioxidant and antibacterial potential of methanolic, hexanic, and aqueous extracts from Adiantum caudatum leaves. Antioxidants. 2015;4:394–409. doi: 10.3390/antiox4020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zain SNDM, Omar WAW. Antioxidant activity, total phenolic content and total flavonoid content of water and methanol extracts of Phyllanthus species from Malaysia. Pharmacognosy Journal. 2018;10(4):677–681. doi: 10.5530/pj.2018.4.111. [DOI] [Google Scholar]
- 45.Al-Rubaye AF, Hameed IH, Kadhim MJ. A review: uses of gas chromatography-mass spectrometry (GC-MS) for analysis of bioactive natural compounds of some plants. Int J Toxicological and Pharmacological Res. 2017;9(1):81–85. doi: 10.25258/ijtpr.v9i01.9042. [DOI] [Google Scholar]
- 46.Pan F, Su TJ, Cai SM, Wu W. Fungal endophyte derived Fritillaria unibracteata var wabuensis diversity antioxidant capacities in vitro and relations to phenolic flavonoid or saponin compounds. Scientific Reports. 2017;7(42008):1–14. doi: 10.1038/srep42008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chowdary K, Kaushik N. Fungal endophyte diversity and bioactivity in the Indian medicinal plant Ocimum sanctum Linn. PLoS ONE. 2015;10(11):1–25. doi: 10.1371/journal.pone.0141444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Not applicable.